Abstract
Although the exact pathogenic processes involved in vitiligo are still unknown, its association with autoimmune disorders and endocrine dysfunction has been reported. One of its associations is with thyroid diseases. The purpose of this retrospective study was to determine the prevalence of thyroid function tests and thyroid autoantibody abnormalities in children diagnosed with vitiligo and compare the results with the literature. The laboratory documents of thyroid function tests (FT3, FT4, and TSH) and thyroid autoantibodies (TgAb and TPOAb) belonging to the pediatric vitiligo patients were studied retrospectively. Thyroid function tests and thyroid autoantibody abnormalities were detected in 20 (25.3%) of the pediatric vitiligo patients. Thirteen (16.4%) patients were evaluated as subclinical hypothyroidism, two (2.5%) were evaluated as hypothyroidism, and five (6.3%) were evaluated as euthyroidism. Thyroid autoantibodies were found to be positive in nine (11.3%) patients. Previously reported prevalence of thyroid disease in children with vitiligo ranged from 10.7 to 24.1%, and the prevalence of 25.3% determined in this study was compatible with the literature. Also, the high rate of subclinical hypothyroidism determined in these patients attracted attention to the probable development of overt hypothyroidism in a long term. Thus, our results suggest that thyroid function tests and thyroid autoantibodies should be analyzed in children with vitiligo.
Keywords: Autoimmunity, child, thyroid function tests, vitiligo
INTRODUCTION
Vitiligo is a generalized autoimmune disease manifesting acquired white patches due to loss of melanocytes. It is the most prevalent pigmentary disorder with an incidence rate between 0.1 and 2% showing multifactorial etiology and polygenic inheritance.[1]
Autoimmune disorders, genetic factors, autotoxic metabolites of melanin synthesis, accumulation of neurochemical substances, biochemical imbalance with defective free radical defense, deficiency in melanocyte growth factors, and an intrinsic defect of structure and function of melanocytes have been proposed to be involved in its pathogenesis.[2]
Vitiligo is frequently associated with various organ-specific autoimmune diseases, such as, Hashimato's thyroiditis, Addison's disease, diabetes mellitus type 1, and pernicious anemia.[3] Autoimmune thyroid diseases with a prevalence of up to 10.7% accompany vitiligo.[4] The aim of this study is to determine the prevalence of thyroid diseases in children with vitiligo and compare it with the literature.
MATERIALS AND METHODS
The study was conducted retrospectively in children with nonsegmental vitiligo, who applied to the Pediatric Dermatology Clinic between April 2008 and January 2010. Laboratory findings of the patients were analyzed for systemic and autoimmune diseases. The patients’ thyroid function tests and thyroid autoantibody results were enrolled without considering the extent of the vitiligo lesions. Free triiodothyronine (FT3) (Reference range: 2.53-5.22 g/ml), free thyroxine (ST4) (Reference range: 0.90-1.67 ng/dl) thyroid stimulating hormone (TSH) (Reference range: 0.66 - 4.14 μlU/ml), thyroglobulin antibody (TgAb) (positive titration >115 IU/ml), and thyroid peroxidase antibody (TPOAb) (positive titration >34 IU/ml) (Roche Diagnostics GmbH, Mannheim, Germany) levels were interpreted.
Descriptive statistics were used to evaluate the findings and the T test was used to compare the rates (SPSS version 16.0, Chicago, IL, USA).
RESULTS
A total of 79 pediatric patients with vitiligo whose ages ranged between two and fifteen were enrolled in the study. Twenty-nine (36.7%) patients were male and 50 (63.3%) patients were female (female:male = 1.7:1). The mean age of the patients was found to be 8.19 ± 3.45 years. Nineteen (24.0%) patients were between the ages of two and five, (45.5%) patients were between the ages of six and ten, and 24 (30.3%) patients were between the ages of 11 and 15 [Table 1].
Table 1.
Age distribution of pediatric patients with vitiligo
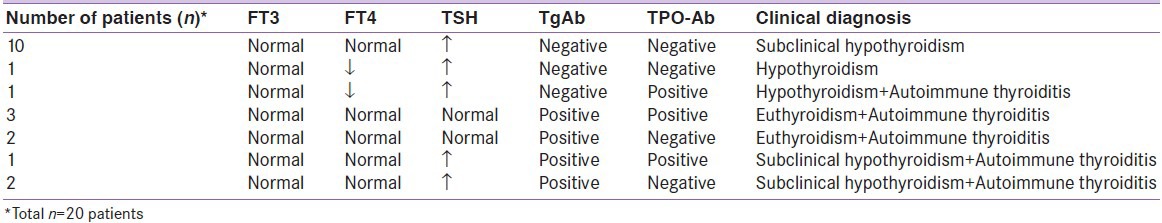
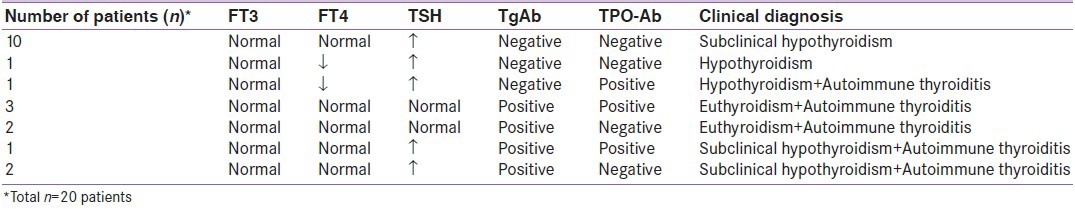
Abnormalities of thyroid function tests and thyroid autoantibodies were found in a total of 20 (25.3%) patients; eight males (40.0%) and 12 (60.0%) females. The abnormality of thyroid autoantibodies was found in a total of nine (11.3%) patients; three (33.3%) males and six (66.7%) females. TgAb positivity was present in eight (10.1%) patients and TPOAb positivity was present in five (6.3%) patients. The positivity of these two antibodies together was present in a total of four (5.1%) patients.
Of the 20 patients in whom thyroid function tests and/or thyroid antibodies were abnormal, ten (12.6%) had a high TSH level and normal FT3 and FT4 levels and were evaluated as subclinical hypothyroidism. One (1.2%) patient had both low TSH and FT4 levels and was evaluated as hypothyroidism. One (1.2%) patient had low FT4 and high TSH levels with TPOAb positivity and was evaluated as hypothyroidism and autoimmune thyroiditis. Five (6.3%) patients had only autoantibody positivity (three patients with both TgAb and TPOAb positivity and two patients with only TgAb positivity) and were evaluated as euthyroidism and autoimmune thyroiditis. Three (3.7%) patients had high TSH levels with autoantibody positivity (one patient with both TgAb and TPOAb positivity and two patients with only TgAb positivity) and were evaluated as subclinical hypothyroidism and autoimmune thyroiditis.
Consequently, subclinical hypothyroidism was the most frequent diagnosis established in 13 (16.4%) of the 79 pediatric patients with vitiligo [Table 2].
Table 2.
Laboratory findings and clinical diagnoses of pediatric vitiligo patients in whom abnormal thyroid function tests and/or thyroid antibodies were detected
Statistically, there was no significant difference between male and female patients when they were compared for thyroid function tests and thyroid antibody abnormalities (P = 0.728). Also, there was no significant difference between male and female patients when they were statistically compared for autoimmune thyroiditis (P = 0.826).
DISCUSSION
Vitiligo is an acquired depigmentary disorder affecting around 1% of the world's population. It is more predominant in younger ages and approximately 50% of the cases have the onset of their disease prior to the age of 14 years.[5,6,7]
Vitiligo affects both the sexes equally, but women more frequently visit the doctor due to cosmetic reasons. In this study of children with vitiligo a female preponderance (female:male = 1.7:1) was present. Vitiligo has been reported to start between 8 and 12 years of age in 51% of the children.[8] This study showed that 45.5% of the children with vitiligo were between 6 and 10 years of age.
Autoimmunity is one hypothesis forwarded to explain nonsegmental vitiligo. A specific cytotoxic T-cell reaction, either of primary origin or resulting from damage to melanocytes by other mechanisms has been suggested to be responsible for destroying the pigment cells. A genetic predisposition to immune dysregulation can lead to aberrant T-cell reactivities that destroy melanocytes. Alternatively, autoreactive antimelanocyte T-cells can arise in response to a challenge to the immune system.[9] Involvement of the immune system in the pathogenesis is evidenced by the effectiveness of immunomodulatory agents, such as, corticosteroids and calcineurin inhibitors.[10,11] On the other hand, segmental vitiligo is explained by the neural theory, which proposes that some chemical released from the peripheral nerve endings causes a decreased production of melanin.[12]
Vitiligo is epidemiologically associated with other autoimmune diseases suggesting that predisposition to other autoimmune diseases involves a shared genetic component.[1,13,14] Particularly, an increased frequency of clinical as well as subclinical thyroid disease appears to be increased in frequency in patients with vitiligo.[15] Increased prevalence of autoimmune disorders in association with vitiligo and detection of various autoantibodies, including anti-thyroid and anti-melanocyte antibodies, in the serum of vitiligo patients and alteration of T-cell population showing decreased T-helper cells, are in favor of this autoimmune theory.[16] Vitiligo frequently precedes the thyroid involvement; thus screening vitiligo patients for thyroid function and thyroid antibody seems plausible.[17]
Autoimmune thyroid diseases accompany vitiligo, from which hypothyroidism is one of the most common disorder. In pediatric patients with vitiligo a significant incidence of thyroid dysfunction has been found. Iacovelli et al., reported a figure of 10.7% in children with nonsegmental vitiligo, especially in females, all of whom had thyroid dysfunction.[4] Hashimato's thyroiditis was reported to be the most common autoimmune disease seen in patients with vitiligo.[18]
In a study of children and adolescents with vitiligo, Hashimato's thyroiditis was found to be two-and-a-half times and hypothyroidism ten times more frequent than in a healthy age-and-sex-matched population and 24.1% of these 54 patients with vitiligo had autoimmune thyroiditis as compared to 9.6% of school-aged children from an iodine-replete area of Greece.[19] Yang et al., reported that 11.8% of 363 pediatric vitiligo patients had abnormal levels of thyroid parameters.[20] The thyroid abnormality prevalence of 25.3% determined in our study is compatible with the literature attracting attention to the association of thyroid disease and vitiligo in childhood. Also, of the 20 patients in whom a thyroid abnormality was determined, 13 (16.4%) had subclinical hypothyroidism and nine (11.3%) had thyroid autoantibody positivity.
Dave et al., showed that the frequency of thyroid disorders were 57.1% in adult patients with vitiligo in comparison to 10% in people without vitiligo. Thirty-four percent of the patients in their study had thyroid antibodies.[21] Manighalam et al., conducted a study on 30 patients with vitiligo, and they found hyperthyroidism and hypothyroidism in 10 and 6.6%, respectively.[22] Another study reported that an antithyroid antibody was detected in 18.1% of the patients with vitiligo in comparison to 7.3% in the control group, with more prevalence in females.[23]
Subclinical hypothyroidism is characterized by an elevated serum TSH level associated with a normal total or free T4 and T3 values.[24] Subclinical hypothyroidism is a laboratory diagnosis with no significant findings or symptoms associated with thyroid dysfunction in patients.[25] Long-term follow up has demonstrated the development of overt hypothyroidism at a rate of 5-20% per year, especially in autoimmune thyroiditis.[26] When we consider that the prevalence of subclinical hypothyroidism is <2% in the pediatric age group,[27] the rate found in our study was higher.
This retrospective study of prevalence attracts attention to the association of vitiligo with thyroid abnormalities. As the risk of developing overt hypothyroidism is high in patients with subclinical hypothyroidism, we suggest that thyroid function tests and thyroid autoantibodies be analyzed in children with vitiligo.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Alkhateeb A, Fain PR, Thody A, Bennett DC, Spritz RA. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208–14. doi: 10.1034/j.1600-0749.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- 2.Ongenae K, Van Geel N, Naeyaert JM. Evidence for an autoimmune pathogenesis of vitiligo. Pigment Cell Res. 2003;16:90–100. doi: 10.1034/j.1600-0749.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- 3.Njoo MD, Westerhof W. Vitiligo. Pathogenesis and treatment. Am J Clin Dermatol. 2001;2:167–81. doi: 10.2165/00128071-200102030-00006. [DOI] [PubMed] [Google Scholar]
- 4.Iacovelli P, Sinagra JL, Vidolin AP, Marenda S, Capitanio B, Leone G, et al. Relevance of thyroiditis and of other autoimmune diseases in children with vitiligo. Dermatology. 2005;210:26–30. doi: 10.1159/000081479. [DOI] [PubMed] [Google Scholar]
- 5.Kovacs SO. Vitiligo. J Am Acad Dermatol. 1998;38:647–66. doi: 10.1016/s0190-9622(98)70194-x. [DOI] [PubMed] [Google Scholar]
- 6.Arican O, Koç K, Ersoy L. Clinical characteristics in 113 Turkish vitiligo patients. Acta Dermatovenerol Alp Panonica Adriat. 2008;17:129–32. [PubMed] [Google Scholar]
- 7.Schwartz RA, Janniger CK. Vitiligo. Cutis. 1997;60:239–44. [PubMed] [Google Scholar]
- 8.Al-Mutairi N, Sharma AK, Al-Sheltwy M, Nour-Eldin O. Childhood vitiligo: A prospective hospital-based study. Australas J Dermatol. 2005;46:150–3. doi: 10.1111/j.1440-0960.2005.00167.x. [DOI] [PubMed] [Google Scholar]
- 9.Rezaei N, Gavalas NG, Weetman AP, Kemp EH. Autoimmunity as an aetiological factor in vitiligo. J Eur Acad Dermatol Venereol. 2007;21:865–76. doi: 10.1111/j.1468-3083.2007.02228.x. [DOI] [PubMed] [Google Scholar]
- 10.Njoo MD, Spuls PI, Bos JD, Westerhof W, Bossuyt PM. Nonsurgical repigmentation therapies in vitiligo: Meta-analysis of the literature. Arch Dermatol. 1998;134:1532–40. doi: 10.1001/archderm.134.12.1532. [DOI] [PubMed] [Google Scholar]
- 11.Lepe V, Moncada B, Castanedo-Cazares JP, Torres-Alvarez MB, Ortiz CA, Torres-Rubalcava AB. A double-blind randomized tiral of 0.1% tacrolimus vs 0.05% clobetasol for the treatment of childhood vitiligo. Arch Dermatol. 2003;139:581–5. doi: 10.1001/archderm.139.5.581. [DOI] [PubMed] [Google Scholar]
- 12.Grimes PE. White patches and bruised souls: Advances in the pathogenesis and treatment of vitiligo. J Am Acad Dermatol. 2004;51:S5–7. doi: 10.1016/j.jaad.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Laberge G, Mailloux CM, Gowan K, Holland P, Bennett DC, Fain PR, et al. Early disease onset and increased risk of other autoimmune diseases in familial generalized vitiligo. Pigment Cell Res. 2005;18:300–5. doi: 10.1111/j.1600-0749.2005.00242.x. [DOI] [PubMed] [Google Scholar]
- 14.Birlea SA, Fain PR, Spritz RA. A Romanian population isolate with high frequency of vitiligo and associated autoimmune diseases. Arch Dermatol. 2008;144:310–6. doi: 10.1001/archderm.144.3.310. [DOI] [PubMed] [Google Scholar]
- 15.Hegedüs L, Heidenheim M, Gervil M, Hjalgrim H, Høier-Madsen M. High frequency of thyroid dysfunction in patients with vitiligo. Acta Derm Venereol. 1994;74:120–3. doi: 10.2340/0001555574120123. [DOI] [PubMed] [Google Scholar]
- 16.Bleehen SS, Anstey AV. Disorders of skin color. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. Oxford, UK: Blackwell Science; 2004. pp. 1–70. [Google Scholar]
- 17.Shong YK, Kim JA. Vitiligo in autoimmune thyroid disease. Thyroidology. 1991;3:89–91. [PubMed] [Google Scholar]
- 18.Kakourou T. Vitiligo in children. World J Pediatr. 2009;5:265–8. doi: 10.1007/s12519-009-0050-1. [DOI] [PubMed] [Google Scholar]
- 19.Kakourou T, Kanaka-Gantenbein C, Papadopoulou A, Kaloumenou E, Chrousos GP. Increased prevalence of chronic autoimmune (Hashimato's) thyroiditis in children and adolescents with vitiligo. J Am Acad Dermatol. 2005;53:20–3. doi: 10.1016/j.jaad.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, Lin X, Fu W, Luo X, Kang K. An approach to the correlation between vitiligo and autoimmune thyroiditis in Chinese children. Clin Exp Dermatol. 2009;35:706–10. doi: 10.1111/j.1365-2230.2009.03671.x. [DOI] [PubMed] [Google Scholar]
- 21.Dave S, D’Souza M, Thappa DM, Reddy KS, Bobby ZB. High frequency of thyroid dysfunction in Indian patients with vitiligo. Indian J Dermatol. 2003;48:68–72. [Google Scholar]
- 22.Manighalam SH, Hajeabdolhamid M, Tosi P, Amirjavanbakht A, Sadat N. Association between vitiligo and thyroid dysfunction. Iran J Endocrinol Metab. 2002;4:165–8. [Google Scholar]
- 23.Daneshpazhooh M, Mostofizadeh GM, Behjati J, Akhyani M, Robati RM. Anti-thyroid peroxidase antibody and vitiligo: A controlled study. BMC Dermatol. 2006;6:3. doi: 10.1186/1471-5945-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDermott MT, Ridgway EC. Subclinical hypothyroidism is mild thyroid failure and should be treated. J Clin Endocrinol Metab. 2001;86:4585–90. doi: 10.1210/jcem.86.10.7959. [DOI] [PubMed] [Google Scholar]
- 25.Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, et al. Subclinical thyroid disease: Scientific review and guidelines for diagnosis and management. JAMA. 2004;291:228–38. doi: 10.1001/jama.291.2.228. [DOI] [PubMed] [Google Scholar]
- 26.Zadik Z. Overuse or misuse of thyroid function tests in pediatrics. J Pediatr Endocrinol Metab. 2009;22:875–6. doi: 10.1515/jpem.2009.22.10.875. [DOI] [PubMed] [Google Scholar]
- 27.Seshadri KG. Subclinical hypothyroidism in children. Indian J Endocrinol Metab. 2012;16:S156–8. doi: 10.4103/2230-8210.104028. [DOI] [PMC free article] [PubMed] [Google Scholar]


