Abstract
Background:
High prevalence of vitamin D deficiency (VDD) has been reported throughout the India for all age groups. Increased awareness about VDD among treating physicians has led to increased prescriptions of vitamin D preparations. Based on our experience of varied clinical and radiological response with different vitamin D formulations, we decided to assess cholecalciferol content of commonly available vitamin D formulations.
Materials and Methods:
We measured cholecalciferol content of 14 commercial preparations (two in the form of tablets and 12 as sachet) available in Indian market. Lab analysis was carried out in Shriram Institute for Industrial Research by high-performance liquid chromatography.
Results:
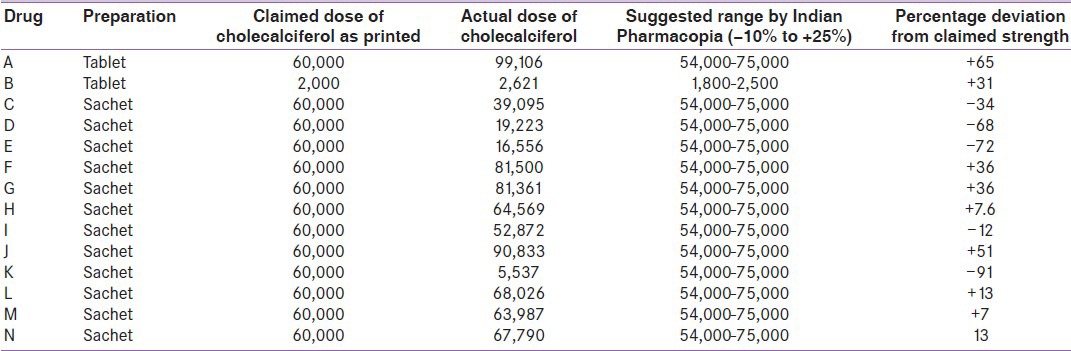
Of the total 14 samples analyzed only 4 (28.57%) were found to be within the acceptable ranges from −90 to +125% as defined by Indian Pharmacopia while 5 (35.7%) had higher and 5 (35.7%) had lower than the acceptable range. The percentage variation in cholecalciferol content as observed from the printed ranged widely from −91% to +65%.
Conclusions:
Our study shows a high degree of variability in cholecalciferol content of commercial preparations available in the Indian pharmaceutical market. This variation has many clinical implications as it may lead both, under treatment as well as vitamin D toxicity.
Keywords: Cholecalciferol, commercial preparations
INTRODUCTION
Vitamin D has received world-wide attention not only for bone health in children and adults, but also for reducing risk for many chronic diseases including autoimmune disorders, diabetes mellitus, heart diseases, cancers and infectious diseases.[1] Recent literature from across the world has documented high prevalence of vitamin D deficiency (VDD) as determined by low circulating levels of serum 25 hydroxy vitamin D (S.25(OH) D) across all age groups and both sexes.[2,3] Holick has gone to the extent of describing it as a pandemic.[4]
High prevalence of VDD has been reported throughout the India for all age groups including neonates, infants and school going children, adolescents, adults, pregnancy, lactating women and senior citizens. This is probably a result of poor sun exposure, dark skin complexion, atmospheric pollution, vegetarian foods habits, absence of food fortification with vitamin D and poor intake of vitamin D supplements.[5]
Increased awareness about VDD in treating physicians has led to increased prescriptions of vitamin D. This increased demand of vitamin D preparations forced pharmaceutical companies to market many oral vitamin D preparations. Most commercial formulations are in the form of sachet, tablets, softgel capsule, syrup and drops, etc.
In view of several reports of hypercalcemia and vitamin D intoxication due to manufacturing and labeling errors in “over the counter” vitamin D supplements[6,7,8,9,10] and based on our varied clinical experience with different formulations of vitamin D containing cholecalciferol, we decided to evaluate the contents of cholecalciferol in order to make sure whether the cholecalciferol contents of different formulations are commensurate with the printed strengths on the formulation.
MATERIALS AND METHODS
The present study, first searched for all vitamin D preparations containing only cholecalciferol available in India, through internet using key words “vitamin D preparation, cholecalciferol, vitamin D3 formulations in India“ and websites, namely “www.drugsupdate.com” and www.cimsasia.com/India. Subsequently we contacted all 35 pharmaceutical shops in the vicinity of the institute and prepared a list of commercial preparations containing cholecalciferol. Preparations containing any other ingredient apart from cholecalciferol were excluded from the study including calcium. Similarly, all preparations containing any other form of vitamin D apart from cholecalciferol were also excluded. We obtained 14 commonly used preparations from these pharmaceutical shops which had a turnover of at least 100 units/month and expiry period of at least 6 months from the date of purchase. Three units of each preparation from the same batch of manufacturing were purchased and put into a dark envelop to conceal the identity. In order to blind the sample from the lab, we labeled each preparation with numbers. For instance – preparation no. 1 were labeled as 1.1, 1.2 and 1.3. The lab in-charge was requested to randomly draw any one from three envelops for each preparation for analysis.
Lab analysis
Lab analysis was carried out in Shriram Institute for Industrial Research, an independent, not-for-profit, self-supporting research organization (NABL accredited, ISO 9001, BIS recognized, accredited for residue monitoring and DGMS approved; www.shriraminstitute.org). Staff involved in the analysis of samples had no information about this project and was provided samples on the day of analysis.
Sample preparation
Weighed amount of sample of vitamin D3 was taken in a 250 ml iodine flask and 20 ml of dimethyl sulphoxide was added and kept at 40°C for 1 h. Extraction was carried out with 25 ml n-hexane (repeated thrice). Anhydrous sodium sulfate was used to remove water and the extract was transferred into rotary evaporator flask. Hexane layer was evaporated and volume was made to 10 ml with mobile phase.
Standard preparation
100 IU dilution of standard vitamin D3 was used in the mobile phase.
High-performance liquid chromatography operating conditions
Agilent HPLC Model 1200 (Agilent Technologies Inc., Santa Clara, California) was used with mobile phase of N-hexane isopropyl alcohol (99:1) and ultraviolet detector at a wavelength of 254 nm. HPLC columns of silica (Phenomenex 250 mm × 4.6 mm, 5 μ) was used at a flow rate of 1 ml/min and injected volume of 20 μl at 35°C.
Analysis
A total of 20 μl of standard solution was injected first and the chromatograms for standard acquired and the area response of peak due to vitamin D peak from the standard chromatograms was obtained. The response factor (R.F.) for vitamin D standard was calculated as per following equation:

A total 20 μL of sample solution was injected and the chromatogram was recorded and the area response of peak due to vitamin D peak from the sample chromatograms was obtained. The concentration of vitamin D in sample was calculated with the following equation:

Cholecalciferol content range
As per suggestions of Indian Pharmacopeia, we used 90-125% as acceptable range for cholecalciferol content in drug formulations in the Indian market.[11]
RESULTS
Cholecalciferol preparations available in India
A total of 46 cholecalciferol preparations of varied strengths were available at the time of initiation of the study. Of these 46 brands, 54 formulations were available as sachet (23), soft gelatin capsule or tablets (16), injectables (11), syrup/liquid (2) and drops (1). The formulation that contained highest cholecalciferol content (600,000 IU/ml) was in the form of injection.
Cholecalciferol content analysis
We analyzed a total of 14 preparations, which included 12 sachets and two in tablet forms. Of these 14 samples, only 4 (28.57%) were found to be within the acceptable range (–90 to +125%) as defined by Indian Pharmacopia while 5 (35.7%) had higher and 5 (35.7%) had lower than the acceptable range [Table 1]. The percentage variation as observed from the printed ranged widely from −91% to +65%.
Table 1.
Cholecalciferol contents of different preparations available in India
DISCUSSION
The present study showed a very high variability in cholecalciferol content between the printed strength and actual level in the preparations as measured by HPLC. Only 28.5% of the formulations tested were within the acceptable range.
Such variability in cholecalciferol content has also been reported by several other investigators from other parts of the world. Recently, Garg et al.[12] subjected to analysis 12 cholecalciferol formulations available in New Zealand market and reported that 50% of these formulations were found to be out of the acceptable range. A percentage variation in the ranges from 8% ± 2% to 201 ± 29% was observed in the printed strength and the actual strength of the formulation as against the accepted norms of 100 ± 10%. Of the six formulations not within the acceptable range, three were on the lower side (29 ± 11%, 8 ± 2%, 21 ± 8%) and the remaining three were on the higher side (201 ± 29%, 156 ± 6%, and 133 ± 9%). It is important to highlight the fact that none of these six formulations were registered as medicine. The two preparations, which have been registered as medicine were within the acceptable range.
In another recently published paper, Leblanc et al.[13] reported a variation of 52-105% in compounded 50,000 IU cholecalciferol tablets and 23-146% in 1000 IU compounded tablets. Only one-third of pills were within 10% of the expected strength as recommended by US Pharmacopeial (USP) convention standard for compounded pills. Furthermore, when one pill was sampled from each of five bottles of the same lot, the potency variation ranged from 57% to 138% of the stated amount. Similar large variation (9-140%) in the potency of the pills was also observed when pills were analyzed from five bottles with different lot numbers. The USP convention standards for over-the counter (OTC) cholecalciferol preparation states that the content of the preparation when analyzed should be within 90-120% of the stated dose; however, the authors reported a percentage variation of 52-135% of stated dose OTC preparations.
The present study differed from the above quoted two studies in the fact that all formulations, so analyzed were registered as “drug” and used for therapeutic purposes. However, using stricter USP convention standards of 90-110%,[14] only 2 out of 14 preparations tested in our study were within the acceptable range.
Similarly, high degree of viability in vitamin D fortified food products has also been reported. Holick et al.[15] found that only 29% (12/42 samples) samples of the 13 brands of vitamin D fortified milk were within the acceptable ranges from 80% to 120% for fortified food. No vitamin D was detected at all in 3 of the 14 samples of skimmed milk tested while seven of the 10 samples of infant formula contained more than 200% of the amount stated on the label; the sample with the highest concentration contained 419% of the stated amount. Another study from United Arab Emirates reported that only 39% of vitamin D fortified milk and milk products were within the acceptable range with 31% were under-fortified and 30% over-fortified.[16]
There are many clinical implications of our study. High degree of variability in cholecalciferol content may result in variability in clinical response to treatment. Use of cholecalciferol preparations with less content (like preparations with s. no. 4, 5 and 11) will not result in an increase in S.25(OH) D level and clinical improvement in patients with VDD. However, treating physician will think that he/she has used adequate doses of cholecalciferol for treatment of VDD, but the subject is still VDD. On the other hand, use of preparations such as 1, 6, 7 and 10 may result in hypercalcemia and vitamin D toxicity,
Hypervitaminosis D or vitamin D intoxication have been reported with vitamin D supplements.[6,7]
Recently, hypercalcemia and/or hypercalciuria have also been reported in various studies following pharmacological as well as a maintenance dose of oral vitamin D.[17,18] Several reports of life threatening complications of vitamin D toxicity with severe hypercalcemia and acute renal failure because of errors in the manufacturing and labeling of dietary supplements have recently emerged in the literature.[8,9,10]
In view of the above reports and cholecalciferol contents of most available commercial preparations in India, not being within the prescribed acceptable range as defined by Indian pharmacopeia, there is a need for quality control and sound manufacturing practices to be adhered to by the companies and stricter regulation by the Government for ensuring acceptable quality and safety of these preparations.
This being the first pilot study, it has several limitations. The important ones are not able to analyze samples in triplicate and evaluate injectable preparations because of financial constraints.
In view of the above, there is an urgent need to undertake a well-planned large sample size study to confirm results of this pilot project.
Footnotes
Source of Support: Nil
Conflict of Interest: No
REFERENCES
- 1.Holick MF. Evidence-based D-bate on health benefits of vitamin D revisited. Dermatoendocrinol. 2012;4:183–90. doi: 10.4161/derm.20015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab. 2012;97:1153–8. doi: 10.1210/jc.2011-2601. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF. Nutrition: D-iabetes and D-eath D-efying vitamin D. Nat Rev Endocrinol. 2012;8:388–90. doi: 10.1038/nrendo.2012.84. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF. The vitamin D deficiency pandemic and consequences for nonskeletal health: Mechanisms of action. Mol Aspects Med. 2008;29:361–8. doi: 10.1016/j.mam.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marwaha RK, Goswami R. Vitamin D deficiency and its health consequences in India. In: Holick MF, editor. Vitamin D: Physiology, Molecular Biology, and Clinical Applications. 2nd ed. New York: Humana Press; 2010. pp. 529–42. [Google Scholar]
- 6.Vanstone MB, Oberfield SE, Shader L, Ardeshirpour L, Carpenter TO. Hypercalcemia in children receiving pharmacologic doses of vitamin D. Pediatrics. 2012;129:e1060–3. doi: 10.1542/peds.2011-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowe H, Cusano NE, Binkley N, Blaner WS, Bilezikian JP. Vitamin D toxicity due to a commonly available “over the counter” remedy from the Dominican Republic. J Clin Endocrinol Metab. 2011;96:291–5. doi: 10.1210/jc.2010-1999. [DOI] [PubMed] [Google Scholar]
- 8.Kaptein S, Risselada AJ, Boerma EC, Egbers PH, Nieboer P. Life-threatening complications of vitamin D intoxication due to over-the-counter supplements. Clin Toxicol (Phila) 2010;48:460–2. doi: 10.3109/15563650.2010.486382. [DOI] [PubMed] [Google Scholar]
- 9.Granado-Lorencio F, Rubio E, Blanco-Navarro I, Pérez-Sacristán B, Rodríguez-Pena R, García López FJ. Hypercalcemia, hypervitaminosis A and 3-epi-25-OH-D3 levels after consumption of an “over the counter” vitamin D remedy. a case report. Food Chem Toxicol. 2012;50:2106–8. doi: 10.1016/j.fct.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Araki T, Holick MF, Alfonso BD, Charlap E, Romero CM, Rizk D, et al. Vitamin D intoxication with severe hypercalcemia due to manufacturing and labeling errors of two dietary supplements made in the United States. J Clin Endocrinol Metab. 2011;96:3603–8. doi: 10.1210/jc.2011-1443. [DOI] [PubMed] [Google Scholar]
- 11.Indian Pharmacopoeia. 5th ed. 2007. [Last accessed on 2013 Mar 25]. Available from: http://www.ipc.nic.in/index2.asp?slid=55&sublinkid=33&lang=1&EncHid .
- 12.Garg S, Sabri D, Kanji J, Rakkar PS, Lee Y, Naidoo N, et al. Evaluation of vitamin D medicines and dietary supplements and the physicochemical analysis of selected formulations. J Nutr Health Aging. 2013;17:158–61. doi: 10.1007/s12603-012-0090-4. [DOI] [PubMed] [Google Scholar]
- 13.Leblanc ES, Perrin N, Johnson JD, Ballatore A, Hillier T. Over-the-counter and compounded vitamin D: Is Potency what we expect? JAMA Intern Med. 2013;11:1–2. doi: 10.1001/jamainternmed.2013.3812. [DOI] [PubMed] [Google Scholar]
- 14.US Pharmacopeia. Pharmaceutical compounding – Nonsterile preparations. 2012. [Last accessed on 2012 Oct 15]. Available from: http://www.pharmacopeia.cn/v29240/usp29nf24s0_c795.html .
- 15.Holick MF, Shao Q, Liu WW, Chen TC. The vitamin D content of fortified milk and infant formula. N Engl J Med. 1992;326:1178–81. doi: 10.1056/NEJM199204303261802. [DOI] [PubMed] [Google Scholar]
- 16.Laleye LC, Wasesa AA, Rao MV. A study on vitamin D and vitamin A in milk and edible oils available in the United Arab Emirates. Int J Food Sci Nutr. 2009;60(Suppl 5):1–9. doi: 10.1080/09637480802054094. [DOI] [PubMed] [Google Scholar]
- 17.Gallagher JC, Sai A, Templin T, 2nd, Smith L. Dose response to vitamin D supplementation in postmenopausal women: A randomized trial. Ann Intern Med. 2012;156:425–37. doi: 10.7326/0003-4819-156-6-201203200-00005. [DOI] [PubMed] [Google Scholar]
- 18.Gallagher JC, Peacock M, Yalamanchili V, Smith LM. Effects of vitamin D supplementation in older African American women. J Clin Endocrinol Metab. 2013;98:1137–46. doi: 10.1210/jc.2012-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]


