Abstract
We are hereby reporting a case of a 72-year-old Indian man, who, in the absence of a detectable tumor, presented with symptomatic hypoglycemia in the postabsorptive state (3-5 h after meal). His serum levels of insulin and C-peptide were very high. He was not taking any hypoglycemic drug. Hypoglycemic episodes completely subsided after withdrawal of pentoprazole and incorporation of small frequent meals in the dietary plan. Six months after the initial presentation, the subject became free of hypoglycemic episodes. Although insulin autoimmune syndrome (IAS) is the third leading cause of spontaneous hypoglycemia in Japan, it is extremely uncommon in the Western Countries. Till 2009, more than 200 cases from Japan and as many as 58 cases outside Asia have been reported. To the best of our knowledge, this is the first case of IAS reported from India.
Keywords: Autoimmune hypoglycemia, Hirata's disease, insulin autoimmune syndrome
INTRODUCTION
Common causes of hypoglycemia in nondiabetic patients are surreptitious use of drugs, insulinoma, extrapancreatic tumors and autoimmune hypoglycemia. Autoimmunity causes hypoglycemia in two ways: (a) insulin-receptor antibodies having insulinomimetic action and (b) insulin autoimmune syndrome (IAS), in which antibodies first bind insulin, forming a complex, and subsequently, regardless of the glucose levels, release it, causing hypoglycemia.[1] IAS has shown strong association with the use of medications containing sulfhydryl group, autoimmune disorders, plasma cell dyscrasias and HLA DR4.[2]
CASE REPORT
A 72-year-old man of Indian origin had first episode of hypoglycemia 2 months earlier when, 4-5 h after taking lunch, he was found to be disoriented. He was taken to the nearby hospital where level of his plasma blood glucose was found to be 20 mg/dL and intravenous 10% dextrose infusion reversed his symptoms. During hospital stay, he continued to have hypoglycemic episodes in the postabsorptive state. He was not a known diabetic and was not taking any drug known to cause hypoglycemia. There was no family history of diabetes. Result of routine investigations, including hemogram and renal, liver and thyroid function tests, were not significant. During one such episode, plasma blood glucose level was 15 mg/dL and the corresponding serum insulin was 123 mU/L. With provisional diagnosis of insulinoma, imaging studies, including ultrasonography, dual phase computed tomography (CT) and magnetic resonance (MR) imaging of abdomen, MR and CT angiography of the abdomen and contrast-enhanced CT of chest, were done sequentially. All these imaging modalities failed to detect any lesion. At this stage, the patient was referred to us. Review of past history revealed that the patient had ischemic cerebral infarct 8 years back when he was detected to have hypertension. One year back, he suffered from coronary ischemia. The patient never had gastrointestinal surgery. At the time of presentation, he was taking drugs – Pantoprazol, Aspirin, Clopidogrel, Torsemide, Isosorbide dinitrate, Atenolol, Atorvastatin and Amlodipine. General and systemic physical examination did not reveal any abnormality. During his stay in the Endocrine Ward, the patient continued to have hypoglycemia in the postabsorptive state. During one such episode, the plasma blood glucose level was 29 mg/dL, serum insulin was 3162 mU/L and serum C-peptide was 19.05 ng/mL (latter two measured by electrochemiluminescence immunoassay, using a Roche Elecsys 2010 immunoassay analyzer, GmBH, Mannheim, Germany], while serum cortisol and growth hormone responses were adequate. Serum proinsulin level was 420 pmol/L (normal fasting value < 20 pmol/L, measured by radioimmunoassay; Millipore Corporation, Merck Millipore Headquarters, 290 Concord Road, Billerica, MA 01821 USA). Trans-esophageal ultrasonography also failed to locate any lesion. Extended oral glucose tolerance test (OGTT) with 75 g of glucose showed paradoxical hyperglycemia followed by hypoglycemia with very high insulin levels [Figure 1]. These findings were highly suggestive of IAS. Other immunological findings of the patient showed elevated rheumatoid factor titers (>256, normal 0-20) and antinuclear antibody titers on Hep-2 substrate (1:80, normal < 1:20). The serum immunoglobulin G (IgG) level was 2416 mg/dL (normal 960-1968 mg/dL), IgM was 473 mg/dL (normal 125-380 mg/dL) and IgA was 146 mg/dL (normal 90-242 mg/dL), as measured by enzyme-linked immunosorbent assay. Samples for human leukocyte antigen (HLA) studies were collected, which showed HLA-A*02, A * 11, B * 35, B * 51, DRB1 * 0403 and DRB1 * 0404.
Figure 1.
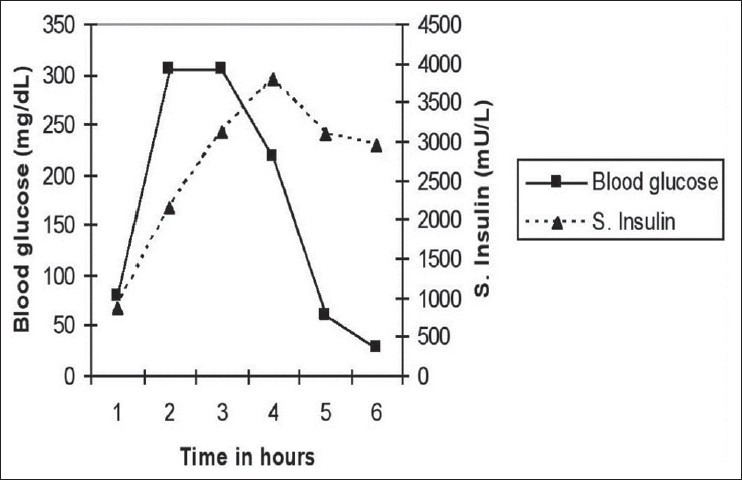
Extended oral glucose tolerance test at the time of initial evaluation
Review of patient's drug intake revealed that he was taking three drugs, viz. Pentoprazol, Clopidogril and Torsemide, which contained sulfur and hydrogen atoms. Under the plan to stop usage of these drugs one by one, to begin with, Pentoprazole was discontinued and the patient was advised to take meals in small quantities frequently. As a follow-up thereof, the patient was observed keenly during the next week for any hypoglycemic symptoms, which were noticed to have subsided gradually. Eventually, the patient was discharged with the said advice. Review after 3 months, with extended OGTT, showed quantum decrease in serum insulin levels along with disappearance of the hyperglycemic phase, which feature was subsisting earlier. In the changed scenario, maximum blood glucose level reached was 183 mg/dL at 1 h [Figure 2]. Subsequent follow-up during the next 6 months revealed that the patient did not have any symptoms of hypoglycemia and was healthy.
Figure 2.
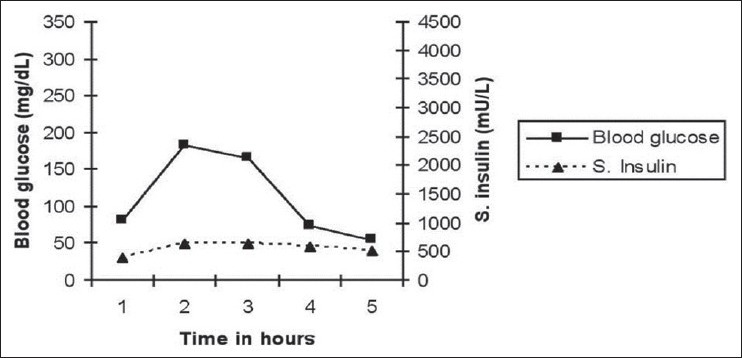
Extended oral glucose tolerance test 3 months after initial evaluation
DISCUSSION
Clinically, IAS presents by way of symptoms of hypoglycemia, which can surface either in the postprandial or in the postabsorptive phases. This disease occurs without any gender predilection. Its onset is, however, quite often dramatic. Paradoxically, hyperglycemia can occur following a meal or glucose challenge.[3] In such patients, total serum insulin levels are very high in comparison with those seen in the patients of insulinoma, in as much as insulin levels are just inappropriate and rarely more than 100 mU/L in the latter category.[3] Besides, serum-free insulin level may be normal or high with incompletely suppressed plasma C-peptide level. In such cases, serum proinsulin levels have also been demonstrated to be very high.[1] Insulin antibodies are typically polyclonal, although monoclonal antibodies have also been described.[4] IAS has been observed to be associated with the usage of drugs like insulin, sulfonylureas; SH-containing medicines (methimazole, captopril, penicillamine etc.), hydralazine, procainamide, isoniazid and α-interferone etc., in association with autoimmune disorders, plasma cell dyscrasias, alcoholic liver disease and organ transplant patients.[4] The disease is self-limiting in most of the cases. Most of the patients respond to small frequent meals – six or more times – in a day. Alfa-glucosidase inhibitors may sometimes be helpful in decreasing the postprandial levels of immunoreactive insulin in the sera and resultantly, reducing postprandial hypoglycemic episodes. Such patients who did not respond to the said treatment were treated with prednisolone, diazoxide, octreotide and plasmapheresis, with variable results.[2] In the backdrop of the foregoing analytic review of the facts described herein above, it may be possible that subsidence of hypoglycemia could have been part of the natural course of the disease, which is corroborated by the fact that there was spontaneous remission in 189 of 226 (83.6%) Japanese patients of IAS.[2,3]
There is a strong genetic predisposition as revealed by HLA association studies; 96% (48 out of 50) of Japanese patients were DR4 (odds ratio 39.9; P < 10−4), while 12 (24%) were DR9 positive. All 48 DR4-positive Japanese IAS patients possessed DQA1 * 0301/DQB1 * 0302 regardless of differences in DR4 alleles. Two Korean and the one Chinese IAS responder patient were positive for DRB1 * 0406/DQA1 * 0301/DQB1 * 0302.[2] These alleles are 10-30 times more prevalent in the Japanese and in the Koreans in comparison with the Caucasians. This distribution tends to explain the higher prevalence of the aforesaid disorder in the Japanese population. The present case was also found to be HLA-DR4 homozygous, and the resolution analysis revealed it to be DRB1 * 0403/*0404.
The exact mechanism of hypoglycemia in IAS has not been found, but it is postulated that sulphydryl group interacts with disulfide bond in the insulin molecule, making the latter more immunogenic.[5] Most of the cases reported from Japan are following the use of sulphhydryl group-containing drugs, while cases reported from other parts of the world are more frequently associated with autoimmune diseases or plasma cell dyscrasias.[1] Our patient was taking drugs, and both rheumatoid factor and antinuclear antibodies were positive. No test could be performed to demonstrate the presence of specific antibody in our case; nonetheless, based on cumulative clinical and biochemical parameters and spontaneous remission of hypoglycemia, it fits well in this syndrome. HLA association is also corroborative in this case. The subject was taking three drugs that contained sulfur and hydrogen atoms, namely Pantoprazole [Figure 3],[6] Clopidogril and Torsemide. Pantoprazole ((5-(Difluoromethoxy 2-(((3,4-dimethoxy-2-pyridinyl) methyl) sulfinyl)-1H-benzimidazole sodium) is a prodrug that requires activation in an acid environment. After absorption into systemic circulation, it diffuses into the parietal cells of the stomach and accumulates in the acidic secretory canaliculi. Here, it is activated by proton-catalyzed formation of a thiophillic sulfenamide or sulfenic acid. This activated form reacts by covalent binding with sulfhydryl group of cysteines, the extracelluar domain of the H + K+-ATPase. We postulate that the activated form of sulfenamide or sulfenic acid may bind with disulfide bond in the insulin molecule, making the latter more immunogenic. The patient, being free of symptoms, did not agree to pantoprazole-rechallenge. All the same, learning points from the case are the clues to suspect IAS, specifically, a very high serum insulin level but no detectable tumor in a patient of endogenous hyperinsulinemic hypoglycemia. Paradoxical hyperglycemia in the glucose tolerance test is also remarkable. Suspecting this rare entity is crucial as it will spare the patient from avoidable aggressive investigations and unwarranted surgical procedures.
Figure 3.
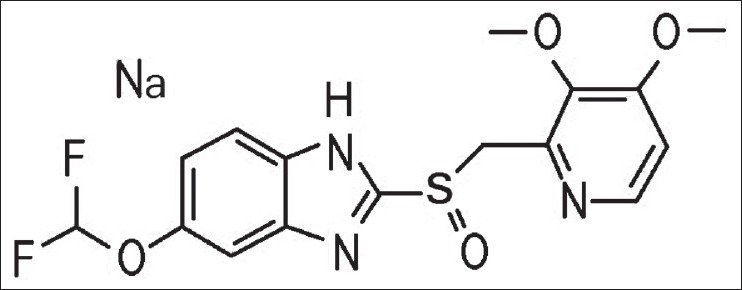
Molecular structure of Pantoprazole (http://www.chemblink.com/products/138786-67-1.htm)
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Lupsa BC, Chong AY, Cochran EK, Soos MA, Semple RK, Gorden P. Autoimmune forms of hypoglycemia. Medicine (Baltimore) 2009;88:141–53. doi: 10.1097/MD.0b013e3181a5b42e. [DOI] [PubMed] [Google Scholar]
- 2.Uchigata Y, Hirata Y. Insulin autoimmune syndrome (IAS, Hirata disease) Ann Med Interne (Paris) 1999;150:245–53. [PubMed] [Google Scholar]
- 3.Redmon JB, Nuttal FQ. Autoimmune hypoglycemia. Endocrinol Metab Clin North Am. 1999;28:603–18. doi: 10.1016/s0889-8529(05)70090-6. [DOI] [PubMed] [Google Scholar]
- 4.Uchigata Y, Tokunaga K, Nepom G, Bannai M, Kuwata S, Dozio N, et al. Differential immunogen determinants of polyclonal insulin autoimmune syndrome (Hirata's disease) and monoclonal insulin autoimmune syndrome. Diabetes. 1995;44:227–32. doi: 10.2337/diab.44.10.1227. [DOI] [PubMed] [Google Scholar]
- 5.Taylor SI, Barbetti F, Accili D, Roth J, Gorden P. Syndromes of autoimmunity and hypoglycemia. Autoantibodies directed against insulin and its receptor. Endocrinol Metab Clin North Am. 1989;18:123–43. [PubMed] [Google Scholar]
- 6.Chem blink online database of chemicals from around the world. [Last accessed on 2012 Feb 6]. Available from: http://www.chemblink.com/products/138786-67-1.htm .


