Abstract
Objective:
The aim and objective was to study the prevalence of gestational diabetes mellitus (GDM) by using National Diabetes Data Group (NDDG) and American Diabetes Association (ADA) (2004) criteria and the correlation of GDM with gestational blood pressure (BP) and maternal age.
Study Design:
This was a cross-sectional study in which 300 pregnant women in 24-28 weeks of pregnancy who screened positive with 1-h glucose load ≥ 140 mg/dL underwent a diagnostic 3-h oral glucose tolerance test (OGTT). BP was obtained by review of the medical records.
Results:
Thirty-seven (12.33%) women were screened positive with 50 g glucose challenge test (GCT) (≥140 mg%) out of the 300 participants. With 100 g 3-h OGTT among these 37 women, none of them fulfilled the NDDG diagnostic criteria for GDM. However, on using the ADA (2004) criteria, three (8.1%) women were diagnosed to have GDM. All three of them had systolic BP between 120 and 139 mmHg; two of them had diastolic BP between 80 and 89 mmHg. Among 37 subjects with GCT > 140 mg%, majority were older than 26 years.
Conclusion:
Using the ADA (2004) guideline, 1% of the total study population had GDM. The BP of these patients fell within the prehypertensive range, thus suggesting an association between GDM and BP.
Keywords: Blood pressure, gestational diabetes mellitus, glucose tolerance test
INTRODUCTION
The prevalence of gestational diabetes mellitus (GDM) among pregnant women in the United States ranges from 3% to 7%, depending on the population studied.[1,2,3] Most women with GDM do not continue to have hyperglycemia after delivery.[4] However, up to 50% of women with a history of GDM will develop type 2 diabetes in the decade following their GDM diagnosis.[5]
Women with both pre-GDM and GDM are at an increased risk for hypertensive disorders of pregnancy and can lead to higher maternal and fetal morbidity. On the reverse, hypertensive disorders of pregnancy can lead to significant increases in the risk for future cardiovascular events in diabetic women and women with a history of gestational diabetes.[6]
Some studies have shown that elevated BP before or during early pregnancy are associated with the development of gestational diabetes. There are no published data on this from North-East. We planned to evaluate the prevalence of GDM and its correlation with maternal age and gestational blood pressure (BP) in a study among women with singleton pregnancy in Manipuri population.
MATERIALS AND METHODS
Study design
This was a cross-sectional study carried out in the Department of Medicine, Regional Institute of Medical Sciences (RIMS), in collaboration with the Department of Obstetrics and Gynaecology, RIMS. The duration of the study is two calendar years from September 2010 to August 2012. Three hundred cases of randomly selected pregnant women from the outpatient unit of the Department of Obstetrics and Gynaecology, RIMS, with spontaneous singleton pregnancy in the 24th to 28th weeks of gestation having no history of GDM before the index pregnancy were made to undergo a detailed clinical examination. Women with a history of GDM in the previous pregnancy, known case of diabetes mellitus and systemic hypertension, and unwilling subjects were excluded from the study.
All the selected participants underwent a glucose challenge test (GCT) where 50 g of glucose was given to them, without considering the timing of the last meal. Cases with blood sugar level ≥140 mg/dL after 1 h proceeded to 100 g 3-h oral glucose tolerance test (OGTT). A patient is considered to have GDM if two or more of the values obtained during the 100 g glucose load 3-h OGTT are abnormal according to either or both the National Diabetes Data Group (NDDG) criteria[7] and American Diabetes Association (ADA) (2004) criteria.[8]
Women were categorized according to BP level recommended by American Heart Association, outside of pregnancy, that is, < 120/80 mmHg as normal, 120-139/80-89 mmHg as prehypertension, > 140/90 mmHg as hypertension.[9]
Pregnancy BP was the BP measured at 24-28 weeks of gestation and pregravid BP is the BP measured closest to but before last menstrual period and no more than 5 years before pregnancy; not included if it was from an emergency visit.
Statistical analysis
The data were analyzed using SPSS v. 16 and correlation was done using Fisher's exact test with a confidence interval of 95% and the significance level at P < 0.05.
RESULTS
One hundred and thirty-three women out of the 300 (43.3%) have both systolic and diastolic BP in the prehypertensive range of above 120/80 mmHg. One hundred and seventy-nine (59.7%) women have systolic BP in the prehypertensive range of 120-139 mmHg and 191 (63.7%) women have diastolic BP in the prehypertensive range of 80-89 mmHg. The various levels of BP in different age groups are shown in Table 1.
Table 1.
Distribution of age and blood pressure during pregnancy
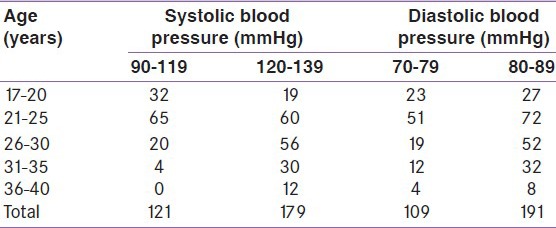
Out of 300 pregnant women screened, 263 (87.67%) had GCT < 140 mg % and 37 (12.33%) subjects had GCT > 140 mg %. The majority of the women with positive screening on 50 g GCT were in the age range 26-30 years [Table 2].
Table 2.
A 50 g glucose challenge test in different age groups
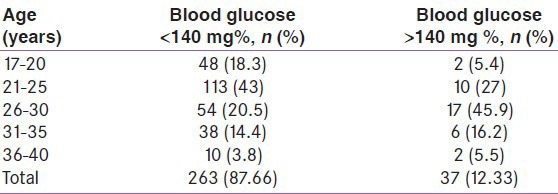
Among these 37 participants screened positive for GCT, there was a statistically significant association with raised systolic BP but similar association was not seen with diastolic BP. The distribution of BP among women with normal or impaired 50 g GCT is shown in Table 3.
Table 3.
Blood pressure and 50 g glucose challenge test
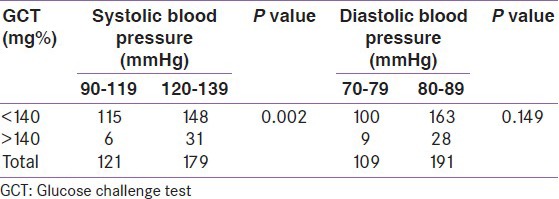
With 100 g 3-h OGTT among the 37 subjects with positive 50 g GCT, the median blood glucose fasting level was 82 ± 12.14 mg%, for 1-h OGTT 170 ± 24.3 mg%, for 2-h OGTT 132 ± 17.7 mg%, and 104 ± 15.8 mg% for 3-h OGTT. None of them fulfilled the NDDG diagnostic criteria of GDM in the 100 g 3-h OGTT, although two patients had fasting blood glucose level above the cutoff level of > 105 mg% [Table 4a].
Table 4a.
A 100 g 3-h oral glucose tolerance test and blood pressure. National diabetes data group criteria
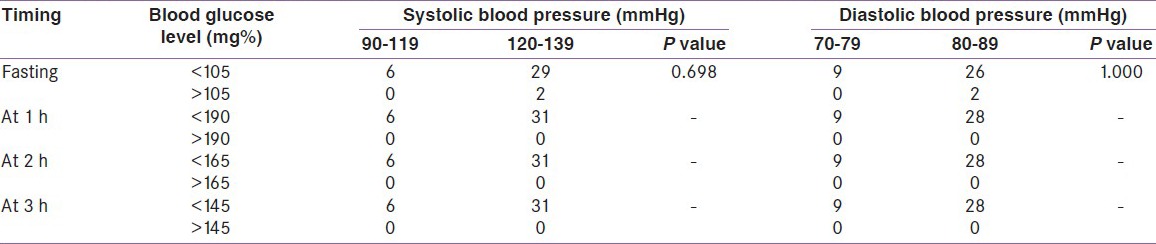
However, on using the ADA (2004) criteria, among the 37 participants with 50 g GCT level >140 mg%, three (8.1%) were diagnosed to have GDM with 3-h 100 g OGTT. This represents 8.1% increase in GDM diagnoses, from 0% (0 of 37) to 8.1% (3 of 37), using the more inclusive criteria. The BP of the three GDM patients was 120/88, 128/80, and 130/88 mmHg, which fell within the prehypertensive range [Table 4b].
Table 4b.
A 100 g 3-h oral glucose tolerance test and blood pressure. ADA (2004) criteria
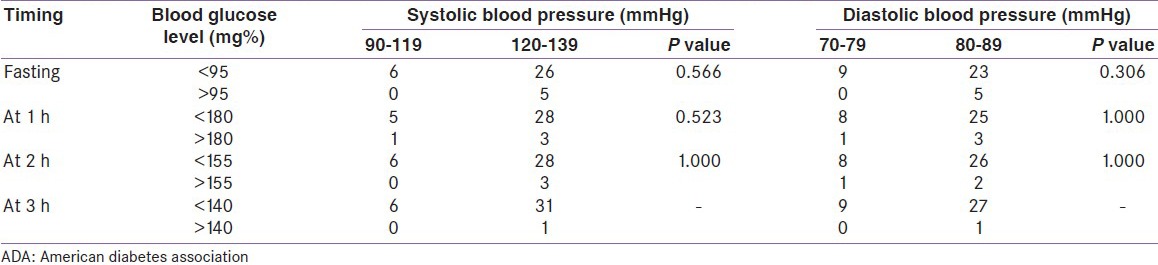
According to the NDDG criteria with the cutoff point for a positive fasting blood sugar level >105 mg%, two (5.4%) participants showed a positive result and both of them had systolic BP within the range 120-139 mmHg and diastolic BP within the range 80-89 mmHg (prehypertensive range). On using the ADA (2004) criteria, five (13.51%) participants showed a positive result at >95 mg% and they all fell in the prehypertensive range of BP, that is, 120-139 mmHg for systolic BP and 80-89 mmHg for diastolic BP.
In the 1 h OGTT, none of the participants reached the cutoff level for positive test, that is, >190 mg% on using the NDDG criteria. But on using the ADA (2004) criteria, four (10.81%) participants have blood sugar level above the cutoff level of >180 mg%. Among these four women, three had a systolic and diastolic BP within the prehypertensive range (120-139 and 80-89 mmHg).
Again in the 2-h OGTT result, none of the participants reached the NDDG cutoff level of blood sugar >165 mg%, whereas on using the ADA criteria, three (8.1%) women had blood sugar level >155 mg%. All three of them had systolic BP between 120 and 139 mmHg; two of them had diastolic BP between 80 and 89 mmHg.
One (2.7%) participant showed a positive 3-h OGTT result of >140 mg% as per the ADA (2004) criteria and her BP fell within the prehypertensive range for both systolic and diastolic pressures. There was no positive result in the 3-h OGTT on using the NDDG criteria.
DISCUSSION
In Manipuri population, the prevalence of diabetes mellitus has been reported to be 4.03% in 1997[10] and the prevalence of gestational diabetes was reported to be 1.6% in 1999.[11]
A recent study in a tertiary care institution from North India has also reported a low prevalence (1.5%) of GDM.[12] Several studies from various parts of India have reported the prevalence of GDM ranging from 3.8% to 17.8% using different criteria.[13,14,15,16,17,18]
In the present study, out of the 300 participants, 37 women had blood glucose level more than 140 mg% in the 50 g GCT with the majority older than 26 years. There was a significant correlation between screening positive in GCT and age.
The same finding was observed by Seshiah et al.[19] who mentioned in their study that age >25 years is one of the independent risk factor for GDM. Similarly, Hoseini et al.[20] have also included maternal age >26 years as one of the significant risk factors for GDM. ADA (2010) guideline also mentioned age <25 years as a low-risk group who do not require screening for GDM unless they have other risk factors for GDM.[21]
The distribution of the BP among the participants in relation to positive screening of GCT showed a significant relationship. Although the participants do not have hypertension, those with positive GCT still have a higher BP range compared with those with negative GCT. Previous other studies have also shown association of BP with impaired GCT. Solomon et al.[22] observed abnormal glucose loading test as a significant predictor of development of hypertension and nonproteinuric hypertension in pregnancy. Vembergre et al.[23] also noted that pregnancy-induced hypertension appears to be linked to the level of glucose intolerance during pregnancy, independently of the other known risk factors of hypertension. During early pregnancy, women with prehypertension had a small increased risk of GDM, and women with hypertension had a twofold increased risk of GDM compared with women with normal BP after adjusting for age, race/ethnicity, gestational week of BP, body mass index, and parity. Similar results were seen among the subset of women with BP levels measured before pregnancy.[24]
Several randomized clinical trials had compared various standard criteria for screening and diagnosis of GDM, but the opinion about the policy of detection and diagnosis has not been settled. The Fourth International Workshop Conference on GDM indicated that the infants of women who meet the lower Carpenter–Coustan criteria are at similar risk for perinatal morbidity as those patients identified using the NDDG criteria.[25]
Thus, using the more inclusive ADA (2004) criteria may help us to identify an additional number of women with GDM who otherwise may have gone undiagnosed and untreated although they have similar risk as that of the treated women.
Mild gestational hyperglycemia (MGH), which is defined as a positive GCT followed by a negative OGTT, is characterized both by insulin resistance and a near-normal pattern of glucose-triggered insulin release.[26] Vemberge et al.[23] defined gestational mild hyperglycemia as one abnormal value of OGTT in their study and showed the rate of pregnancy-induced hypertension in GDM, gestational mild hyperglycemia, and control were, respectively, 17.0%, 10.8%, and 4.6%. Pregnancy-induced hypertension was linked to the level of glucose intolerance during pregnancy, independently of the other known risk factors of hypertension. Gonsalves et al.[27] had also mentioned that women with gestational hyperglycemia and GDM have higher risk of hypertension in addition to that of type 2 diabetes. Similarly, Retnakaran et al.[28] have concluded in their study that both GDM and mild glucose intolerance predict an increased likelihood of metabolic syndrome at three months postpartum, supporting the concept that women with gestational dysglycemia may have an underlying latent metabolic syndrome.
On that note, our study has observed 37 participants with abnormal GCT but having normal 3-h OGTT according to the NDDG criteria. Two participants showed an abnormal fasting blood sugar level but their 3-h OGTT remained within the normal limit. These two women may, therefore be labeled as having MGH. Apart from those who met two of the cutoff values in the 3-h OGTT to be diagnosed as GDM as per the ADA (2004) guideline, an additional five women met one of the cutoff values among the three OGTT cutoffs. Three of them had a fasting blood glucose level >95mg% and another two had a 1-h OGTT level >180 mg%.
So, in the present study, out of the 300 women, three were diagnosed with GDM, five women have gestational mild hyperglycemia and 37 women have MGH. Women with gestational diabetes are at an increased risk for hypertensive disorders of pregnancy.
CONCLUSION
In the present study, three (1%) women were diagnosed with gestational diabetes on using the ADA (2010) criteria. Although none of the women in the study have hypertension, 83.8% of those who screened positive for gestational diabetes have systolic BP in the prehypertensive range; 16.2% of them have diastolic BP in the prehypertensive range showing an association of BP with gestational dysglycemia in Manipuri women.
LIMITATIONS OF THE STUDY
Because of the small sample size and cross-sectional nature of the study, there is limitation in the interpretation of this study. Also, with 11.33% women detected to have gestational dysglycemia, a larger study with adequate sample size, follow-up, and duration is needed to give us more insight about the prevalence of gestational diabetes and its correlation with BP, its effect on the maternal and fetal outcome, and also the impact of diagnosing and treating the women who were diagnosed only on using the more inclusive criteria.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Albrecht SS, Kuklina EV, Bansil P, Jamieson DJ, Whiteman MK, Kourtis AP, et al. Diabetes trends among delivery hospitalizations in the US, 1994-2004. Diabetes Care. 2010;33:768–73. doi: 10.2337/dc09-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Getahun D, Nath C, Ananth CV, Chavez MR, Smulian JC. Gestational diabetes in the United States: Temporal trends 1989 through 2004. Am J Obstet Gynecol. 2008;198:525. doi: 10.1016/j.ajog.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Kim SY, England L, Wilson HG, Bish C, Satten GA, Dietz P. Percentage of gestational diabetes mellitus attributable to overweight and obesity. Am J Public Health. 2010;100:1047–52. doi: 10.2105/AJPH.2009.172890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bottalico JN. Recurrent gestational diabetes: Risk factors, diagnosis, management, and implications. Semin Perinatol. 2007;31:176–84. doi: 10.1053/j.semperi.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: A systematic review. Diabetes Care. 2002;25:1862–8. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan SD, Umans JG, Ratne R. Hypertension complicating diabetic pregnancies: Pathophysiology, management and controversies. J Clin Hypertens (Greenwich) 2011;13:275–84. doi: 10.1111/j.1751-7176.2011.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Diabetes Association: Gestational diabetes mellitus. Diabetes Care. 1998;21:S60–1. [Google Scholar]
- 8.American Diabetes Association: Gestational diabetes mellitus (Position Statement) Diabetes Care. 2004;27(Suppl 1):S88–90. doi: 10.2337/diacare.27.2007.s88. [DOI] [PubMed] [Google Scholar]
- 9.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, et al. Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 10.Singh TP, Singh AD, Singh TB. Prevalence of diabetes mellitus in Manipur. J Diabet Assoc India. 1997;37:41–6. [Google Scholar]
- 11.Singh TP, Dkhar A, Singh TB, Singh KT. Gestational diabetes mellitus among the manipuri women: The prevalence and risk factors. J Diabet Assoc India. 1999;39:41–6. [Google Scholar]
- 12.Tripathi R, Tolia N, Gupta VK, Mala YM, Ramji S, Tyagi S. Screening for gestational diabetes mellitus: A prospective study in a tertiary care institution of North India. J Obstet Gynaecol Res. 2012;38:351–7. doi: 10.1111/j.1447-0756.2011.01706.x. [DOI] [PubMed] [Google Scholar]
- 13.Zargar AH, Sheikh MI, Bashir MI, Masoodi SR, Laway BA, Wani AI, et al. Prevalence of gestational diabetes mellitus in Kashmiri women from the Indian subcontinent. Diabetes Res Clin Pract. 2004;66:139–45. doi: 10.1016/j.diabres.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 14.Swami SR, Mehetre R, Shivane V, Bandgar TR, Menon PS, Shah NS. Prevalence of carbohydrate intolerance of varying degrees in pregnant females in western India (Maharashtra)–A hospital-based study. J Indian Med Assoc. 2008;106:712–4. 735. [PubMed] [Google Scholar]
- 15.Seshiah V, Balaji V, Balaji MS, Paneerselvam A, Arthi T, Thamizharasi M, et al. Prevalence of gestational diabetes mellitus in South India (Tamil Nadu): A community based study. J Assoc Physicians India. 2008;56:329–33. [PubMed] [Google Scholar]
- 16.Wahi P, Dogra V, Jandial K, Bhagat R, Gupta R, Gupta S, et al. Prevalence of gestational diabetes mellitus (GDM) and its outcomes in Jammu region. J Assoc Physicians India. 2011;59:227–30. [PubMed] [Google Scholar]
- 17.Balaji V, Balaji M, Anjalakshi C, Cynthia A, Arthi T, Seshiah V. Diagnosis of gestational diabetes mellitus in Asian-Indian women. Indian J Endocrinol Metab. 2011;15:187–90. doi: 10.4103/2230-8210.83403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seshiah V, Balaji V, Shah SN, Joshi S, Das AK, Sahay BK, et al. Diagnosis of gestational diabetes mellitus in the community. J Assoc Physicians India. 2012;60:15–7. [PubMed] [Google Scholar]
- 19.Seshiah V, Balaji V, Balaji MS, Paneerselvam A, Kapur A. Pregnancy and diabetes scenario around the world: India. Int J Gynaecol Obstet. 2009;104:35–8. doi: 10.1016/j.ijgo.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 20.Hoseini SS, Hantoushzadeh S, Shoar S. Evaluating the extent of pregravid risk factors of gestational diabetes mellitus in women in Tehran. Iran Red Crescent Med J. 2011;13:407–14. [PMC free article] [PubMed] [Google Scholar]
- 21.American diabetes association. Standards of Medical Care in Diabetes 2010. Diabetes Care. 2010;33(Suppl 1):S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solomon CG, Graves SW, Greene MF, Seely EW. Glucose intolerance as a predictor of hypertension in pregnancy. Hypertension. 1994;23:717–21. doi: 10.1161/01.hyp.23.6.717. [DOI] [PubMed] [Google Scholar]
- 23.Vambergue A, Nuttens MC, Goeusse P, Biausque S, Lepeut M, Fontaine P. Pregnancy induced hypertension in women with gestational carbohydrate intolerance: The diagest study. Eur J Obstet Gynecol Reprod Biol. 2002;102:31–5. doi: 10.1016/s0301-2115(01)00556-5. [DOI] [PubMed] [Google Scholar]
- 24.Hedderson MM, Ferrara A. High blood pressure before and during early pregnancy is associated with an increased risk of gestational diabetes mellitus. Diabetes Care. 2008;31:2362–7. doi: 10.2337/dc08-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berggren EK, Boggess KA, Stuebe AM, Jonsson Funk M. National diabetes data group vs carpenter–coustan criteria to diagnose gestational diabetes. Am J Obstet Gynecol. 2011;205:253.e1–7. doi: 10.1016/j.ajog.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weijers R, Bekedam DJ, Smulders YM. Determinants of mild gestational hyperglycemia and gestational diabetes mellitus in a large Dutch multiethnic cohort. Diabetes Care. 2002;25:72–7. doi: 10.2337/diacare.25.1.72. [DOI] [PubMed] [Google Scholar]
- 27.Gonsalves LC, Silva MR, Pera Asoli JC, de Silveria LV, Padovani CR, de Pimenta WP. Hypertension after gestational hyperglycaemia. Arq Bras Endocrinol Metabol. 2005;49:265–70. doi: 10.1590/s0004-27302005000200013. [DOI] [PubMed] [Google Scholar]
- 28.Retnakaran R, Qi Y, Connelly PW, Sermer M, Zinman B, Hanley AJ. Glucose intolerance in pregnancy and postpartum risk of metabolic syndrome in young women. J Clin Endocrinol Metab. 2010;95:670–7. doi: 10.1210/jc.2009-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


