Abstract
Statins are the revolutionary drugs in the cardiovascular pharmacotherapy. But they also possess several adverse effects like myopathy with elevation of hepatic transaminases (>3 times the upper limit of normal) or creatine kinase (>10 times the upper limit of normal) and some rare side-effects, including peripheral neuropathy, memory loss, sleep disturbances, and erectile dysfunction. Due to these adverse effects, patients abruptly withdrew statins without consulting physicians. This abrupt discontinuation of statins is termed as statin intolerance. Statin-induced myopathy constitutes two third of all side-effects from statins and is the primary reason for statin intolerance. Though statin intolerance has considerably impacted cardiovascular outcomes in the high-risk patients, it has been well effectively managed by prescribing statins either as alternate-day or once weekly dosage regimen, as combination therapy with a non-statin therapy or and by dietary intervention. The present article reviews the causes, clinical implications of statin withdrawal and management of statin intolerance.
Keywords: Adverse effects of stains, myopathy, rhabdomyolysis, statins, statin intolerance
INTRODUCTION
Statins or 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors have revolutionized cardiovascular pharmacotherapy. Though they have confirmed safety and efficacy profile, statins have reportedly been associated with several adverse effects forcing abrupt discontinuation without consulting their physician.[1] This is termed as statin intolerance. Statins were shown to adversely affect fatty acid oxidation, decrease sarcolemal cholesterol, deplete isoprenoid, disturb calcium homeostasis and lipid metabolism, cause autoimmune phenomena, increase intracellular lipids, and cause mitochondrial dysfunction due to inhibition of ubiquinone or coenzyme Q10 production, leading to emergence of statin-induced adverse effects [Table 1].[2] The major statin-induced adverse effects are muscle-related complications (myalgia, myopathy, rhabdomyolysis), hepatotoxicity, nephrotoxicity (proteinuria), headache, rash, and gastrointestinal discomforts.[3,4] These adverse effects manifest more severely in patients aged over 80 years, with liver and muscle disorders, chronic renal insufficiency with diabetes or hypothyroidism, or co-prescription of agents that compete with statins for metabolism such as fibrates, cyclosporine, warfarin, azole antifungals, macrolides, Human Immunodeficiency Virus (HIV) protease inhibitors, verapamil or amiodarone.[4] Predominantly, myalgia is considered as the leading cause of statin intolerance.[5,6] Both myopathy and rhabdomyolysis constitute two thirds of statin adverse effects that warrant serious attention. Fatal incidence of rhabdomyolysis was considered as the major reason of cerivastatin withdrawal in 2001.[7] As per Food and Drug Administration, myopathy is defined as elevation of creatinine kinase (CK) up to 10-fold, myalgia as any pain in a muscle or group of muscles, and rhabdomyolysis as CK elevation >50-fold with severe skeletal muscle injury and lysis and myoglobinuria. Other rare adverse effects of statins-peripheral neuropathy, memory loss, erectile dysfunction, and sleep disturbances may also lead to statin intolerance [Table 3].
Table 1.
Mechanism of major statin-induced adverse effects
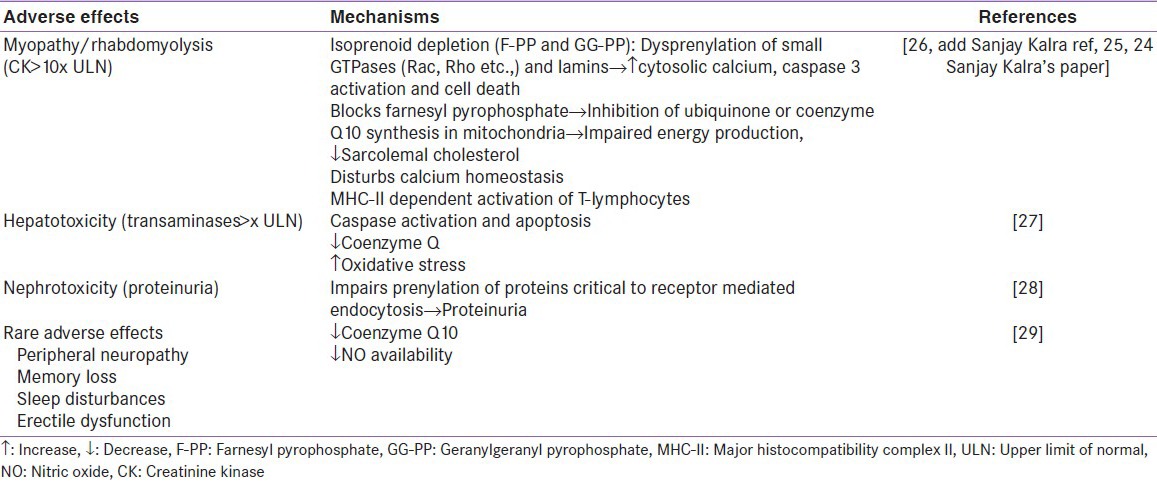
Table 3.
Effects of statin withdrawal
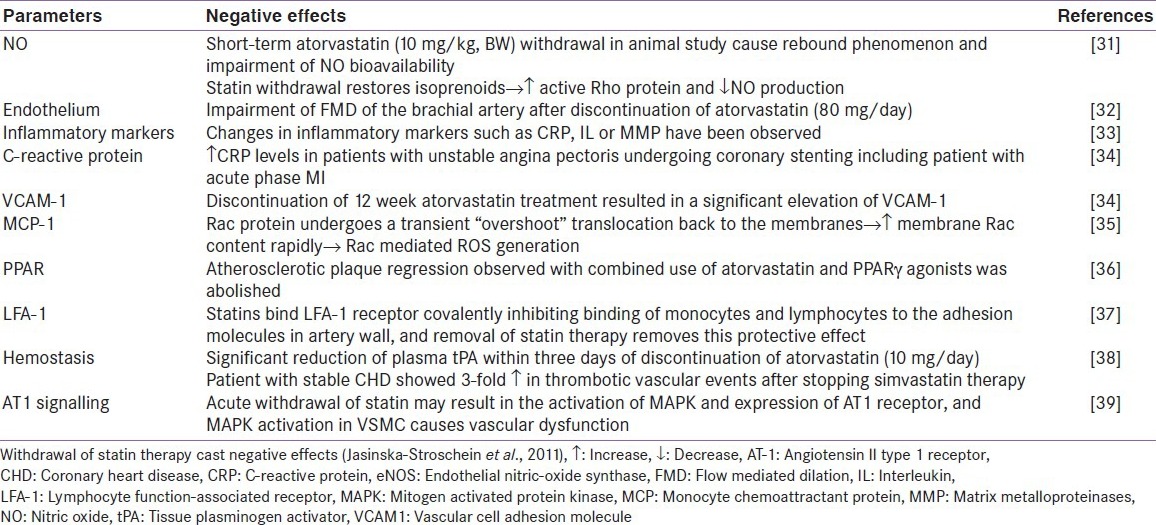
In clinical practice, statin intolerance poses a great hindrance to effective treatment in patients afflicted with cardiovascular disease, leading to statin discontinuation in 28.9% of patients within the first year of initiation of statin therapy.[8]
HOW TO IDENTIFY STATIN INTOLERANCE
Both statin-induced elevation of CK levels (defined as >10 times the Upper Limit of Normal [ULN]) and hepatic transaminases (defined as >3 times the ULN) have been reported as good predictors of serious statin-adverse effects.[9]
Assessment of creatine kinase in statin-induced myopathy
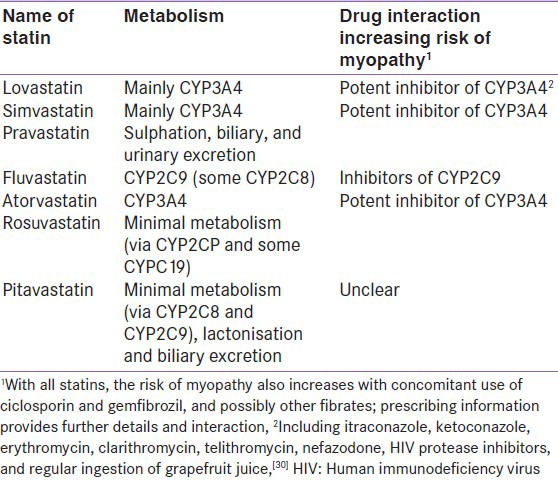
Statin-induced myopathy is a class effect of statins which tends to progress to rhabdomyolysis at higher doses of statins. Myopathy/rhabdomyolysis is usually precipitated when statins are co-prescribed with interacting drugs, including fibrates and the inhibitors and inducers of hepatic microsomal enzymes (cytochrome P450) which included cyclosporine A, azole antifungals, fibrates, niacin, protease inhibitors, macrolide antibiotics, calcium channel blockers, warfarin, and grapefruit juice.[10] Routine CK assessment was not found useful in detecting rare cases of myopathy in patients who were on the standard statin doses.[11] Statin product information mentions that patients who experienced any new or unexplained muscle pain or weakness must report to their physician and recommended CK assessment in these patients [Figure 1]. In addition, patients with muscle weakness or bilateral proximal muscle pain, excluding other known causes have also been recommended to have their CK assessed. Patients with CK level >10 times the ULN most likely suffer from myopathy and have brown discoloration of urine, which serves as an indicator of overall elevation of myoglobin in the blood. These patients with myopathy have elevated levels of transaminases which normalise on improvement of myopathy. With increase in awareness of drug interactions, statin intolerance could be prevented [Table 2].
Figure 1.
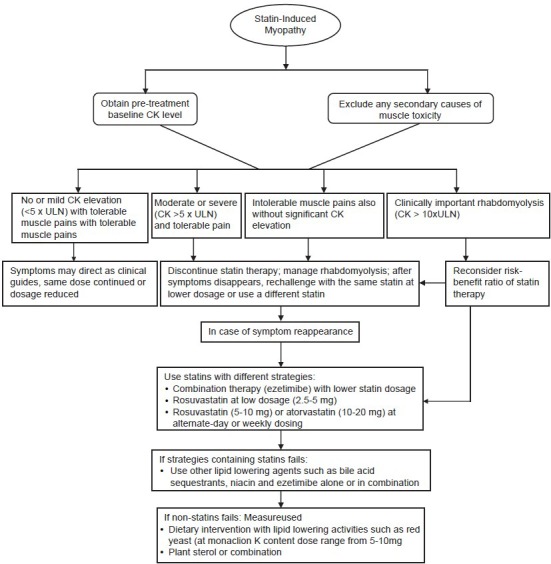
Statin-induce myopathy management
Table 2.
Important statin drug interactions causing statin intolerance
Assessment of hepatic transaminases
Product information of statins mentions the baseline measurement of hepatic enzymes such as ALT (Alanine aminotransferase) and AST (aspartate aminotransferase) and forbids use of statins in active liver diseases.[12] Therefore, patients with baseline liver abnormalities and active disease should refrain from using statins. Generally, at a clinical dose of statins, there is rare (<1%) elevation of hepatic enzymes but at higher doses the level of hepatic enzyme increases with differing intensity depending on the type of statin.
Routine assessment of hepatic transaminases is no longer required for simvastatin, pravastatin, or lovastatin up to 40 mg/day but is recommended for other statins and for higher doses of statins. If ALT or AST levels are >3 times the ULN in an asymptomatic patient with other liver abnormalities, the liver function test should be done within a week and statins must be stopped temporarily if ALT level did not fall to the normal level.[12] In 2006, the Liver Expert Panel reported that they did not find any proof that statins caused life-threatening liver damage and elevation of hepatic enzymes reversed after stopping statin. The panel did not recommend routine evaluation but suggested repetition of the test in case the transaminases are >3 times ULN and further evaluation if it remains elevated. The statins should be discontinued only in the presence of any objective liver injury.[13]
MANAGEMENT OF STATIN INTOLERANCE
The first step in the strategy to manage statin intolerance is to rule out extraneous factors that may increase the risk of myopathy/rhabdomyolysis or elevate hepatic transaminases. The National Lipid Association Statin Safety Task Force has prescribed guidelines to manage statin intolerance [Figure 1].
Other strategies used to manage statin intolerance are switching therapy, alternate day dosing, non-statin lipid-lowering drugs, lipid lowering nutraceuticals, and specific pharmacotherapies which are detailed below.
Switching therapy
This strategy of switching therapy is effective in only some patients since the criteria to select the new statins are not clearly delineated.[14,15] Switching from 1) Mild to high lipophilic statin 2) from cytochrome P450 metabolised to non-cytochrome P450 metabolised statin, and 3) to a lower dosage of a more potent statin have been utilised. A prospective, open-label pilot study evaluated the effectiveness and safety profile of rosuvastatin (newer and a highly potent non-cytochrome P450 metabolised statin) at 5 and 10 mg/day doses in patients with primary high LDL-C (mean, 177 mg/dL) and intolerance to other statins due to myalgia.[16]
In moderately high risk patient 5 mg/day rosuvastatin was prescribed and 10 mg/day was prescribed to high or very high risk patients. It was noted after 36 weeks that 5 mg/day rosuvastatin dose reduced LDL-C by 42% and 10 mg/day by 39%. Only one patient who was receiving 10 mg/day rosuvastatin dose discontinued rosuvastatin treatment due to unilateral muscular pain after 4 weeks with no significant elevation in hepatic enzymes.
Alternate day dosing
Statins with longer half-life maintain lipid lowering effect over a longer period of time, enabling alternate day dosing strategy with statin. Atorvastatin with a mean half-life of 14 hrs is metabolised into two active metabolites-orthohydroxy and parahydroxy forms. Both these active metabolites contribute to 70% activity of atorvastatin and have a half-life of 20 to 30 hrs.[17] This pharmacokinetic parameter of atorvastatin makes it suitable for an alternate-day dosage regimen and continues its lipid lowering activity for considerably a longer period of time. Rosuvastatin, a third generation statin, possess a long half-life period of around 19hrs. Two patients who developed intolerance to daily atorvastatin therapy, due to myalgia, were subsequently treated with rosuvastatin (at a dosage of 2.5 mg and 5 mg) on 1st, 3rd, and 5th days of the week, respectively. At both the doses, the level of LDL-C decreased by 38% and 20%, respectively with the resolution of adverse effects after six weeks of treatment.[18] In 2008, Gadarla et al., reported use of rosuvastatin (5 and 10 mg), two-times a week (on the first and fourth day of the week) for a period longer than three weeks in patients aged 62-70 years who developed myopathy due to other lipid-lowering therapy.[19] The rosuvastatin dosage regimen was well accepted by 80% of the patients with significant 26% LDL-C reduction from the baseline. In another study, eight patients who were intolerant to daily statin responded well with once-weekly dosage of rosuvastatin (5-20 mg) and reported a mean LDL-C reduction of 29%.[20] The apparent reasons for weekly statin regimen tolerance could be due to either lowering of overall plasma concentration of statins or psychological reasons. However, this alternate day dosing strategy had certain limitation. First, alternate day statin dose administration causes a lower LDL-C reduction (up to 10-15% less) compared to daily dose regimen in high-risk patients. However, this LDL-C reduction was much lower than the ideal LDL-C target set for each high-risk patient. Second, alternate-day dosing strategy has not been established through clinical trials. Thus alternate day dosing strategy should be used only as a secondary option in specific high-risk patients who are intolerant to lower statin dosage.
Non statin lipid-lowering drugs
Non-statin lipid lowering drugs include a bile acid sequestrant (colesevelam), an intestinal cholesterol absorption inhibitor (ezetimibe), fibrates, and niacin which may be either used alone or in combination. These drugs have been considered in cases of unmanaged statin intolerance. Co-administration of ezetimibe and bile acid sequestrants (colesevelam, colestipol, or cholestyramine) yields additional reduction of LDL-C levels without any adverse effects in comparison to stable bile acid sequestrant regimen alone. Addition of ezetimibe to nicotinic acid lowers LDL-C levels without modifying nicotinic acid-induced increase of HDL-C. The triple therapy i.e. bile acid sequestrant, statin, and ezetimibe or nicotinic acid further reduces LDL-C levels. However, no clinical outcome studies with these combinations have been performed. Functional food containing phytosterols or plant sterol containing tablets have been reported to reduce LDL-C levels up to 5-10% in patients taking a stable dose of a statin. This combination of plant sterol and statins was have been reported as well tolerated and safe.[21,22] Since no clinical trials with combination of plant sterols and other lipid-lowering drugs have been established for CVD outcomes, their efficacy in CVD risk reduction remains speculative.
Use of lipid lowering nutraceuticals
Various dietary interventions, including foods low in saturated fat and high in viscous fibers (e.g., oats and barley), plant sterols, vegetable protein foods (soy), and nuts (e.g., almonds) have been used in patients who cannot tolerate statins. The efficacy of these dietary interventions was further strengthened by addition of nutraceuticals such as red yeast.[23] In 2003, Jenkins and colleagues reported that dietary portfolio of cholesterol-lowering foods caused cholesterol lowering effect comparable to a statin. But due to its limitation of palatability, dietary supplements were used as an alternative option.[24] Chinese red yeast rice is a dietary supplement made by fermenting the yeast, Monascus purpureus, over rice. Monascus yeast produces a family of substances called monacolins capable of inhibiting the enzyme HMG-CoA reductase and also contains unsaturated fatty acids and phytosterols. Red yeast rice offers only modest LDL-C lowering (up to 20%) and has been prescribed to only low-risk individuals or in whom LDL-C level is not far from the target.[25]
Specific Pharmacotherapies
Presently, there is a lack of consensus on the use of specific pharmacotherapy for statin-induced myopathy. Coenzyme Q10 (CoQ10) deficiency has been correlated with the development of myopathy. Various studies have reported significant improvement in statin-induced adverse effects-myopathy, myalgia, peripheral neuropathy, fatigue, dyspnoea, and memory loss if coenzyme Q10 was given as a co-therapy with statins.[26,27,28,29,30] In 2009, Kalra et al.,[5] reported that coenzyme Q10 (200 mg/day) supplementation in statin-treated patients would help in preventing statin-induced adverse effects, leading to low statin intolerance and maximal benefits of statin.
Vitamin deficiency has been associated with myalgia and poor muscle function and its supplementation have shown ameliorative effects in statin induced myopathy. A recent trial has shown that 92% of patients become myalgia free after three months of vitamin D supplementation.[31]
CONCLUSION
In recent years, increasing complaints of intolerance to statins have considerably impacted outcomes, especially in high-risk patients. Furthermore, several studies have reported negative consequences of acute statin withdrawal on cardiac patients, especially the patients with ACS and stroke. Increased understanding of statin-induced adverse-effects and awareness of clinical implications of acute statin withdrawal has discouraged patients from abrupt discontinuation of statin therapy. Statin intolerance has been effectively managed either by the use of alternate-day statin regimen with longer half lives like atorvastatin and rosuvastatin, combination of statins with non-statins, dietary interventions, or by specific pharmacotherapies.
ACKNOWLEDGEMENT
Max Neeman International, New Delhi, was responsible for preparation of the review article.
Footnotes
Source of Support: Astra Zeneca wrt to medical writing support
Conflict of Interest: None declared.
REFERENCES
- 1.Gotto AM. Statins, cardiovascular disease, and drug safety. Am J Cardiol. 2006;97:3C–5. doi: 10.1016/j.amjcard.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Abd TT, Jacobson TA. Statin induced myopathy: A review and myopathy. Expert Opin Drug Saf. 2011;10:373–87. doi: 10.1517/14740338.2011.540568. [DOI] [PubMed] [Google Scholar]
- 3.Beltowski J, Wojcicka G, Jamroz-Wisniewska A. Adverse effects of statins-mechanism and consequences. Curr Drug Saf. 2009;4:209–28. doi: 10.2174/157488609789006949. [DOI] [PubMed] [Google Scholar]
- 4.Stone NJ. Stopping statins. Circulation. 2004;110:2280–2. doi: 10.1161/01.CIR.0000145140.06171.3D. [DOI] [PubMed] [Google Scholar]
- 5.Kalra S, Agrawal N, Kalra B, Sharma A, Kamboj R. The role of Coenzyme Q10 in statin associated myopathy. Electron Physic. 2009;1:2–8. [Google Scholar]
- 6.Jacobson TA. Toward pain free statin prescribing algorithm for diagnosis and management of mayalgia. Mayo Clin Proc. 2008;83:687–700. doi: 10.4065/83.6.687. [DOI] [PubMed] [Google Scholar]
- 7.Staffa JA, Chang J, Green L. Cerivastatin and reports of fatal rhabdomyolysis [letter] N Engl J Med. 2002;346:539–40. doi: 10.1056/NEJM200202143460721. [DOI] [PubMed] [Google Scholar]
- 8.Kamal-Bahl SJ, Burke T, Watson D, Wentworth C. Discontinuation of lipid modifying drugs among commercially insured United States patients in recent clinical practice. Am J Cardiol. 2007;99:530–4. doi: 10.1016/j.amjcard.2006.08.063. [DOI] [PubMed] [Google Scholar]
- 9.Mancini GB, Baker S, Bergeron J, Fitchett D, Frohlich J, Genest J, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: Proceedings of a canadian working group consensus conference. Canad J Cardiol. 2011;27:635–62. doi: 10.1016/j.cjca.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Bellosta S, Paoletti R, Corsinin A. Safety of statins: Focus on clinical pharmacokinetics and drug interaction. Circulation. 2004;109:III50–7. doi: 10.1161/01.CIR.0000131519.15067.1f. [DOI] [PubMed] [Google Scholar]
- 11.Armitage J. The safety of statins in clinical practice. Lancet. 2007;370:1781–90. doi: 10.1016/S0140-6736(07)60716-8. [DOI] [PubMed] [Google Scholar]
- 12.Argo CK, Loria P, Caldwell SH, Lonardo A. Statins in liver disease: A molehill, an iceberg, or neither. Hepatology. 2008;48:662–9. doi: 10.1002/hep.22402. [DOI] [PubMed] [Google Scholar]
- 13.Cohen DE, Anania FA, Chalasani N. Assessment of statin safety by hepatologists. Am J Cardiol. 2006;97:77c–81. doi: 10.1016/j.amjcard.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Hansen KE, Hildebrand JP, Ferguson EE, Stein JH. Outcomes in 45 patients with statin-associated myopathy. Arch Intern Med. 2005;165:2671–6. doi: 10.1001/archinte.165.22.2671. [DOI] [PubMed] [Google Scholar]
- 15.Arca M, Pigna G. Treating statin intolerant patients. Diab Metab Synd Obes Targ Ther. 2011;4:1555–66. doi: 10.2147/DMSO.S11244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glueck CJ, Aregawi D, Agloria M, Khalil Q, Winiarska M, Munjal J, et al. Rosuvastatin 5 and 10 mg/d: A pilot study of the effects in hypercholesterolemic adults unable to tolerate other statins and reach LDL. Clin Ther. 2006;28:933–42. doi: 10.1016/j.clinthera.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Lins RL, Matthys KE, Verpooten GA, Peeters PC, Dratwa M, Stolear JC, et al. Pharmacokinetics of atorvastatin and its metabolites after single and multiple dosing in hypercholesterolaemic haemodialysis patients. Nephro Dial Transplant. 2003;18:967–76. doi: 10.1093/ndt/gfg048. [DOI] [PubMed] [Google Scholar]
- 18.Mackie BD, Satija S, Nell C, Miller J, Sperling LS. Monday, wednesday and friday dosing of rosuvastatin in patients intolerant to statin therapy. Am J Cardiol. 2007;99:291. doi: 10.1016/j.amjcard.2006.07.093. [DOI] [PubMed] [Google Scholar]
- 19.Gadarla M, Kearns AK, Thompson PD. Efficacy of rosuvastatin (5 mg and 10 mg) twice a week in patients intolerant to daily statins. Am J Cardiol. 2008;101:1747–48. doi: 10.1016/j.amjcard.2008.02.061. [DOI] [PubMed] [Google Scholar]
- 20.Bakes JM, Vernero CV, Gibson CA, Ruisinger JF, Howard PA, Thompson PD, et al. Effectiveness and tolerability of every-other-day rosuvastatin dosing in patients with prior statin intolerance. Ann Pharmacother. 2008;42:341–6. doi: 10.1345/aph.1K604. [DOI] [PubMed] [Google Scholar]
- 21.Abumweis SS, Barake R, Jones PJ. Plant sterols/stanols as cholesterol lowering agents: A meta-analysis of randomized controlled trials. Food Nutr Res. 2008;52 doi: 10.3402/fnr.v52i0.1811. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ESC/EAS Guidelines for the management of dyslipidaemias. The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur Heart J. 2011;32:1769–18. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 23.Carter M, O’Keefe JH. Is red yeast rice a suitable alternative for statins. Mayo Clin Proc. 2008;83:1294. doi: 10.4065/83.11.1294. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins DJ, Kendall CW, Marchie A, Faulkner DA, Wong JM, de Souza R, et al. Effects of a dietary portfolio of cholesterol-lowering foods vs lovastatin on serum lipids and C-reactive protein. JAMA. 2003;290:502–10. doi: 10.1001/jama.290.4.502. [DOI] [PubMed] [Google Scholar]
- 25.Ma J, Li Y, Ye Q, Li J, Hua Y, Ju D, et al. Constituents of red yeast rice, a traditional Chinese food and medicine. J Agric Food Chem. 2000;48:5220–5. doi: 10.1021/jf000338c. [DOI] [PubMed] [Google Scholar]
- 26.Thibault A, Samid D, Tompkins AC, Figg WD, Cooper MR, Hohl RJ, et al. Phase I study of lovastatin, an inhibitor of the mevalonate pathway, in patients with cancer. Clin Cancer Res. 1996;2:483–91. [PubMed] [Google Scholar]
- 27.Kim WS, Kim MM, Choi HJ, Yoon SS, Lee MH, Park K, et al. Phase II study of high-dose lovastatin in patients with advanced gastric adenocarcinoma. Invest New Drugs. 2001;19:81–3. doi: 10.1023/a:1006481423298. [DOI] [PubMed] [Google Scholar]
- 28.Langsjoen PH, Langsjoen AM, Lucas LA. Treatment of statin adverse effects with supplementation of Coenzyme Q 10 and statin drug discontinuation. Bio Factors. 2005;25:147–52. doi: 10.1002/biof.5520250116. [DOI] [PubMed] [Google Scholar]
- 29.Schaars CF, Stalenhoef AF. Effects of ubiquinone (Coenzyme Q10) in myopathy in statin users. Curr Opin Lipidol. 2008;19:553–7. doi: 10.1097/MOL.0b013e3283168ecd. [DOI] [PubMed] [Google Scholar]
- 30.Kelly P, Vasu S, Getato M, McNurlan M, Lawson WE. Coenzyme Q10 improves myopathic pain in statin treated patients. J Am Coll Cardiol. 2005;45:3A. [Google Scholar]
- 31.Ahmed W, Khan N, Glueck CJ, Pandey S, Wang P, Goldenberg N, et al. Low serum 25 (OH) vitamin D levels (32 ng/mL) are associated with reversible myositis myalgia in statin-treated patients. Transl Res. 2009;153:11–6. doi: 10.1016/j.trsl.2008.11.002. [DOI] [PubMed] [Google Scholar]


