Abstract
Pregnancy is associated with normal physiological changes in endocrine system that assists fetal survival as well as preparation of labor. The pituitary gland is one of the most affected organs in which major changes in anatomy and physiology take place. Due to overlapping clinical and biochemical features of pregnancy, sometimes the diagnosis of pituitary disorders may be challenging. It is important to know what normal parameters of changes occur in endocrine system in order to diagnose and manage complex endocrine problems in pregnancy. In our present review, we will focus on pituitary disorders that occur exclusively during pregnancy like Sheehan's syndrome and lymphocytic hypophysitis and pre-existing pituitary disorders (like prolactinoma, Cushing's disease and acromegaly), which poses significant challenge to endocrinologists.
Keywords: Cushing's disease, growth hormone, lymphocytic hypophysitis, pituitary disorders, placenta, pregnancy, prolactinoma, Sheehan's syndrome
INTRODUCTION
Pregnancy is a normal altered physiological state in which profound anatomic and physiological changes occur in almost every organ. Anterior pituitary undergoes two- to three-fold enlargement during pregnancy, because of hyperplasia and hypertrophy of lactotroph cells. In contrast to lactotrophs, the size of other anterior pituitary cells remains unchanged or decrease. The function of most endocrine glands is altered partly because of placental hormones and increase in binding proteins. In current review, the anatomical and physiological changes in pituitary during pregnancy and management of pituitary disorders like Cushing's disease, acromegaly and prolactinoma will be discussed. We will also review Sheehan's syndrome and lymphocytic hypophysitis which occur in context of pregnancy.
CHANGES IN SIZE AND FUNCTION OF PITUITARY
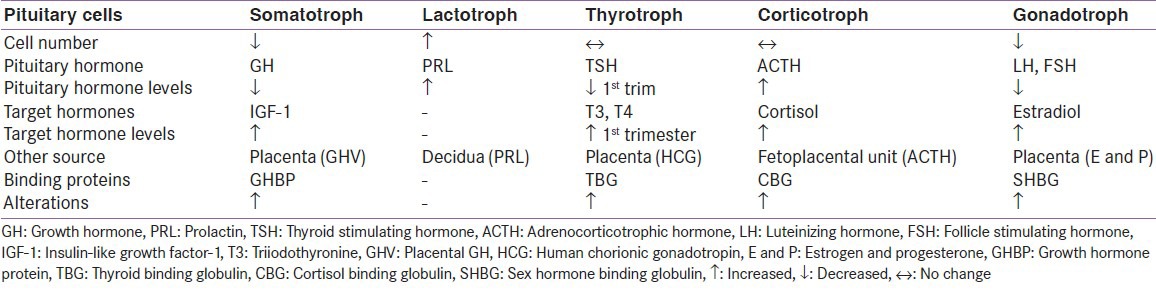
The weight of the gland increases by approximately one-third during pregnancy.[1] These changes have been studied using MR imaging. Mean height of normal gland is 9.6-10 mm during pregnancy, 10.2-12 mm in immediate postpartum period and regains normal pre-pregnancy size in about 6 months post-partum.[2,3] During pregnancy, the maternal pituitary gland undergoes remarkable hemodynamic changes. Two important processes alter the hormonal milieu of the body, first the increase in the levels of binding proteins and second production of many hormones both from pituitary and placenta.[4] Summary of important changes in trophic and target hormone levels is given in Table 1. In view of this, the diagnosis of any endocrine disorder becomes challenging.
Table 1.
Hormone changes during pregnancy
Prolactin
During pregnancy, the percentage of lactotrophs increases up to 40% in response to elevated maternal estrogen and at the end of pregnancy, prolactin (PRL) level may increase up to or more than 200 ng/ml.[5,6] In presence of estrogen, more blood supply to the pituitary is derived from systemic circulation, which has a low dopamine concentration and lesser from the hypothalamo-pituitary portal circulation, which is high in dopamine.[7] Role of progesterone on PRL secretion has also been suggested.[8] Although baseline serum PRL levels are elevated, response to stimuli like TRH and sleep are maintained.[9]
Thyroid axis
During pregnancy, a number of physiological changes occur in the size of the thyroid gland and level of thyroid hormones. High levels of estrogen increase the binding proteins and the bound form of thyroid hormone increases. There is almost 50% physiological increase in total thyroxine (T4) although the free form remains unchanged.[10] Because of thyroid stimulating hormone (TSH)-like activity of human chorionic gonadotropin (HCG) during first trimester, there is increased production of thyroid hormones and a marginal decrease in TSH level.[11] During the second and third trimester, the HCG stimulation of thyroid gland decreases while maternal levels of total T4 continue to be above, and TSH below the reference values of non-pregnant state.[12] Because of increase in maternal blood volume, renal iodine clearance and metabolism of thyroid hormones by placenta, thyroid gland size and vascularity increases.[13]
Gonadotropin axis
Gonadotrophs constitute 7 to 15% of anterior pituitary cells and are located in the lateral portion of the gland. Gonadotroph cells decrease during pregnancy and normalize at one year after delivery.[14] Basal level of gonadotropins decrease starting from 6-7 weeks and remain undetectable thereafter.[14] Response of gonadotropins to gonadotroph releasing hormone (GnRH) is also blunted in parallel with the decrease in gonadotropin levels. These changes are due to feedback inhibition from elevated circulating levels of estrogen, progesterone and PRL.[15]
Growth hormone axis
During pregnancy there is decrease in the number of somatotroph cells. This results in decreased circulating GH levels as measured by conventional radio immunoassay (RIA) and immunoradiometric assay (IRMA) methods. Despite this, maternal levels of insulin like growth factor (IGF) 1 are slightly elevated.[16,17] Many clinicians are aware that pregnancy is associated with pseudo-acromegaloid state. This is because of production of placental GH (GHV) from syncytiotrophoblast, which binds to hepatic GH receptor and stimulates IGF 1. GHV decreases pituitary GH production by stimulating IGF1 production.[18] If GH-like activity is measured during pregnancy, only 3% of the activity is explained by maternal GH and 12% by placental lactogen.[19] In presence of increasing amount of estrogen during pregnancy, an enhanced GH response to provocative testing would be expected since estrogen facilitates GH secretion.[20] However, high levels of estrogen block the normal stimulation of IGF-1 production by GH.[21,22]
Pituitary adrenal axis
In pregnancy, maternal hypothalamic-pituitary axis is activated, which leads to an increase in plasma adrenocorticotropic hormone (ACTH), cortisol (both free and total) and urinary free cortisol. ACTH level progressively increases followed by final surge during labor.[23] Placenta also produces ACTH, but is impermeable to maternal ACTH.[24] The contribution of pituitary and placental ACTH during various phases of pregnancy is not known. However, the ACTH present in plasma is highly pulsate and maintains diurnal variation suggesting that majority of ACTH is from pituitary source.[22] The response of adrenals to stimulation and suppression during pregnancy are different. Whereas there is hyper response to stimulation of adrenals, there is incomplete suppression in response to administration of dexamethasone.[25,26] The range of plasma cortisol concentration in pregnancy overlaps the level seen in Cushing's syndrome although diurnal variation is preserved.[27,28]
Posterior pituitary
Major shifts of fluid during normal pregnancy produce a decreased plasma osmolality of about 10 mosmol/kg and decrease in plasma sodium of 4-5 mEq/L. The shift in osmotic threshold appears at about 5 to 8 weeks of gestation and persists throughout pregnancy, returning to normal by 2 weeks after delivery.[29,30] Though the set point for release of AVP is decreased, peripheral plasma vasopressin level and its response to changes in plasma osmolality remain unchanged.[31,32]
PROLACTINOMAS AND PREGNANCY
Prolactinoma are the commonest form of pituitary tumors accounting for 50% of the functioning pituitary adenomas.[33] These are predominantly benign tumors and classified into microprolactinomas (size <10 mm) or macroprolactinomas (size ≥10 mm). Macroprolactinomas are less common and seen more in men. With the availability of medical treatment for hyperprolactinemia and amenorrhea, pregnancy in these women is quite common. Dopamine agonists (DA) rapidly restore fertility in infertile women with hyperprolactinemia and amenorrhea Estrogen derived from placenta stimulates prolactin secretion and causes hypertrophy of lactotrophs.[34] Because of this there is fear of increase in size of prolactinomas during pregnancy.
Dopamine agonists during pregnancy
Dopamine binds to receptors on lactotroph cells in the anterior pituitary and downregulates adenyl cyclase resulting in inhibition of PRL secretion. All DA cross placenta.[35] Among DA, there is maximum experience with the use of bromocriptine during pregnancy. In more than 6000 pregnancies where bromocriptine was used at conception, the incidence of abortions, ectopic/multiple pregnancies and congenital malformations in the offspring were not higher than from the control population.[36] The first trimester of pregnancy is the period when teratogenicity is highest with any drug, at the same time growth of prolactinoma is the least. It is recommended to use barrier contraception until the first 2-3 cycles have occurred and to stop the drug once pregnancy is confirmed.[37] In this way the gestational exposure to bromocriptine will be limited to 3-4 weeks only. Data on safety of cabergoline is accumulating. Recent data of 789 pregnant women is available. The incidence of abortions, stillbirths, ectopic pregnancy, hydatidiform moles, low birth weight babies and neonatal malformations are comparable to that of general population. The incidence of congenital malformation in patients taking cabergoline is reported around 2.2% compared to 1.8% in bromocriptine and 3% in general population.[38] Lebbe et al. recently reproduced the early findings on the safety of cabergoline on 100 pregnancies treated with cabergoline.[39] Given the safety profile of cabergoline, it is now increasingly being used as first line treatment in women with hyperprolactinemia who desire pregnancy.
Effect of pregnancy on prolactinoma and its management
Microprolactinoma
Microadenomas follow a being course and tumor regrowth occurs in about 1.3% of patients.[37] DA need to be stopped once the pregnancy is confirmed. Patients are followed monthly for symptoms of tumor expansion like headache and visual disturbances and every trimester for visual field testing. In case of symptoms and appearance of visual field defects, a non-contrast MRI is to be performed after the first trimester. If tumor expansion is documented, DA can be restarted and an eye is kept on visual symptoms and field of vision. Women who had previously responded to DA and had received it for a sufficient time before pregnancy are good indicators of response during pregnancy.[40] Surgical intervention is not required for microprolactinoma.[41]
Macroprolactinoma
Enlargement of macroprolactinoma is a significant issue and is quite common especially in women who did not have surgery or radiation before conception. In a large series, 26% of women without a previous surgery had significant tumor enlargement during pregnancy against 3% of those who previously had received surgery or radiation.[42] There are two options for management of women with macroprolactinoma during pregnancy. First is to stop DA after confirmation of pregnancy and monitor closely during pregnancy. Monitoring includes looking for appearance of headache and visual symptoms every month, visual field examination every two months and non-contrast MRI after the first trimester. If there is increase in tumor size, DA is to be restarted. The second option is to continue DA throughout pregnancy and monitor as in macroadenoma. This option is preferred if tumor is outside the sellar boundaries. If MRI after first trimester reveals further enlargement of the mass despite continuation of DA, either delivery if the term is nearer or trans-sphenoid surgery (TSS) if term is not close are the choices, keeping in mind that there is increased risk of abortions with TSS.[40,41] Some experience about monitoring of serum PRL levels during pregnancy has been published from India. In two cases of macroprolactinoma after monthly monitoring of serum PRL levels, bromocriptine was started in fifth month when serum PRL level was >250 ng/ml. It is suggested that in situations where facility for doing periodic MRI is not available, monthly monitoring of PRL levels and timely institution of bromocriptine is an option.[42] At present, however, serum PRL is not considered as a tool for monitoring prolactinoma growth, because PRL normally rises as pregnancy advances; also PRL may not increase in all patients of prolactinoma.[43] After delivery, it is recommended to measure serum PRL after two months and repeat an MRI two months after the end of lactation.[44,45] DA can be stopped if woman wishes to breastfeed the baby, if women have to be continued on DA to prevent tumor re-growth it should be continued though lactation may be impaired.[46]
PREGNANCY AND CUSHING'S SYNDROME
Pregnancy rarely occurs in untreated Cushing's syndrome (CS) because hypercortisolism exerts profound effect on reproductive axis preventing normal follicular development and ovulation.[47] Spontaneous exacerbation and amelioration of symptoms during pregnancy or post-partum have been reported.[48] A few cases of true pregnancy-induced CS are also reported which completely reversed after pregnancy.[49] Cushing's disease, if left untreated, can cause high blood pressure, diabetes, preeclampsia, infection, pulmonary edema, premature birth and intrauterine growth retardation.[50] Because of profound physiological changes, diagnosis of CD during pregnancy may be difficult and high clinical suspicion is needed. Striae, hypertension and gestational diabetes are the most common signs of CS in pregnant women; however, these occur in normal pregnancy also. Furthermore, proximal muscle weakness, spontaneous bruising and wide purple striae favor diagnosis of CS.[51] Adrenal etiology is the commonest cause of CS followed by pituitary and less commonly an ectopic tumor.[52] Biochemical evaluation of CS during pregnancy is complicated, because estrogen-induced production of cortisol binding globulin (CBG) coupled with increase in placental production of ACTH and CRH lead to increase in total and free cortisol level.[53] There are some suggestions while evaluating a woman for CS. Diurnal variation in cortisol is altered in women with CS but not in normal pregnancy and late night cortisol of >5 μg/dl or >50% of morning cortisol are suggestive of CS.[27] Similarly urinary free cortisol greater than three times the upper limit in the last two trimesters indicates CS. In addition, increase in urinary 17-hydroxycorticosteroid excretion may be because of CS and not because of pregnancy.[54] There is little role of low-dose dexamethasone test in evaluating a woman with suspected CS. Plasma ACTH levels may be high normal in pregnant women with pituitary-dependent Cushing's disease, and detectable levels do not rule out adrenal etiology.[52] A high dose dexamethasone suppression test may be helpful in differentiating between CD and ectopic CS.[28] If pituitary disease is suspected, MRI can be performed. Adrenal CT should be avoided and ultrasound can be used for evaluation of adrenals but is less helpful.[55]
Treatment of CS during pregnancy must be individualized. During first trimester, the first line of treatment is medical with surgical resection to follow. Among drugs, either metyrapone or ketoconazole can be used though both have side effects, but metyrapone is preferred.[55,56] Mitotane is teratogenic and therapeutic abortion is required in case of exposure. Data from India regarding CS and pregnancy is limited to single case reports. Kriplani et al. reported a 25-year-old woman in whom CD was diagnosed during pregnancy. Pregnancy was complicated by pregnancy-induced hypertension, glucose intolerance, prematurity and IUGR.[57] In one of recent case report, Raju et al. reported a case of pregnancy and CS, which was complicated by transient adrenal insufficiency in the neonate.[58]
PREGNANCY AND ACROMEGALY
Pregnancy is rare in acromegaly, because expanding tumor mass decreases gonadotroph and or GnRH reserve and secretion. Simultaneous occurrence of hyperprolactinemia also impairs fertility.[46] Acromegaly occurring during pregnancy is associated with increased risk of pregnancy-related diabetes and hypertension; increased birth weight has also been reported in some women.[59] Maternal effects are more often seen in women in whom GH and IGF levels were not controlled before pregnancy. The enlargement of pituitary mass occurs less frequently and is mostly limited to macroadenomas. In a recent study, only 3 out of 27 cases of acromegaly had increase in size of pituitary mass whereas 22 remained stable during pregnancy.[60] An increased risk of pituitary hemorrhage has been reported during pregnancy.[61] Diagnosis of acromegaly during pregnancy is difficult because of increased production of GHV from placenta and subsequent generation of IGF-1. Two important features of pituitary GH differentiate it from placental variant, pituitary GH is highly pulsatile and responds to TRH stimulation.[62] Treatment options of acromegaly during pregnancy include careful observation; medical treatment or surgery are less favorable options. Bromocriptine suppresses GH level less than 5 μg/L in not more than 15% of these patients and is usually effective in case of co-secretion of GH and PRL.[18] Uncomplicated delivery has been reported when bromocriptine treatment was continued during pregnancy.[63] Although octreotide is assigned to category B drug in pregnancy, safety data about this drug is accumulating.[64] Octreotide has been successfully used in pregnant patients until confirmation of pregnancy.[65] There are also reports of octreotide use during early pregnancy.[66] Recently lanreotide has been used in pregnant women without any deleterious side effect on fetus.[67] Koshy et al. recently reported a 30-year-old woman who presented with sudden diminution of vision. On investigations was found to have biochemical evidence of GH excess, and MRI revealed a pituitary macroadenoma. She was operated in 22nd week of pregnancy and delivered a normal baby. She was later on treated with gamma knife after delivery.[68]
LYMPHOCYTIC HYPOPHYSITIS
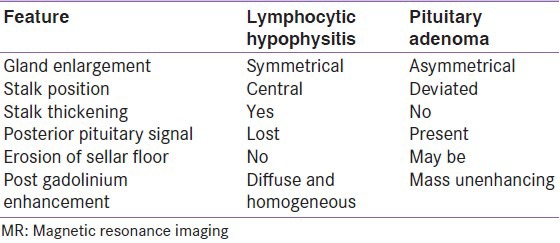
Lymphocytic hypophysitis (LH) also called autoimmune hypophysitis is the most common among inflammations affecting pituitary gland. LH is six times more common in women and shows a striking association with pregnancy;[69] patients present in last month of pregnancy or immediately after delivery; although, it can occur in men as well as in children.[70] It can involve predominantly anterior pituitary (lymphocytic adenohypophysis) or posterior pituitary (infundibuloneurohypophysitis). LH may be associated with other autoimmune disorders especially Hashimoto's thyroiditis.[71] Though many autoimmune diseases go into remission during pregnancy, LH manifests during pregnancy. The cause of this paradox is not completely understood but there are some explanations. First pituitary enlarges during pregnancy, which may lead to release of pituitary antibodies.[1] During pregnancy, pattern of pituitary blood-flow changes such that it derives more blood from systemic circulation and less from hypothalamic-pituitary portal system; it is thus possible that pituitary becomes more accessible to the immune system.[72,73] Clinical picture of LH is variable and may present either symptoms related to sellar compression, hypopituitarism, diabetes insipidus and hyperprolactinemia. The cause of this paradox is possibly related to increased exposure of pituitary to systemic blood and consequently more accessibility to immune system.[72,73] The natural history of LH is that of progression from inflammation to atrophy seen as empty sella on MRI.[74] It is usually important to differentiate these conditions from Sheehan's syndrome because both occur in postpartum period. Common differentiating features between LH and Sheehan's syndrome (SS) are given in Table 2. LH should be suspected if following three features are present: 1) Symptoms occur during or soon after pregnancy; 2) ACTH and/or TSH deficiency is present with normal gonadotropin and GH secretion and 3) diffuse contrast enhancement following gadolinium on MR imaging.[75] LH can sometimes be confused with pituitary adenoma on imaging; some differentiating features between the two are given in Table 3. Once diagnosis of LH is convincingly supported, pituitary hormone deficiencies should be replaced. Since LH is an autoimmune disease, treatment with steroids is advocated, which often resolves sellar mass and improves endocrine dysfunction. Surgery should be opted if patient has visual impairment. Transsphenoidal surgery may require to confirm diagnosis and to relieve symptoms of compression. There are a few reported cases of lymphocytic hypophysitis from India. In most of the cases, diagnosis has been established in surgical specimen biopsies.[76,77,78,79,80] In one of the cases, LH was believed to be recurrent and associated with hypercortisolism.[81]
Table 2.
Common differentiating features between Sheehan's syndrome and lymphocytic hypophysitis
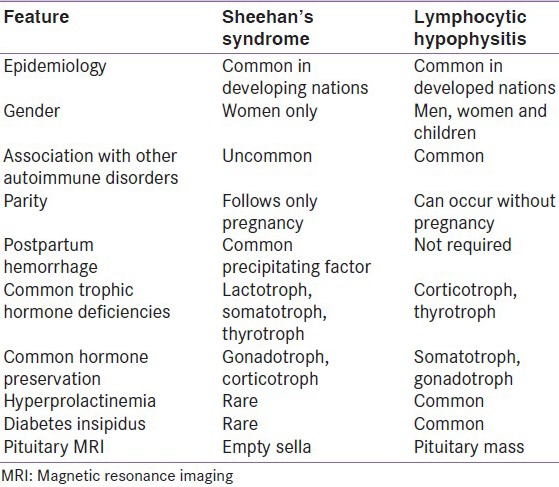
Table 3.
Differentiating features on MR imaging between lymphocytic hypophysitis and pituitary adenoma
SHEEHAN'S SYNDROME
SS means deficiency of anterior pituitary hormone resulting from infarction and necrosis of the physiologically enlarged pituitary gland of pregnancy, usually preceded by postpartum hemorrhage (PPH). Till 1938 the disease was known as Simmond's disease and was believed to be due to thrombosis or bacterial emboli in hypophyseal arteries. Sheehan later discovered that Simmond's disease was due to necrosis of anterior pituitary following PPH.[82,83] Though disease is now rare in developed world, it continues to be the common cause of hypopituitarism in developing countries.[84,85] Pituitary enlargement during pregnancy results in compression of superior hypophyseal artery, any hypotension around childbirth causes arterial spasm in smaller vessels, apoplexy and subsequent pituitary necrosis.[86] Pathogenesis of SS is not clear. Role of autoimmunity in the development of hypopituitarism has been suggested. It is believed that tissue necrosis could release sequestered antigens, triggering pituitary autoimmunity and delayed hypopituitarism.[87]
Variable patterns of pituitary hormone deficiency have been observed in patients with SS. Disease presents with lactation failure, failure of resumption of menstrual cycles, symptoms of hypothyroidism and hypoadrenal state.[88] Preservation of one or more of the anterior pituitary hormone secretion can occur. Sparing or recovery of gonadotroph function and consequent pregnancy has been reported in some cases.[89,90] GH and PRL producing cells are mostly confined to the lower and lateral regions of the pituitary gland and are most susceptible to damage following ischemic necrosis. Because of this, an absence of PRL rise after TRH was considered to be the most sensitive screening test in patients with SS.[91]
One of the first presentations of SS is failure of lactation; however, SS can be associated with hyperprolactinemia also.[92,93] Rarely PRL function can recover after initial failure.[94] Classically SS presents with all the anterior pituitary hormone deficiencies. Clinical features in these patients include severe hypothyroidism, fine wrinkling around mouth suggestive of GH deficiency, hypotension and hypopigmentation suggestive of ACTH deficiency and loss of secondary sex characters suggestive of gonadotroph deficiency [Figure 1]. In one series, from our center 100% of patients had GH deficiency and 85% had corticotroph deficiency documented on insulin tolerance test. Gonadotroph, lactotroph and thyrotroph failure was detected in 98%, 94% and 70% of patients, respectively.[84] Selective preservation of some of the trophic hormones has also been reported and patients could rarely have a normal lactation, regular cycles and subsequent pregnancy.[95,96] Posterior pituitary dysfunction leading to diabetes insipidus is considered rare; however, partial defects have recently been reported.[97,98] Little attention has been given to so-called non-endocrine features, which may be the only presentation in these patients. Anemia and other cytopenias are common in these patients. We recently demonstrated that around 80% of these patients have normocytic and normochromic anemia, which responds to replacement of thyroxine and prednisolone.[99] These patients may also present with pancytopenia with hypocellular marrow, and replacement of thyroxine and prednisolone results complete reversibility.[100] Cardiac abnormalities thought to be common in patients with primary hypothyroidism have also been reported in patients with SS; dilated cardiomyopathy in these patients has been reported and we believe other cardiac abnormalities are as common in these patients as in patients with primary hypothyroidism.[101] These patients may either present with psychiatric manifestations or later may appear after replacement of glucocorticoids.[102] There is limited data available on the prevalence of SS in general community. In a previous study from this place, 3% of 8730 parous females aged 20-40 years were suspected to have SS, which was proved in one-third of them.[85]
Figure 1.
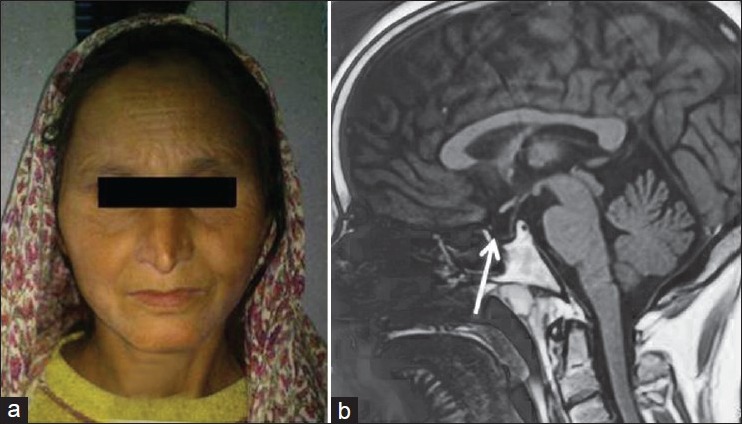
(a) Facial features of Sheehan's syndrome showing fine wrinkling of face, loss of eyebrows laterally, skin hypopigmentation. (b) T1-weighted sagittal MRI image showing pituitary gland filled with cerebrospinal fluid and stalk touching the base of floor features indicative of empty sella
Diagnosis of SS is simple in an area where it is common. Clinical hypopituitarism associated with low or inappropriately normal basal pituitary hormones in the context of previous pregnancy, without any pituitary mass lesion on imaging makes a diagnosis of disorder likely. Imaging studies preferably MRI reveal a small pituitary with partial or complete empty sella [Figure 1]. The diagnostic criteria for Sheehan's syndrome have recently been proposed. These include: (a) History of postpartum hemorrhage or failure of lactation and/or amenorrhea following last child birth; (b) more than one anterior pituitary hormone deficiency and (c) empty sella on MR imaging.[88]
Treatment of SS consists of replacement of deficient hormones. Glucocorticoids are replaced without the need for fludrocortisones and are to be started before replacing thyroxine; hypogonadism increases the risk of osteoporosis and causes decrease in secondary sex characters, so replacement is needed especially in premenopausal women. GH replacement has recently been shown to improve quality of life in these patients.[103]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Gonzalez JG, Elizondo G, Saldivar D, Nanez H, Todd LE, Villarreal JZ. Pituitary gland growth during normal pregnancy: An in vivo study using magnetic resonance imaging. Am J Med. 1988;85:217–20. doi: 10.1016/s0002-9343(88)80346-2. [DOI] [PubMed] [Google Scholar]
- 2.Dinc H, Esen F, Demirci A, Sari A, Resit GH. Pituitary dimensions and volume measurements in pregnancy and post-partum MR assessment. Acta Radiol. 1998;39:64–9. doi: 10.1080/02841859809172152. [DOI] [PubMed] [Google Scholar]
- 3.Elster AD, Sander TH, Vines FS, Chen MY. Size and shape of the pituitary gland during pregnancy and postpartum measurement with MR imaging. Radiology. 1991;181:531–5. doi: 10.1148/radiology.181.2.1924800. [DOI] [PubMed] [Google Scholar]
- 4.Buster JE, Abrahm GE. The hormone applications of steroid hormone radioimmunoassay to clinical obstetrics. Obstet Gynecol. 1975;46:489–99. [PubMed] [Google Scholar]
- 5.Horseman ND. Prolactin. In: De Groo LJ, Jameson JL, editors. Endocrinology. 6th ed. Philadelphia: Saunders; 2010. pp. 165–78. [Google Scholar]
- 6.Rigg LA, Lein A, Yen SS. Pattern of increase in circulating prolactin levels during human gestation. Am J Obstet Gynecol. 1977;129:454–6. doi: 10.1016/0002-9378(77)90594-4. [DOI] [PubMed] [Google Scholar]
- 7.Elias KA, Weiner RI. Direct arterial vascularization of estrogen-induced prolactin-secreting anterior pituitary tumors. Proc Natl Acad Sci U S A. 1984;81:4549–53. doi: 10.1073/pnas.81.14.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rakoff JS, Yen SS. Progesterone incuces acute release of prolactin in estrogen primed ovariectomized women. J Clin Endocrinol Metab. 1978;47:918–21. doi: 10.1210/jcem-47-4-918. [DOI] [PubMed] [Google Scholar]
- 9.Quigley ME, Ishizuka B, Ropert JF, Yen SS. The food-entrained prolactin and cortisol release in late pregnancy and prolactinoma patients. J Clin Endocrinol Metab. 1982;54:1109–12. doi: 10.1210/jcem-54-6-1109. [DOI] [PubMed] [Google Scholar]
- 10.Glinoer D, de Nayer P, Bourdoux P, Lemone M, Robyn C, Van Steirteghem A, et al. Regulation of maternal thyroid during pregnancy. J Clin Endocrinol Metab. 1990;71:276–87. doi: 10.1210/jcem-71-2-276. [DOI] [PubMed] [Google Scholar]
- 11.Berghout A, Wiessinga W. Thyroid size and thyroid function during pregnancy: An analysis. Eur J Endocrinol. 1998;138:536–42. doi: 10.1530/eje.0.1380536. [DOI] [PubMed] [Google Scholar]
- 12.Boas M, Forman JL, Juul A, Feldt-Rasmussen U, Skakkebaek NE, Hilsted L, et al. Narrow intra-individual variation of maternal thyroid function in pregnancy based on a longitudinal study on 132 women. Eur J Endocrinol. 2009;161:903–10. doi: 10.1530/EJE-09-0579. [DOI] [PubMed] [Google Scholar]
- 13.Glinoer D, Soto MF, Bourdoux P, Lejeune B, Delange F, Lemone M, et al. Pregnancy in patients with mild thyroid abnormalities: Maternal and neonatal repercussions. J Clin Endocrinol Metab. 1991;73:421–7. doi: 10.1210/jcem-73-2-421. [DOI] [PubMed] [Google Scholar]
- 14.Reyes FI, Winter JS, Faiman C. Pituitary gonadotropin function during human pregnancy: Serum FSH and LH levels before and after LHRH administration. J Clin Endocrinol Metab. 1976;42:590–2. doi: 10.1210/jcem-42-3-590. [DOI] [PubMed] [Google Scholar]
- 15.Foyouzi N, Frisbaek Y, Norwitz ER. Pituitary gland and pregnancy. Obstet Gynecol Clin North Am. 2004;31:873–92. doi: 10.1016/j.ogc.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Daughaday WH, Trivedi B, Winn HN, Yan H. Hypersomatotropism in pregnant women, as measured by a human liver radioreceptor assay. J Clin Endocrinol Metab. 1990;70:215–21. doi: 10.1210/jcem-70-1-215. [DOI] [PubMed] [Google Scholar]
- 17.Merimee TJ, Zapf J, Froesch ER. Insulin-like growth factors in the fed and fasted states. J Clin Endocrinol Metab. 1982;55:999–1002. doi: 10.1210/jcem-55-5-999. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson L, Frankenne F, Edèn S, Hennen G, Von Schoultz B. Growth hormone 24-h serum profiles during pregnancy: Lack of pulsatility for the secretion of the placental variant. Br J Obstet Gynaecol. 1989;96:949–53. doi: 10.1111/j.1471-0528.1989.tb03352.x. [DOI] [PubMed] [Google Scholar]
- 19.Feldt-Rasmussen U, Mathiesen ER. Endocrine disorders in pregnancy: Physiological and hormonal aspects of pregnancy. Best Pract Res Clin Endocrinol Metab. 2011;25:875–84. doi: 10.1016/j.beem.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Stone RT, Maurer RA, Gorski J. Effect of estradiol-17 beta on preprolactin messenger ribonucleic acid activity in the rat pituitary gland. Biochemistry. 1977;16:4915–21. doi: 10.1021/bi00641a027. [DOI] [PubMed] [Google Scholar]
- 21.Frantz AG, Rabkin MT. Effect of estrogen and sex differences on secretion of human growth hormone. J Clin Endocrinol Metab. 1965;25:1470–80. doi: 10.1210/jcem-25-11-1470. [DOI] [PubMed] [Google Scholar]
- 22.Clemmons DR, Underwood LE, Ridgway EC, Kliman B, Kjellberg RN, Van Wyk JJ. Estradiol treatment of acromegaly. Reduction of immunoreactive somatomedin-C and improvement in metabolic status. Am J Med. 1980;69:571–5. doi: 10.1016/0002-9343(80)90470-2. [DOI] [PubMed] [Google Scholar]
- 23.Carr BR, Parker CR, Jr, Madden JD, MacDonald PC, Porter JC. Maternal plasma adrenocorticotropin and cortisol relationships throughout human pregnancy. Am J Obstet Gynecol. 1981;139:416–22. doi: 10.1016/0002-9378(81)90318-5. [DOI] [PubMed] [Google Scholar]
- 24.Demey-Ponsart E, Foidart JM, Sulon J, Sodoyez JC. Serum CBG, free and total cortisol and circadian patterns of adrenal function in normal pregnancy. J Steroid Biochem. 1982;16:165–9. doi: 10.1016/0022-4731(82)90163-7. [DOI] [PubMed] [Google Scholar]
- 25.Nolten WE, Rueckert PA. Elevated free cortisol index in pregnancy: Possible regulatory mechanisms. Am J Obstet Gynecol. 1981;139:492–8. doi: 10.1016/0002-9378(81)90331-8. [DOI] [PubMed] [Google Scholar]
- 26.Rees LH, Burke CW, Chard T, Evans SW, Letchworth AT. Possible placental origin of ACTH in normal human pregnancy. Nature. 1975;254:620–2. doi: 10.1038/254620b0. [DOI] [PubMed] [Google Scholar]
- 27.Lindsay JR, Nieman LK. The hypothalamic-pituitary-adrenal axis in pregnancy: Challenges in disease detection and treatment. Endocr Rev. 2005;26:775–99. doi: 10.1210/er.2004-0025. [DOI] [PubMed] [Google Scholar]
- 28.Cousins L, Rigg L, Hollingsworth D, Meis P, Halberg F, Brink G, et al. Qualitative and quantitative assessment of the circadian rhythm of cortisol in pregnancy. Am J Obstet Gynecol. 1983;145:411–6. doi: 10.1016/0002-9378(83)90309-5. [DOI] [PubMed] [Google Scholar]
- 29.Davison JM, Vallotton MB, Lindheimer MD. Plasma osmolality and urinary concentration and dilution during and after pregnancy: Evidence that lateral recumbency inhibits maximal urinary concentrating ability. Br J Obstet Gynaecol. 1981;88:472–9. doi: 10.1111/j.1471-0528.1981.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 30.Hime MC, Richardson JA. Diabetes insipidus and pregnancy: Case report, incidence and review of literature. Obstet Gynecol Surv. 1978;33:375–9. doi: 10.1097/00006254-197806000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Davison JM, Gilmore EA, Dürr J, Robertson GL, Lindheimer MD. Altered osmotic thresholds for vasopressin secretion and thirst in human pregnancy. Am J Physiol. 1984;246:F105–9. doi: 10.1152/ajprenal.1984.246.1.F105. [DOI] [PubMed] [Google Scholar]
- 32.Robertson GL. Diabetes insipidus. Endocrinol Metab Clin North Am. 1995;24:549–72. [PubMed] [Google Scholar]
- 33.Hashim IA, Aston R, Butler J, McGregor AM, Smith CR, Norman M. The proportion of glycosylated prolactin in serum is decreased in hyperprolactinemic states. J Clin Endocrinol Metab. 1990;71:111–5. doi: 10.1210/jcem-71-1-111. [DOI] [PubMed] [Google Scholar]
- 34.Karaca Z, Tanriverdi F, Unluhizarci K, Kelestimur F. Pregnancy and pituitary disorders. Eur J Endocrinol. 2010;162:453–75. doi: 10.1530/EJE-09-0923. [DOI] [PubMed] [Google Scholar]
- 35.Heaney AP, Fernando M, Melmed S. Functional role of estrogen in pituitary tumor pathogenesis. J Clin Invest. 2002;109:277–83. doi: 10.1172/JCI14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krupp P, Monka C. Bromocriptine in pregnancy: Safety aspects. Klin Wochenschr. 1987;65:823–7. doi: 10.1007/BF01727477. [DOI] [PubMed] [Google Scholar]
- 37.Casanueva FF, Molitch ME, Schlechte JA, Abs R, Bonert V, Bronstein MD, et al. Guidelines of the pituitary society for the diagnosis and management of prolactinomas. Clin Endocrinol (Oxf) 2006;65:265–73. doi: 10.1111/j.1365-2265.2006.02562.x. [DOI] [PubMed] [Google Scholar]
- 38.Molitch ME. Prolactinoma in pregnancy. Best Pract Res Clin Endocrinol Metab. 2011;25:885–96. doi: 10.1016/j.beem.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 39.Lebbe M, Hubinont C, Bernard P, Maiter D. Outcome of 100 pregnancies initiated under treatment with cabergoline in hyperprolactinaemic women. Clin Endocrinol (Oxf) 2010;73:236–42. doi: 10.1111/j.1365-2265.2010.03808.x. [DOI] [PubMed] [Google Scholar]
- 40.Motivala S, Gologorsky Y, Kostandinov J, Post KD. Pituitary disorders during pregnancy. Endocrinol Metab Clin North Am. 2011;40:827–36. doi: 10.1016/j.ecl.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Musolino NR, Bronstein MD. Prolactinomas pregnancy. In: Bronstein MD, editor. Pituitary tumors in pregnancy. Boston: Kluwer Academic Publishers; 2001. pp. 91–108. [Google Scholar]
- 42.Kulshreshtha B, Jyotsna VP, Kriplani A, Kumar S, Seith S, Ammini AC. Management of macroprolactinomas during pregnancy-report of two cases. Indian J Endocrinol Metab. 2007;11:35–7. [Google Scholar]
- 43.Divers WA, Jr, Yen SS. Prolactin-producing microadenoma in pregnancy. Obstet Gynecol. 1983;62:425–9. [PubMed] [Google Scholar]
- 44.Molitch ME. Management of prolactinomas during pregnancy. J Reprod Med. 1999;44(12 Suppl):S1121–6. [PubMed] [Google Scholar]
- 45.Christin-Maître S, Delemer B, Touraine P, Young J. Prolactinoma and estrogens: Pregnancy, contraception and hormonal replacement therapy. Ann Endocrinol (Paris) 2007;68:106–12. doi: 10.1016/j.ando.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Bronstein MD, Salgado LR, de Castro Musolino NR. Medical management of pituitary adenomas: The special case of management of the pregnant woman. Pituitary. 2002;5:99–107. doi: 10.1023/a:1022364514971. [DOI] [PubMed] [Google Scholar]
- 47.Lado-Abeal J, Rodriguez-Arnao J, Newell-Price JD, Perry LA, Grossman AB, Besser GM, et al. Menstrual abnormalities in women with Cushing's disease are correlated with hypercortisolemia rather than raised circulating androgen levels. J Clin Endocrinol Metab. 1998;83:3083–8. doi: 10.1210/jcem.83.9.5084. [DOI] [PubMed] [Google Scholar]
- 48.Guilhaume B, Sanson ML, Billaud L, Bertagna X, Laudat MH, Luton JP. Cushing's syndrome and pregnancy: Aetiologies and prognosis in twenty-two patients. Eur J Med. 1992;1:83–9. [PubMed] [Google Scholar]
- 49.Miyoshi T, Otsuka F, Suzuki J, Inagaki K, Takeda M, Kano Y, et al. Periodic secretion of adrenocorticotropin in a patient with Cushing's disease manifested during pregnancy. Endocr J. 2005;52:287–92. doi: 10.1507/endocrj.52.287. [DOI] [PubMed] [Google Scholar]
- 50.Sheeler LR. Cushing's syndrome and pregnancy. Endocrinol Metab Clin North Am. 1994;23:619–27. [PubMed] [Google Scholar]
- 51.Chico A, Manzanares JM, Halperin I, Martinez de Osaba MJ, Adelantado J, Webb SM. Cushing's disease and pregnancy: Report of six cases. Eur J Obstet Gynecol Reprod Biol. 1996;64:143–6. doi: 10.1016/0301-2115(95)02258-9. [DOI] [PubMed] [Google Scholar]
- 52.Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, et al. The diagnosis of Cushing's syndrome: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93:1526–40. doi: 10.1210/jc.2008-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kreines K, Perin E, Salzer R. Pregnancy in cushing's syndrome. J Clin Endocrinol Metab. 1964;24:75–9. doi: 10.1210/jcem-24-1-75. [DOI] [PubMed] [Google Scholar]
- 54.Bevan JS, Gough MH, Gillmer MD, Burke CW. Cushing's syndrome in pregnancy: The timing of definitive treatment. Clin Endocrinol (Oxf) 1987;27:225–33. doi: 10.1111/j.1365-2265.1987.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 55.Connell JM, Cordiner J, Davies DL, Fraser R, Frier BM, McPherson SG. Pregnancy complicated by Cushing's syndrome: Potential hazard of metyrapone therapy: Case report. Br J Obstet Gynaecol. 1985;92:1192–5. doi: 10.1111/j.1471-0528.1985.tb03037.x. [DOI] [PubMed] [Google Scholar]
- 56.Leiba S, Weinstein R, Shindel B, Lapidot M, Stern E, Levavi H, et al. The protracted effect of o, p’-DDD in Cushing's disease and its impact on adrenal morphogenesis of young human embryo. Ann Endocrinol (Paris) 1989;50:49–53. [PubMed] [Google Scholar]
- 57.Kriplani A, Buckshee K, Ammini AC. Cushing syndrome complicating pregnancy. Aust N Z J Obstet Gynaecol. 1993;33:428–30. doi: 10.1111/j.1479-828x.1993.tb02130.x. [DOI] [PubMed] [Google Scholar]
- 58.Gopal RA, Acharya SV, Bandgar TR, Menon PS, Shah NS. Cushing disease with pregnancy. Gynecol Endocrinol. 2012;28:533–5. doi: 10.3109/09513590.2011.632789. [DOI] [PubMed] [Google Scholar]
- 59.Caron P, Broussaud S, Bertherat J, Borson-Chazot F, Brue T, Cortet-Rudelli C, et al. Acromegaly and pregnancy: A retrospective multicenter study of 59 pregnancies in 46 women. J Clin Endocrinol Metab. 2010;95:4680–7. doi: 10.1210/jc.2009-2331. [DOI] [PubMed] [Google Scholar]
- 60.Herman-Bonert V, Seliverstov M, Melmed S. Pregnancy in acromegaly: Successful therapeutic outcome. J Clin Endocrinol Metab. 1998;83:727–31. doi: 10.1210/jcem.83.3.4635. [DOI] [PubMed] [Google Scholar]
- 61.Beckers A, Stevenaert A, Foidart JM, Hennen G, Frankenne F. Placental and pituitary growth hormone secretion during pregnancy in acromegalic women. J Clin Endocrinol Metab. 1990;71:725–31. doi: 10.1210/jcem-71-3-725. [DOI] [PubMed] [Google Scholar]
- 62.Frankenne F, Closset J, Gomez F, Scippo ML, Smal J, Hennen G. The physiology of growth hormones (GHs) in pregnant women and partial characterization of the placental GH variant. J Clin Endocrinol Metab. 1988;66:1171–80. doi: 10.1210/jcem-66-6-1171. [DOI] [PubMed] [Google Scholar]
- 63.Luboshitzky R, Dickstein G, Barzilai D. Bromocriptine-induced pregnancy in an acromegalic patient. JAMA. 1980;244:584–6. [PubMed] [Google Scholar]
- 64.Yap AS, Clouston WM, Mortimer RH, Drake RF. Acromegaly first diagnosed in pregnancy: The role of bromocriptine therapy. Am J Obstet Gynecol. 1990;163:477–8. doi: 10.1016/0002-9378(90)91178-f. [DOI] [PubMed] [Google Scholar]
- 65.Takano T, Saito J, Soyama A, Ito H, Iizuka T, Yoshida T, et al. Normal delivery following an uneventful pregnancy in a Japanese acromegalic patient after discontinuation of octreotide long acting release formulation at an early phase of pregnancy. Endocr J. 2006;53:209–12. doi: 10.1507/endocrj.53.209. [DOI] [PubMed] [Google Scholar]
- 66.Mozas J, Ocon E, López de la Torre M, Suarez AM, Miranda JA, Herruzo AJ. Successful pregnancy in a woman with acromegaly treated with somatostatin analog (octreotide) prior to surgical resection. Int J Gynaecol Obstet. 1999;65:71–3. doi: 10.1016/s0020-7292(98)00221-5. [DOI] [PubMed] [Google Scholar]
- 67.Ben Salem Hachmi L, Kammoun I, Bouzid C, Smida H, Nagi S, Turki Z, et al. Management of acromegaly in pregnant woman. Ann Endocrinol (Paris) 2010;71:60–3. doi: 10.1016/j.ando.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 68.Koshy T, Rajaratnam S, Mathews JE, Rajshekhar V. Acromegaly in pregnancy. Indian J Endocrinol Metab. 2012;16:1029–31. doi: 10.4103/2230-8210.103033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scheithauer BW, Sano T, Kovacs KT, Young WF, Jr, Ryan N, Randall RV. The pituitary gland in pregnancy: A clinicopathologic and immunohistochemical study of 69 cases. Mayo Clin Proc. 1990;65:461–74. doi: 10.1016/s0025-6196(12)60946-x. [DOI] [PubMed] [Google Scholar]
- 70.Asa SL, Bilbao JM, Kovacs K, Josse RG, Kreines K. Lymphocytic hypophysitis of pregnancy resulting in hypopituitarism: A distinct clinicopathologic entity. Ann Intern Med. 1981;95:166–71. doi: 10.7326/0003-4819-95-2-166. [DOI] [PubMed] [Google Scholar]
- 71.Goudie RB, Pinkerton PH. Anterior hypophysitis and Hashimoto's disease in a woman. J Pathol Bacteriol. 1962;83:584–5. [PubMed] [Google Scholar]
- 72.Asa SL, Penz G, Kovacs K, Ezrin C. Prolactin cells in the human pituitary. A quantitative immunocytochemical analysis. Arch Pathol Lab Med. 1982;106:360–3. [PubMed] [Google Scholar]
- 73.Van Rodd JJ, Eernisse JG, van Leeuwen A. Leukocyte antibodies in sera from pregnant women. Nature. 1958;181:1735–6. doi: 10.1038/1811735a0. [DOI] [PubMed] [Google Scholar]
- 74.Vanbesien J, Schiettecatte J, Anckaert E, Smitz J, Velkeniers B, De Schepper J. Circulating anti-prolactin auto-antibodies must be considered in the differential diagnosis of hyperprolactinaemia in adolescents. Eur J Pediatr. 2002;161:373–6. doi: 10.1007/s00431-002-0967-z. [DOI] [PubMed] [Google Scholar]
- 75.Unlühizarci K, Bayram F, Colak R, Oztürk F, Selçuklu A, Durak AC, et al. Distinct radiological and clinical appearance of lymphocytic hypophysitis. J Clin Endocrinol Metab. 2001;86:1861–4. doi: 10.1210/jcem.86.5.7440. [DOI] [PubMed] [Google Scholar]
- 76.Naik RG, Ammini A, Shah P, Sarkar C, Mehta VS, Berry M. Lymphocytic hypophysitis. Case report. J Neurosurg. 1994;80:925–7. doi: 10.3171/jns.1994.80.5.0925. [DOI] [PubMed] [Google Scholar]
- 77.Prasad A, Madan VS, Sethi PK, Prasad ML, Buxi TB, Kanwar CK. Lymphocytic hypophysitis: Can open exploration of the sella be avoided? Br J Neurosurg. 1991;5:639–42. doi: 10.3109/02688699109002889. [DOI] [PubMed] [Google Scholar]
- 78.Rao S, Rajkumar A, Kuruvilla S. Sellar lesion: Not always a pituitary adenoma. Indian J Pathol Microbiol. 2008;51:269–70. doi: 10.4103/0377-4929.41688. [DOI] [PubMed] [Google Scholar]
- 79.Rumana M, Kirmani A, Khursheed N, Besina S, Khalil M. Lymphocytic hypophysitis with normal pituitary function mimicking a pituitary adenoma: A case report and review of literature. Clin Neuropathol. 2010;29:26–31. doi: 10.5414/npp29026. [DOI] [PubMed] [Google Scholar]
- 80.Gundgurthi A, Kharb S, Garg MK, Brar KS, Bharwaj R, Gupta S, et al. Combined granulomatous and lymphocytic hypophysitis presenting as pituitary incidentaloma in a middle-aged woman. Indian J Endocrinol Metab. 2012;16:846–9. doi: 10.4103/2230-8210.100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Biswas K, Goyal R, Ammini AC, Karak AK, Sarkar C, Mishra NK, et al. Recurrent lymphocytic hypophysitis in a women 27 year after subtotal adrenalectomy for hypercortisolism possibly of autoimmune origin. J Assoc Physicians India. 2005;53:1066–9. [PubMed] [Google Scholar]
- 82.Sheehan HL. Postpartum necrosis of anterior pituitary. J Pathol Bacteriol. 1937;45:189–214. [Google Scholar]
- 83.Sheehan HL. Simmond's disease due to postpartum necrosis of anterior pituitary. Q J Med. 1939;8:277–309. [Google Scholar]
- 84.Zargar AH, Masoodi SR, Laway BA, Shah NA, Salahuddin M, Siddiqi MA, et al. Clinical spectrum of Sheehan's syndrome. Ann Saudi Med. 1996;16:338–41. doi: 10.5144/0256-4947.1996.338. [DOI] [PubMed] [Google Scholar]
- 85.Zargar AH, Singh B, Laway BA, Masoodi SR, Wani AI, Bashir MI. Epidemiologic aspects of postpartum pituitary hypofunction (Sheehan's syndrome) Fertil Steril. 2005;84:523–8. doi: 10.1016/j.fertnstert.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 86.Dejager S, Gerber S, Foubert L, Turpin G. Sheehan's syndrome: Differential diagnosis in acute phase. J Intern Med. 1988;244:261–6. doi: 10.1046/j.1365-2796.1998.00370.x. [DOI] [PubMed] [Google Scholar]
- 87.Goswami R, Kochupillai N, Crock PA, Jaleel A, Gupta N. Pituitary autoimmunity in patients with Sheehan's syndrome. J Clin Endocrinol Metab. 2002;87:4137–41. doi: 10.1210/jc.2001-020242. [DOI] [PubMed] [Google Scholar]
- 88.Kelestimur F. Sheehan's syndrome. Pituitary. 2003;6:181–8. doi: 10.1023/b:pitu.0000023425.20854.8e. [DOI] [PubMed] [Google Scholar]
- 89.Laway BA, Ganie MA, Wani IR, Butt TP, Zargar AH. Multiple spontaneous pregnancies in Sheehan syndrome with preserved gonadotrophin function. Endocrinologist. 2009;19:253–4. [Google Scholar]
- 90.Zargar AH, Masoodi SR, Laway BA, Sofi FA, Wani AI. Pregnancy in Sheehan's syndrome a report of three cases. J Assoc Physicians India. 1998;46:476–8. [PubMed] [Google Scholar]
- 91.Shahmanesh M, Ali Z, Pourmand M, Nourmand I. Pituitary function tests in Sheehan's syndrome. Clin Endocrinol (Oxf) 1980;12:303–11. doi: 10.1111/j.1365-2265.1980.tb02714.x. [DOI] [PubMed] [Google Scholar]
- 92.Kelestimur F. Hyperprolactinemia in a patient with Sheehan's syndrome. South Med J. 1992;85:1008–10. doi: 10.1097/00007611-199210000-00019. [DOI] [PubMed] [Google Scholar]
- 93.Laway BA, Ganie MA, Bashir MI, Kotwal SK, Mir SA, Gojwari T, et al. Sheehan's syndrome with hyperprolactinemia. Turk Jem. 2010;14:47–9. [Google Scholar]
- 94.Laway BA, Mir SA, Zargar AH. Recovery of prolactin functions following spontaneous pregnancy in a woman with Sheehan's syndrome. Indian J Endocrinol Metab. 2012 doi: 10.4103/2230-8210.123571. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Laway BA, Mir SA, Gojwari T, Shah TR, Zargar AH. Selective preservation of anterior pituitary functions in patients with Sheehan's syndrome. Indian J Endocrinol Metab. 2011;15(Suppl 3):S238–41. doi: 10.4103/2230-8210.84874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zargar AH, Wani AI, Laway BA, Masoodi SR, Salahuddin M. Regular ovulatory cycles in a case of Sheehan's syndrome. J Assoc Physicians India. 1998;46:474–5. [PubMed] [Google Scholar]
- 97.Atmaca H, Tanriverdi F, Gokce C, Unluhizarci K, Kelestimur F. Posterior pituitary function in Sheehan's syndrome. Eur J Endocrinol. 2007;156:563–7. doi: 10.1530/EJE-06-0727. [DOI] [PubMed] [Google Scholar]
- 98.Laway BA, Mir SA, Dar MI, Zargar AH. Sheehan's syndrome with central diabetes insipidus. Arq Bras Endocrinol Metabol. 2011;55:171–4. doi: 10.1590/S0004-27302011000200010. [DOI] [PubMed] [Google Scholar]
- 99.Laway BA, Mir SA, Bashir MI, Bhat JR, Samoon J, Zargar AH. Prevalence of hematological abnormalities in patients with Sheehan's syndrome: Response to replacement of glucocorticoids and thyroxine. Pituitary. 2011;14:39–43. doi: 10.1007/s11102-010-0255-2. [DOI] [PubMed] [Google Scholar]
- 100.Laway BA, Bhat JR, Mir SA, Khan RS, Lone MI, Zargar AH. Sheehan's syndrome with pancytopenia: Complete recovery after hormone replacement (case series and review) Ann Hematol. 2010;89:305–8. doi: 10.1007/s00277-009-0804-9. [DOI] [PubMed] [Google Scholar]
- 101.Laway BA, Alai MS, Gojwari T, Ganie MA, Zargar AH. Sheehan syndrome with reversible dilated cardiomyopathy. Ann Saudi Med. 2010;30:321–4. doi: 10.4103/0256-4947.65269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Laway BA, Shah TR, Bashir MI, Dada AH, Zargar AH. Acute onset psychosis following steroid replacement in Sheehan's syndrome. Acta Endocrinologica (Buc) 2010;6:533–8. [Google Scholar]
- 103.Kelestimur F, Jonsson P, Molvalilar S, Gomez JM, Auernhammer CJ, Colak R, et al. Sheehan's syndrome: Baseline characteristics and effect of 2 years of growth hormone replacement therapy in 91 patients in KIMS-Pfizer International Metabolic Database. Eur J Endocrinol. 2005;152:581–7. doi: 10.1530/eje.1.01881. [DOI] [PubMed] [Google Scholar]


