Abstract
Polyphenols such as epigallocatechin gallate (EGCG) and resveratrol have received a great deal of attention because they may contribute to the purported neuroprotective action of the regular consumption of green tea and red wine. Many studies, including those published by our group, suggest that this protective action includes their abilities to prevent the neurotoxic effects of beta-amyloid, a protein whose accumulation likely plays a pivotal role in Alzheimer's disease. Moreover, the scavenging activities of polyphenols on reactive oxygen species and their inhibitory action of cyclooxygenase likely explain, at least in part, their antioxidant and anti-inflammatory activities. Besides these well-documented properties, the modulatory action of these polyphenols on intracellular signaling pathways related to cell death/survival (e.g., protein kinase C, PKC) has yet to be investigated in detail. Using rat hippocampal neuronal cells, we aimed to investigate here the effects of EGCG and resveratrol on cell death induced by GF 109203X, a selective inhibitor of PKC. The MTT/resazurin and spectrin assays indicated that EGCG and resveratrol protected against GF 109203X-induced cell death and cytoskeleton degeneration, with a maximal effect at 1 and 3 μM, respectively. Moreover, immunofluorescence data revealed that cells treated with these polyphenols increased PKC gamma (γ) activation and promoted neuronal interconnections. Finally, we found that the protective effects of both polyphenols on the cytoskeleton and synaptic plasticity were mediated by the PKCγ subunit. Taken together, the results suggest that PKC, and more specifically its γ subunit, plays a critical role in the protective action of EGCG and resveratrol on neuronal integrity.
Keywords: PKC, polyphenols, neuroprotection, resveratrol, epigallocatechin gallate, hippocampal cultured cells
Introduction
It is well established that the regular consumption of fruit, vegetables, and beverages such as green tea and red wine (in moderation) reduces the risk of developing age-related neurological disorders such as Alzheimer's disease (AD) (Orgogozo et al., 1997; Truelsen et al., 2002; Luchsinger et al., 2004; Dai et al., 2006). Polyphenols present in high amounts in fruit, vegetables, tea, and red wine likely contribute to their beneficial effects.In support of this hypothesis, two follow-up studies reported that regular consumption of polyphenols was inversely correlated with the risk of dementia and cognitive decline (Commenges et al., 2000; Letenneur et al., 2007). Moreover, in vitro and animal studies reported that epigallocatechin gallate (EGCG), the most abundant polyphenol present in green tea, and resveratrol, a stilbene enriched in red wine, exerts neuroprotective actions against the toxicity induced by β-amyloid (Aβ) (Choi et al., 2001; Han et al., 2004; Marambaud et al., 2005; Rezai-Zadeh et al., 2005; Bastianetto et al., 2006; Haque et al., 2008; Lee et al., 2009), a protein whose accumulation plays a pivotal role in AD-related cognitive symptoms (Selkoe, 2000).
The modulation of intracellular effectors has been proposed to explain, at least partly, the effects of polyphenols in neurodegenerative processes. Among other effects, we have shown that protein kinase C (PKC) enzymes are involved in the neuroprotective effect of resveratrol against Aβ-induced neurotoxicity (Han et al., 2004; Bastianetto et al., 2007), while other groups have suggested that EGCG promotes the release of nonamyloidogenic soluble precursor through a PKC-dependent pathway (Levites et al., 2003). Moreover, Levites et al. (2002) have shown that activation of PKC by EGCG is linked to cell survival in Parkinson's disease, suggesting that PKC plays an important role in the neuroprotective action of theses polyphenols.
Recently, among the 12 isoforms of PKC (Newton, 1997), our group has reported that gamma (γ) activity is linked to successful cognitive aging (Menard and Quirion, 2012), while another group found that protein levels of PKCγ are lower in an AD mouse model (Dehvari et al., 2007). It is thus hypothesized that the purported cognitive enhancing properties of polyphenols in memory-impaired animals (Cherniack, 2012) could be due to their stimulatory effects on PKCγ activity. Accordingly, we investigate here the effects of resveratrol and EGCG on PKCγ activation in hippocampal neuronal cells exposed to a broad spectrum inhibitor of PKC known as dihydrochloride3-{1-[3-(dimethylamino) propyl]-1H-indol-3yl}-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione (GF 109203X). Both compounds prevent GF 109203X-induced neuronal death and the disruption of cytoskeleton organization. Interestingly, resveratrol and EGCG increase not only PKCγ (Thr674) phosphorylation, but also the expression of the kinase, suggesting an active role for this enzyme in neuronal cell survival promoted by these polyphenols.
Material and methods
Material
Materials used for cell cultures were obtained from Invitrogen-Gibco BRL (Burlington, Ontario, Canada). Resveratrol, EGCG and other chemicals were purchased from Sigma Chemical Co. (Oakville, Ontario, Canada). All drugs were freshly prepared on the day of the experiment in a final concentration of either ethanol or DMSO not exceeding 0.01%. These solvents at 0.01% (v/v) have no effect by themselves on cell survival (data not shown).
Neuronal hippocampal cell cultures
Enriched rat hippocampal cell cultures were prepared from E19 fetuses obtained from Sprague-Dawley rats (Charles River Canada, St-Constant, Quebec, Canada) as described previously (Bastianetto et al., 2000). Animal care complied with protocols and guidelines of the McGill University Animal Care Committee and the Canadian Council for Animal Care. Hippocampal cells were plated at day 0 at a density of approximately 12 × 104 viable cells per well in 96-well plates. In brief, hippocampal neuronal cells were grown in Dulbecco's modified Eagles medium (D-MEM) high glucose supplemented with 20 mM KCl, 15 mM HEPES and 1% (v/v) serum-free growth medium N2 (final composition: 5 μg/ml insulin, 100 μM putrescine, 20 nM progesterone, 1.0 μg/ml transferrin, 30 nM selenium), and maintained at 37°C in a 95% air/5% CO2 humidified atmosphere. Medium was removed at day 3 and replaced with the same medium until the day of experiment (day 6).
GF 109203X-induced toxicity
GF 109203X (dihydrochloride3-[1-[3-(dimethylamino) propyl]-1H-indol-3yl]-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione) is a potent and selective inhibitor of PKC (Toullec et al., 1991) that has been shown to produce neuronal hippocampal cell death (Han et al., 2004). Briefly, cells were incubated in HEPES-buffered DMEM high-glucose medium and co-treated with GF 109203X (5 μ M) and either EGCG (1–10 μ M) or resveratrol (1–10 μ M). After a 24 h incubation period, cell viability was determined using the MTT and resazurin colorimetric assays (see below).
Assessment of neuronal survival
Neuronal survival was estimated using MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide], a dye that measures the mitochondrial activity of living cells (Bastianetto et al., 2000; Zhang et al., 2004). Cell survival was measured in parallel with resazurin, a widely used indicator of cell viability in mammalian cell cultures (Anoopkumar-Dukie et al., 2005). The resazurin assay is based on the ability of viable, metabolically active cells to reduce resazurin to resorufin and dihydro-resorufin. Toxic insults that impair cell viability affect the capacity to reduce resazurin, and the rate of dye reduction is directly proportional to the number of viable cells present (Vega-Avila and Pugsley, 2011). Cell survival estimated by the MTT and resazurin assays was spectrophotometrically determined at 570 nm. Regarding resazurin assay, background optical density (OD) at 600 nm was subtracted from OD at 570 nm for optimal result. Colorimetric assays were performed using a micro-plate reader (Bio-Tek Instruments® Inc., Ville St-Laurent, Quebec, Canada).
Immunofluorescence
On day 0, hippocampal neurons were plated on poly-d-lysine (25 μ g/mL)-coated 12 mm glass coverslips (Fisher, Nepean, On, Canada) placed in multiwell plates and grown in the same medium as described above. On day 3, the medium was removed, hippocampal cells were washed in PBS (pH 7.4) for 5 min, then fixed in 4% paraformaldehyde. After several PBS washes, cells were permeabilized with 0.2% Tween 20 in PBS for 10 min at room temperature, then processed for immunofluorescence labeling. In brief, sections were first incubated in 10% normal goat serum diluted in 0.1 M PBS with 0.05% Tween 20 (PBST) and 1% BSA for 60 min at room temperature, followed by overnight incubation with primary antibodies at 4°C in a solution of 1% serum and 1% BSA in 0.1 M PBST. The anti-rabbit PKCγ (Thr674) and PKCγ antibodies were purchased from Abcam (Cambridge, MA, USA) and used at 1/100 dilution. To reveal the cytoskeleton structure, a spectrin antibody (1/250 dilution, produced in mouse) was purchased from Cell Signaling (Danvers, MA, USA). After 3 washes in PBS, sections were incubated with corresponding secondary antibodies (1:500, Invitrogen, Carlsbad, CA, USA) conjugated with Alexa Fluor 488 or Alexa Fluor 568 in 1% BSA in PBST for 2 h at room temperature in the dark. Sections were washed three times with PBS for 5 min each in the dark. Nuclei were stained with Hoechst solution (2 μg/ml, Invitrogen, Carlsbad, CA, USA) for 5 min and subsequently the sections were washed and coverslipped with Fluoromount-G (Southern Biotech, Birmingham, AL, USA). Pictures were taken at 40x magnification with an Axio Observer microscope with Apotome (Carl Zeiss MicroImaging GmbH, Germany).
Statistical analyses
OD values reflecting MTT and resazurin reduction were proportional to the number of viable cells. The OD of the control group (vehicle alone) was regarded as 100%. The rate of surviving cells treated with various drugs was expressed as a percentage of control groups. Statistical analysis was performed using One-Way ANOVA followed by a Dunnett's multiple comparison test, with p < 0.05 being considered statistically significant.
Results
Effects of resveratrol and EGCG against toxicity induced by GF 109203X
As described previously (Han et al., 2004), a 24 h treatment with the PKC inhibitor GF 109203X (5 μM) resulted in about 30% hippocampal neuronal cell death, as monitored using MTT and resazurin colorimetric assays (Figures 1A,B). Cell death was strongly reduced, in a concentration-dependent manner, in the presence of resveratrol with maximal effect obtained at 3 μM (Figures 1A). The effect of resveratrol was shared by EGCG, which offered maximal and almost complete protection at 1 μM, as estimated by both colorimetric assays (Figures 1B). Moreover, 24 h exposure to either resveratrol or EGCG in the initial medium (i.e., without GF 109203X) exerted a protective and maximal effect per se at 1–3 μM (MTT and resazurin), and higher concentrations tended to be less effective (Figures 1A,B).
Figure 1.
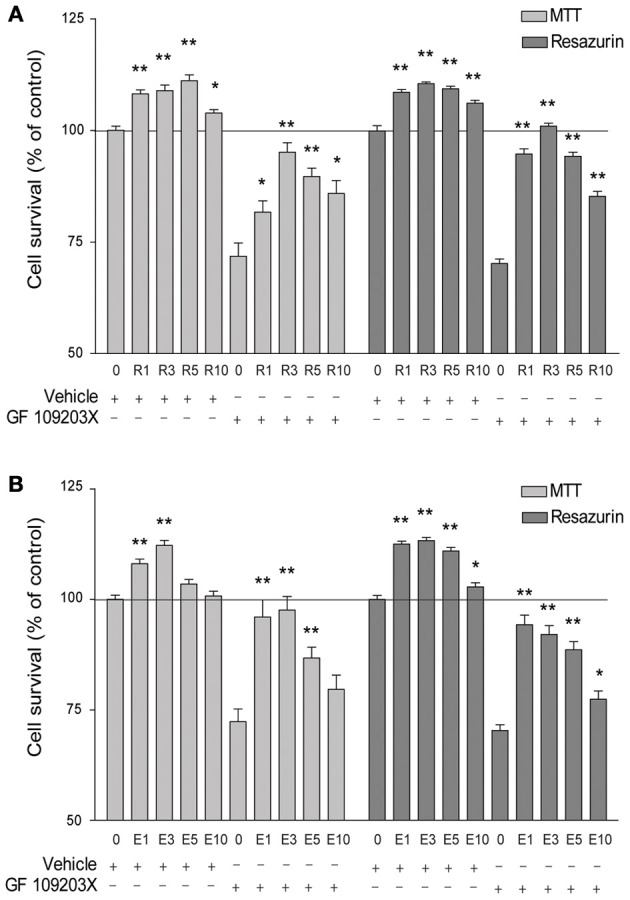
Resveratrol (R) and epigallocatechin gallate (E) increase hippocampal cell survival, whether exposed or not to a PKC inhibitor. (A) Cell survival is significantly enhanced by resveratrol treatment in comparison with the vehicle alone, with a maximal effect at 3 μM, as assessed by MTT and resazurin assays. The selective PKC inhibitor GF 109230X (5 μM) induces significant cell death that is rescued by co-treatment with resveratrol, these effects being maximal at 3 μM. (B) Similarly, cell survival is increased by EGCG treatment alone, with a maximal effect at 3 μM. Cell death produced by GF 109230X (5 μM) can be attenuated by EGCG, with a maximal effect at 1 μM. Data represent mean ± SEM from three (resazurin assay) to four (MTT assay) separate cultures and are expressed as percentages of the vehicle alone. Values were compared to the vehicle or GF 109203X alone for statistical analysis, *p < 0.05, **p < 0.01.
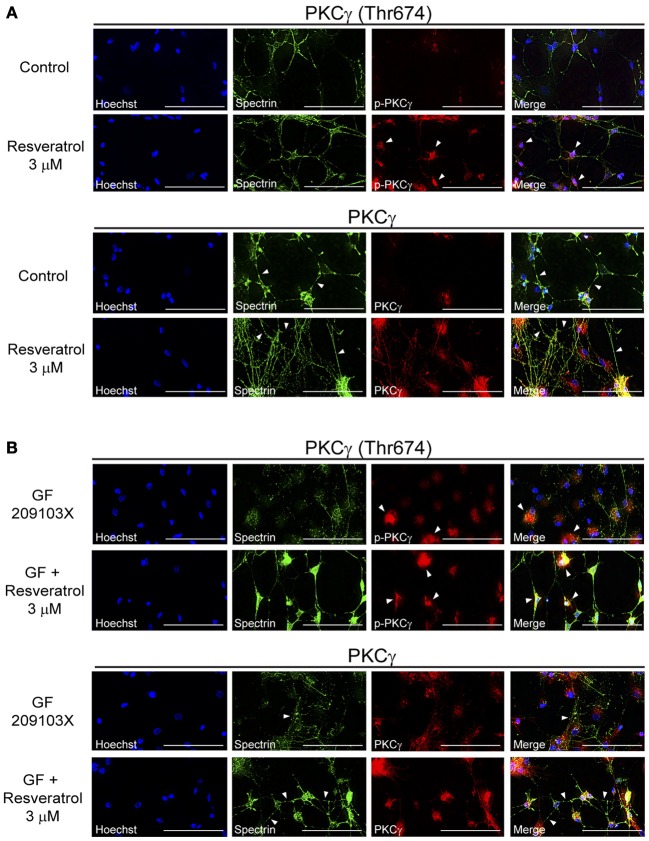
Polyphenols activate PKCγ enzyme, increase neuronal interconnections and prevent cytoskeleton degeneration
Neuroprotective effects have been associated with PKCγ activation and signaling (Zhang et al., 2011) but have not yet been explored for polyphenols. Using co-immunofluorescence, we evaluated the effects of resveratrol and EGCG treatments on PKCγ activation and cell morphology. Indeed, PKCγ interact with proteins associated with neurite elongation, formation and maintenance of synaptic contacts (Zhang et al., 2011). As shown in Figure 2A, resveratrol (3 μM) increased both PKCγ (Thr674) phosphorylation and expression in hippocampal cultured cells. Moreover, cells treated with this polyphenol showed higher interconnections, as revealed by spectrin staining and indicated by arrows (Figure 2A). On the other hand, cells exposed to GF 109203X were characterized by altered synaptic connexions and cytoskeleton degeneration (Figure 2B). Few cells showed PKCγ activation, suggesting a compensatory mechanism to reduce cell death. PKCγ was phosphorylated on the Thr674 residue in multiple hippocampal cells treated with resveratrol following PKC inhibition (Figure 2B).
Figure 2.
Resveratrol treatment increases PKCγ activation, neuronal interconnections and maintains cytoskeleton integrity. (A) Application of resveratrol (3 μM) activated PKCγ by phosphorylation at threonine 674 residue, increased PKCγ expression and neuronal interconnections in hippocampal cells. (B) Treatment with the PKC inhibitor GF 109203X (5 μM) disrupted the cellular cytoskeleton, as shown by spectrin immunofluorescence, and activation of PKCγ by resveratrol prevented this neurodegenerative process. Scale bar is set at 100 μm. Pictures are representative of average observations from three separate culture preparations.
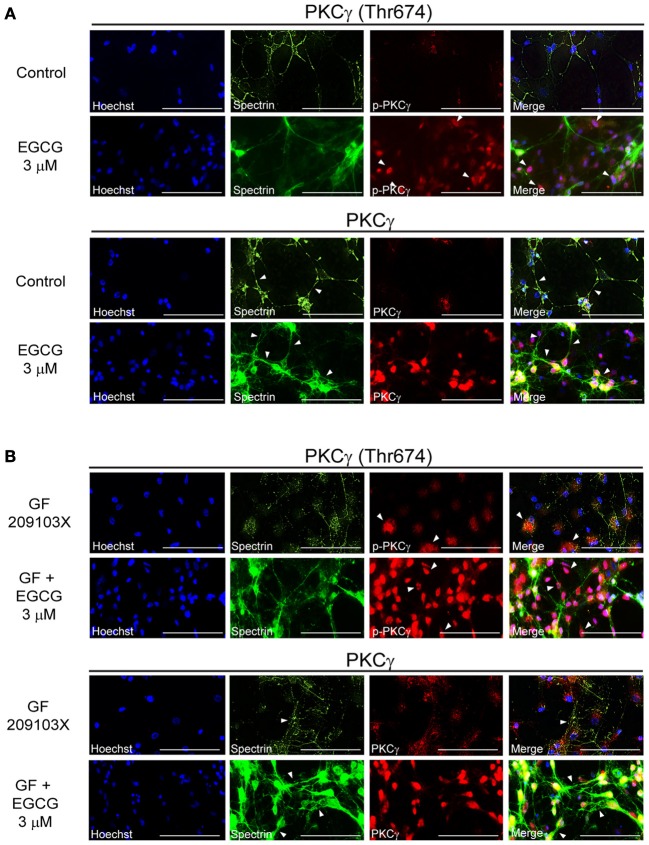
Like resveratrol, EGCG (3 μM) alone seemed to promote neuronal interconnections and clearly increased PKCγ phosphorylation (Figure 3A). PKCγ enzymes were seen most prominently around the nuclei of cells treated with EGCG (Figure 3A), while it was evenly distributed in the cytoplasm after resveratrol application (Figure 2A). EGCG strongly increased PKCγ activity in GF109203X-treated cells and prevented cytoskeleton degeneration (Figure 3B).
Figure 3.
EGCG enhances synaptic plasticity through PKCγ activation. (A) EGCG (3 μM) treatment increased PKCγ expression, phosphorylation at the Thr674 site and facilitated neuronal interconnections. (B) This polyphenol can also prevent cytoskeleton neurodegeneration induced by GF 109203X (5 μM) by modulating PKCγ level and activity. Scale bar is set at 100 μm. Pictures are representative of average observations from three separate culture preparations.
Discussion
In this study, EGCG and resveratrol, two active ingredients found in tea and wine respectively, were shown to, concentration (low μM)-dependently, protect against GF109203X-induced toxicity in cultured hippocampal neurons. These findings confirm and extend previous studies suggesting that the neuroprotective actions of resveratrol and EGCG involve the modulation of kinases such as PKC and cell death mediators (Miloso et al., 1999; Bastianetto et al., 2007; Bastianetto and Quirion, 2010; Bastianetto et al., 2011; Zhao et al., 2011).
Earlier, our group showed that resveratrol can reduce PKCδ phosphorylation in cultured hippocampal neuronal cells exposed to Aβ, suggesting that PKCδ is likely involved in resveratrol-mediated protection against Aβ-induced neurotoxicity (Han et al., 2004). We report here on the potential role of another isoform, PKCγ, in the neuroprotective effects of resveratrol and EGCG. It is well established that this isoenzyme is involved in synaptic development and neuronal plasticity. Indeed, PKCγ has been shown to interact with proteins associated with neurite elongation and formation, and the maintenance of synaptic contacts (Zhang et al., 2011). Moreover, it has been reported that PKCγ levels decline after ischemic preconditioning (Shamloo and Wieloch, 1999), and a down-regulation of PKCγ membrane translocation may be involved in a mouse model of hippocampal cell death mediated by oxygen-glucose deprivation (Liu et al., 2008). Finally, insulin-mediated inhibition of cortical neuronal necrosis following hypoxia has been reported to be associated with the translocation of PKCγ (Hamabe et al., 2005).
Both polyphenols increased PKCγ (Thr674) phosphorylation and its expression in presence of the PKC inhibitor. These effects occurred in the same concentration range as those protecting neuronal cells, suggesting an involvement of this PKC isoform in the neuroprotective action of the polyphenols. This hypothesis is supported by the finding that both polyphenols were able to prevent altered synaptic connexions and cytoskeleton degeneration induced by GF 109203X. Moreover, in the absence of the toxic agent, EGCG and resveratrol per se increased cell survival, accompanied by stimulation of PKCγ phosphorylation and maintenance of cytoskeleton architecture and neuronal plasticity. The effect of resveratrol on PKCγ was somewhat surprising, since we previously reported a lack of effects of resveratrol on PKCγ phosphorylation (Han et al., 2004). This apparent discrepancy may be explained by the concentrations used in the two studies (20 μM vs 3 μM in the present study).
These findings are of particular interest, since it is known that cytosolic PKCγ interacts with synapsin, a protein associated with synaptic vesicle release, which also binds with high affinity to actin, an essential component of the architecture of the cytoskeleton (Zhang et al., 2011). PKCγ can regulate cytoskeleton assembly (Rosenberg and Ravid, 2006) and has been reported to be actively involved in glutamate receptor trafficking, suggesting an important role for this kinase in synaptic development and plasticity (Patten and Ali, 2009). N-methyl-d-aspartate (NMDA) receptors are known to control dendritic spine motility, synaptogenesis and synapse stabilization (Gambrill and Barria, 2011). The stimulation of both ionotropic and metabotropic glutamate receptors can activate PKCγ, thus facilitating intracellular signaling and gene expression associated with learning and memory processes (Codazzi et al., 2006). Recently, (Huang et al., 2012) reported that reduction of the PKCγ-ERK (extracellular signal-regulated protein kinase) signaling pathway activation was involved in hippocampal neurodegeneration and persistent learning and memory impairments induced by ketamine, a NMDA receptor antagonist. Interestingly, the present results suggest that resveratrol and EGCG can facilitate hippocampal plasticity and interconnections through PKCγ activation, a process that could be associated with several higher brain functions, and that these polyphenols also prevent the suppression of PKCγ phosphorylation associated with hippocampal neurodegeneration (Huang et al., 2012).
Several missense mutations in the PKCγ gene have been found in spinocerebellar ataxia type 14 (SCA14), an autosomal dominant neurodegenerative disease (Yamamoto et al., 2010; Ueda et al., 2013). Mutant PKCγ kinases act as a dominant negative regulator on wild-type PKCγ enzymes, disrupting synaptic pruning, plasticity and transmission (Shuvaev et al., 2011). Mutant cytosolic PKCs have a tendency to aggregate, resulting in neuronal cell death (Seki et al., 2005; Asai et al., 2009). Interestingly, Yamamoto et al. (2010) reported that stimulation of autophagy provoked by treatment with rapamycin can promote the degradation of mutant PKCγ enzymes, while normal kinases are not affected. Moreover, aggregate formation and the cytotoxicity induced by mutant γPKCs has been reported to be inhibited in SH-SY5Y cells by congo red, a dye known to inhibit amyloid oligomers and fibril formation of misfolded proteins (Seki et al., 2010). Various groups, including ours, have shown that EGCG and resveratrol can stimulate autophagy in various types of cells, including macrophages (Li et al., 2011; Kim et al., 2013; Pallauf and Rimbach, 2013), and prevent the formation of Aβ oligomers and fibrils (Bastianetto et al., 2006, 2008; Cheng et al., 2013), suggesting that polyphenols may be beneficial in the treatment of SCA14 patients.
The concentrations of EGCG (165–275 μ M) and resveratrol (19–34 μ M) present in tea extracts and red wine (Sato et al., 1997; Wang et al., 2003; Del Rio et al., 2004; Hashim et al., 2013) are much higher than those required to produce their in-vitro protective effects as seen in the present study. Although polyphenols such as EGCG and resveratrol undergo significant metabolism and conjugation during absorption in humans (Goldberg et al., 2003; Spencer, 2009; Timmers et al., 2012), several animal studies have shown that they are able to cross the blood–brain barrier. For example, peripheral administration of EGCG (50 mg/kg i.p.) (Lee et al., 2004; Park et al., 2010) and resveratrol (20 mg/kg, i.p.) (Raval et al., 2006; Sakata et al., 2010) exerted neuroprotective actions in rodent models of ischemia (Nagai et al., 2002; Bastianetto and Quirion, 2010) and transgenic models of AD (Rezai-Zadeh et al., 2005; Karuppagounder et al., 2009). Moreover, a significant (one-third) portion of administered [3H]EGCG was found in the mouse brain after a single administration of the radioligand, suggesting that regular consumption of green tea may enable the brain to maintain a fairly high level of EGCG (Suganuma et al., 1998). It is therefore likely that regular consumption of tea (black or green) provides sufficient amounts of phenolic compounds to offer neuroprotection. In support of this hypothesis, various epidemiological studies have reported that tea consumption (2 or more cups /day) reduces (from 28 to 60%) the risk of Parkinson's disease (Checkoway et al., 2002; Tan et al., 2003) and attenuates (up to 43%) the rate of cognitive decline (Ng et al., 2008; Arab et al., 2011), whereas red wine (in moderation) reduces the risk of developing dementia (Orgogozo et al., 1997; Truelsen et al., 2002; Luchsinger et al., 2004; Dai et al., 2006).
The exact mechanisms involved in the activation of PKCγ remain to be established. It has been shown that PKCγ is upstream of several signaling pathways, including ERK and the mammalian target of rapamycin (mTOR) (Menard and Quirion, 2012), which are involved in several neurological disorders, including AD (Klafki et al., 2009; Ma et al., 2010). Interestingly, it has been reported that resveratrol can facilitate cell survival in the ischemic heart by modulating ERK signaling (Das et al., 2006) and decreasing the volume of infarcts in rats (Sinha et al., 2002). It is also known that serum deprivation reduces intercellular communication that can be attenuated by EGCG through ERK activation in endothelial cells (Zhao et al., 2011). In contrast, EGCG restores Aβ-induced cognitive impairments through the inhibition of ERK in mice (Lee et al., 2009). Moreover, opposite effects of polyphenols on the mTOR signaling pathway have been reported. While the inhibition of inflammation by resveratrol in microglial cells requires mTOR activation (Zhong et al., 2012), polyphenols are generally considered inhibitors of this pathway (Menendez et al., 2011). It would thus be worthwhile to explore further the effects of polyphenols on ERK and mTOR signaling following PKC inhibition in neural cells.
Taken together, these results highlight a role for PKCγ in cell survival, maintenance of the cytoskeleton architecture and neuronal plasticity. Interestingly, treatment with polyphenols protects hippocampal neurons against GF 109203X-induced cell death and cytoskeleton degeneration through stimulation of PKCγ phosphorylation. Thus, regular consumption of green tea and red wine, which are enriched in EGCG and resveratrol respectively, may activate neuroprotective signaling pathways, confirming their relevance in preventing age-related neurological disorders.
Author contributions
Caroline Menard, Stéphane Bastianetto and Rémi Quirion designed research; Caroline Menard and Stéphane Bastianetto performed research; Caroline Menard and Stéphane Bastianetto analyzed data; Caroline Menard, Stéphane Bastianetto and Rémi Quirion wrote the paper.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported by a grant from the Canadian Institutes of Health Research (CIHR) to Rémi Quirion (Grant #MOP-8580). Caroline Menard is the recipient of a CIHR fellowship. The authors would like to thank Drs Salah El Mestikawy and Gregory Dal-Bo for their technical assistance and Kathe Lieber for editing this manuscript.
References
- Anoopkumar-Dukie S., Carey J. B., Conere T., O'Sullivan E., Van Pelt F. N., Allshire A. (2005). Resazurin assay of radiation response in cultured cells. Br. J. Radiol. 78, 945–947 10.1259/bjr/54004230 [DOI] [PubMed] [Google Scholar]
- Arab L., Biggs M. L., O'Meara E. S., Longstreth W. T., Crane P. K., Fitzpatrick A. L. (2011). Gender differences in tea, coffee, and cognitive decline in the elderly: the cardiovascular health study. J. Alzheimer's dis. 27, 553–566 10.3233/JAD-2011-110431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai H., Hirano M., Shimada K., Kiriyama T., Furiya Y., Ikeda M., et al. (2009). Protein kinase C gamma, a protein causative for dominant ataxia, negatively regulates nuclear import of recessive-ataxia-related aprataxin. Hum. Mol. Genet. 18, 3533–3543 10.1093/hmg/ddp298 [DOI] [PubMed] [Google Scholar]
- Bastianetto S., Quirion R. (2010). Heme oxygenase 1: another possible target to explain the neuroprotective action of resveratrol, a multifaceted nutrient-based molecule. Exp. Neurol. 225, 237–239 10.1016/j.expneurol.2010.06.019 [DOI] [PubMed] [Google Scholar]
- Bastianetto S., Brouillette J., Quirion R. (2007). Neuroprotective effects of natural products: interaction with intracellular kinases, amyloid peptides and a possible role for transthyretin. Neurochem. Res. 32, 1720–1725 10.1007/s11064-007-9333-x [DOI] [PubMed] [Google Scholar]
- Bastianetto S., Krantic S., Quirion R. (2008). Polyphenols as potential inhibitors of amyloid aggregation and toxicity: possible significance to Alzheimer's disease. Mini Rev. Med. Chem. 8, 429–435 10.2174/138955708784223512 [DOI] [PubMed] [Google Scholar]
- Bastianetto S., Krantic S., Chabot J. G., Quirion R. (2011). Possible involvement of programmed cell death pathways in the neuroprotective action of polyphenols. Curr. Alzheimer Res. 8, 445–451 10.2174/156720511796391854 [DOI] [PubMed] [Google Scholar]
- Bastianetto S., Ramassamy C., Dore S., Christen Y., Poirier J., Quirion R. (2000). The Ginkgo biloba extract (EGb 761) protects hippocampal neurons against cell death induced by beta-amyloid. Eur. J. Neurosci. 12, 1882–1890 10.1046/j.1460-9568.2000.00069.x [DOI] [PubMed] [Google Scholar]
- Bastianetto S., Yao Z. X., Papadopoulos V., Quirion R. (2006). Neuroprotective effects of green and black teas and their catechin gallate esters against beta-amyloid-induced toxicity. Eur. J. Neurosci. 23, 55–64 10.1111/j.1460-9568.2005.04532.x [DOI] [PubMed] [Google Scholar]
- Checkoway H., Powers K., Smith-Weller T., Franklin G. M., Longstreth W. T., Jr., Swanson P. D. (2002). Parkinson's disease risks associated with cigarette smoking, alcohol consumption, and caffeine intake. Am. J. Epidemiol. 155, 732–738 10.1093/aje/155.8.732 [DOI] [PubMed] [Google Scholar]
- Cheng B., Gong H., Xiao H., Petersen R. B., Zheng L., Huang K. (2013). Inhibiting toxic aggregation of amyloidogenic proteins: a therapeutic strategy for protein misfolding diseases. Biochim. Biophys. Acta 1830, 4860–4871 10.1016/j.bbagen.2013.06.029 [DOI] [PubMed] [Google Scholar]
- Cherniack E. P. (2012). A berry thought-provoking idea: the potential role of plant polyphenols in the treatment of age-related cognitive disorders. Br. J. Nutr. 108, 794–800 10.1017/S0007114512000669 [DOI] [PubMed] [Google Scholar]
- Choi Y. T., Jung C. H., Lee S. R., Bae J. H., Baek W. K., Suh M. H., et al. (2001). The green tea polyphenol (-)-epigallocatechin gallate attenuates beta-amyloid-induced neurotoxicity in cultured hippocampal neurons. Life Sci. 70, 603–614 10.1016/S0024-3205(01)01438-2 [DOI] [PubMed] [Google Scholar]
- Codazzi F., Di Cesare A., Chiulli N., Albanese A., Meyer T., Zacchetti D., et al. (2006). Synergistic control of protein kinase Cgamma activity by ionotropic and metabotropic glutamate receptor inputs in hippocampal neurons. J. Neurosci. 26, 3404–3411 10.1523/JNEUROSCI.0478-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commenges D., Scotet V., Renaud S., Jacqmin-Gadda H., Barberger-Gateau P., Dartigues J. F. (2000). Intake of flavonoids and risk of dementia. Eur. J. Epidemiol. 16, 357–363 10.1023/A:1007614613771 [DOI] [PubMed] [Google Scholar]
- Dai Q., Borenstein A. R., Wu Y., Jackson J. C., Larson E. B. (2006). Fruit and vegetable juices and Alzheimer's disease: the Kame Project. Am. J. Med. 119, 751–759 10.1016/j.amjmed.2006.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Tosaki A., Bagchi D., Maulik N., Das D. K. (2006). Potentiation of a survival signal in the ischemic heart by resveratrol through p38 mitogen-activated protein kinase/mitogen- and stress-activated protein kinase 1/cAMP response element-binding protein signaling. J. Pharmacol. Exp. Therap. 317, 980–988 10.1124/jpet.105.095133 [DOI] [PubMed] [Google Scholar]
- Dehvari N., Cedazo-Minguez A., Isacsson O., Nilsson T., Winblad B., Karlstrom H., et al. (2007). Presenilin dependence of phospholipase C and protein kinase C signaling. J. Neurochem. 102, 848–857 10.1111/j.1471-4159.2007.04571.x [DOI] [PubMed] [Google Scholar]
- Del Rio D., Stewart A. J., Mullen W., Burns J., Lean M. E., Brighenti F., et al. (2004). HPLC-MSn analysis of phenolic compounds and purine alkaloids in green and black tea. J. Agric. Food Chem. 52, 2807–2815 10.1021/jf0354848 [DOI] [PubMed] [Google Scholar]
- Gambrill A. C., Barria A. (2011). NMDA receptor subunit composition controls synaptogenesis and synapse stabilization. Proc. Natl. Acad. Sci. U.S.A. 108, 5855–5860 10.1073/pnas.1012676108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D. M., Yan J., Soleas G. J. (2003). Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin. Biochem. 36, 79–87 10.1016/S0009-9120(02)00397-1 [DOI] [PubMed] [Google Scholar]
- Hamabe W., Fujita R., Ueda H. (2005). Insulin receptor-protein kinase C-gamma signaling mediates inhibition of hypoxia-induced necrosis of cortical neurons. J. Pharmacol. Exp. Ther. 313, 1027–1034 10.1124/jpet.104.082735 [DOI] [PubMed] [Google Scholar]
- Han Y. S., Zheng W. H., Bastianetto S., Chabot J. G., Quirion R. (2004). Neuroprotective effects of resveratrol against beta-amyloid-induced neurotoxicity in rat hippocampal neurons: involvement of protein kinase C. Br. J. Pharmacol. 141, 997–1005 10.1038/sj.bjp.0705688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque A. M., Hashimoto M., Katakura M., Hara Y., Shido O. (2008). Green tea catechins prevent cognitive deficits caused by Abeta1-40 in rats. J. Nutr. Biochem. 19, 619–626 10.1016/j.jnutbio.2007.08.008 [DOI] [PubMed] [Google Scholar]
- Hashim S. N., Schwarz L. J., Boysen R. I., Yang Y., Danylec B., Hearn M. T. (2013). Rapid solid-phase extraction and analysis of resveratrol and other polyphenols in red wine. J. Chromatogr. 1313, 284–290 10.1016/j.chroma.2013.06.052 [DOI] [PubMed] [Google Scholar]
- Huang L., Liu Y., Jin W., Ji X., Dong Z. (2012). Ketamine potentiates hippocampal neurodegeneration and persistent learning and memory impairment through the PKCgamma-ERK signaling pathway in the developing brain. Brain Res. 1476, 164–171 10.1016/j.brainres.2012.07.059 [DOI] [PubMed] [Google Scholar]
- Karuppagounder S. S., Pinto J. T., Xu H., Chen H. L., Beal M. F., Gibson G. E. (2009). Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer's disease. Neurochem. Int. 54, 111–118 10.1016/j.neuint.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. S., Montana V., Jang H. J., Parpura V., Kim J. A. (2013). Epigallocatechin Gallate (EGCG) stimulates autophagy in vascular endothelial cells: A POTENTIAL ROLE FOR REDUCING LIPID ACCUMULATION. J. Biol. Chem. 288, 22693–22705 10.1074/jbc.M113.477505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klafki H. W., Lewczuk P., Kamrowski-Kruck H., Maler J. M., Muller K., Peters O., et al. (2009). Measurement of ERK 1/2 in CSF from patients with neuropsychiatric disorders and evidence for the presence of the activated form. J. Alzheimer's Dis. 18, 613–622 10.3233/JAD-2009-1167 [DOI] [PubMed] [Google Scholar]
- Lee H., Bae J. H., Lee S. R. (2004). Protective effect of green tea polyphenol EGCG against neuronal damage and brain edema after unilateral cerebral ischemia in gerbils. J. Neurosci. Res. 77, 892–900 10.1002/jnr.20193 [DOI] [PubMed] [Google Scholar]
- Lee J. W., Lee Y. K., Ban J. O., Ha T. Y., Yun Y. P., Han S. B., et al. (2009). Green tea (-)-epigallocatechin-3-gallate inhibits beta-amyloid-induced cognitive dysfunction through modification of secretase activity via inhibition of ERK and NF-kappaB pathways in mice. J. Nutr. 139, 1987–1993 10.3945/jn.109.109785 [DOI] [PubMed] [Google Scholar]
- Letenneur L., Proust-Lima C., Le Gouge A., Dartigues J. F., Barberger-Gateau P. (2007). Flavonoid intake and cognitive decline over a 10-year period. Am. J. Epidemiol. 165, 1364–1371 10.1093/aje/kwm036 [DOI] [PubMed] [Google Scholar]
- Levites Y., Amit T., Youdim M. B., Mandel S. (2002). Involvement of protein kinase C activation and cell survival/cell cycle genes in green tea polyphenol (−)-epigallocatechin 3-gallate neuroprotective action. J. Biol. Chem. 277, 30574–30580 10.1074/jbc.M202832200 [DOI] [PubMed] [Google Scholar]
- Levites Y., Amit T., Mandel S., Youdim M. B. (2003). Neuroprotection and neurorescue against Abeta toxicity and PKC-dependent release of nonamyloidogenic soluble precursor protein by green tea polyphenol (-)-epigallocatechin-3-gallate. FASEB J. 17, 952–954 10.1096/fj.02-0881fje [DOI] [PubMed] [Google Scholar]
- Li W., Zhu S., Li J., Assa A., Jundoria A., Xu J., et al. (2011). EGCG stimulates autophagy and reduces cytoplasmic HMGB1 levels in endotoxin-stimulated macrophages. Biochem. Pharmacol. 81, 1152–1163 10.1016/j.bcp.2011.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Li J., Yang J., Ji F., Bu X., Zhang N., et al. (2008). Inhibition of PKCgamma membrane translocation mediated morphine preconditioning-induced neuroprotection against oxygen-glucose deprivation in the hippocampus slices of mice. Neurosci. Lett. 444, 87–91 10.1016/j.neulet.2008.08.014 [DOI] [PubMed] [Google Scholar]
- Luchsinger J. A., Tang M. X., Siddiqui M., Shea S., Mayeux R. (2004). Alcohol intake and risk of dementia. J. Am. Geriatr. Soc. 52, 540–546 10.1111/j.1532-5415.2004.52159.x [DOI] [PubMed] [Google Scholar]
- Ma T., Hoeffer C. A., Capetillo-Zarate E., Yu F., Wong H., Lin M. T., et al. (2010). Dysregulation of the mTOR pathway mediates impairment of synaptic plasticity in a mouse model of Alzheimer's disease. PloS ONE 5:e12845 10.1371/journal.pone.0012845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marambaud P., Zhao H., Davies P. (2005). Resveratrol promotes clearance of Alzheimer's disease amyloid-beta peptides. J. Biol. Chem. 280, 37377–37382 10.1074/jbc.M508246200 [DOI] [PubMed] [Google Scholar]
- Menard C., Quirion R. (2012). Successful cognitive aging in rats: a role for mGluR5 glutamate receptors, homer 1 proteins and downstream signaling pathways. PloS ONE 7:e28666 10.1371/journal.pone.0028666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez J. A., Vellon L., Oliveras-Ferraros C., Cufi S., Vazquez-Martin A. (2011). mTOR-regulated senescence and autophagy during reprogramming of somatic cells to pluripotency: a roadmap from energy metabolism to stem cell renewal and aging. Cell cycle 10, 3658–3677 10.4161/cc.10.21.18128 [DOI] [PubMed] [Google Scholar]
- Miloso M., Bertelli A. A., Nicolini G., Tredici G. (1999). Resveratrol-induced activation of the mitogen-activated protein kinases, ERK1 and ERK2, in human neuroblastoma SH-SY5Y cells. Neurosci. Lett. 264, 141–144 10.1016/S0304-3940(99)00194-9 [DOI] [PubMed] [Google Scholar]
- Nagai K., Jiang M. H., Hada J., Nagata T., Yajima Y., Yamamoto S., et al. (2002). (-)-Epigallocatechin gallate protects against NO stress-induced neuronal damage after ischemia by acting as an anti-oxidant. Brain Res. 956, 319–322 10.1016/S0006-8993(02)03564-3 [DOI] [PubMed] [Google Scholar]
- Newton A. C. (1997). Regulation of protein kinase C. Curr. Opin. Cell Biol. 9, 161–167 10.1016/S0955-0674(97)80058-0 [DOI] [PubMed] [Google Scholar]
- Ng T. P., Feng L., Niti M., Kua E. H., Yap K. B. (2008). Tea consumption and cognitive impairment and decline in older Chinese adults. Am. J. Clin. Nutr. 88, 224–231 [DOI] [PubMed] [Google Scholar]
- Orgogozo J. M., Dartigues J. F., Lafont S., Letenneur L., Commenges D., Salamon R., et al. (1997). Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev. Neurol. 153, 185–192 [PubMed] [Google Scholar]
- Pallauf K., Rimbach G. (2013). Autophagy, polyphenols and healthy ageing. Ageing Res. Rev. 12, 237–252 10.1016/j.arr.2012.03.008 [DOI] [PubMed] [Google Scholar]
- Park J. W., Hong J. S., Lee K. S., Kim H. Y., Lee J. J., Lee S. R. (2010). Green tea polyphenol (-)-epigallocatechin gallate reduces matrix metalloproteinase-9 activity following transient focal cerebral ischemia. J. Nutr. Biochem. 21, 1038–1044 10.1016/j.jnutbio.2009.08.009 [DOI] [PubMed] [Google Scholar]
- Patten S. A., Ali D. W. (2009). PKCgamma-induced trafficking of AMPA receptors in embryonic zebrafish depends on NSF and PICK1. Proc. Natl. Acad. Sci. U.S.A. 106, 6796–6801 10.1073/pnas.0811171106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval A. P., Dave K. R., Perez-Pinzon M. A. (2006). Resveratrol mimics ischemic preconditioning in the brain. J. Cereb. Blood Flow Metab. 26, 1141–1147 10.1038/sj.jcbfm.9600262 [DOI] [PubMed] [Google Scholar]
- Rezai-Zadeh K., Shytle D., Sun N., Mori T., Hou H., Jeanniton D., et al. (2005). Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J. Neurosci. 25, 8807–8814 10.1523/JNEUROSCI.1521-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Ravid S. (2006). Protein kinase Cgamma regulates myosin IIB phosphorylation, cellular localization, and filament assembly. Mol. Biol. Cell 17, 1364–1374 10.1091/mbc.E05-07-0597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata Y., Zhuang H., Kwansa H., Koehler R. C., Dore S. (2010). Resveratrol protects against experimental stroke: putative neuroprotective role of heme oxygenase 1. Exp. Neurol. 224, 325–329 10.1016/j.expneurol.2010.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Suzuki Y., Okuda T., Yokotsuka K. (1997). Contents of resveratrol, piceid, and their isomers in commercially available wines made from grapes cultivated in Japan. Biosci. Biotechnol. Biochem. 61, 1800–1805 10.1271/bbb.61.1800 [DOI] [PubMed] [Google Scholar]
- Seki T., Adachi N., Ono Y., Mochizuki H., Hiramoto K., Amano T., et al. (2005). Mutant protein kinase Cgamma found in spinocerebellar ataxia type 14 is susceptible to aggregation and causes cell death. J. Biol. Chem. 280, 29096–29106 10.1074/jbc.M501716200 [DOI] [PubMed] [Google Scholar]
- Seki T., Takahashi H., Yamamoto K., Ogawa K., Onji T., Adachi N., et al. (2010). Congo red, an amyloid-inhibiting compound, alleviates various types of cellular dysfunction triggered by mutant protein kinase cgamma that causes spinocerebellar ataxia type 14 (SCA14) by inhibiting oligomerization and aggregation. J. Pharmacol. Sci. 114, 206–216 10.1254/jphs.10170FP [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. (2000). Toward a comprehensive theory for Alzheimer's disease. Hypothesis: Alzheimer's disease is caused by the cerebral accumulation and cytotoxicity of amyloid beta-protein. Ann. N.Y. Acad. Sci. 924, 17–25 10.1111/j.1749-6632.2000.tb05554.x [DOI] [PubMed] [Google Scholar]
- Shamloo M., Wieloch T. (1999). Rapid decline in protein kinase Cgamma levels in the synaptosomal fraction of rat hippocampus after ischemic preconditioning. Neuroreport 10, 931–935 10.1097/00001756-199904060-00007 [DOI] [PubMed] [Google Scholar]
- Shuvaev A. N., Horiuchi H., Seki T., Goenawan H., Irie T., Iizuka A., et al. (2011). Mutant PKCgamma in spinocerebellar ataxia type 14 disrupts synapse elimination and long-term depression in Purkinje cells in vivo. J. Neurosci. 31, 14324–14334 10.1523/JNEUROSCI.5530-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha K., Chaudhary G., Gupta Y. K. (2002). Protective effect of resveratrol against oxidative stress in middle cerebral artery occlusion model of stroke in rats. Life Sci. 71, 655–665 10.1016/S0024-3205(02)01691-0 [DOI] [PubMed] [Google Scholar]
- Spencer J. P. (2009). The impact of flavonoids on memory: physiological and molecular considerations. Chem. Soc. Rev. 38, 1152–1161 10.1039/b800422f [DOI] [PubMed] [Google Scholar]
- Suganuma M., Okabe S., Oniyama M., Tada Y., Ito H., Fujiki H. (1998). Wide distribution of [3H](-)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis 19, 1771–1776 10.1093/carcin/19.10.1771 [DOI] [PubMed] [Google Scholar]
- Tan E. K., Tan C., Fook-Chong S. M., Lum S. Y., Chai A., Chung H., et al. (2003). Dose-dependent protective effect of coffee, tea, and smoking in Parkinson's disease: a study in ethnic Chinese. J. Neurol. Sci. 216, 163–167 10.1016/j.jns.2003.07.006 [DOI] [PubMed] [Google Scholar]
- Timmers S., Auwerx J., Schrauwen P. (2012). The journey of resveratrol from yeast to human. Aging 4, 146–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toullec D., Pianetti P., Coste H., Bellevergue P., Grand-Perret T., Ajakane M., et al. (1991). The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J. Biol. Chem. 266, 15771–15781 [PubMed] [Google Scholar]
- Truelsen T., Thudium D., Gronbaek M. (2002). Amount and type of alcohol and risk of dementia: the copenhagen city heart study. Neurology 59, 1313–1319 10.1212/01.WNL.0000031421.50369.E7 [DOI] [PubMed] [Google Scholar]
- Ueda T., Seki T., Katanazaka K., Sekiguchi K., Kobayashi K., Kanda F., et al. (2013). A novel mutation in the C2 domain of protein kinase C gamma associated with spinocerebellar ataxia type 14. J. Neurol. 260, 1664–1666 10.1007/s00415-013-6916-0 [DOI] [PubMed] [Google Scholar]
- Vega-Avila E., Pugsley M. K. (2011). An overview of colorimetric assay methods used to assess survival or proliferation of mammalian cells. Proc. West. Pharmacol. Soc. 54, 10–14 [PubMed] [Google Scholar]
- Wang X., Song K. S., Guo Q. X., Tian W. X. (2003). The galloyl moiety of green tea catechins is the critical structural feature to inhibit fatty-acid synthase. Biochem. Pharmacol. 66, 2039–2047 10.1016/S0006-2952(03)00585-9 [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Seki T., Adachi N., Takahashi T., Tanaka S., Hide I., et al. (2010). Mutant protein kinase C gamma that causes spinocerebellar ataxia type 14 (SCA14) is selectively degraded by autophagy. Genes Cells 15, 425–438 10.1111/j.1365-2443.2010.01395.x [DOI] [PubMed] [Google Scholar]
- Zhang H. X., Du G. H., Zhang J. T. (2004). Assay of mitochondrial functions by resazurin in vitro. Acta Pharmacol. Sin. 25, 385–389 [PubMed] [Google Scholar]
- Zhang N., Yin Y., Han S., Jiang J., Yang W., Bu X., et al. (2011). Hypoxic preconditioning induced neuroprotection against cerebral ischemic injuries and its cPKCgamma-mediated molecular mechanism. Neurochem. Int. 58, 684–692 10.1016/j.neuint.2011.02.007 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Yu L., Xu S., Qiu F., Fan Y., Fu G. (2011). Down-regulation of connexin43 gap junction by serum deprivation in human endothelial cells was improved by (-)-Epigallocatechin gallate via ERK MAP kinase pathway. Biochem. Biophys. Res. Commun. 404, 217–222 10.1016/j.bbrc.2010.11.096 [DOI] [PubMed] [Google Scholar]
- Zhong L. M., Zong Y., Sun L., Guo J. Z., Zhang W., He Y., et al. (2012). Resveratrol inhibits inflammatory responses via the mammalian target of rapamycin signaling pathway in cultured LPS-stimulated microglial cells. PloS ONE 7:e32195 10.1371/journal.pone.0032195 [DOI] [PMC free article] [PubMed] [Google Scholar]