 |
Philip Andrew is a clinical cardiologist in private practice.
Guyton's venous return curve (VRC) should continue to be taught for two reasons:
It uniquely describes the preeminent role of the vasculature in determining cardiac filling pressures, right atrial pressure (RAP) the primary focus herein. Specifically, RAP varies (a) inversely with cardiac output (CO), (b) inversely with total vascular resistance, (c) directly with mean systemic pressure (MSP), the pressure starting point of a static systemic circulation (CO zero). MSP in turn varies directly with blood volume and inversely with arterial and venous compliance (Levy & Pappano, 2007).
Plotting the VRC and the Frank-Starling CO curve on the same axis co-ordinates (Fig. 1) illustrates how RAP and CO each respond when the other is the independent variable, their point of intersection determining RAP and CO at steady state. This forms Guyton's Graphical Analysis (GGA). Fully capable of modelling any and all physiological and pathophysiological central circulatory states, GGA is the most explicit, complete, and useful clinical teaching model of the central circulation.
Figure 1.
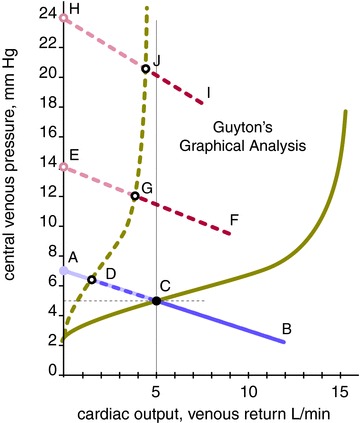
The pathogenetic phases of HF, abbreviations per text, modelled by GGA, after (Guyton et al. 1973) with permission from Elsevier. Modifications include transposed axes to favour conceptualization and clinical utility over scientific convention. The term VRC is used in this legend to comport with conventional terminology; all such usage can and should be superseded by the term ‘Guyton-Levy venous pressure curve’ (see text). HF pressures and flows illustrative. Purple: systemic VRC. Red: pulmonary VRC. Green: LV Frank-Starling CO–VR curve. Right ventricular CO–VR curve: not shown. Filled lines and dots: normal. Dashes and open dots: effects of impaired LV pumping capacity. Black: steady state points: black dot C, normal; black dot C to circle D, Phase I HF; D to G, Phase II HF; G to J+, Phase III HF. Shaded: virtual pressures, that is, all venous pressures exceeding steady state. Shaded dot A: normal mean systemic pressure. Shaded lines: virtual components of VRCs: A–C, normal; A–D, Phase I HF; E–G, Phase II HF; H–J, Phase III HF. Shaded open dots E and H: mean pulmonary pressure of Phase II and III HF, respectively.
However, some claim that in heart failure (HF) the VRC/GGA fails to show:
“redistribution of CO among the various vascular beds”;
“how the high RAP in HF is a consequence of blood accumulating on the inlet side of the failing pump”;
“retention of salt and water”;
“an increase [in] arterial resistance” (Beard & Feigl, 2011).
A closer look reveals this not to be the case. The pathogenesis of HF comprises three phases, all fully modelled by GGA (Fig. 1), each addressing one or more of these claims:
Phase I, vascular redistribution
All else equal, reduced ventricular pumping capacity lowers CO and mean arterial pressure by Hagen-Poiseuille's/Ohm's Law. With the circulation a closed zero-sum loop, blood volume incrementally distending the systemic arterial bed at its preceding higher mean pressure had to be ‘borrowed’ from its upstream systemic venous bed. At the new lower mean arterial pressure it is ‘repaid’ to that bed, raising RAP.
Phase II, ventricular re-equilibration
In HF ventricular dysfunction typically affects the left ventricle (LV) more that the right (RV). This causes blood to accumulate in the pulmonary vascular bed upstream to the weaker LV, blood again borrowed from the upstream systemic venous reservoir. Accordingly, mean pulmonary pressure, the pulmonary VRC, left atrial pressure, and LV output all increase. Simultaneously, MSP, the systemic VRC, RAP, and RV output decrease (not shown). With LV output rising from its initial nadir and RV output falling from normal, biventricular output re-equilibrates at an intermediate point below normal. Phases I and II are complete within a few seconds.
Phase III, neurohormonal activation
The remaining CO shortfall (Fig. 1, CO at point C minus CO at point G) activates systemic neurohormonal mechanisms to retain salt and water (expand extracellular volume), reduce venous capacitance and increase vascular resistance. This increases MSP, RAP, and RV output (not shown), in turn obligating an increase in mean pulmonary pressure, left atrial pressure and LV output to maintain balanced ventricular output and restore CO to normal. If this is achieved HF is compensated. Severe LV dysfunction, where CO cannot be compensated at sub-lethal left atrial pressure (Fig. 1), causes incessant neurohormonal activation, inexorably raising RAP and further distending the RV within the pericardial space it shares with the LV. The distended RV compresses the LV, decreasing its effective (transmural) filling pressure/preload and stroke volume (Atherton et al. 1997). This bends the Frank-Starling curve back toward the pressure axis (formerly known as the ‘descending limb of the CO curve,’ not shown), stimulating even greater neurohormonal activation in a futile and counterproductive attempt to restore normal CO. The high RAP typical of severe left heart dysfunction with bi-ventricular HF is the result of neurohormonal activation.
Increased vascular resistance, a feature of Phase II and III HF, steepens the slope of the VRC (Fig. 1; Guyton et al. 1973).
Clearly, GGA fully models all phases and severities of HF. It has also modelled exercise, changes in posture, shock, tissue hypoxia, arteriovenous fistulas, patent ductus arteriosus, respiration, the open chest, cardiac tamponade, the artificial heart, and others (Guyton et al. 1973) – more than enough to justify continued teaching of its requisite component, the VRC.
The VRC has but one flaw, the name affixed to it. The ‘venous return curve’ is in fact no such thing … it is a venous pressure curve. This simple misnomer has been a major obstacle to the understanding and acceptance of the VRC, its derivative GGA, and of a fundamental characteristic of circulatory physiology – the co-identity of CO and venous return (VR) at steady state (Levy, 1979). That is, at steady state CO and VR are jointly and severally represented by the same curve – the Frank-Starling curve. By conjoining CO and VR into the flow symbol Q (Q = CO = VR), Levy inadvertently obscured this mutual identity (Levy, 1979), as does the ongoing near-universal omission of VR from the flow axis label. An exception to the CO–VR identity is made for fleeting imbalances where, say, an independent increase in VR increases CO (Levy & Pappano, 2007). These fleeting imbalances provide the all-important physiological and pathophysiological supply–demand transitions to which the circulation must respond, and are modelled by GGA.
With the venous pressure curve misrepresented as the venous return curve and the Frank-Starling CO curve also representing VR, appearances are of two quite distinct curves representing the same entity, VR. It is not surprising, then, that (a) one could be construed to control the other, in other words that VR can control itself (Levy characterized this as ‘circular reasoning’ but did not elaborate on the misnomer, dual representation, and labelling aspects), or that (b) CO and VR may actually diverge at steady state, another spurious premise.
An example of circular reasoning: ‘If one wishes to say … that venous return governs cardiac output, then … there is little to quarrel with’ (Reddi & Carpenter, 2005). But as Levy and Pappano partly model (Levy & Pappano, 2007), what is characterized as an independent change in VR in fact registers haemodynamically as a change in intravenous volume to which MSP/RAP/CO are directly proportionate. RAP, the final common pathway of transitions in circulatory demand, represents the net effect of all variables determining RAP (see point (1) above), not just VR/intravascular volume. The RAP/venous pressure intermediary further obviates strained attempts to rationalize self-regulation: ‘one [should] not try to be too specific about what exactly venous return actually is … what units, for example, are we to measure venous return in?’ (Reddi & Carpenter, 2005). Merely renaming the VRC the venous pressure curve resolves ‘venous return governs cardiac output, …’ to ‘venous pressure governs cardiac output …,’ a simple iteration of Starling's Law.
An example of divergent reasoning is the celebrated but apocryphal inference that Guyton represented HF as ‘both a decrease in forward output and an increase in venous return … a physical impossibility’ (Packer, 1990; Kass et al. 2011). In fact, although Guyton's VRC misnomer set the stage for divergent reasoning, there is no evidence he actually fell prey to it. Guyton did misperceive:
RAP as the variable controlling CO in his original VRC experiment. Actually, CO, as permitted by a Starling resistor acting as an adjustable tricuspid stenosis, inversely controlled RAP (Levy, 1979).
MSP minus RAP as the pressure gradient for systemic VR; it is mean arterial pressure minus RAP (Levy, 1979).
RAP as a ‘backpressure’ effect on his perceived but non-existent pressure gradient for systemic VR (Levy, 1979) – non-existent because at any CO above zero MSP is a virtual, not a physical quantity.
But in no way do these misperceptions conflate VR with venous pressure. And in no way do they compromise the integrity or utility of his venous pressure curve or its derivative GGA, which, properly understood, is the definitive clinical teaching model of central haemodynamics.
Guyton's venous pressure curve must continue to be taught. It should also be formally renamed the ‘Guyton–Levy venous pressure curve’ in apt symmetry to the ‘Frank-Starling CO–VR curve’ and its historical contribution, and as a break with misperceptions of the past. Levy, who hailed GGA as ‘a monumental contribution to the analysis of cardiovascular control’ (Levy, 1979), preferred ‘vascular function curve’ but, like VR, ‘vascular function’ is not a quantity it represents.
Call for comments
Readers are invited to give their views on this and the accompanying CrossTalk articles in this issue by submitting a brief comment. Comments may be posted up to 6 weeks after publication of the article, at which point the discussion will close and authors will be invited to submit a ‘final word'. To submit a comment, go to http://jp.physoc.org/letters/submit/jphysiol;591/23/5791
Glossary
Abbreviations
- CO
cardiac output
- GGA
Guyton's graphical analysis
- HF
heart failure
- LV
left ventricle/ventricular
- MSP
mean systemic pressure
- RAP
right atrial pressure
- RV
right ventricle/ventricular
- VR
venous return
- VRC
venous return curve
References
- Atherton JJ, Moore TD, Lele SS, Thomson HL, Galbraith AJ, Belenkie I, Tyberg JV, Frenneaux MP. Diastolic ventricular interaction in chronic heart failure. Lancet. 1997;349:1720–1724. doi: 10.1016/S0140-6736(96)05109-4. [DOI] [PubMed] [Google Scholar]
- Beard DA, Feigl EO. Understanding Guyton's venous return curves. Am J Physiol Heart Circ Physiol. 2011;301:H629–H633. doi: 10.1152/ajpheart.00228.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton AC, Jones CE, Coleman TG. Circulatory Physiology 1; Cardiac Output and its Regulation. Philadelphia, PA, USA: Saunders; 1973. [Google Scholar]
- Kass DA, Tyberg JV, Beard DA, Feigl EO. Understanding Guyton's venous return curves - podcast. Am J Physiol Heart Circ Physiol. 2011;301:H629–H633. doi: 10.1152/ajpheart.00228.2011. http://ajpheart.podbean.com/2011/07/18/understanding-guytons-venous-return-curves/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy MN. The cardiac and vascular factors that determine systemic blood flow. Circ Res. 1979;44:739–747. doi: 10.1161/01.res.44.6.739. [DOI] [PubMed] [Google Scholar]
- Levy MN, Pappano AJ. Cardiovascular Physiology. Philadelphia, PA, USA: Mosby; 2007. [Google Scholar]
- Packer M. Abnormalities of diastolic function as a potential cause of exercise intolerance in chronic heart failure. Circulation. 1990;81:III78–86. [PubMed] [Google Scholar]
- Reddi BA, Carpenter RH. Venous excess: a new approach to cardiovascular control and its teaching. J Appl Physiol. 2005;98:356–364. doi: 10.1152/japplphysiol.00535.2004. [DOI] [PubMed] [Google Scholar]


