Abstract
Although there is general agreement that mucosal 5-hydroxytryptamine (5-HT) can initiate peristaltic reflexes in the colon, recent studies have differed as to whether or not the role of mucosal 5-HT is critical. We therefore tested the hypothesis that the secretion of 5-HT from mucosal enterochromaffin (EC) cells is essential for the manifestation of murine colonic peristaltic reflexes. To do so, we analysed the mechanisms underlying faecal pellet propulsion in isolated colons of mice lacking tryptophan hydroxylase 1 (Tph1−/− mice), which is the rate-limiting enzyme in the biosynthesis of mucosal but not neuronal 5-HT. We used video analysis of faecal pellet propulsion, tension transducers to record colonic migrating motor complexes (CMMCs) and intracellular microelectrodes to record circular muscle activity occurring spontaneously or following intraluminal distension. When compared with control (Tph1+/+) mice, Tph1−/− animals exhibited: (1) an elongated colon; (2) larger faecal pellets; (3) orthograde propulsion followed by retropulsion (not observed in Tph1+/+ colon); (4) slower in vitro propulsion of larger faecal pellets (28% of Tph1+/+); (5) CMMCs that infrequently propagated in an oral to anal direction because of impaired descending inhibition; (6) reduced CMMCs and inhibitory responses to intraluminal balloon distension; (7) an absence of reflex activity in response to mucosal stimulation. In addition, (8) thin pellets that propagated along the control colon failed to do so in Tph1−/− colon; and (9) the 5-HT3 receptor antagonist ondansetron, which reduced CMMCs and blocked their propagation in Tph1+/+ mice, failed to alter CMMCs in Tph1−/− animals. Our observations suggest that mucosal 5-HT is essential for reflexes driven by mucosal stimulation and is also important for normal propagation of CMMCs and propulsion of pellets in the isolated colon.
Key points
Previous studies have indicated that neither neuronal nor mucosal 5-hydroxytryptamine (5-HT) are important for colonic migrating motor complexes (CMMCs) or faecal pellet propulsion. Therefore, tryptophan hydroxylase 1 knockout (TPH1KO) mice were used to examine the role of mucosal 5-HT in generating CMMCs and faecal pellet propulsion, as TPH1 is the regulatory enzyme necessary for the synthesis of 5-HT in enterochromaffin cells in the mucosa.
Control mice generated a robust CMMC when the mucosa was mechanically stimulated, which was blocked by ondansetron (5-HT3 antagonist), and could propagate faecal pellets that did not significantly distend the bowel, suggesting that they were propelled by mucosal reflexes in the absence of stretch reflexes.
TPH1KO mice exhibited no mucosal reflexes, reduced responses to intraluminal distension and propelled only larger faecal pellets, suggesting that they relied upon stretch reflexes alone.
In control mice, CMMCs, which can propel a faecal pellet, propagated in an oral to anal direction, whereas, in TPH1KO mice, they rarely propagated.
Both the propagation and amplitude of CMMCs were reduced by ondansetron in control mice, whereas this drug did not affect CMMCs in TPH1KO mice.
This suggests that 5-HT release from the mucosa and stretch reflexes are important for normal colonic propulsion.
Introduction
5-Hydroxytryptamine (5-HT, serotonin) is highly abundant in the gut (∼95% of the 5-HT in the body) and participates in neurocrine, paracrine and endocrine signalling (Gershon, 2012, 2013). Enteric sources of 5-HT include mucosal enterochromaffin (EC) cells, mast cells in rats and mice, and myenteric serotonergic neurons, which are descending interneurons that also project to the submucous plexus (Sang et al. 1997; Lomax & Furness, 2000). Evidence suggests that altered serotonergic signalling plays an important role in the pathophysiology of gastrointestinal disorders, including diarrhea induced by cholera toxin or bile salts, as well as irritable bowel syndrome (Lesurtel et al. 2008; Li et al. 2011; Faure et al. 2010). Secretion of 5-HT from EC cells can activate intrinsic primary afferent neurons (IPANs) that initiate motility and secretomotor reflexes (Bülbring & Crema, 1959a; Pan & Gershon, 2000; Heredia et al. 2009; Bayguinov et al. 2010). Colonic descending serotonergic interneurons, which activate inhibitory motor neurons, produce tonic inhibition of the circular muscle and promote secretion (Dickson et al. 2010b; Heredia et al. 2012; Okamoto et al. 2012). 5-HT3 receptor antagonists are effective against irritable bowel syndrome with diarrhea (Mangel & Northcutt, 1999), whereas 5-HT4 receptor agonists are effective against irritable bowel syndrome with constipation (Prather et al. 2000). 5-HT4 receptors are located on colonic EC cells, epithelial cells and goblet cells (Hoffman et al. 2012); moreover, 5-HT4 receptor agonists are more effective at stimulating faecal pellet propulsion when applied intraluminally rather than serosally (Jin et al. 1999; Hoffman et al. 2012). Despite the important role of mucosal 5-HT in enteric pathophysiology, its role in the generation of peristaltic reflexes is still controversial.
Bülbring and co-workers first showed that increased intraluminal pressure simultaneously released 5-HT from EC cells and initiated peristaltic reflexes in vivo and in vitro (Bülbring & Crema, 1958, 1959a,b; Bülbring et al. 1958; reviewed by Gershon, 2012, 2013). They also showed that anaesthesia, asphyxiation or removal of the mucosa abolished the reflex, implying that it is mucosa dependent; moreover, luminal, but not serosal, application of 5-HT initiated the reflex and lowered the threshold for its pressure-induced manifestation. Bülbring concluded that it was likely that pressure caused EC cells to secrete 5-HT, which stimulated IPANs that evoked the peristaltic reflex. However, Bülbring was unable to decide whether mucosal 5-HT is ‘of primary importance’ in the initiation of the peristaltic reflex because she was unable to deplete mucosal 5-HT stores completely with reserpine (available at that time) or to block 5-HT biosynthesis (Bülbring & Crema, 1959b). Boullin (1964) later emphasized this uncertainty when he placed rats on a tryptophan-deficient diet and achieved a severe depletion of intestinal 5-HT that failed to abolish the peristaltic reflex.
Bertrand (2006) reported that 5-HT release from the mucosa followed, rather than initiated, a peristaltic contraction in the small intestine. The concerning aspect of these studies is that a variety of agents that blocked smooth muscle contraction or elicited contraction also dramatically inhibited or enhanced 5-HT release, respectively. A possibility is that the fine electrodes used in these amperometry studies elicited spurious oxidation currents as a result of movement of the fine tip rather than because of the release of 5-HT. Using a similar technique, others have found that, ‘The rise and decay in the current are influenced by the movement of the electrode..’ (Marcelli & Patel, 2010). This possibility is also supported by the observations that electrical slow waves recorded with extracellular electrodes follow movement and are abolished when movement is suppressed, but not when recorded with intracellular microelectrodes in paralysed tissues (Bayguinov et al. 2011).
More recent studies have also not succeeded in clarifying the role played by mucosal 5-HT in the initiation of peristaltic reflexes (Smith et al. 2010). Some experiments have supported Bülbring's suggestion that the peristaltic reflex is 5-HT and mucosa dependent (Heredia et al. 2009, Bayguinov et al. 2010; Dickson et al. 2010a,b), whereas others have not (Keating & Spencer, 2010; Spencer et al. 2011, 2013; Sia et al. 2013a,b). Differences in stimuli used to evoke peristaltic reflexes may partially explain apparent contradictions between studies. Clearly, when fluid is injected into the small intestine or a faecal pellet is present in the colon, it can stretch the bowel wall and exert pressure on the mucosa that releases 5-HT (Bülbring & Crema, 1959a; Heredia et al. 2009). Receptors able to evoke peristaltic contractions, however, are not limited to pressure on the mucosa; distension of the gut and circumferential stretch can also do so (Frigo & Lecchini, 1970; Mackenna & McKirdy, 1972; Brookes et al. 1999; Spencer et al. 2000, 2001; Heredia et al. 2009). One can therefore bypass EC cells and still evoke peristaltic reflexes, by activating components of the reflex arc that are distal to the EC cell–primary afferent neuron junction.
At issue is whether peristaltic reflexes can be initiated in preparations following removal of the mucosa. The apparent conflict may be caused by the multiple means by which peristaltic reflexes can be evoked. Circumferential stretch and mechanical stimulation of the mucosa can each elicit similar peristaltic reflexes through mechanosensitive interneurons and IPANs, respectively (Smith et al. 1991, 1992, 2007; Spencer & Smith, 2004; Spencer et al. 2006; Heredia et al. 2009; Bayguinov et al. 2010).
An eliciting stimulus might thus appear to be independent of the mucosa and its 5-HT if it were to activate peristaltic reflexes only through stretch receptors. Localized mucosal stimulation also evokes peristaltic reflexes that are similar to those evoked by stretch (Smith & Furness, 1988; Smith et al. 1991, 1992; Spencer & Smith, 2001; Heredia et al. 2009). Mucosal stimulation enhances the peristaltic reflexes activated by circumferential stretch, although stretch does not reciprocally enhance the effects of mucosal stimulation. These observations imply that IPANs, which are in the myenteric and submucous plexus, are activated by mucosal 5-HT (Kirchgessner et al. 1992; Bertrand et al. 2000; Pan & Gershon, 2000; Bayguinov et al. 2010), synapse with stretch-sensitive interneurons in the myenteric plexus (Smith et al. 1991, 2007; Spencer & Smith, 2004). If the faecal pellets are large enough to stretch the bowel wall, they activate stretch receptors and initiate peristaltic reflexes that propel them down the bowel even after the mucosa has been removed (Spencer et al. 2011; Sia et al. 2013a,b). Such a stimulus essentially short-circuits EC cells and activates stretch receptors to elicit propulsive reflexes.
The current study was carried out to test the hypothesis that mucosal secretion of 5-HT is necessary for the initiation of peristaltic reflexes and normal faecal pellet transit in the isolated murine colon. To do this, we compared the reflex responses and faecal pellet propagation in isolated preparations of colon from control (Tph1+/+) mice with those from mice in which tryptophan hydroxylase 1 (Tph1−/−) was genetically deleted. TPH1 is the rate-limiting enzyme for 5-HT biosynthesis in mucosal EC and mast cells, and so Tph1−/− mice lack mucosal 5-HT (Cote et al. 2003; Yadav et al. 2010; Li et al. 2011; Gross et al. 2012). Because TPH2 is the rate-limiting enzyme for 5-HT biosynthesis in central and myenteric serotonergic neurons, neuronal 5-HT is still present in Tph1−/− mice.
Methods
Animals
Male Tph1−/− mice (90–120 days old) and age-matched male littermates (Tph1+/+) were bred on a C57BL/6 background (Li et al. 2011). Animals were air freighted to the University of Nevada, Reno where they were housed in the transgenic facility at the University of Nevada School of Medicine, Reno. All mice were housed under pathogen-free conditions on a 12 h light/dark cycle with food and water ad libitum. Mice were euthanized by inhaling a 5% concentration of isoflurane, followed by cervical dislocation. A ventral midline incision was made and the whole colon (proximal colon and distal colon) was carefully excised. These procedures were in accordance with National Institutes of Health guidelines for the care and use of laboratory animals and approved by the Animal Ethics Committee at the University of Nevada, Reno. The animal experimentation policies and procedures comply with those in the UK, as outlined by Drummond (2009).
Faecal pellets expelled from both types of mice in vivo were collected over each 24 h period for 1 week, and their number was averaged to give the daily faecal output. Faecal pellets were expelled from the isolated colon with a syringe filled with oxygenated Krebs’ solution. They were then weighed when just expelled and after drying in an oven. Their dimensions were measured with a graticle on a dissecting microscope. Approximately five faecal pellets per colon were analysed.
Preparations
Spontaneous emptying of a full colon
The entire isolated colon, containing faecal pellets, was pinned in an organ bath lined with Sylgard (WPI Inc., Sarosota, FL, USA). One pin in the oral opening and one pin in the anal opening were placed to maintain the orientation of the colon for video imaging.
Artificial pellet propulsion
The entire emptied colon was placed in the same organ bath; however, two pins were placed in the oral opening to allow epoxy-coated or artificial faecal pellets to be inserted. In other experiments, faecal pellets of different diameters were used. Pellets were constructed by moulding and baking modelling polymer clay to different diameters (1–3 mm; length, 5–6 mm) (Polyform, Cool Tool, Fort Atkinson, WI, USA). The pellet was inserted into the oral end, and the movement was recorded with a video camera (model WV-BP330, Panasonic CCTV) directly to a computer (iMac, Apple, Cupertino, CA, USA) and analysed with in-house Volumetry software (G7). Using this software, spatiotemporal maps were generated by compressing a single frame vertically and stacking them in a descending order according to time. The maps were then rotated 90 deg counter clockwise so that the viewer could read them from right to left. This orientation of the maps statically illustrates the moving pellet (top to bottom) over time (left to right), not contraction. On these maps the oral and anal ends of the colon are fixed throughout the length (left to right) as the top and bottom of the map, respectively. The object shown in these maps is the location of a pellet in the bowel over time, the location being visualized by a light band of grey moving from upper left (oral at time begin) to lower right (anal at time end) (Heredia et al. 2009, 2012).
Tension recordings of colonic migrating motor complexes (CMMCs)
A glass micropipette (outer diameter, 1 mm; length, 8 cm; Sutter Instruments, Novato, CA, USA) was inserted through and glued to an epoxy-coated faecal pellet (diameter, 2.7 mm, length, 6 mm). The pipette was inserted through the lumen and secured to the floor of the organ bath using ‘U’-shaped pins at each end; these held the pipette in position with the fixed pellet located in the middle of the colon (Fig. 4). Three isometric tension transducers (model TST125C; Biopac Systems Inc., Santa Barbara, CA, USA) were attached by suture silk at regular intervals to the colonic wall in order to measure the tension of the circular muscle; the silk was glued to the colon by a bead of Gluture (WPI Inc.) and the initial resting tension was set to 8 mN, so as to not stretch the colon significantly. Three tension transducers were placed at ∼10–15 mm from the oral end of the colon (proximal), at the colonic flexure (middle) and at 10–15 mm from the anal end (anal). Tissues were equilibrated for 20–30 min, until regular spontaneous CMMCs were observed (see Fig. 4).
Figure 4. Colonic migrating motor complexes (CMMCs) and the effect of a 5-HT3 antagonist.
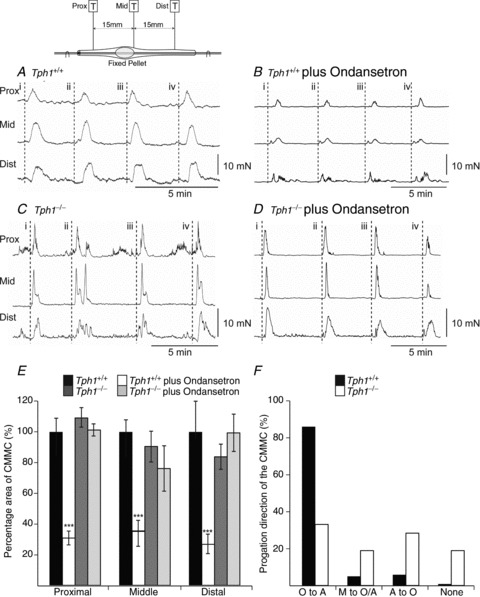
A, isometric tension recording from the proximal, middle and distal colon of spontaneous CMMCs occurring in the Tph1+/+ mouse colon. Lines i–iv indicate propagation in the oral to anal direction. B, spontaneous CMMCs occurring in the Tph1+/+ mouse colon in the presence of ondansetron (3 μm). Lines i, ii and iv indicate no propagation of the CMMC; line iii indicates CMMC propagation in the anal to oral direction. C, spontaneous CMMCs occurring in the Tph1−/− colon. Lines ii and iv indicate CMMC propagation in the oral to anal direction; lines i and iii indicate no CMMC propagation. D, spontaneous CMMCs occurring in the Tph1−/− colon in the presence of ondansetron (3 μm). Line i indicates CMMC oral to anal propagation; lines ii and iii indicate no propagation of the CMMC; line iv indicates CMMC propagation in the anal to oral direction. E, plot summarizing the area under the CMMC (relative to spontaneous CMMCs in Tph1+/+) in Tph1+/+ (n= 6) and Tph1−/− (n= 5) mice before and after ondansetron (3 μm). F, plot summarizing the propagation direction of CMMCs in Tph1+/+ and Tph1−/− mice: oral to anal, O to A; originating in mid-colon and propagating in both an oral and anal direction, M to O/A; anal to oral propagation, A to O; None, no discernible propagation direction, i.e. they occurred synchronously. ***P < 0.001.
Balloon distension
An inflatable balloon was substituted for the fixed pellet when the effects of luminal distension were analysed. A small balloon was placed midway between the upper and middle transducers (Fig. 7). A syringe filled with Krebs’ solution was attached to the tube and used to inflate the balloon which, when inflated, had the diameter (2.5–3 mm) of a faecal pellet. Inflatable balloons were manufactured by placing a 5 mm by 5 mm piece of latex over a polyethylene tube (outer diameter, 1.22 mm; inner diameter, 0.76 mm; Clay Adams, Parsippany, NJ, USA) and securing it with suture silk. The frequency, duration and amplitude of contractile complexes were measured using Acknowledge 3.2.6 (Biopac Systems, Inc., Goleta, CA, USA).
Figure 7. Colonic migrating motor complexes (CMMCs) evoked by activation of reflexes in Tph1−/− mice.
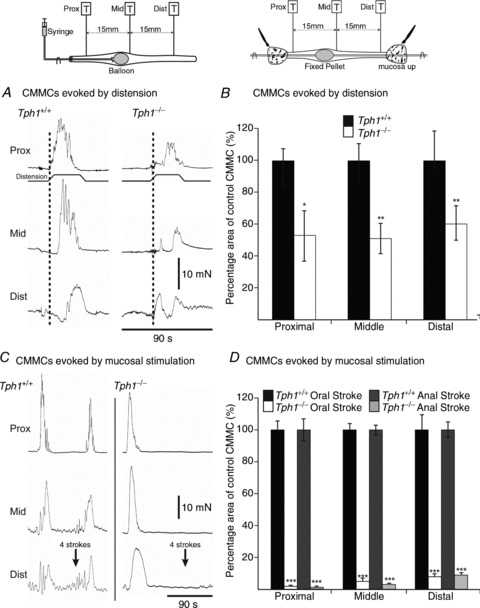
A, CMMC evoked by balloon distension in Tph1+/+ and Tph1−/− colon. B, plot summarizing CMMCs evoked by balloon distension in Tph1+/+ and Tph1−/− mice as a percentage of spontaneous CMMC area in the Tph1+/+ colon. Dotted line indicates oral to anal CMMC propagation in Tph1+/+ but not in Tph1−/− mice. C, mucosal stimulation (four brush strokes applied anally) evoked a CMMC in Tph1+/+ colon, but not in Tph1−/− colon. D, plot summarizing CMMC area evoked by oral and anal mucosal stimulation in Tph1+/+ and Tph1−/− colon.
Mucosal reflexes
A small section of mucosa (5 mm × 10 mm) was pinned facing upwards on the proximal and distal ends for brush stimulation, applied either as a gentle stroke to the mucosa or by pressing on the mucosa with a brush (Fig. 5; Heredia et al. 2009).
Figure 5. Effects of mucosal and electric field stimulation (EFS) following ondansetron.
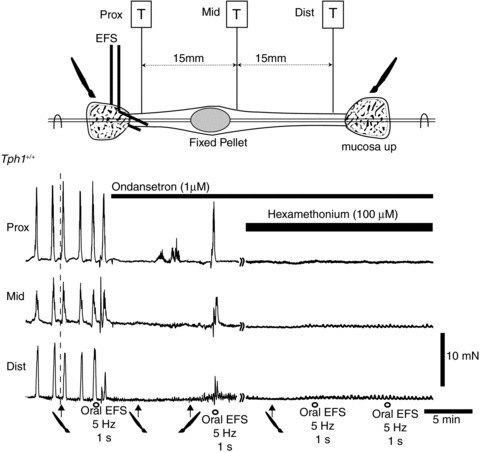
Mucosal stimulation applied at the anal or oral end of the colon evokes a premature colonic migrating motor complex (CMMC) in the Tph1+/+ colon. Similarly, EFS (0.5 ms, 5 Hz for 1 s, 20 V) applied at the oral end (0) also evokes a premature propagating CMMC. Spontaneous CMMCs and the responses to mucosal stimulation are blocked by ondansetron (1 μm), which does not, however, affect the propagating CMMC evoked by EFS. The CMMC evoked by EFS is, however, blocked by hexamethonium.
Elongation of the colon
Elongation of the colon whilst recording tension was achieved by removing tension from the transducers, unpinning a segment of the colon and repinning it at its new length. The tension was then restored to its original resting level (Heredia et al. 2010).
Electrophysiological recordings
The distal colon was cut open along the mesentery and pinned with the mucosa against the base of the organ bath. An inflatable balloon was inserted into the proximal opening and placed in the centre of the colon for distension experiments, approximately 10 mm from the distal recording site (Fig. 8). Smooth muscle cells were impaled by advancing a microelectrode through the outer longitudinal muscle into the underlying circular muscle, as described previously (Heredia et al. 2009, 2010; Dickson et al. 2010a,b).
Figure 8. Effects of balloon distention.
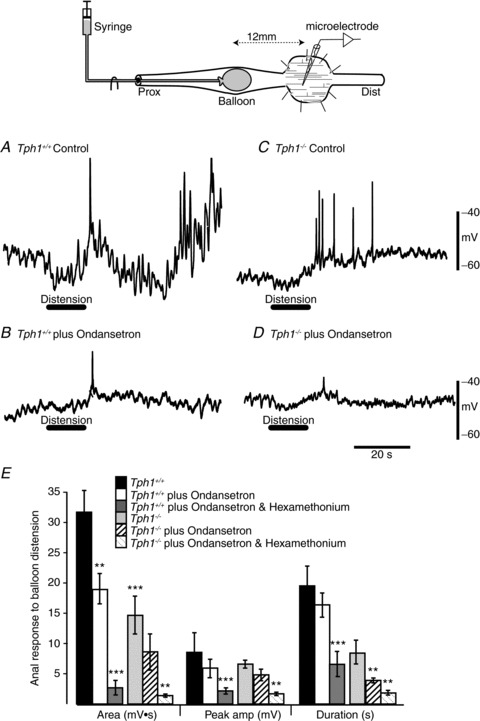
Intracellular microelectrode recordings were made from the circular muscle anal of the site of distension (Upper panel). A, Balloon distension evokes a burst of IJPs/hyperpolarization in the circular muscle of Tph1+/+. B, Balloon distension evokes a smaller response after ondansetron (3 μM) in Tph1+/+. C, Response to balloon distension in Tph1−/− colon. D, Response to balloon distension in Tph1−/− colon after ondansetron (3 μM). E, Plot summarizing area, peak amplitude and duration of the hyperpolarization activated by balloon distension in Tph1+/+ and Tph1−/− colon, before and after ondansetron and following the further addition of hexamethonium (100 μM). Note that ondansetron and hexamethonium also reduced the duration of the inhibitory response in Tph1−/− colon. **P < 0.01, ***P < 0.001.
Analysis of data
The apparent conduction velocity of CMMCs was measured at the half amplitude of contraction and tabulated with the known distances between recording transducers. The effects of drugs were evaluated over a 40 min time period following the maximal drug effect. Microelectrode recordings were made with an Axoprobe 1A amplifier (Molecular Devices, Sunnyvale, CA, USA) and recorded onto a PC with Axoscope 9.0 software (Molecular Devices). Tests for statistical significance were made using Sigma Plot 5.0 (Jandel Scientific, San Rafael, CA, USA).
Statistical methods
Statistical comparisons of data were performed using Student's (paired or unpaired) t tests or ANOVA, and P < 0.05 was considered as statistically significant. n refers to the number of animals from which colons were taken. All data are presented as means ± SEM. In the figures, *P < 0.05, **P < 0.01 and ***P < 0.001.
Drugs and solutions
Hexamethonium bromide and ondansetron were purchased from Sigma-Aldrich (St. Louis, MO, USA). Krebs’ solution contained (mm): NaCl, 120.35; KCl, 5.9; NaHCO3, 15.5; NaH2PO4, 1.2; MgCl2, 1.2; CaCl2, 2.5; glucose, 11.5 (continuously gassed with 3% CO2–97% O2, pH 7.3–7.4).
Results
Phenotypic changes in the colons of TPH1−/− mice
TPH1−/− (29.5 ± 1.1 g, n= 8) mice were similar in weight to their age-matched TPH1+/+ (28.7 ± 0.5 g, n= 8) littermates (P > 0.05). The length of the Tph1−/− colon was significantly longer than that of age-matched controls (Tph1+/+): 61.7 ± 3.4 mm full, 49.5 ± 1.0 mm empty versus 50.8 ± 0.9 mm full, 40.1 ± 1.2 mm empty, respectively (P < 0.001, n= 8) (Fig. 1A and C). Most faecal pellets were larger in Tph1−/− than in Tph1+/+ mice (Fig. 1B); moreover, although the wet weights of stool were similar the dry weights were significantly increased in Tph1−/− mice (Fig. 1B and E). Faecal pellets from Tph1−/− mice were generally wider (diameter: Tph1−/−, 2.8 ± 0.2 mm; Tph1+/+, 1.8 ± 0.1 mm; P < 0.05) and longer (length: Tph1−/−, 6.4 ± 0.2 mm; Tph1+/+, 4.8 ± 0.3 mm; P < 0.05; 20 pellets, n= 5) than those in Tph1+/+ mice (Fig. 1B).
Figure 1. Colonic length and faecal pellets.
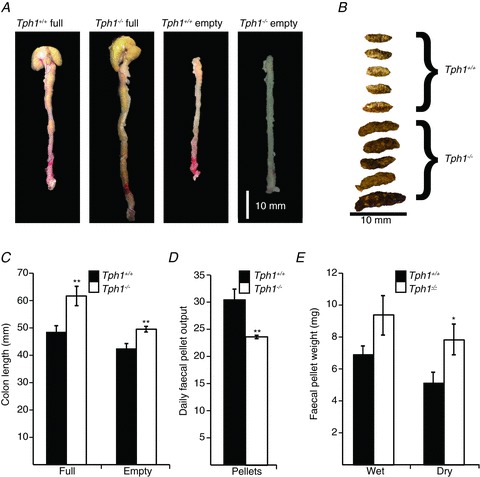
A, comparison of full and empty colons of Tph1+/+ and Tph1−/− mice. B, comparison of faecal pellets taken from Tph1+/+ and Tph1−/− mice. C, plot summarizing colonic length in full and empty colons taken from Tph1+/+ and Tph1−/− mice. D, plot of daily faecal pellet output from Tph1+/+ and Tph1−/− mice. E, plot of average single faecal pellet weight, both wet and dry, in Tph1+/+ and Tph1−/− mice.
Daily output of faecal pellets was lower in Tph1−/− (23.1 ± 0.3 pellets per day) than in Tph1+/+ (28.8 ± 1.3 pellets per day) animals (P < 0.001, n= 8); however, the daily faecal output was greater in Tph1−/− than in Tph1+/+ mice.
The average faecal pellet wet weight in mg × daily faecal pellet output was 9 mg × 23.1 = 207.9 mg in Tph1−/− and 6.8 mg × 28.8 = 195.8 mg in Tph1+/+ mice (Fig. 1D and E). This suggests that Tph1−/− mice ingested larger quantities of food (∼11%) than did Tph1+/+ mice.
Faecal pellet propagation
An epoxy-coated faecal pellet (diameter, 3 mm; length, 6 mm), which was similar to the larger faecal pellets in the Tph1−/− mouse, was used to study the propulsion of pellets down the isolated colon. Such pellets propagated along the length of the whole isolated colon, but did so more slowly in Tph1−/− (0.30 ± 0.04 mm s−1) than in Tph1+/+ (0.90 ± 0.10 mm s−1; P < 0.001, n= 8; Fig. 2) colons. This reduction in pellet velocity was observed throughout both the distal colon and rectum (Fig. 2D). In addition to the overall slower pellet transit in Tph1−/− colons (Fig. 2B), retrograde peristalsis was regularly observed in Tph1−/− but not in Tph1+/+ colon. Orthograde propulsion often alternated with retrograde propulsion that was not, however, sufficient to block overall faecal pellet transit. Faecal pellet propulsion was always continuous in Tph1+/+ colons (Fig. 2A and C).
Figure 2. Pellet transit in the colon.
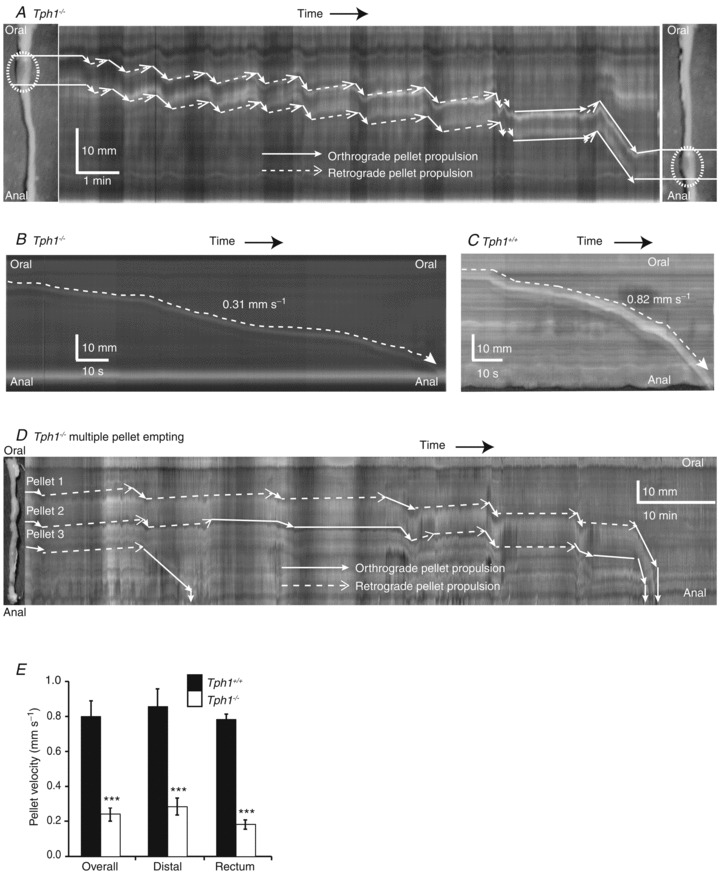
A, transit of an artificial faecal pellet (diameter, 3 mm; length, 6 mm; dotted ellipse) in the Tph1−/− mouse with the pellet outlined to show both orthograde and retrograde propulsion in the spatiotemporal map; distance is from top (oral) to bottom (anal), whereas time begins on the left and ends on the right. Whitish streaks indicate pellet movement in an oral to anal direction, not contraction. White lines indicate outer edges of the pellet. Note that orthograde propulsion is indicated by a continuous line preceding a solid arrow and retrograde propulsion is indicated by a dotted line preceding a hollow arrow. B and C, comparison of artificial pellet propulsion in Tph1−/− and Tph1+/+ mice. D, spontaneous emptying of faecal contents in a Tph1−/− mouse. E, plot summarizing overall faecal pellet velocity through the proximal colon, distal colon and rectum of Tph1+/+ and Tph1−/− mice. ***P < 0.001
When the colon full of faecal pellets was taken from the animal and mounted in an organ bath, the time required for complete evacuation was greater in Tph1−/− (89.3 ± 5 min, n= 3) than in Tph1+/+ (35.6 ± 3 min, n= 3; Fig. 2D) colons.
Propagation of faecal pellets of different diameters
Following removal of the mucosa, large faecal pellets, which probably activate only stretch reflexes, have been shown to propagate down an isolated guinea-pig colon (Spencer et al. 2011; Sia et al. 2013a,b). Conversely, we determined whether mucosal reflexes alone, in the absence of distension, could propagate faecal pellets.
Artificial faecal pellets (diameter, 1–3 mm; length, 6 mm) propagated down isolated Tph1+/+ colons. The velocity of pellet propagation was relatively dependent upon the pellet diameter (Fig. 3). Thin faecal pellets usually, but not always, propagated more slowly than those of wider diameter, possibly because thin pellets failed to exert sufficient pressure on the mucosa to release enough 5-HT to initiate a peristaltic reflex (Fig. 3D; Bülbring & Crema, 1959a). The propagation of a thin pellet (diameter, 1–2 mm) down the Tph1+/+ colon was always blocked on reaching an area from which the mucosa had been removed (10 mm long; n= 4; Fig. 1B). In contrast, wider faecal pellets (diameter ≥ 2.5 mm) eventually propagated through the mucosa-stripped zone, although they sometimes lingered for over 40 min proximal to the lesion (Fig. 3C; six passes, n= 4). Pellets with a diameter of 1.0–2.0 mm failed to propagate down isolated Tph1−/− colons at all; furthermore, although pellets that were 2.5 mm or more did propagate down isolated Tph1−/− colons, the velocity of their movement was much slower than that in Tph1+/+ colon (Fig. 3D).
Figure 3. Propagation of pellets of different diameters.

A, propagation of an artificial thin (diameter, 1.5 mm; length, 5 mm) pellet, which did not noticeably distend the bowel, along the Tph1+/+ colon. Dotted ellipses show the location of the pellet along the colon, pictured at either end of the spatiotemporal map. Note that the thin pellet propagated at a velocity of 0.6 mm s−1 (white box). B, propagation (velocity, 0.82 mm s−1) of a thin pellet along the Tph1+/+ colon was blocked on reaching a 10 mm zone of colon from which the mucosa had been removed (dotted lines across spatiotemporal map). Filled rectangle (expanded above) shows spatiotemporal map of faecal pellet propagation. C, a larger (diameter, 2.5 mm; length, 5 mm) faecal pellet, which distended the bowel, also propagated (velocity, 0.5 mm s−1) down the Tph1+/+ colon. However, when this faecal pellet entered the zone from which the mucosa had been removed, it stopped, but continued on its way after 40 min, but at a reduced velocity (0.22 mm s−1). Black rectangles show faecal pellet propagation. D, plot of velocity of pellet propagation versus the diameter of a faecal pellet, as well as the percentage increase in the resting diameter generated by a faecal pellet, in Tph1+/+ and Tph1−/− mice (average of three to five passes of each pellet, n= 5). Vertical dotted lines show the average diameter of a faecal pellet in Tph1+/+ (1.7 mm) and Tph1−/− (2.8 mm) colon. ***P < 0.001
Colonic migrating motor complexes (CMMCs)
CMMCs occur in most mammals (Sarna, 1991; Bywater et al. 1989). In humans they are often referred to as high-amplitude propagating contractions (Sood & Rudolf, 2007). CMMCs appear to be important for the propulsion of faecal pellets in the isolated colon (Heredia et al. 2009). When a faecal pellet is held at a fixed position in the colon, CMMCs propagate mainly in an oral to anal direction, because a faecal pellet strongly activates descending neural inhibition (Heredia et al. 2009). Under these conditions, CMMCs resemble the in vivo‘law of the intestine’ or peristaltic reflex (oral contraction/anal relaxation) described first by Bayliss and Starling (1899) and characterized by Bülbring and Crema (1959b).
CMMCs occurred spontaneously in both Tph1+/+ and Tph1−/− mice. The frequency of CMMCs in Tph1−/− mice was similar (0.3 ± 0.1 c min−1; n= 8) to that in Tph1+/+ mice (0.4 ± 0.1 c min−1; P < 0.01; n= 8; Fig. 4A and C). The normal propagation of the CMMC in an oral to anal direction, however, was compromised in Tph1−/− colons, despite the presence of a faecal pellet. Oral to anal propagation occurred in 83% of Tph1+/+ colons; 7% originated in the middle of the colon and propagated in the oral to anal direction, 9% originated anally and propagated in an anal to oral direction, whereas 1% did not show propagation. In contrast, CMMCs propagated in an oral to anal direction in only 33% of Tph1−/− colons; 19% originated in the middle of the colon, 28% propagated in an anal to oral direction and 20% did not propagate (90 CMMCs, n= 8; Fig. 4F). Those CMMCs that propagated in an oral to anal direction in Tph1−/− colons, however, had a faster apparent conduction velocity than those in Tph1+/+ mice: Tph1−/−, 1.2 ± 0.1 mm s−1; Tph1+/+, 0.8 ± 0.3 mm s−1 (30 CMMCs, P < 0.05, n= 5). This suggests that the inhibition preceding a CMMC is compromised in Tph1−/− mice.
Effect of blocking 5-HT3 receptors on spontaneous CMMCs inTPH1−/− mice
Bath applications of ondansetron (5-HT3 antagonist; 3 μm) significantly reduced the area of the CMMC contraction in Tph1+/+ colons, but did not affect the CMMC in Tph1−/− mice (Fig. 4A–E). Following ondansetron (3 μm), CMMCs in Tph1+/+ colon often occurred synchronously along the organ, i.e. they showed little propagation, which was reflected in their increase in apparent conduction velocity (1.2 ± 0.1 mm s−1, 80 CMMCs; n= 6; Fig. 4B). In contrast, ondansetron did not affect the apparent conduction velocity of CMMCs in Tph1−/− colons (1.3 ± 0.2 mm s−1, 60 CMMCs; P > 0.01, n= 5; Fig. 4D). This demonstrated that ondansetron not only reduced the amplitude of CMMCs in colons of Tph1+/+ mice, but also reduced the preceding inhibition, which is responsible for their propagation (Heredia et al. 2009, 2012).
In three other experiments with colons of Tph1+/+ mice, ondansetron (1 μm) blocked propagating CMMCs and also the reflex response to mucosal stimulation, applied either at the oral or anal end of the colon (Fig. 5). Ondansetron, however, did not block the propagating CMMC evoked by electric field stimulation (EFS; duration, 0.5 ms; 20 V; 5 Hz for 1 s; n= 5) applied either at the oral or anal end of the tissue. After ondansetron, hexamethonium (100 μm; n= 5; Fig. 5), however, completely blocked EFS-evoked CMMCs.
The nicotinic antagonist hexamethonium (100 μm; n= 5) blocked spontaneous CMMCs in Tph1−/− colons (Fig. 6A and B), as it did in Tph1+/+ colons (n= 6) (Bywater et al. 1989; Fida et al. 1997; Heredia et al. 2009).
Figure 6. Effect of blocking nicotinic receptors and elongation reflexes.
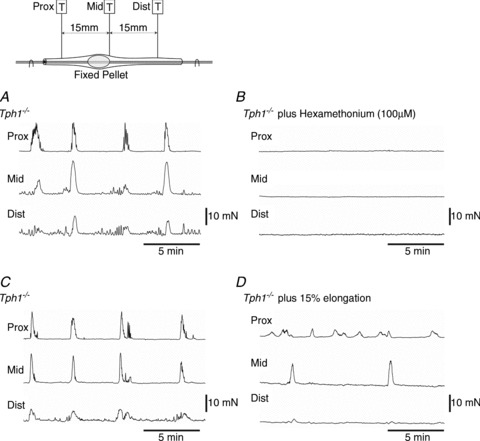
A, isometric tension recording of spontaneous colonic migrating motor complexes (CMMCs) occurring in a Tph1−/− colon. B, hexamethonium (100 μm) blocked spontaneous CMMC activity in a Tph1−/− colon. C, spontaneous CMMCs occurring in a Tph1−/− colon. D, addition of 15% colonic elongation (to activate the occult reflex) reduced spontaneous CMMCs in the Tph1−/− mouse.
Activation of intrinsic neural reflexes
Elongation of the isolated colon activates a dominant inhibitory ‘occult reflex’ which reduces the amplitude of spontaneous CMMCs in Tph1+/+ colon (Heredia et al. 2010). Similar effects of elongation were also observed in Tph1−/− colon (Fig. 7C and D; n= 3); therefore, because the colons of these mice are elongated, an ongoing inhibitory ‘occult reflex’ would be expected to contribute to slow faecal pellet transit in vivo (Heredia 2010, 2012).
A CMMC can be activated in response to circumferential stretch or mechanical stimulation of the mucosa with an artist's brush; the ensuing mucosal response is substantially reduced or blocked by 5-HT3 antagonists (Heredia et al. 2009; Bayguinov et al. 2010).
Inflation of an intraluminal balloon, which would normally be expected to activate both stretch and mucosal reflexes, applied between the proximal and middle tension transducer, evoked a robust premature CMMC in Tph1+/+ colon, but a significantly reduced response in colons of TPH1KO mice (Fig. 7A and B). Balloon distension of the Tph1−/− colon evoked responses that were ∼50% of the magnitude of those observed in Tph1+/+ colon (Fig. 8B).
Brushing the colonic mucosa with an artist's brush, applied either to the mucosa at the oral end of the proximal colon or to the rectal mucosa at the anal end, also elicited a robust premature CMMC in Tph1+/+ colon; these CMMCs were blocked by ondansetron (1 μm; n= 5). Mucosal stimulation of the Tph1−/− colon, however, unlike distension, failed to evoke CMMCs (Fig. 7C and D; n= 5).
Electrophysiological responses to intraluminal distension
The circular smooth muscle of the colon has been shown to be under tonic inhibition; i.e. spontaneous inhibitory junction potentials (IJPs) occur between CMMCs (Spencer et al. 1998, 2005; Heredia et al. 2009, 2012; Dickson et al. 2010a,b). Serotonergic interneurons synapse with inhibitory motor neurons that innervate the circular muscle (Heredia et al. 2009, 2012; Dickson et al. 2010b).
In the present study we observed spontaneous IJPs in the circular muscle of both Tph1+/+ and Tph1−/− mice (Fig. 8A and B). Their frequency in Tph1−/− colon (19.3 ± 2.7 c min−1) was significantly higher than that in Tph1+/+ colon (16.3 ± 3.5 c min−1) (P < 0.01, n= 8). In Tph1−/− colons, spontaneous IJPs had an amplitude of 5.6 ± 0.4 mV, which was lower than that of IJPs in Tph1+/+ colons (10.6 ± 0.7 mV). The resting membrane potentials of the circular muscle in Tph1+/+ (57.3 ± 1.0 mV) and Tph1−/− (55.4 ± 1.0 mV) mice were not significantly different (P > 0.05).
In both Tph1−/− and Tph1+/+ colon, oral intraluminal balloon distension resulted in a prolonged burst of IJPs/hyperpolarization of the circular muscle at distal recording sites (descending inhibition) which was sometimes followed by a CMMC (Fig. 8A and B). Both the duration (Tph1−/−, 8.5 ± 1.8 s; Tph1+/+, 20.8 ± 2.2 s, P < 0.001; n= 5) and area under the hyperpolarization (Tph1−/−, 14.8 ± 3.2 mV-s; Tph1+/+, 32.8 ± 2.3 mV-s; P < 0.001, n= 5) were significantly smaller in Tph1−/− than in Tph1+/+ mice. The peak amplitude of the evoked hyperpolarization was similar in Tph1−/− and Tph1+/+ mice (Tph1−/−, 6.6 ± 0.6 mV; Tph1+/+, 8.5 ± 1.5 mV; P > 0.05). Ondansetron (3 μm) significantly reduced both the duration and area under the evoked hyperpolarization in both Tph1−/− and Tph1+/+ colon (Fig. 8). Although ondansetron reduced the peak amplitude of the evoked hyperpolarization in Tph1−/− colon, its effect on this parameter in colons of Tph1+/+ mice was not significant (Fig. 8). The addition of hexamethonium (100 μm) further reduced the area, peak amplitude and duration of the hyperpolarization in both Tph1−/− and Tph1+/+ mice (Fig. 8E).
Discussion
Neither the deletion nor pharmacological inhibition of TPH1 has been reported to alter murine total gut transit time, gastric emptying, small bowel propulsion or rate of expulsion of beads inserted into the rectum (Yadav et al. 2010; Li et al. 2011). These observations have led to the suggestion that mucosal 5-HT may not be essential for constitutive gastrointestinal motility. In apparent support of this idea, we found that the daily faecal output of Tph1−/− mice was equal to or greater than that of control mice. In contrast, however, faecal pellet propulsion in the isolated colon was only 28% of that of Tph1+/+ mice; moreover, in comparison with the Tph1+/+ colon, the Tph1−/− colon: (1) was longer; (2) contained larger faecal pellets; (3) had a longer emptying time; (4) demonstrated retrograde propulsion of faecal pellets; (5) only rarely propagated CMMCs in an oral to anal direction, because of a lack of descending inhibition; (6) exhibited spontaneous CMMCs in the absence of mucosal stimulation; (7) manifested no mucosal reflexes; (8) exhibited reduced CMMCs and inhibitory responses to intraluminal distension; and (9) manifested CMMCs that were resistant to ondansetron (5-HT3 antagonist). Clearly, in vitro colonic motility is compromised in Tph1−/− mice, which lack mucosal 5-HT. The elongated colon, large faecal pellets and lack of mucosal reflexes suggest that these mice display signs of slow transit constipation (see Heredia et al. 2012). The apparently normal transit seen in vivo is probably a result of adaptive compensatory changes in these mice, which have abnormal cardiac function (Cote et al. 2003), and behavioural changes (see below, and Trowbridge et al. (2011).
In support of the concept that mucosal 5-HT is important for normal colonic propulsion of faecal pellets, we found that thin pellets, which did not significantly distend the colon, propagated down the isolated Tph1+/+ colon, but not the Tph1−/− colon, implying that mucosal reflexes alone, without stretch reflexes, are capable of propelling faecal pellets. Frigo and Lecchini (1970), who found, in studies of isolated colons from cat and guinea-pig, that ‘…the peristaltic reflex could not be elicited from areas from which the mucosal and submucosal layers had been removed…’ similarly concluded that, ‘these layers are essential for the triggering of the peristaltic reflex and for the propulsion of solid contents in the colon’. Mucosa- and stretch-activated reflexes probably both function in the normal propagation of faecal pellets in the colon in vivo. These reflexes would be expected to act in concert with mucosal reflexes, enhancing stretch reflexes (Smith et al. 1991). Moreover, the two types of reflex would be expected to be self-reinforcing, providing a fail-safe mechanism that would enable propulsive reflexes to continue to be manifest, even if one type of reflex becomes compromised, perhaps as a result of reduced muscle tone, which reduces activity in IPANs (Kunze et al. 1998; Smith et al. 2003), or mucosal damage. Under such circumstances, one or the other peristaltic mechanism would probably assume dominance.
It cannot be concluded that the persistence of gastrointestinal transit in Tph1−/− mice proves that mucosal 5-HT, which is absent in these animals, makes no contribution to peristaltic reflexes in the unpertrubed mouse colon. On the contrary, our observations imply that mucosal 5-HT makes a significant contribution to normal peristaltic reflexes in the murine colon. Importantly, our studies also suggest that 5-HT is the major, if not the only, motility-related paracrine factor released from EC cells by mechanical stimulation of the mucosa, as implied previously (Heredia et al. 2009; Bayguinov et al. 2010).
CMMCs in Tph1−/− mice
A fixed faecal pellet normally releases 5-HT from the mucosa, which simulates IPANs that, in turn, activate descending (serotonergic) inhibitory nerve pathways (Heredia et al. 2009, 2012). Descending inhibition is generated by the robust activation of distal-projecting serotonergic neurons which activate inhibitory motor neurons via 5-HT3 receptors (Heredia et al. 2009, 2012; Dickson et al. 2010b). CMMCs have an apparent conduction velocity of ∼0.8 mm s−1 (Heredia et al. 2009), which is ∼400 times slower than conduction along axons (∼0.3 m s−1) within the myenteric plexus (Schemann et al. 2002). Consequently, during the initial phase of the CMMC, inhibitory and excitatory muscle responses are probably activated almost simultaneously and summate at the level of the muscle (Bayguinov et al. 2010). If not for the preceding inhibitory response in the muscle, which delays the onset of the excitatory response, CMMCs would be expected to occur almost synchronously along the colon (Heredia et al. 2009, 2012), as they do in the presence of ondansetron. 5-HT is likely to be a primary neurotransmitter in this pathway, mediating the preceding relaxation (Dickson et al. 2010b), as suggested by recent immunohistochemical studies (Sharrad et al. 2013).
The faster apparent oral to anal conduction velocity of CMMCs in Tph1−/− mice indicates that IPANs are not effectively activating the inhibition that precedes the CMMC because they are not being excited by mucosal 5-HT (Heredia et al. 2009; Bayguinov et al. 2010; Dickson et al. 2010b). A rapid conduction velocity suggests that there is not enough time for relaxation of the muscles in front of a faecal pellet, leading to a premature CMMC contraction that overlaps and traps a faecal pellet. This could explain the relatively short distances traversed by a faecal pellet in Tph1−/− mice in contrast with the longer distances a pellet travels in the control colon.
Spontaneous CMMCs were observed in Tph1−/− mice despite the absence of mucosal 5-HT, as they are in mice constipated as a result of a minor outlet obstruction, in which an up-regulation of prostaglandins blocks mucosal reflexes because it inhibits the release of 5-HT (Heredia et al. 2012). These observations suggest that the colon adapts to a lack of mucosal 5-HT by increasing the excitability of IPANs, as it does in constipated mice (N. Grainger & T. K. Smith, unpublished observations).
Tph1−/− mice exhibited CMMCs, the amplitude of which was unaffected by ondansetron, which reduced or blocked CMMCs and their propagation in wild-type mice (Bush et al. 2001; Heredia et al. 2009). CMMCs in Tph1−/− mice, however, were completely blocked by hexamethonium, as they were in control mice, suggesting that they are activated by ascending excitatory nerve pathways that rely on acetylcholine acting on nicotinic receptors (Heredia et al. 2009, 2012).
Role of 5-HT3 receptors in generating CMMCs
We have shown previously that removal of the mucosa abolishes CMMCs, but not spontaneous IJPs, neuronal firing or CMMCs evoked by EFS, suggesting that the neural circuits in the myenteric plexus remain intact after the mucosa is removed (Heredia et al. 2009; Bayguinov et al. 2010; Dickson et al. 2010a,b). Spontaneous CMMCs are sensitive to the blockade of 5-HT3 receptors with ondansetron (Bush et al. 2001; Heredia et al. 2009), which also block the preceding hyperpolarization (Dickson et al. 2010b). In contrast with spontaneous CMMCs, those evoked by EFS are insensitive to ondansetron (Dickson et al. 2010b), suggesting that the drug does not inhibit CMMC generation.
Spontaneous CMMCs and pellet propulsion have been reported to occur in mice and guinea-pigs treated with reserpine to lower intestinal 5-HT levels (Sia et al. 2013a,b; Spencer et al. 2013). Interestingly, ondansetron (Spencer et al. 2013) and the 5-HT4 antagonist SDZ-205-557 (Sia et al. 2013b) have been found to reduce CMMCs and faecal pellet propulsion dose dependently in reserpine-treated mice and guinea-pigs, respectively, even when the mucosa is removed. The presence of CMMCs in reserpine-treated gut has been used to support a hypothesis that neither EC cell nor neuronal 5-HT play a role in initiating or mediating CMMCs. Reserpine, however, is an irreversible inhibitor of the vesicular monoamine transporter, and thus interferes only with the transport of 5-HT from cytosol to vesicles (Henry & Scherman, 1989). Reserpine does not prevent the hydroxylation of tryptophan; therefore, 5-HT biosynthesis continues in reserpine-treated tissue, although newly synthesized 5-HT is exposed to monoamine oxidase (MAO), which restricts the size of the cytosolic 5-HT pool. Inhibition of MAO thus reverses the effects of reserpine (Spector et al. 1960); moreover, cytosolic 5-HT can be secreted (Kuhn et al. 1985) and, if MAO is inhibited, can cause a generalized 5-HT-mediated ‘serotonin syndrome’ (Sternbach, 1991). Reserpine, even in massive doses, does not eliminate 5-HT from the bowel (Bülbring & Crema, 1959b; Bülbring & Gershon, 1967), although the low level of residual cytosolic 5-HT would not be detected by an assay, such as immunocytochemistry, which lacks sufficient sensitivity.
Because 5-HT is still present in the bowel and can be secreted after animals have been treated with reserpine, 5-HT remains a potential mediator of CMMCs that occur in reserpine-treated gut. Indeed, the observations that 5-HT3 and 5-HT4 antagonists inhibit CMMCs and peristaltic contractions despite treatment with reserpine (Spencer et al. 2013; Sia et al. 2013a,b) would be explained more plausibly by the idea that these compounds block the effects of newly synthesized cytosolic 5-HT, which reserpine-treated EC cells or neurons continue to secrete in response to the eliciting stimulus of distension. One need not postulate never before demonstrated non-specific effects of ondansetron or SDZ-205-557 (Sia et al. 2013a,b; Spencer et al. 2013).
We therefore believe that ondansetron inhibits the generation of spontaneous CMMCs in our control mice by antagonizing 5-HT3 receptor activation on the mucosal processes of IPANs (Bertrand et al. 2000; Heredia et al. 2009; Bayguinov et al. 2010; Dickson et al. 2010b), and perhaps by inhibiting ascending interneurons in excitatory nerve pathways, as these may be activated by descending serotonergic neurons (Spencer et al. 2005), as has been shown for the small intestine (Yuan et al. 1994; Neal & Bornstein 2007).
Intraluminal responses to distension in Tph1−/− colon
Responses to intraluminal distension, including CMMCs and anal bursts of IJPs, in Tph1−/− colon were less robust than those in isolated Tph1+/+ colon, suggesting that responses to intraluminal distension are at least partially dependent on the secretion of 5-HT from EC cells. Descending serotonergic neurons, acting on the 5-HT3 receptors expressed by inhibitory motor neurons, may also contribute to the responses to distension in both Tph1−/− and Tph1+/+ mice.
Retrograde propulsion in TPH1KO colon
Orthograde propulsion was often followed by retrograde faecal pellet propulsion in the isolated Tph1−/− colon. Because such a sequence was not observed in Tph1+/+ mice, there is a clear dysfunction in the coordination of CMMC in Tph1−/− colon. The retrograde propulsion is probably caused by CMMC contractions that propagate in the anal to oral direction. CMMCs in the Tph1+/+ colon propagate in an oral to anal direction over 95% of the time, whereas fewer than 35% of CMMCs in the Tph1−/− colon propagate orally to anally. The larger faecal pellets of Tph1−/− mice is consistent with the idea that faecal material spends more time in vivo in the colon of Tph1−/− than in that of Tph1+/+ mice. This possibility is consistent with the observation that pellets are propelled more slowly in isolated Tph1−/− than in Tph1+/+ colon. Pellet formation in vivo normally occurs around the mid-region of the colon and the propulsion of pellets in vitro is more severely retarded in the Tph1−/− distal colon and rectum of the isolated colon. The phenomenon of retrograde propulsion, were it also to occur in vivo, might also contribute to the formation of the large faecal pellets of Tph1−/− mice. The large faecal pellets, excessively elongated colons and rare propagation of anally directed CMMCs found in Tph1−/− mice are phenomena that would be expected if slow transit constipation were to be present in Tph1−/− mice (Heredia et al. 2012). Indeed, the absence of mucosally initiated peristaltic reflexes in Tph1−/− mice might force pellet size to increase to where they are large enough to engage stretch-activated reflexes in order for propulsion to occur.
In vivo versus in vitro motility
The fine movements of the bowel are not monitored when in vivo measurements are made. Instead, only the final outcome is monitored, which is an aggregate of these movements. Measurements are made of how long it takes for a marker to traverse the whole bowel, empty from the stomach or be expelled from the rectum. Those are relatively gross measurements and do not provide information about specific reflexes or, indeed, any insight into how the gut might behave to specific types of perturbation, such as mucosal stimulation or distension. The in vitro measurements, in contrast, provide information about how the gut responds to particular stimuli, but they do not reveal whether or how much these particular stimuli are present in vivo. A full assessment of motility therefore requires both in vivo and in vitro observations. The current work suggests that mucosal 5-HT contributes a great deal to mucosa-driven reflex activity of the bowel. In contrast, distension-driven reflexes can evidently occur independently of mucosal 5-HT. It is likely that this distinction accounts for some of the discrepant conclusions in the literature about the role of mucosal 5-HT. It is also likely that, in a totally unchallenged state, propulsion can occur in the gut even when, as in a Tph1−/− gut, the bowel is unable to manifest mucosa-driven peristaltic reflexes normally. Unperturbed Tph1−/− animals survive and their total gastrointestinal transit time is normal, even though in vitro experiments reveal that their bowel is unable to mount certain types of reflex activity. It is now important to try to discover what situations in vivo require the manifestation of the type of 5-HT-dependent reflex activity that the current in vitro studies indicate is deficient in Tph1−/− mice.
It is interesting that the ability of Tph1−/− mice to eject beads from the rectum is comparable with that of Tph1+/+ mice (Li et al. 2011). The bead used in these studies had a diameter of 3 mm, which is similar to the diameter of natural faecal pellets observed in Tph1−/− mice. The expulsion rate of beads from the rectum of Tph1−/− mice in vivo may thus appear normal even though mucosal reflexes are absent, because 5-HT-independent stretch reflexes are still present. It may be necessary to examine the expulsion of beads of different sizes to find differences between Tph1+/+ and Tph1−/− mice.
The difference between the demonstrated mucosal 5-HT dependence of the in vitro peristaltic reflex in the colon and the evident mucosal 5-HT independence of the in vivo constitutive gastrointestinal transit time suggests that intestinal behaviour analogous to the classic peristaltic reflex first described by Bayliss and Starling (1899) in the small intestine is not essential for constitutive gastrointestinal motility. Multiple enteric motility patterns have been described in vivo (Wood, 1999); the pattern that perhaps comes closest to the peristaltic reflex is power propulsion, which may not be manifest in vivo unless the bowel is distended or the mucosa is stimulated by a challenge, such as infection or obstruction. Other forms of motility may be able to sustain propulsion even when mucosal reflex activity is lost.
It seems likely that Tph1−/− mice generate enough propulsive CMMCs in vivo, perhaps as a result of distension of the bowel wall by the abnormally large faecal pellets which, in the Tph−/− colon, maintain transit. The Tph1−/− gut can thus propel material adequately to support survival if circumstances are ideal. Challenge with a pathogen or another adverse environmental circumstance in vivo might evoke a peristaltic reflex-like intestinal behaviour, such as power propulsion or power retropulsion. If so, the survival or function of a Tph1−/− animal, which may be unable to mount such a response, might be compromised.
Conclusions
Our in vitro studies of isolated mouse colon suggest that there is considerable dysfunction in the regulation of colonic motility in Tph1−/− mice which is not evident in the gross measurement of the total gastrointestinal transit time in the murine gut in vivo. This difference may be a result of the integrative nature of the measurements of transit, which do not reveal the nature of underlying reflexes or motor patterns; alternatively, compensatory mechanisms may be able to maintain transit at an adequate basal level even when the complex mechanisms of paracrine regulation of intrinsic neuronal reflexes are compromised.
Acknowledgments
Imaging was performed in a Core Laboratory funded by a National Institutes of Health Grant COBRE 8P20GM103513-09.
Glossary
Abbreviations
- CMMC
colonic migrating motor complex
- EC
enterochromaffin
- EFS
electric field stimulation
- 5-HT
5-hydroxytryptamine
- IJP
inhibitory junction potential
- IPAN
intrinsic primary afferent neuron
- MAO
monoamine oxidase
- TPH1KO
tryptophan hydroxylase 1 knockout
Additional information
Competing interests
There are no conflicts of interest to disclose.
Author contributions
D.J.H. was responsible for tension recordings, electrophysiology, pellet propulsion, data analysis and figure construction. S.D.K., R.D.C. and T.O. contributed to tension and pellet propulsion experiments and interpretation of the data. T.K.S. directed the overall project, data analysis and construction of figures. M.D.G. bred, genotyped and provided the TPH1KO mice, and provided important insights into the interpretation of the data. M.D.G., T.K.S., D.J.H. and S.D.K. edited and wrote the final manuscript. All authors read and approved the manuscript.
Funding
This study was funded by grants from the National Institutes of Health: RO1 DK45713 (T.K.S.), PO1DK 41315 (S.D.K.) and NS 12969, NS 15547 (M.D.G.).
References
- Bayguinov O, Hennig GW, Sanders KM. Movement based artifacts may contaminate extracellular electrical recordings from GI muscles. Neurogastroenterol Motil. 2011;23:1029–1042. doi: 10.1111/j.1365-2982.2011.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayguinov PO, Hennig GW, Smith TK. Calcium activity in different classes of myenteric neurons underlying the migrating motor complex in the murine colon. J Physiol. 2010;588:399–421. doi: 10.1113/jphysiol.2009.181172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss WM, Starling EH. The movements and innervation of the small intestine. J Physiol. 1899;24:99–143. doi: 10.1113/jphysiol.1899.sp000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand PP. Real-time measurement of serotonin release and motility in guinea pig ileum. J Physiol. 2006;577:689–704. doi: 10.1113/jphysiol.2006.117804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand PP, Kunze WA, Furness JB, Bornstein JC. The terminals of myenteric intrinsic primary afferent neurons of the guinea-pig ileum are excited by 5-hydroxytryptamine acting at 5-hydroxytryptamine-3 receptors. Neuroscience. 2000;101:459–469. doi: 10.1016/s0306-4522(00)00363-8. [DOI] [PubMed] [Google Scholar]
- Boullin DJ. Observations on the significance of 5-hydroxytryptamine in relation to the peristaltic reflex of the rat. Br J Pharmacol. 1964;23:14–33. doi: 10.1111/j.1476-5381.1964.tb01563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes SJ, Chen BN, Costa M, Humphreys CM. Initiation of peristalsis by circumferential stretch of flat sheets of guinea-pig ileum. J Physiol. 1999;516:525–538. doi: 10.1111/j.1469-7793.1999.0525v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E, Crema A. Observations concerning the action of 5-hydroxytryptamine on the peristaltic reflex. Br J Pharmacol. 1958;13:444–457. doi: 10.1111/j.1476-5381.1958.tb00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E, Crema A. The release of 5-hydroxytryptamine in relation to pressure exerted on the intestinal mucosa. J Physiol. 1959a;146:18–28. doi: 10.1113/jphysiol.1959.sp006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E, Crema The action of 5-hydroxytryptamine, 5-hydroxytryptophan and reserpine on intestinal peristalsis in anaesthetized guinea-pigs. J Physiol. 1959b;146:29–53. doi: 10.1113/jphysiol.1959.sp006176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E, Gershon MD. 5-Hydroxytryptamine participation in the vagal inhibitory innervation of the stomach. J Physiol. 1967;192:823–846. doi: 10.1113/jphysiol.1967.sp008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E, Lin RCY, Schofield G. An investigation of the peristaltic reflex in relation to anatomical observations. Q J Exp Physiol. 1958;43:26–37. doi: 10.1113/expphysiol.1958.sp001305. [DOI] [PubMed] [Google Scholar]
- Bush TG, Spencer NJ, Watters N, Sanders KM, Smith TK. Effects of alosetron on spontaneous migrating motor complexes in murine small and large bowel in vitro. Am J Physiol Gastrointest Liver Physiol. 2001;281:974–983. doi: 10.1152/ajpgi.2001.281.4.G974. [DOI] [PubMed] [Google Scholar]
- Bywater RA, Small RC, Taylor GS. Neurogenic slow depolarizations and rapid oscillations in the membrane potential of circular muscle of mouse colon. J Physiol. 1989;413:505–519. doi: 10.1113/jphysiol.1989.sp017666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote F, Thevenot E, Fligny C, Fromes Y, Darmon M, Ripoche MA, Bayard E, Hanoun N, Saurini F, Lechat P, Dandolo L, Hamon M, Mallet J, Vodjdani G. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci U S A. 2003;100:13525–13530. doi: 10.1073/pnas.2233056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson EJ, Heredia DJ, Hennig GW, Smith TK. Mechanisms underlying the colonic migrating motor complex in both wild-type and nNOS knockout mice. Am J Physiol Gastrointest Liver Physiol. 2010a;298:222–232. doi: 10.1152/ajpgi.00399.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson EJ, Heredia DJ, Smith TK. Critical role of 5-HT1A, 5-HT3 and 5-HT7 receptor subtypes in the initiation, generation, and propagation of the murine colonic migrating motor complex. Am J Physiol Gastrointest Liver Physiol. 2010b;299:144–157. doi: 10.1152/ajpgi.00496.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure C, Patey N, Gauthier C, Brooks EM, Mawe GM. Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterology. 2010;139:249–258. doi: 10.1053/j.gastro.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fida R, Lyster DJ, Bywater RA, Taylor GS. Colonic migrating motor complexes (CMMCs) in the isolated mouse colon. Neurogastroenterol Motil. 1997;9:99–107. doi: 10.1046/j.1365-2982.1997.d01-25.x. [DOI] [PubMed] [Google Scholar]
- Frigo GM, Lecchini S. An improved method for studying the peristaltic reflex in the isolated colon. Br J Pharmacol. 1970;39:346–356. doi: 10.1111/j.1476-5381.1970.tb12898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD. Serotonin is a sword and a shield of the bowel: serotonin plays offense and defense. Trans Am Clin Climatol Assoc. 2012;123:268–280. [PMC free article] [PubMed] [Google Scholar]
- Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes. 2013;20:14–21. doi: 10.1097/MED.0b013e32835bc703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross ER, Gershon MD, Margolis KG, Gertsberg ZV, Li Z, Cowles RA. Neuronal serotonin regulates growth of the intestinal mucosa in mice. Gastroenterology. 2012;143:408–417. doi: 10.1053/j.gastro.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia DJ, Dickson EJ, Bayguinov PO, Hennig GW, Smith TK. Localized release of serotonin (5-hydroxytryptamine) by a fecal pellet regulates migrating motor complexes in murine colon. Gastroenterology. 2009;136:1328–1338. doi: 10.1053/j.gastro.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia DJ, Dickson EJ, Bayguinov PO, Hennig GW, Smith TK. Colonic elongation inhibits pellet propulsion and migrating motor complexes in the murine large bowel. J Physiol. 2010;588(Pt 15):2919–2934. doi: 10.1113/jphysiol.2010.191445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia DJ, Grainger N, McCann CJ, Smith TK. Insights from a novel model of slow-transit constipation generated by partial outlet obstruction in the murine large intestine. Am J Physiol Gastrointest Liver Physiol. 2012;303:1004–1016. doi: 10.1152/ajpgi.00238.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J, Scherman D. Radioligands of the vesicular monoamine transporter and their use as markers of monoamine storage vesicles. Biochem Pharmacol. 1989;38:2395–2404. doi: 10.1016/0006-2952(89)90082-8. [DOI] [PubMed] [Google Scholar]
- Hoffman JM, Tyler K, MacEachern SJ, Balemba OB, Johnson AC, Brooks EM, Zhao H, Swain GM, Moses PL, Galligan JJ, Sharkey KA, Greenwood-Van Meerveld B, Mawe GM. Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology. 2012;142:844–854. doi: 10.1053/j.gastro.2011.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JG, Foxx-Orenstein AE, Grider JR. Propulsion in guinea pig colon induced by 5-hydroxytryptamine (HT) via 5-HT4 and 5-HT3 receptors. J Pharmacol Exp Ther. 1999;288:93–97. [PubMed] [Google Scholar]
- Keating DJ, Spencer NJ. Release of 5-hydroxytryptamine from the mucosa is not required for the generation or propagation of colonic migrating motor complexes. Gastroenterology. 2010;138:659–670. doi: 10.1053/j.gastro.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL, Tamir H, Gershon MD. Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity. J Neurosci. 1992;12:235–248. doi: 10.1523/JNEUROSCI.12-01-00235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn DM, Wolf WA, Youdim MB. 5-Hydroxytryptamine release in vivo from a cytoplasmic pool: studies on the 5-HT behavioural syndrome in reserpinized rats. Br J Pharmacol. 1985;84:121–129. [PMC free article] [PubMed] [Google Scholar]
- Kunze WAA, Furness JB, Bertrand PP, Bornstein JC. Intracellular recording from myenteric neurons of the guineapig ileum that respond to stretch. J Physiol. 1998;506:827–842. doi: 10.1111/j.1469-7793.1998.827bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesurtel M, Soll C, Graf R, Clavien PA. Role of serotonin in the hepato-gastrointestinal tract: an old molecule for new perspectives. Cell Mol Life Sci. 2008;65:940–952. doi: 10.1007/s00018-007-7377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Chalazonitis A, Huang YY, Mann JJ, Margolis KG, Yang QM, Kim DO, Côté F, Mallet J, Gershon MD. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci. 2011;31:8998–9009. doi: 10.1523/JNEUROSCI.6684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax AE, Furness JB. Neurochemical classification of enteric neurons in the guinea-pig distal colon. Cell Tissue Res. 2000;302:59–72. doi: 10.1007/s004410000260. [DOI] [PubMed] [Google Scholar]
- Mackenna BR, McKirdy HC. Peristalsis in the rabbit distal colon. J Physiol. 1972;220:33–54. doi: 10.1113/jphysiol.1972.sp009693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel AW, Northcutt AR. Review article: the safety and efficacy of alosetron, a 5-HT3 receptor antagonist, in female irritable bowel syndrome patients. Aliment Pharmacol Ther. 1999;13:77–82. doi: 10.1046/j.1365-2036.1999.00010.x. [DOI] [PubMed] [Google Scholar]
- Marcelli G, Patel BA. Understanding changes in uptake and release of serotonin from gastrointestinal tissue using a novel electroanalytical approach. Analyst. 2010;135:2340–2347. doi: 10.1039/c0an00260g. [DOI] [PubMed] [Google Scholar]
- Neal KB, Bornstein JC. Mapping 5-HT inputs to enteric neurons of the guinea-pig small intestine. Neuroscience. 2007;145:556–567. doi: 10.1016/j.neuroscience.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Bayguinov PO, Broadhead MJ, Smith TK. Ca2+ transients in submucous neurons during the colonic migrating motor complex in the isolated murine large intestine. Neurogastroenterol Motil. 2012;24:769–778. doi: 10.1111/j.1365-2982.2012.01934.x. [DOI] [PubMed] [Google Scholar]
- Pan H, Gershon MD. Activation of intrinsic afferent pathways in submucosal ganglia of the guinea pig small intestine. J Neurosci. 2000;20:3295–3309. doi: 10.1523/JNEUROSCI.20-09-03295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather CM, Camilleri M, Zinsmeister AR, McKinzie S, Thomforde G. Tegaserod accelerates colocecal transit in patients with constipation-predominant irritable bowel syndrome. Gastroenterology. 2000;118:463–468. doi: 10.1016/s0016-5085(00)70251-4. [DOI] [PubMed] [Google Scholar]
- Sang Q, Williamson S, Young HM. Projections of chemically identified myenteric neurons of the small and large intestine of the mouse. J Anat. 1997;190:209–222. doi: 10.1046/j.1469-7580.1997.19020209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarna SK. Physiology and pathophysiology of colonic motor activity (1) Dig Dis Sci. 1991;36:827–862. doi: 10.1007/BF01311244. [DOI] [PubMed] [Google Scholar]
- Schemann M, Michel K, Peters S, Bischoff SC, Neunlist M. Cutting-edge technology. III. Imaging and the gastrointestinal tract: mapping the human enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2002;282:919–925. doi: 10.1152/ajpgi.00043.2002. Review. [DOI] [PubMed] [Google Scholar]
- Sharrad DF, Chen BN, Brookes SJ. Neurochemical coding compared between varicose axons and cell bodies of myenteric neurons in the guinea-pig ileum. Neurosci Lett. 2013;534:171–176. doi: 10.1016/j.neulet.2012.10.049. [DOI] [PubMed] [Google Scholar]
- Sia TC, Flack N, Robinson L, Kyloh M, Nicholas SJ, Brookes SJ, Wattchow DA, Dinning P, Oliver J, Spencer NJ. Is serotonin in enteric nerves required for distension-evoked peristalsis and propulsion of content in guinea-pig distal colon. Neuroscience. 2013a;240:325–335. doi: 10.1016/j.neuroscience.2013.02.061. [DOI] [PubMed] [Google Scholar]
- Sia TC, Whiting M, Kyloh M, Nicholas S, Brookes SJ, Oliver J, Dinning P, Wattchow DA, Spencer NJ. 5-HT3 and 5-HT4 antagonists inhibit peristaltic contractions in guinea-pig distal colon by mechanisms independent of endogenous 5-HT. Front Neurosci. 2013b;7:1–10. doi: 10.3389/fnins.2013.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood MR, Rudolf CD. Gastrointestinal motility disorders in adolescent patients: transitioning to adult care. Gastroenterol Clin N Am. 2007;36:749–763. doi: 10.1016/j.gtc.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Smith TK, Bornstein JC, Furness JB. Interactions between reflexes evoked by distension and mucosal stimulation: electrophysiological studies of guinea-pig ileum. J Auton Nerv Syst. 1991;34:69–75. doi: 10.1016/0165-1838(91)90009-r. [DOI] [PubMed] [Google Scholar]
- Smith TK, Bornstein JC, Furness JB. Convergence of reflex pathways excited by distension and mechanical stimulation of the mucosa onto the same myenteric neurons of the guinea-pig small intestine. J Neurosci. 1992;12:1502–1510. doi: 10.1523/JNEUROSCI.12-04-01502.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Dickson EJ, Heredia DJ, Hennig GW, Bayguinov PO. Controversies involving the role of 5-hydroxytryptamine in generating colonic migrating motor complexes: what is spontaneous. Gastroenterology. 2010;138:1213–1214. doi: 10.1053/j.gastro.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Furness JB. Reflex changes in circular muscle activity elicited by stroking the mucosa: an electrophysiological analysis in the isolated guinea-pig ileum. J Auton Nerv Syst. 1988;25:205–218. doi: 10.1016/0165-1838(88)90025-2. [DOI] [PubMed] [Google Scholar]
- Smith TK, Oliver GR, Hennig GW, O'Shea DM, Vanden Berghe P, Kang SH, Spencer NJ. A smooth muscle tone-dependent stretch-activated migrating motor pattern in isolated guinea-pig distal colon. J Physiol. 2003;551:955–969. doi: 10.1113/jphysiol.2003.049163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Spencer NJ, Hennig GW, Dickson EJ. Recent advances in enteric neurobiology: mechanosensitive interneurons. Neurogastroenterol Motil. 2007;19:869–878. doi: 10.1111/j.1365-2982.2007.01019.x. Review. [DOI] [PubMed] [Google Scholar]
- Spector S, Shore PA, Brodie BB. On the reported inhibition of monoamine oxidase by an agent with sedative properties. Science. 1960;132(3429):735. doi: 10.1126/science.132.3429.735. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Bywater RA, Taylor GS. Disinhibition during myoelectric complexes in the mouse colon. J Auton Nerv Syst. 1998;71:37–47. doi: 10.1016/s0165-1838(98)00063-0. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Dickson EJ, Hennig GW, Smith TK. Sensory elements within the circular muscle are essential for mechanotransduction of ongoing peristaltic reflex activity in guinea-pig distal colon. J Physiol. 2006;576:519–531. doi: 10.1113/jphysiol.2006.109561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Hennig GW, Dickson E, Smith TK. Synchronization of enteric neuronal firing during the murine colonic MMC. J Physiol. 2005;564:829–847. doi: 10.1113/jphysiol.2005.083600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Nicholas SJ, Robinson L, Kyloh M, Flack N, Brookes SJ, Zagorodnyuk VP, Keating DJ. Mechanisms underlying distension-evoked peristalsis in guinea pig distal colon: is there a role for enterochromaffin cells. Am J Physiol Gastrointest Liver Physiol. 2011;301:519–527. doi: 10.1152/ajpgi.00101.2011. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Nicholas SJ, Sia TC, Staikopoulos V, Kyloh M, Beckett EA. By what mechanism does ondansetron inhibit colonic migrating motor complexes: does it require endogenous serotonin in the gut wall. Neurogastroenterol Motil. 2013;25:677–685. doi: 10.1111/nmo.12136. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Smith TK. Simultaneous intracellular recordings from longitudinal and circular muscle during the peristaltic reflex in guinea-pig distal colon. J Physiol. 2001;533(Pt 3):787–799. doi: 10.1111/j.1469-7793.2001.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Smith TK. Mechanosensory S-neurons rather than AH-neurons appear to generate a rhythmic motor pattern in guinea-pig distal colon. J Physiol. 2004;558(Pt 2):577–596. doi: 10.1113/jphysiol.2004.063586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Walsh M, Smith TK. Purinergic and cholinergic neuro-neuronal transmission underlying reflexes activated by mucosal stimulation in the isolated guinea-pig ileum. J Physiol. 2000;522:321–331. doi: 10.1111/j.1469-7793.2000.t01-1-00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148:705–713. doi: 10.1176/ajp.148.6.705. [DOI] [PubMed] [Google Scholar]
- Trowbridge S, Narboux-Nême N, Gaspar P. Genetic models of serotonin (5-HT) depletion: what do they tell us about the developmental role of 5-HT. Anat Rec (Hoboken) 2011;294:1615–1623. doi: 10.1002/ar.21248. [DOI] [PubMed] [Google Scholar]
- Wood JD. Enteric nervous control of motility in the upper gastrointestinal tract in defensive states. Dig Dis Sci. 1999;44:44S–52S. [PubMed] [Google Scholar]
- Yadav VK, Balaji S, Suresh PS, Liu XS, Lu X, Li Z, Guo XE, Mann JJ, Balapure AK, Gershon MD, Medhamurthy R, Vidal M, Karsenty G, Ducy P. Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nat Med. 2010;16:308–312. doi: 10.1038/nm.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan SY, Bornstein JC, Furness JB. Investigation of the role of 5-HT3 and 5-HT4 receptors in ascending and descending reflexes to the circular muscle of guinea-pig small intestine. Br J Pharmacol. 1994;112:1095–1000. doi: 10.1111/j.1476-5381.1994.tb13196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Spencer NJ. Localization of the sensory neurons and mechanoreceptors required for stretch evoked colonic migrating motor complexes in mouse colon. Front Physiol. 2011;2:1–8. doi: 10.3389/fphys.2011.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]


