Abstract
We examined the effect of growth hormone (GH) on connective tissue of tendon and skeletal muscle during immobilisation and re-training in humans. Young men (20–30 years; n= 20) were randomly assigned to daily recombinant human GH (rhGH) (33–50 μg kg−1 day−1) or placebo (Plc), and had one leg immobilised for 2 weeks, followed by 6 weeks of strength training. The cross-sectional area (CSA), maximal muscle strength (maximal voluntary contraction, MVC) and biomechanical properties of the quadriceps muscle and patellar tendon were determined. Muscle and tendon biopsies were analysed for mRNA of collagen (COL1A1/3A1), insulin-like growth factors (IGF-1Ea/Ec), lysyl oxidase (LOX), matrix metalloproteases (MMP-2 and MMP-9), decorin and tenascin-C. Fibril morphology was analysed by transmission electron microscopy (TEM) to detect changes in the fibril diameter distribution. In muscle, CSA and MVC declined with immobilisation and recovered with rehabilitation similarly in both groups. Likewise, both groups showed increased IGF-1Ea/Ec and COL1A1/3A1 expression in muscle during re-training after immobilisation compared with baseline, and the increase was more pronounced when subjects received GH. The tendon CSA did not change during immobilisation, but increased in both groups during 6 weeks of rehabilitation (∼14%). A decline in tendon stiffness after immobilisation was observed only in the Plc group, and an increase during 6 weeks of rehabilitation was observed only in the GH group. IGF-1Ea and COL1A1/3A1 mRNA increased with immobilisation in the GH group only, and LOX mRNA was higher in the GH group than in the Plc group after immobilisation. Both groups showed an increase in MMP-2 with immobilisation, whereas no changes in MMP-9, decorin and tenascin-C were observed. The tendon fibril diameter distribution remained unchanged in both groups. In conclusion, GH stimulates collagen expression in both skeletal muscle and tendon, abolishes the normal inactivity-related decline in tendon stiffness and LOX, and results in increased tendon CSA and stiffness during rehabilitation. GH has a matrix-stabilising effect during periods of inactivity and rehabilitation in humans.
Key points
Loss of muscle and tendon function during periods of immobilisation and rehabilitation represents a challenge in clinical medicine.
It is not known to what extent growth hormone (GH) supplementation is able to counteract the absence of mechanical loading during immobilisation in musculo-tendinous connective tissue.
This study examines the effect of GH in musculo-tendinous connective tissue in humans during immobilisation and subsequent rehabilitation.
The main study findings were the observation that GH supplementation stimulates collagen expression in musculo-tendinous tissue and abolishes the normal decline in tendon stiffness and lysyl oxidase during immobilisation. Furthermore, GH supplementation results in an increased tendon size and stiffness during rehabilitation.
GH supplementation has a matrix-stabilising effect during periods of inactivity and rehabilitation in humans.
Introduction
Counteracting the loss of muscle and tendon function during periods of immobilisation and recovering from this catabolic situation represent a challenge in clinical medicine. It is well known that inactivity of leg musculature over 2–3 weeks causes a 10–15% loss of skeletal muscle and a 20–25% reduction in muscle strength (Berg & Tesch, 1996; de Boer et al. 2007a; Suetta et al. 2009). Furthermore, in tendon tissue, it has been demonstrated that lower limb leg inactivity over just a few weeks results in a loss of tendon stiffness and a marked reduction in collagen synthesis, but without any change in tendon size (de Boer et al. 2007b; Seynnes et al. 2008; Couppe et al. 2012). Whether or not pharmacological intervention with special focus on both matrix and contractile musculo-tendinous tissue adaptation can counteract the loss of tissue quality and mass, or accelerate the effect of rehabilitation, is unknown.
The growth hormone/insulin-like growth factor-1 (GH/IGF-1) axis is known to play a central role in the regulation of human collagen turnover in musculo-tendinous tissue (Doessing et al. 2010b). In both patients with acromegaly (high GH/IGF-1 levels) and those with GH deficiency, or in healthy humans receiving GH supplementation, it has been shown that high GH and IGF-1 levels in the blood are associated with increased local mRNA expression of both IGF-1 and collagen in tendon and skeletal muscle, suggesting a stimulating role of GH/IGF-1 in human musculo-tendinous connective tissue (Trepp et al. 2008; Doessing et al. 2010a, b). Although GH supplementation has been shown to have a positive anabolic effect on musculo-skeletal tissues in both animals (Wilson et al. 1995) and humans with GH deficiency (Claessen et al. 2013), the anabolic effect of GH/IGF-1 levels on healthy human adults under a challenged situation (e.g. inactivity, malnutrition) is less clear, and its interaction with mechanical loading remains unexplained.
Studies on humans have revealed that mechanical loading increases peritendinous and intramuscular collagen turnover (Langberg et al. 1999; Heinemeier et al. 2003; Miller et al. 2005). However, it is not known to what extent elevated GH/IGF-1 axis activity – through GH administration to humans – will be able to counteract the absence of mechanical loading during inactivity in connective tissue adaptation.
GH supplementation in healthy subjects has not been shown to have any anabolic effect on muscle growth and strength (Yarasheski et al. 1992; Lange et al. 2002; Rennie, 2003), and these findings are supported by a study in which 2 weeks of GH supplementation in healthy young males did not affect myofibrillar protein synthesis (Doessing et al. 2010a). Rats receiving a single bolus injection of IGF-1 showed an increase in muscle mass and strength compared with the placebo group and, furthermore, an additive effect on muscle protein was observed when combining IGF-1 injection with strength training (Lee et al. 2004). The same study examined how IGF-1 affected muscle in a period of de-training for 12 weeks after 8 weeks of strength training. The study showed that the loss of muscle mass and strength with de-training was attenuated by IGF-1 expression, indicating that IGF-1 could diminish the loss of muscle mass and function during inactivity of rats (Lee et al. 2004).
It is hypothesized that systemic GH supplementation will reduce the degradation of connective tissue (collagen) and thus affect tendon biomechanical properties during inactivity via the stimulation of local IGF-1 and collagen expression in the connective tissue of the skeletal muscle and adjacent tendon, and that GH supplementation will have a stimulating effect on the same parameters during rehabilitation.
The aim of this study was to determine the effect of GH supplementation on the musculo-tendinous tissue during inactivity and rehabilitation.
Methods
Study design
Subjects
Twenty young, healthy, physically untrained men (age ± SD, 23 ± 2 years) with a body mass index between 18.5 and 25 were recruited in this study. All subjects were healthy non-smokers and none took any kind of prescribed medication. Furthermore, all subjects had no known injuries to their lower extremities. The study design was a double-blind, randomised, placebo-controlled trial. The study (H-4-2010-010) was approved by the local Ethics Committee of Region Copenhagen in accordance with the Declaration of Helsinki. All subjects were informed of the risks associated with the study and gave written informed consent.
Intervention protocol
All participants were subjected to 2 weeks of unilateral (randomly selected limb) lower limb cast immobilisation from the hip to the ankle, followed by 6 weeks of supervised unilateral strength training (three times a week). All measurements described below were conducted at baseline prior to the immobilisation procedure (Pre), after 2 weeks of immobilisation (Post immob) and again after 2 weeks and 6 weeks of heavy resistance training. Measurements in the training period were always performed 48 h after the last training session.
Recombinant human GH (rhGH) administration
Subjects were randomly assigned in a double-blind fashion to receive either rhGH (Norditropin, Novo Nordisk, Denmark) (rhGH; n= 10) or placebo (Plc; n= 10). rhGH/Plc was administered by daily subcutaneous injection in the thigh for the 8 week intervention period. The rhGH dosage was 33.3 μg kg−1 day−1 for the first week and, if there were no signs of side effects, the dosage was up-regulated to 50 μg kg−1 day−1 for the remaining 7 weeks. After detailed instructions, the subjects were able to perform the injections by themselves at home. The participants visited the institute once every week during the intervention to ensure that no side effects had occurred. If side effects were present (pitting leg oedema, carpal tunnel syndrome, trigger fingers, weight gain or transient atrial fibrillation from fluid retention), the rhGH dosage was adjusted to a tolerable concentration. If possible thereafter, the dose was gradually increased again up to the maximum of 50 μg kg−1 day−1.
Immobilisation protocol
Immobilisation was performed for 2 weeks using a lightweight fibre cast applied from just above the malleoli to just below the groin, which, in other studies, has been proven to induce substantial muscle atrophy in short-term immobilisation studies involving young individuals (Suetta et al. 2009). The cast was positioned at 50° of knee joint flexion to minimize walking ability of the casted limb, and the subjects were carefully instructed to perform all activities on crutches and to abstain from ground contact, as well as to perform isometric contractions of the quadriceps of the immobilised leg. During the 2 weeks of immobilisation, the subjects were contacted every second day by mail or telephone and were carefully instructed to perform passive movements around the ankle joint (venous pump exercises) several times a day to prevent the potential formation of deep venous thrombosis.
Re-training procedure
After removal of the cast, the subjects received rehabilitation training for 6 weeks, three sessions per week (18 training sessions in total). The re-training protocol was a supervised unilateral strength training programme focusing only on the immobilised leg, which has been proven previously to elicit increases in muscle size and maximal muscle strength in young individuals (Suetta et al. 2009). After a 5 min warm-up on a stationary bike, the subjects performed supervised knee extension and leg press exercises. To induce a sufficient response in the thigh musculature, the training intensity was 3–4 sets × 12 repetitions [15 repetitions maximum (RM)] in week 1, 4 sets × 10 repetitions (12 RM) in weeks 2–4 and 4 × 8 repetitions (10 RM) in weeks 5–8. The training load was adjusted on a weekly basis by the use of 5 RM tests.
Measurements
The structural measurements [magnetic resonance imaging (MRI) of the quadriceps muscle and patellar tendon, maximal voluntary contraction (MVC) and patellar tendon mechanical properties] were performed on both sides at baseline (Pre) and after 6 weeks of strength training, but only on the immobilised leg after immobilisation (Post immob) and after 2 weeks of re-training.
MRI of skeletal muscle
All participants were scanned in a 1.5 T Philips Intera (Eindhoven, the Netherlands) or a General Electric (GE) Signa Horizon (Milwaukee, WI, USA) MRI scanner using the following protocol. With the subject in the supine position, and both limbs extended and relaxed using the body array coil, a scout localizer centred mid-femur was performed to ensure that the femurs and knee joints were included in the scanners field of view (FOV = 48 cm). Dependent on the femur length of the subject, seven to eight T1-weighted axial scans of the femur [echo time (TE) = 17 ms; repetition time (TR) = 500 ms; matrix, 864 × 864; FOV = 420 mm], with a slice thickness of 10 mm and an interslice gap of 50 mm, were performed. The first slice was always positioned just below the femur condyles where the tibia plateau was visible to ensure the same scan position between examination time points. The cross-sectional area (CSA) of the quadriceps muscle was analysed as described in detail previously (Suetta et al. 2009). The slice equivalent to 50% of the femur length was used to calculate the CSA, and the measurements were performed three times by a blind trained person using the imaging software Osirix 2.7.5 (Osirix Imaging Medical, Geneva, Switzerland). The mean value of the three measurements was used as the result, and the coefficient of variation for acceptance between measurements was set to <5%.
MRI of tendon
The patellar tendon was scanned in the same 1.5-T MRI scanner as above with the patient supine using a dedicated knee coil and an axial and sagittal T1-weighted turbo spin echo sequence (TE = 17 ms; TR = 500 ms; matrix, 512 × 512; FOV = 150 mm; slice thickness, 3 mm). The knee was slightly flexed (12%) as a result of the built-in supportive pillow in the coil, which ensured the same slight stretch on the patellar tendon between examinations. The axial slices of the patellar tendon were positioned orthogonal to the length in the sagittal plane covering the distal patellar pole to the tibial insertion. The procedure for analysing the patellar tendon has been described in detail elsewhere (Kongsgaard et al. 2007; Couppe et al. 2008). The patellar tendon CSA was measured using the axial slice just distal to the patellar insertion, the slice just proximal to the tibia insertion and midway between these two sites (Couppe et al. 2008). The patellar tendon length was obtained by measuring the distance from the most dorsal insertion part at the patella apex to the dorsal insertion on the tibia. Patellar tendon CSA and length were manually outlined by a blind examiner using the imaging software Osirix 2.7.5 (Osirix Imaging Medical), described in detail elsewhere (Kongsgaard et al. 2007; Couppe et al. 2008). All measurements were performed three times by the same observer who was blind to the chronological MRI time points and treatment groups (GH or Plc). The mean value of these measurements was used as the result, and the coefficient of variation for acceptance between measurements was set to <5%.
Maximal muscle strength (MVC) and mechanical properties of the patellar tendon
Details of the MVC measurement and tendon mechanical properties, including the reliability of the method in our laboratory, have been reported previously (Kongsgaard et al. 2007; Couppe et al. 2008). Within-day correlation coefficient and typical error percentage results for repeated measures were 0.95 and 9.9% for tendon stiffness, 0.97 and 5.5% for tendon strain, and 0.94 and 9.4% for Young's modulus. The subjects were asked to refrain from strenuous exercise 48 h prior to the experiments. The subjects performed a 5-min warm-up on a stationary bike to ensure appropriate preconditioning of the tendon before testing. Subsequently, the subjects were seated in a custom-made rigid chair with both hips and knees flexed to an angle of 90°. A leg cuff, which was connected to a strain gauge (Noraxon Inc., Scottsdale, AZ, USA) through a rigid steel rod perpendicular to the lower leg, was mounted on the leg just above the medial malleolus. A Hitachi EUB-6500 ultrasound scanner (Hitachi Medical Corporation, Tokyo, Japan), equipped with a 10 MHz, 100 mm long, linear array B-mode transducer (Hitachi, Model: EUP-L53L), was fitted into a custom-made rigid cast that was secured to the skin above the patellar tendon in the sagittal plane. The ultrasound probe and cast were positioned so that the distal patella, the entire patellar tendon and the proximal tibia were all visible within the FOV throughout the performed isometric ramp contractions.
The subjects performed four to five slow isometric knee extensions ramps by applying gradually increasing force up to a maximum over a 10 s period during which patellar tendon displacement and knee extension force were measured simultaneously. All measurements were performed on one side (immobilised). The maximal muscle strength of the quadriceps muscles (MVC) was determined as the maximum knee extensor moment during a 10 s ramp contraction. During the ramps, ultrasound S-VHS video images were sampled at 25 Hz using frame-by-frame capturing software (Matrox Morphis Dual frame grabber and Matrox Imaging Library software, Matrox Electronic Systems Ltd., Montreal, QC, Canada). Custom-made frame-by-frame tracking software, using a pyramidal implementation of the Lukas–Kanade optical flow estimation, was used to assess the tendon deformation from the ultrasound videos. The accuracy and reproducibility of this tracking software have been assessed previously (Magnusson et al. 2003). A trigger signal (Pulse generator, PG 58AA, GouldAdvance, Hainault, Essex, UK) initiated the recording of force and ultrasound video, thus allowing for subsequent synchronization of recorded data during the ramp contractions.
The tibia moment arm was measured (from the leg cuff to the lateral epicondyle of the knee) to calculate the knee extensor moment. The force applied to the patellar tendon was calculated by dividing the measured knee extensor moment by the internal moment arm, which was estimated from individually measured femur lengths (Kongsgaard et al. 2007; Couppe et al. 2008). Tendon deformation was defined as the change in distance between the patellar apex and tibia (Kongsgaard et al. 2007; Couppe et al. 2008). The tendon stress was calculated by dividing the tendon force with the average tendon CSA (mean of proximal, middle and distal CSA from MRI). The tendon strain was calculated as the change in length related to the initial tendon length. Polynomial functions (second and third order) were fitted to each single force–deformation curve. The tendon stiffness (Δ force/Δ deformation) and Young's modulus (Δ stress/Δ strain) were calculated at the final 10% of the force–deformation and stress–strain curves, respectively.
Tissue biopsy procedure
Muscle biopsies were taken at baseline, just after immobilisation and again after 2 weeks and 6 weeks of rehabilitation. Tendon biopsies were taken at baseline at the control leg and at the immobilised leg after the immobilisation period to avoid potential re-biopsy effects, and again from both sides after 6 weeks of re-training. After initiating the present study, we analysed biopsies from another study and found that a 6 week ‘washout period’ was insufficient to avoid a repeated biopsy effect on mRNA for several growth and matrix factors (such as IGF-1 and collagen) (Doessing et al. 2010a). On the basis of these data, the biopsies from the 6 weeks re-training were not used in the statistical analysis of time or group interactions.
The muscle and tendon biopsy procedures have been described in detail elsewhere (Hansen et al. 2009; Kongsgaard et al. 2009). In brief, the sample sites were prepared after sterilisation with local anaesthetics (lidocaine, 1%). Muscle tissue was taken from the vastus lateralis muscle using a 5 mm Bergström needle with suction (Bergstrom, 1975), and tendon tissue was taken from the patellar tendon (Bard Magnum Biopsy Instrument, C.R. Bard, Inc., Covington, GA, USA) with a 14 g needle (Movin, 2000). The total wet weight of the muscle sample was around 80–100 mg and the total wet weight of the tendon sample was around 8–10 mg. The biopsies were cleared of external adipose tissue and blood, frozen in liquid nitrogen and stored at −80°C for subsequent analysis.
Hormone measurements
Blood samples drawn from the antecubital vein were separated (3200 g, 4°C) and serum was stored at −80°C. Serum GH and serum IGF-1 concentrations were determined in duplicate with GH ELISA, RMEE022 (Biovendor, Heidelberg, Germany) and IGF-1 ELISA, RMEE20 (Biovendor).
Subjects performed the injections before bedtime and blood samples were drawn early the next day during follow-up.
Muscle and tendon mRNA measurements
Tendon and muscle were homogenized in TriReagent (Molecular Research Center, Cincinnati, OH, USA) using a bead-mixer with steel beads (Biospec Products, Bartlesville, OK, USA). Following homogenization, bromo-chloropropane (Molecular Research Center) was added in order to separate the samples into aqueous and organic phases. Glycogen was added to the tendon samples to improve RNA precipitation. Following isolation of the aqueous phase, RNA was precipitated using isopropanol, washed in ethanol and dissolved in RNAse-free water. All tissue samples were weighed prior to RNA extraction. Muscle RNA concentrations were determined by spectroscopy and tendon RNA concentrations were determined using RiboGreen assay (Molecular Probes, Eugene, OR, USA). Good RNA quality was ensured by gel electrophoresis.
The amount of mRNA for collagen 1 alpha 1 (COL1A1), collagen 1 alpha 3 (COL3A1), insulin-like growth factor-1Ea (IGF-1Ea), insulin-like growth factor-1Ec (IGF-1Ec), lysyl oxidase (LOX), matrix metalloprotease-2 (MMP-2), matrix metalloprotease-9 (MMP-9), decorin, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and 60S acidic ribosomal protein P0 (RPLP0) was determined and measured using reverse transcription real-time polymerase chain reaction (RT-qPCR), as described in detail elsewhere (Doessing et al. 2010a). Primer sequences are given in Doessing et al. (2010a), except for LOX (CGC TGT GAC ATT CGC TAC ACA GGA C, CAT TGG GAG TTT TGC TTT GCC TTC T), MMP-2 (CCG CCT TTA ACT GGA GCA AAA ACA, TTG GGG AAG CCA GGA TCC ATT T), MMP-9 (AGC GAG GTG GAC CGG ATG TT, AGA AGC GGT CCT GGC AGA AAT AG) and decorin (GGT GGG CTG GCA GAG CAT AAG T, TGT CCA GGT GGG CAG AAG TCA). RPLP0 was used for normalisation. To validate RPLP0 as an unregulated mRNA useful for normalisation, we also measured GAPDH. Ideally, the ratio between GAPDH and RPLP0 should be unity in all groups. Unfortunately, after immobilisation, there is a slight decrease in GAPDH relative to RPLP0 in the Plc group, indicating that either GAPDH decreases or RPLP0 increases at this time point (Fig. S1). It is not possible to distinguish between the two possibilities, but, during immobilisation, we believe that it is more likely that the metabolic marker GAPDH will decrease than that protein synthesis (RPLP0) will increase, and we have therefore chosen to use RPLP0 for normalisation.
Transmission electron microscopy (TEM) and stereological analysis (tendon)
The procedure for making TEM images from the tendon biopsies for stereological analyses of tendon fibrillar morphology has been described in detail previously (Kongsgaard et al. 2010). In brief, after the tendon biopsies had been rinsed in 0.15 m sodium cacodylate buffer (pH 7.2), the specimens were fixed in 1% OsO4 in 0.15 m sodium cacodylate buffer (pH 7.2) for 2 h and subsequently dehydrated in ethanol, transferred to propylene oxide and embedded in EPON resin 828/862 mix (Hexion Specialty Chemicals, Rotterdam, the Netherlands) according to standard procedures. After this, sections were cut using a Reichert-Jung Ultracut E microtome (Reichert, Depew, NY, USA). Semi-thin sections, for preanalytical evaluation of the samples, were stained with toluidine blue and visualized (digital images) using a Nikon Coolpix 990 (Nikon Nordic ABl, København, Denmark). When an appropriate condition and orientation of the sample within the EPON block had been confirmed, ultra-thin sections were cut and collected on a one-hole copper grid with Formvar supporting membranes and stained with uranyl acetate and lead citrate. From each biopsy sample cross-section, a simple random sample of 10 digitized TEM images was obtained from the intercellular space.
The stereological analyses of the TEM images were completed on a computer monitor onto which the digitized TEM images were merged with a graphic representation of the stereological test system (C.A.S.T.-grid software, The International Stereology Centre at Olympus, Ballerup, Denmark). The collagen fibrils were counted and measured at a magnification of 210,000. The counting frames covered 3% of the TEM image area, and the TEM images covered approximately 0.5% of the biopsy sample cross-section (∼5 μm2). On average, 449 fibrils (range, 184–1006) were analysed per cross-section. The fibril area was calculated from measurements of the fibril diameter. The fibril diameter was measured as the largest diameter perpendicular to the longest axis of each fibril cross-section, thereby eliminating the influence of sectioning angle. The fibril density was expressed as the absolute number of fibrils per square micrometre and the fibril volume fraction denotes the area occupied by fibrils within the sample area. A single experienced and blind investigator Jytte Overgaard Larsen (JOL) performed all stereological analyses.
Statistics
Data were analysed in SigmaPlot v11 using two-way repeated-measures analysis of variance with Student–Newman–Keuls post-hoc test. Data are presented as means ± SEM. mRNA data from RT-qPCR were log transformed before statistical analysis and presented as the geometric mean ± backtransformed SEM. P < 0.05 was considered to be significant.
Results
Subjects
All 20 subjects participating completed the study. Of the 10 subjects receiving rhGH, a total of three experienced mild side effects (all three experienced mild carpal tunnel syndrome and, one of these, trigger fingers), and the rhGH dose was reduced from 50 to 33 μg kg−1 day−1 until the side effects disappeared (7–10 days).
There were no differences at baseline in age (mean ± SD: GH, 23.2 ± 2.1 years; Plc, 22.1 ± 2.2 years), height (GH, 183 ± 8 cm; Plc, 182 ± 8 cm), weight (GH, 76.4 ± 9.9 kg; Plc, 74.7 ± 5.9 kg) or body mass index (GH, 23.4 ± 2.2 kg m−2; Plc, 22.6 ± 2.2 kg m−2) between the GH and Plc groups.
Systemic GH and IGF-1
Serum GH was increased by approximately threefold during immobilisation and by approximately 10-fold during re-training with rhGH supplementation. Serum IGF-1 was increased by approximately threefold during immobilisation and the increase by threefold was maintained during re-training with rhGH supplementation (Fig. 1).
Figure 1. Circulating serum concentrations of growth hormone (GH) and insulin-like growth factor-1 (IGF-1) during 2 weeks of immobilisation and 6 weeks of re-training in young males (n= 20) with either recombinant human GH (rhGH) (n= 10) or placebo (n= 10).
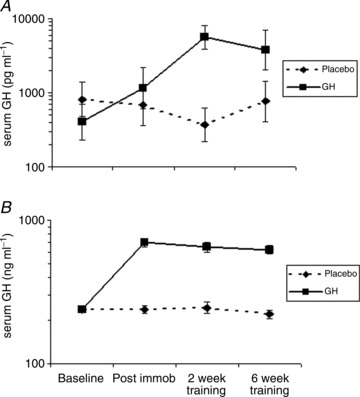
A, serum GH. B, serum IGF-1. Data are geometric means ± SEM.
Quadriceps muscle and patellar tendon CSA
There was no difference between the experimental groups in quadriceps muscle CSA before the intervention (baseline). After immobilisation (Post immob), quadriceps CSA decreased in both groups (mean: GH, 8.8%; Plc, 10.6%; P < 0.05) and both groups showed a similar increase in muscle CSA in response to re-training. Already, after 2 weeks of re-training, both groups had reached their initial baseline values, and both groups achieved a further increase after 6 weeks of re-training (Fig. 2A).
Figure 2. Changes in cross-sectional area (CSA) (muscle and patellar tendon) and maximal muscle strength during 2 weeks of immobilisation and 6 weeks of re-training in young males (n= 20) with either recombinant human growth hormone (rhGH) (n= 10) or placebo (n= 10).
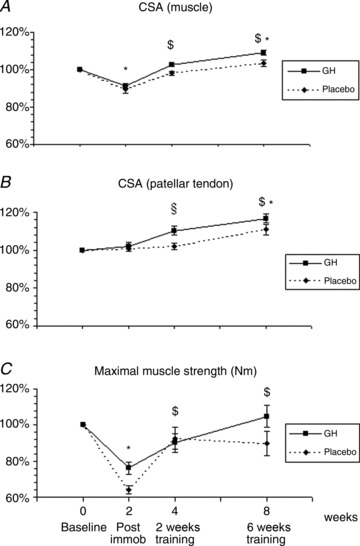
A, CSA of the quadriceps muscle; time effect (P < 0.01) and group interaction (P= 0.09). B, CSA of the patellar tendon; time effect (P < 0.01) and group interaction (P < 0.05). C, maximal isometric muscle strength; time effect (P < 0.01) and group interaction (P= 0.09). *Time effect, P < 0.05, compared with baseline (both groups). $Time effect, P < 0.05, compared with Post immob (both groups). §Time effect, P < 0.05, baseline/Post immob. vs. 2 weeks of re-training (GH). Data are means ± SEM.
For average patellar tendon CSA, both a time effect and a group interaction were demonstrated (P < 0.05). No group differences in average patellar tendon CSA were demonstrated prior to immobilisation (baseline), and no changes were found during immobilisation (Fig. 2B). Both groups showed a significant increase in average patellar tendon CSA after 6 weeks of re-training (GH, +17%; Plc, +11%; P < 0.05 compared with baseline; Fig. 2B) and, in the GH group, a significant response to re-training had occurred already after 2 weeks of re-training (GH, +10%; P < 0.05 compared with baseline; Fig. 2B).
Maximal muscle strength (MVC)
There was no difference at baseline in quadriceps strength between the GH and Plc groups. After 2 weeks of immobilisation, the maximal quadriceps strength was reduced in both the GH and Plc groups (24% and 36%, respectively; P < 0.01) and, in both groups, muscle strength was regained during re-training with no differences between the groups (Fig. 2C).
Tendon biomechanical properties
Patellar tendon stiffness revealed a significant time and group interaction (P < 0.05). In the Plc group, tendon stiffness decreased during the 2 weeks of immobilisation (P < 0.05) and returned to baseline level during re-training (Fig. 3A, Table 1). In contrast, in the GH group, tendon stiffness was maintained during the 2 weeks of immobilisation and was found to increase significantly after re-training (P < 0.05; Fig. 3A, Table 1). After 6 weeks of re-training, a significant difference between the groups was found (P < 0.05; Fig. 3A, Table 1). Young's modulus of the patellar tendon showed a significant time effect (P < 0.05; Fig. 3B) and a different tendency between the two groups (P= 0.06; Fig. 3B). In the Plc group, Young's modulus tended to decrease during immobilisation (P= 0.06; Fig. 3B) and returned to baseline values during re-training. In contrast, Young's modulus remained stable throughout the study in the GH group (Fig. 3B, Table 1).
Figure 3. Changes in patellar tendon biomechanical properties during 2 weeks of immobilisation and 6 weeks of re-training in young males (n= 20) with either recombinant human growth hormone (rhGH) (n= 10) or placebo (n= 10).
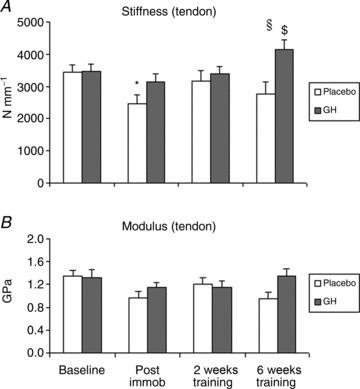
A, patellar tendon stiffness; time effect (P < 0.05) and group interaction (P < 0.05). B, patellar tendon Young's modulus; time effect (P < 0.05) and group interaction (P= 0.06). *Time effect, P < 0.05, compared with baseline (Plc). $Time effect, P < 0.05, from baseline, Post immob and 2 weeks of training (GH). §Group effect, P < 0.05, GH vs. Plc within time point. Data are means ± SEM.
Table 1.
Tendon mechanical properties during 2 weeks of immobilisation and 6 weeks of re-training in young males (n= 20) with either recombinant human growth hormone (rhGH) (n= 10) or placebo (Plc) (n= 10)
| GH (n= 10) | Plc (n= 10) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 2 wk immob | Δ (%) | 2 wk train | Δ (%) | 6 wk train | Δ (%) | Baseline | 2 wk immob | Δ (%) | 2 wk train | Δ (%) | 6 wk train | Δ (%) | |
| Deformation (mm) | 3.2±0.2 | 2.6±0.2 | −18.7±5.2 | 2.9±0.2 | −6.1±8.6 | 2.8±0.2 | −11.0±6.1 | 3.1±0.2 | 2.4±0.2 | −14.1±9.2 | 2.65±0.2 | −7.3 | 3.1±0.2 | 10.8±13.9 |
| Strain (%) | 7.8±0.4 | 6.2±0.5 | −19.7±5.6 | 7.2±0.7 | −6.8±8.8 | 6,9±0.5 | −11.5±6.8 | 7.2±0.7 | 5.6±0.4 | −14.5±9.5 | 5.9±0.3 | −16.6±13.4 | 7.2±0.5 | 11.6±14.8 |
| Stress (MPa) | 44.3±4.2 | 31.8±2.2 | −24.2±6.2 | 36.3±2.7 | −14.2±7.6 | 40.9±3.1 | −3.3±9.1 | 41.7±3.4 | 26.3±2.3 | −35.5±5.4 | 35.7±2.4 | −19.9±10.5 | 32.7±2.6 | −20.9±6.3 |
| Stiffness (N mm-1) | 3479±218 | 3143±218 | −8.4±6.1 | 3392±228 | − 0.2±7.7 | 4147±218 | 22.4±10.6† | 3451±218 | 2465±228 | −27.6±8.0 * | 3178±223 | −6.2±10.0 | 2762±390 | −19.8±10.0† |
| Modulus (GPa) | 1.3±0.1 | 1.2±0.1 | −8.8±5.8 | 1.16±0.1 | −8.5±7.2 | 1.4±0.1 | 6.5±10.2 | 1.4±0.1 | 0.97±0.1 | −26.0±9.2 | 1.1±0.1 | −16.6±14.4 | 0.96±0.1 | −27.4±8.9 |
All values are means ± SEM. 2 wk immob, 2 weeks of immobilisation; 2 wk train, 2 weeks of re-training; 6 wk train, 6 weeks of re-training. *Time effect, P < 0.05, compared with baseline. †Group effect, P < 0.05, GH vs. Plc within time point.
In all other determined tendon biomechanical parameters, there was a time effect (P < 0.05), but no group interaction with respect to tendon deformation, strain or stress throughout the immobilisation and re-training period (Table 1).
IGF-1Ea, IGF-1Ec, COL1A1 and COL3A1 mRNA in skeletal muscle and tendon
In skeletal muscle, there was an effect of both time and treatment (P < 0.05). Both IGF-1Ea and IGF-1Ec mRNA were increased after 2 weeks of immobilisation and during re-training with GH supplementation (P < 0.05; Fig. 4A, B), and both IGF-1Ea and IGF-1Ec showed an increase relative to the Plc group after both 2 weeks of immobilisation and re-training (P < 0.05; Fig. 4A, B). There was also a stimulating effect of training on IGF-1Ea and IGF-1Ec mRNA in the Plc group (P < 0.05; Fig. 4B). Relative to the Plc group, GH supplementation increased COL1A1 mRNA in muscle during immobilisation and, especially, during re-training (P < 0.05; Fig. 4C), and COL3A1 mRNA during re-training (P < 0.05; Fig. 4D). Training alone affected COL1A1 and COL3A1 positively (P < 0.05; Fig. 4C, D).
Figure 4. Muscle insulin-like growth factor-1 (IGF-1) and collagen (COL) mRNA expression during 2 weeks of immobilisation and 6 weeks of re-training in young males (n= 20) with either recombinant human growth hormone (rhGH) (n= 10) or placebo (n= 10).
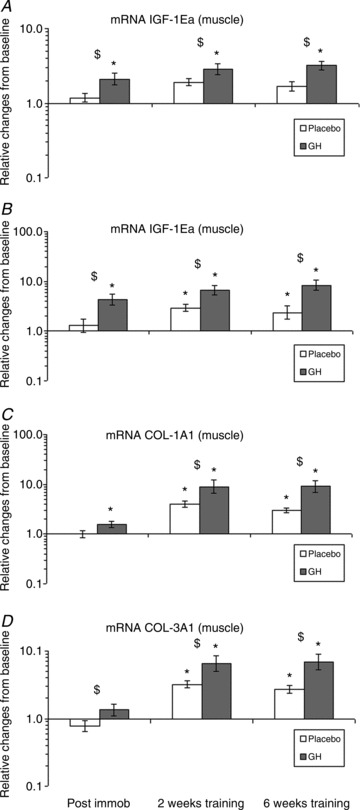
A, IGF-1Ea mRNA (muscle); time effect (P < 0.05) and group interaction (P < 0.05). B, IGF-1Ec mRNA (muscle); time effect (P < 0.05) and group interaction (P < 0.05). C, COL1A1 mRNA (muscle); time effect (P < 0.05) and group interaction (P < 0.05). D, COL3A1 mRNA (muscle); time effect (P < 0.05) and group interaction, (P < 0.05). *Time effect, P < 0.05, compared with baseline. $Group effect, P < 0.05, GH vs. Plc within time point. Data are geometric means ± SEM.
Tendon IGF-1Ea mRNA was increased after 2 weeks of immobilisation with GH supplementation compared with both baseline and the Plc group (P < 0.05; Fig. 5A). IGF-1Ec mRNA concentration was below the detection limit in the majority of the tendon biopsies and no further analysis was made on the samples. Tendon COL1A1 and COL3A1 mRNAs were increased after 2 weeks of immobilisation with GH supplementation compared with baseline and the Plc group (P < 0.05; Fig. 5B, C).
Figure 5. Tendon insulin-like growth factor-1 (IGF-1) and collagen (COL) mRNA expression during 2 weeks of immobilisation in young males (n= 20) with either recombinant human growth hormone (rhGH) (n= 10) or placebo (n= 10).
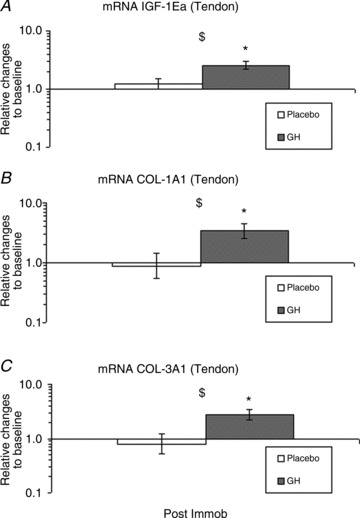
A, IGF-1Ea mRNA (tendon); time effect (P < 0.05) and group interaction (P < 0.05). B, COL1A1 mRNA (tendon); time effect (P= 0.07) and group interaction (P < 0.05). C, COL3A1 (tendon); time effect (P= 0.1) and group interaction, (P < 0.05). *Time effect, P < 0.05, compared with baseline (GH). $Group effect, P < 0.05, GH vs. Plc within time point. Data are geometric means ± SEM.
LOX, MMP-2, MMP-9, decorin and tenascin-C mRNA (tendon)
After 2 weeks of immobilisation, an increase in LOX mRNA in the GH group was observed, whereas a decrease in the Plc group was found, when compared with baseline (P < 0.05; Fig. 6A). At baseline, no significant differences in MMP-2 and MMP-9 mRNAs were seen between the groups. After 2 weeks of immobilisation, the expression of MMP-2 mRNA was increased in both groups (P < 0.05), whereas the expression of MMP-9 mRNA remained unchanged (Fig. 6B, C). At baseline, no significant differences in decorin or tenascin-C mRNA were seen between the groups. After 2 weeks of immobilisation, the gene expression of both decorin and tenascin-C mRNA remained unchanged compared with baseline and between the groups (Fig. 6D, E).
Figure 6. Tendon lysyl oxidase (LOX), matrix metalloprotease-2 (MMP-2), matrix metalloprotease-9, decorin and tenascin-C mRNA expression during 2 weeks of immobilisation in young males (n= 20) with either recombinant human growth hormone (rhGH) (n= 10) or placebo (n= 10).
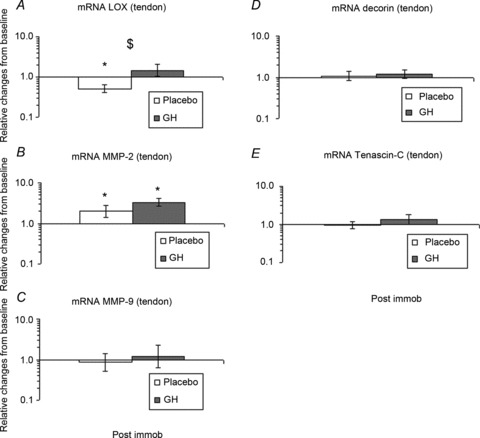
A, LOX mRNA (tendon); time effect (P= 0.5) and group interaction (P < 0.05). B, MMP-2 mRNA (tendon); time effect (P < 0.05) and group interaction (P= 0.2). C, MMP-9 mRNA (tendon); time effect (P= 0.9) and group interaction (P= 0.7). D, decorin (tendon); time effect (P= 0.5) and group interaction (P= 0.8). E, tenascin-C (tendon); time effect (P= 0.7) and group interaction (P= 0.6). *Time effect, P < 0.05, compared with baseline. $Group effect, P < 0.05, GH vs. Plc. within time point. Data are geometric means ± SEM.
Tendon fibril morphology
There were no significant differences at baseline, after 2 weeks of immobilisation or after 6 weeks of re-training in the GH or Plc groups or between groups in fibril volume fraction, fibril density, mean fibril diameter or mean fibril area (Fig. S2). With regard to the fibril diameter distribution (number of fibrils per square micrometre), no significant differences over time or between groups were seen in the GH or Plc groups at baseline (Fig. 7A), after 2 weeks of immobilisation (Fig. 7B) or after 6 weeks of re-training (Fig. 7C).
Figure 7. Absolute tendon fibril diameter distribution during 2 weeks of immobilisation and after 6 weeks of re-training in young males (n= 20) with either recombinant human growth hormone (rhGH) (n= 10) or placebo (n= 10).
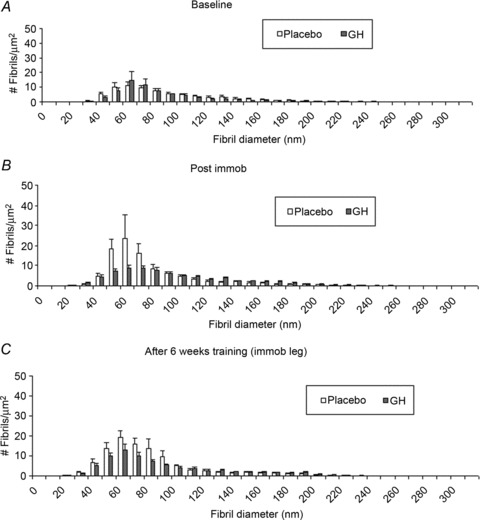
A, fibril diameter distribution of the GH and placebo groups at baseline. B, fibril diameter distribution of the GH and placebo groups after 2 weeks of immobilisation (Post immob). C, fibril diameter distribution of the GH and placebo groups after 6 weeks of training. No time effect or group interactions were seen within time points. Data are means ± SEM.
Discussion
In this study, we demonstrate that GH administration during a period with lower limb inactivity in humans stimulates collagen expression in both skeletal muscle and tendon, and abolishes the normal inactivity-related decline in tendon stiffness and in tendon LOX expression. Further, GH results in an increased tendon CSA and stiffness compared with the control group during a rehabilitation period. Taken together, the study supports the idea that GH has a matrix-stabilising effect during periods of inactivity and rehabilitation in humans. In contrast, skeletal muscle contractile protein does not seem to be influenced by GH administration during either disuse atrophy or rehabilitation in younger males.
The fact that tendon stiffness in the present study did not decrease during the inactivity period, and increased more during rehabilitation when GH was administered relative to controls (Fig. 3A), suggests that the GH/IGF-1 axis could be involved in the observed changes in tendon mechanical properties. In addition to these findings, a tendency towards an effect of GH administration on the tendon modulus during inactivity was found (P= 0.064, Fig. 3B) (GH, −9%; Plc, −26%; Table 1). Furthermore, a slight increase in tendon modulus was observed during the 6 weeks re-training with GH, whereas a decrease was found in the Plc group (GH, +7%; Plc, −27%; Table 1). The decrease in tendon stiffness and, to some extent, in modulus without affecting the tendon size in the Plc group fits with the findings in earlier human studies (de Boer et al. 2007a; Seynnes et al. 2008; Couppe et al. 2012). Taken together, the demonstrated decline in stiffness during immobilisation seems to be diminished with overexpressed IGF-1 levels, indicating a matrix-stabilizing effect of the GH/IGF-1 axis on connective tissue during periods of reduced mechanical loading.
The current findings are in line with observations in mice overexpressing IGF-1, where elevated IGF-1 levels were associated with the overexpression of LOX, as well as an increase in enzymatic cross-link formation (Reiser et al. 1996). Further, in rat vascular cells grown on matrices, IGF-1 (plus hyaluronic acid fragments) resulted in an increased density of cross-links within cell layers (Kothapalli & Ramamurthi, 2008). In our study, the differences between GH administration and the control groups with regard to tissue stiffness were associated with an increased expression of LOX (Fig. 6A). LOX is known to be associated with the formation of enzymatic cross-links (Siegel, 1976; Reiser et al. 1992). Further support for IGF-1 having a stimulating effect on LOX expression is indirectly provided by a study in rat oral tissues, where the application of a mixture of both IGF-1 and Fibroblast growth factor-2 (FGF-2) resulted in increased LOX expression in the oral connective tissues (Trackman et al. 1998).
In our study, we did not systematically analyse the mRNA expression results at the end of the 6 weeks of re-training because of a possible repeated biopsy effect. However, with GH administration during re-training, we found an increased expression of LOX relative to the control group (data not shown). A further up-regulation of IGF-1 and collagen was also seen during re-training, especially when GH was administered (data not shown), supporting the idea of GH/IGF-1 having a stimulating effect on LOX and collagen expression, which could explain the increase in tendon stiffness and modulus during re-training. Taken together, it is likely that IGF-1 stimulates both LOX and collagen expression, and this could result in an increased formation of cross-links, leading to altered mechanical properties of the tendon. It was not possible to measure the formation of cross-links in this study, and therefore we can only speculate on whether increased LOX and collagen expression leads to an increased formation of cross-links.
GH administration was associated with elevated local tissue IGF-1 expression, together with an increase in collagen expression, during both inactivity and rehabilitation (Figs 4 and 5). This is in accordance with findings in animals, where changes in the level of physical activity were associated with a change in IGF-1 and collagen expression (Heinemeier et al. 2009). Likewise, in humans, it has been found that GH administration leads to increased expression of both IGF-1 and collagen, which occur in parallel (Doessing et al. 2010a). More recent data have indicated that animals with overexpression of GH or with GH-receptor blockade demonstrate an overexpression or underexpression of collagen, respectively (Nielsen et al., unpublished data). In our study, we did not determine the protein synthesis rate for collagen directly, but only collagen expression, and thus we cannot state definitively the amount of new collagen that was synthesized. However, the TEM images showed no shift towards an increased number of smaller diameter fibrils after immobilization or during rehabilitation (Fig. 7). This is supported by data showing no change in decorin (Fig. 6D), which is expected to be involved in new fibril formation (Zhang et al. 2006). The possible ultrastructural changes (fibril morphology) as a result of immobilisation and loading are still unclear. In some animal studies, unloading causes a decrease in fibril size (Nakagawa et al. 1989; Majima et al. 2003), whereas others have found no changes during unloading (Lavagnino et al. 2005; Zhou et al. 2007). Mechanical loading has been shown previously in animal studies to yield decreased (Patterson-Kane et al. 1997), increased (Michna, 1984) or unchanged (Patterson-Kane et al. 1998) collagen fibril diameters. A recent human study examining the effect of loading on fibrillar morphology in patients with patellar tendinopathy did not find any changes in fibril diameter or density in the control group (normal tendons) as a result of loading, but an increase in fibril density and a decrease in mean fibril size was seen in the group with patellar tendinopathy (Kongsgaard et al. 2010).
GH administration results in a significant increase in tendon CSA during rehabilitation (Fig. 2B). Although this indicates an accumulation of more collagen fibrils, it cannot be excluded that part of the increase in CSA is a result of fluid accumulation in the tendons following GH administration, as this has been observed in other parts of the body during GH treatment (Lange et al. 2001; Ehrnborg et al. 2005). In support of the latter, an increase in tendon CSA with rehabilitation was also observed in the Plc group. Another explanation regarding the discrepancy between the lack of changes in fibril morphology and increase in tendon CSA during re-training could be stimulation of extracellular matrix proteins other than collagen fibrils.
Loss of skeletal muscle mass in response to inactivity was not influenced by the administration of GH in the present study (Fig. 1A). This indicates that elevated GH/IGF-1 axis activity does not inhibit the effects on skeletal muscle during inactivity. GH has been used previously in intensive care patients and has been shown to have a large protein retention effect in patients receiving both low-dose GH (serum IGF-1 level normalised) (Zhang et al. 2007; Duska et al. 2008) and supraphysiological GH doses (elevated serum IGF-1) (Ziegler et al. 1990; Takala et al. 1999), and thereby contributes to counteract net muscle loss, an effect that potentially could also be present in the present study. We did not determine protein turnover directly in this study, but animal studies have concluded that proteolysis is largely up-regulated during inactivity and can explain the muscle loss that appears during inactivity in animals (Tucker et al. 1981; Thomason & Booth, 1990; Sacheck et al. 2007). Data on humans indicate that muscle loss in disuse atrophy with inactivity can be explained by a marked reduction in myofibrillar protein synthesis (Paddon-Jones et al. 2006; de Boer et al. 2007). Thus, a lack of influence of GH on muscle protein synthesis – as shown in normally active individuals (Doessing et al. 2010a) – would explain the fact that the administration of GH in the present study did not influence the loss of muscle mass with inactivity. Nor did we observe any beneficial effect of GH on skeletal muscle growth during rehabilitation (Fig. 2A). This fits with earlier findings indicating that neither GH (Yarasheski et al. 1992; Lange et al. 2002; Doessing et al. 2010a) nor the combination of training and GH (Yarasheski et al. 1992; Lange et al. 2002; Doessing et al. 2010a) resulted in any protein synthesis-stimulating effect in skeletal muscle.
Conclusions
In conclusion, when GH is administered to young healthy individuals, it stimulates collagen expression in both skeletal muscle and tendon, and abolishes the normal inactivity-related decline in patellar tendon mechanical properties (stiffness and modulus) and the expression of tendon LOX, and results in an increased tendon size and stiffness compared with the control group after rehabilitation with training over 6 weeks. This demonstrates that GH has a matrix-stabilising effect during periods of inactivity and rehabilitation in humans. In contrast, skeletal muscle contractile protein does not seem to be influenced by GH administration during either disuse atrophy or rehabilitation.
Acknowledgments
We wish to express our gratitude to the participants for their time and commitment to the study. In addition, we thank Jesper Løvind Andersen, Anja Jokipii, Ann-Christina Reimann, Camilla Sørensen, Caroline Bøjstrup, Ann-Marie Sedstrøm, Jytte Larsen, Christina Eenberg Hansen and the MRI staff at the Department of Radiology Bispebjerg and Frederiksberg Hospitals for their technical assistance.
Glossary
Abbreviations
- COL1A1
collagen 1 alpha 1
- COL3A1
collagen 3 alpha 1
- CSA
cross-sectional area
- FOV
field of view
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GH
growth hormone
- IGF-1Ea
insulin-like growth factor-1Ea
- IGF-1Ec
insulin-like growth factor-1Ec
- LOX
lysyl oxidase
- MMP
matrix metalloproteinase
- MRI
magnetic resonance imaging
- MVC
maximal voluntary contraction
- rhGH
recombinant human growth hormone
- RM
repetitions maximum
- RPLP0
60S acidic ribosomal protein P0
- TEM
transmission electron microscopy
Additional information
Competing interests
None declared.
Author contributions
Conception and design of the study: APB, MK and HL. Collection, analysis and interpretation of the data: APB, KD, PS, CC, SPM, MB, MK and HL. Writing and critical revision of the manuscript: APB, KD, PS, CC, SPM, MB, MK and HL. All authors approved the final version of the manuscript.
Funding
This study was supported by the Danish Medical Research Council, Novo-Nordisk Foundation, Lundbeck Foundation and Nordea Foundation (Healthy Ageing Grant).
Supplementary material
Fig. S1
Fig. S2
References
- Berg HE, Tesch PA. Changes in muscle function in response to 10 days of lower limb unloading in humans. Acta Physiol Scand. 1996;157:63–70. doi: 10.1046/j.1365-201X.1996.476217000.x. [DOI] [PubMed] [Google Scholar]
- Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35:609–616. [PubMed] [Google Scholar]
- de Boer MD, Maganaris CN, Seynnes OR, Rennie MJ, Narici MV. Time course of muscular, neural and tendinous adaptations to 23 day unilateral lower-limb suspension in young men. J Physiol. 2007a;583:1079–1091. doi: 10.1113/jphysiol.2007.135392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer MD, Selby A, Atherton P, Smith K, Seynnes OR, Maganaris CN, Maffulli N, Movin T, Narici MV, Rennie MJ. The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. J Physiol. 2007b;585:241–251. doi: 10.1113/jphysiol.2007.142828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claessen KM, Appelman-Dijkstra NM, Adoptie DM, Roelfsema F, Smit JW, Biermasz NR, Pereira AM. Metabolic profile in growth hormone-deficient (GHD) adults after long-term recombinant human growth hormone (rhGH) therapy. J Clin Endocrinol Metab. 2013;98:352–361. doi: 10.1210/jc.2012-2940. [DOI] [PubMed] [Google Scholar]
- Couppe C, Kongsgaard M, Aagaard P, Hansen P, Bojsen-Moller J, Kjaer M, Magnusson SP. Habitual loading results in tendon hypertrophy and increased stiffness of the human patellar tendon. J Appl Physiol. 2008;105:805–810. doi: 10.1152/japplphysiol.90361.2008. [DOI] [PubMed] [Google Scholar]
- Couppe C, Suetta C, Kongsgaard M, Justesen L, Hvid LG, Aagaard P, Kjaer M, Magnusson SP. The effects of immobilization on the mechanical properties of the patellar tendon in younger and older men. Clin Biomech (Bristol, Avon) 2012;27:949–954. doi: 10.1016/j.clinbiomech.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Doessing S, Heinemeier KM, Holm L, Mackey AL, Schjerling P, Rennie M, Smith K, Reitelseder S, Kappelgaard AM, Rasmussen MH, Flyvbjerg A, Kjaer M. Growth hormone stimulates the collagen synthesis in human tendon and skeletal muscle without affecting myofibrillar protein synthesis. J Physiol. 2010a;588:341–351. doi: 10.1113/jphysiol.2009.179325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doessing S, Holm L, Heinemeier KM, Feldt-Rasmussen U, Schjerling P, Qvortrup K, Larsen JO, Nielsen RH, Flyvbjerg A, Kjaer M. GH and IGF1 levels are positively associated with musculotendinous collagen expression: experiments in acromegalic and GH deficiency patients. Eur J Endocrinol. 2010b;163:853–862. doi: 10.1530/EJE-10-0818. [DOI] [PubMed] [Google Scholar]
- Duska F, Fric M, Pazout J, Waldauf P, Tuma P, Pachl J. Frequent intravenous pulses of growth hormone together with alanylglutamine supplementation in prolonged critical illness after multiple trauma: effects on glucose control, plasma IGF-I and glutamine. Growth Horm IGF Res. 2008;18:82–87. doi: 10.1016/j.ghir.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Ehrnborg C, Ellegard L, Bosaeus I, Bengtsson BA, Rosen T. Supraphysiological growth hormone: less fat, more extracellular fluid but uncertain effects on muscles in healthy, active young adults. Clin Endocrinol (Oxf) 2005;62:449–457. doi: 10.1111/j.1365-2265.2005.02240.x. [DOI] [PubMed] [Google Scholar]
- Hansen M, Miller BF, Holm L, Doessing S, Petersen SG, Skovgaard D, Frystyk J, Flyvbjerg A, Koskinen S, Pingel J, Kjaer M, Langberg H. Effect of administration of oral contraceptives in vivo on collagen synthesis in tendon and muscle connective tissue in young women. J Appl Physiol. 2009;106:1435–1443. doi: 10.1152/japplphysiol.90933.2008. [DOI] [PubMed] [Google Scholar]
- Heinemeier KM, Langberg H, Olesen JL, Kjaer M. Role of TGF-beta1 in relation to exercise-induced type I collagen synthesis in human tendinous tissue. J Appl Physiol. 2003;95:2390–2397. doi: 10.1152/japplphysiol.00403.2003. [DOI] [PubMed] [Google Scholar]
- Heinemeier KM, Olesen JL, Haddad F, Schjerling P, Baldwin KM, Kjaer M. Effect of unloading followed by reloading on expression of collagen and related growth factors in rat tendon and muscle. J Appl Physiol. 2009;106:178–186. doi: 10.1152/japplphysiol.91092.2008. [DOI] [PubMed] [Google Scholar]
- Kongsgaard M, Kovanen V, Aagaard P, Doessing S, Hansen P, Laursen AH, Kaldau NC, Kjaer M, Magnusson SP. Corticosteroid injections, eccentric decline squat training and heavy slow resistance training in patellar tendinopathy. Scand J Med Sci Sports. 2009;19:790–802. doi: 10.1111/j.1600-0838.2009.00949.x. [DOI] [PubMed] [Google Scholar]
- Kongsgaard M, Qvortrup K, Larsen J, Aagaard P, Doessing S, Hansen P, Kjaer M, Magnusson SP. Fibril morphology and tendon mechanical properties in patellar tendinopathy: effects of heavy slow resistance training. Am J Sports Med. 2010;38:749–756. doi: 10.1177/0363546509350915. [DOI] [PubMed] [Google Scholar]
- Kongsgaard M, Reitelseder S, Pedersen TG, Holm L, Aagaard P, Kjaer M, Magnusson SP. Region specific patellar tendon hypertrophy in humans following resistance training. Acta Physiol (Oxf) 2007;191:111–121. doi: 10.1111/j.1748-1716.2007.01714.x. [DOI] [PubMed] [Google Scholar]
- Kothapalli CR, Ramamurthi A. Benefits of concurrent delivery of hyaluronan and IGF-1 cues to regeneration of crosslinked elastin matrices by adult rat vascular cells. J Tissue Eng Regen Med. 2008;2:106–116. doi: 10.1002/term.70. [DOI] [PubMed] [Google Scholar]
- Langberg H, Skovgaard D, Petersen LJ, Bulow J, Kjaer M. Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J Physiol. 1999;521:299–306. doi: 10.1111/j.1469-7793.1999.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange KH, Andersen JL, Beyer N, Isaksson F, Larsson B, Rasmussen MH, Juul A, Bulow J, Kjaer M. GH administration changes myosin heavy chain isoforms in skeletal muscle but does not augment muscle strength or hypertrophy, either alone or combined with resistance exercise training in healthy elderly men. J Clin Endocrinol Metab. 2002;87:513–523. doi: 10.1210/jcem.87.2.8206. [DOI] [PubMed] [Google Scholar]
- Lange KH, Isaksson F, Rasmussen MH, Juul A, Bulow J, Kjaer M. GH administration and discontinuation in healthy elderly men: effects on body composition, GH-related serum markers, resting heart rate and resting oxygen uptake. Clin Endocrinol (Oxf) 2001;55:77–86. doi: 10.1046/j.1365-2265.2001.01344.x. [DOI] [PubMed] [Google Scholar]
- Lavagnino M, Arnoczky SP, Frank K, Tian T. Collagen fibril diameter distribution does not reflect changes in the mechanical properties of in vitro stress-deprived tendons. J Biomech. 2005;38:69–75. doi: 10.1016/j.jbiomech.2004.03.035. [DOI] [PubMed] [Google Scholar]
- Lee S, Barton ER, Sweeney HL, Farrar RP. Viral expression of insulin-like growth factor-I enhances muscle hypertrophy in resistance-trained rats. J Appl Physiol. 2004;96:1097–1104. doi: 10.1152/japplphysiol.00479.2003. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Hansen P, Aagaard P, Brond J, Dyhre-Poulsen P, Bojsen-Moller J, Kjaer M. Differential strain patterns of the human gastrocnemius aponeurosis and free tendon, in vivo. Acta Physiol Scand. 2003;177:185–195. doi: 10.1046/j.1365-201X.2003.01048.x. [DOI] [PubMed] [Google Scholar]
- Majima T, Yasuda K, Tsuchida T, Tanaka K, Miyakawa K, Minami A, Hayashi K. Stress shielding of patellar tendon: effect on small-diameter collagen fibrils in a rabbit model. J Orthop Sci. 2003;8:836–841. doi: 10.1007/s00776-003-0707-x. [DOI] [PubMed] [Google Scholar]
- Michna H. Morphometric analysis of loading-induced changes in collagen-fibril populations in young tendons. Cell Tissue Res. 1984;236:465–470. doi: 10.1007/BF00214251. [DOI] [PubMed] [Google Scholar]
- Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K, Rennie MJ. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol. 2005;567:1021–1033. doi: 10.1113/jphysiol.2005.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movin T. Tendon tissue sampling. Scand J Med Sci Sports. 2000;10:368–371. doi: 10.1034/j.1600-0838.2000.010006368.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Totsuka M, Sato T, Fukuda Y, Hirota K. Effect of disuse on the ultrastructure of the Achilles tendon in rats. Eur J Appl Physiol Occup Physiol. 1989;59:239–242. doi: 10.1007/BF02386194. [DOI] [PubMed] [Google Scholar]
- Paddon-Jones D, Sheffield-Moore M, Cree MG, Hewlings SJ, Aarsland A, Wolfe RR, Ferrando AA. Atrophy and impaired muscle protein synthesis during prolonged inactivity and stress. J Clin Endocrinol Metab. 2006;91:4836–4841. doi: 10.1210/jc.2006-0651. [DOI] [PubMed] [Google Scholar]
- Patterson-Kane JC, Firth EC, Parry DA, Wilson AM, Goodship AE. Effects of training on collagen fibril populations in the suspensory ligament and deep digital flexor tendon of young thoroughbreds. Am J Vet Res. 1998;59:64–68. [PubMed] [Google Scholar]
- Patterson-Kane JC, Wilson AM, Firth EC, Parry DA, Goodship AE. Comparison of collagen fibril populations in the superficial digital flexor tendons of exercised and nonexercised thoroughbreds. Equine Vet J. 1997;29:121–125. doi: 10.1111/j.2042-3306.1997.tb01653.x. [DOI] [PubMed] [Google Scholar]
- Reiser K, McCormick RJ, Rucker RB. Enzymatic and nonenzymatic cross-linking of collagen and elastin. FASEB J. 1992;6:2439–2449. doi: 10.1096/fasebj.6.7.1348714. [DOI] [PubMed] [Google Scholar]
- Reiser K, Summers P, Medrano JF, Rucker R, Last J, McDonald R. Effects of elevated circulating IGF-1 on the extracellular matrix in ‘high-growth’ C57BL/6J mice. Am J Physiol. 1996;271:R696–R703. doi: 10.1152/ajpregu.1996.271.3.R696. [DOI] [PubMed] [Google Scholar]
- Rennie MJ. Claims for the anabolic effects of growth hormone: a case of the emperor's new clothes. Br J Sports Med. 2003;37:100–105. doi: 10.1136/bjsm.37.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacheck JM, Hyatt JP, Raffaello A, Jagoe RT, Roy RR, Edgerton VR, Lecker SH, Goldberg AL. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J. 2007;21:140–155. doi: 10.1096/fj.06-6604com. [DOI] [PubMed] [Google Scholar]
- Seynnes OR, Maffiuletti NA, Maganaris CN, deBoer MD, Pensini M, di Prampero PE, Narici MV. Soleus T reflex modulation in response to spinal and tendinous adaptations to unilateral lower limb suspension in humans. Acta Physiol (Oxf) 2008;194:239–251. doi: 10.1111/j.1748-1716.2008.01874.x. [DOI] [PubMed] [Google Scholar]
- Siegel RC. Collagen cross-linking. Synthesis of collagen cross-links in vitro with highly purified lysyl oxidase. J Biol Chem. 1976;251:5786–5792. [PubMed] [Google Scholar]
- Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, Ortenblad N, Magnusson SP, Kjaer M, Aagaard P. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol. 2009;107:1172–1180. doi: 10.1152/japplphysiol.00290.2009. [DOI] [PubMed] [Google Scholar]
- Takala J, Ruokonen E, Webster NR, Nielsen MS, Zandstra DF, Vundelinckx G, Hinds CJ. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med. 1999;341:785–792. doi: 10.1056/NEJM199909093411102. [DOI] [PubMed] [Google Scholar]
- Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol. 1990;68:1–12. doi: 10.1152/jappl.1990.68.1.1. [DOI] [PubMed] [Google Scholar]
- Trackman PC, Graham RJ, Bittner HK, Carnes DL, Gilles JA, Graves DT. Inflammation-associated lysyl oxidase protein expression in vivo, and modulation by FGF-2 plus IGF-1. Histochem Cell Biol. 1998;110:9–14. doi: 10.1007/s004180050259. [DOI] [PubMed] [Google Scholar]
- Trepp R, Fluck M, Stettler C, Boesch C, Ith M, Kreis R, Hoppeler H, Howald H, Schmid JP, Diem P, Christ ER. Effect of GH on human skeletal muscle lipid metabolism in GH deficiency. Am J Physiol Endocrinol Metab. 2008;294:E1127–E1134. doi: 10.1152/ajpendo.00010.2008. [DOI] [PubMed] [Google Scholar]
- Tucker KR, Seider MJ, Booth FW. Protein synthesis rates in atrophied gastrocnemius muscles after limb immobilization. J Appl Physiol. 1981;51:73–77. doi: 10.1152/jappl.1981.51.1.73. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Rattray M, Thomas CR, Moreland BH, Schulster D. Growth hormone increases IGF-I, collagen I and collagen III gene expression in dwarf rat skeletal muscle. Mol Cell Endocrinol. 1995;115:187–197. doi: 10.1016/0303-7207(95)03690-3. [DOI] [PubMed] [Google Scholar]
- Yarasheski KE, Campbell JA, Smith K, Rennie MJ, Holloszy JO, Bier DM. Effect of growth hormone and resistance exercise on muscle growth in young men. Am J Physiol. 1992;262:E261–E267. doi: 10.1152/ajpendo.1992.262.3.E261. [DOI] [PubMed] [Google Scholar]
- Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV, Birk DE. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem. 2006;98:1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
- Zhang MM, Wu XT, Zhou Y, Qian K, Zheng YM. Short-term application of low-dose growth hormone in surgical patients: effects on nitrogen balance and blood glucose. World J Gastroenterol. 2007;13:452–456. doi: 10.3748/wjg.v13.i3.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Koike Y, Uhthoff HK, Trudel G. Quantitative histology and ultrastructure fail to explain weakness of immobilized rabbit Achilles’ tendons. Arch Phys Med Rehabil. 2007;88:1177–1184. doi: 10.1016/j.apmr.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Ziegler TR, Young LS, Ferrari-Baliviera E, Demling RH, Wilmore DW. Use of human growth hormone combined with nutritional support in a critical care unit. JPEN J Parenter Enteral Nutr. 1990;14:574–581. doi: 10.1177/0148607190014006574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


