Abstract
Mitochondrial dysfunction and reactive oxygen species (ROS) have been implicated in the aetiology of skeletal muscle insulin resistance, although there is considerable controversy regarding these concepts. Mitochondrial function has been traditionally assessed in the presence of saturating ADP, but ATP turnover and the resultant ADP is thought to limit respiration in vivo. Therefore, we investigated the potential link between submaximal ADP-stimulated respiration rates, ROS generation and skeletal muscle insulin sensitivity in a model of type 2 diabetes mellitus, the ZDF rat. Utilizing permeabilized muscle fibres we observed that submaximal ADP-stimulated respiration rates (250–2000 μm ADP) were lower in ZDF rats than in lean controls, which coincided with decreased adenine nucleotide translocase 2 (ANT2) protein content. This decrease in submaximal ADP-stimulated respiration occurred in the absence of a decrease in electron transport chain function. Treating ZDF rats with resveratrol improved skeletal muscle insulin resistance and this was associated with elevated submaximal ADP-stimulated respiration rates as well as an increase in ANT2 protein content. These results coincided with a greater ability of ADP to attenuate mitochondrial ROS emission and an improvement in cellular redox balance. Together, these data suggest that mitochondrial dysfunction is present in skeletal muscle insulin resistance when assessed at submaximal ADP concentrations and that ADP dynamics may influence skeletal muscle insulin sensitivity through alterations in the propensity for mitochondrial ROS emission.
Key points
Disparity exists within the literature surrounding mitochondrial dysfunction and insulin resistance and previous reports have primarily examined mitochondrial function as a capacity measurement.
We show that submaximal ADP-stimulated respiration rates are lower in ZDF rats, which coincides with decreased adenine nucleotide translocase 2 (ANT2) protein content.
Supplementation of ZDF rats with resveratrol improves skeletal muscle insulin sensitivity, increases submaximal ADP-stimulated respiration rates and increases ANT2 protein content.
Improvements in the ability of ADP to attenuate mitochondrial reactive oxygen species (ROS) emission and cellular redox balance were also observed following resveratrol supplementation.
These data suggest that mitochondrial dysfunction is present in skeletal muscle insulin resistance when assessed at submaximal ADP concentrations and that ADP dynamics may influence skeletal muscle insulin sensitivity through alterations in the propensity for ROS formation.
Introduction
Skeletal muscle, by virtue of its mass and overall rate of insulin-stimulated glucose disposal, is a highly important tissue in the aetiology of insulin resistance and type II diabetes mellitus (T2DM). The mechanisms that result in skeletal muscle insulin resistance remain poorly elucidated and controversial, although a number of hypotheses have been proposed. For example, mitochondrial dysfunction has been suggested to be a cause of skeletal muscle insulin resistance, and is classically defined as a reduction in mitochondrial content and/or an intrinsic impairment within mitochondria (Kelley et al. 2002; Lowell & Shulman, 2005), but there is significant discrepancy within the literature surrounding this concept (Kelley et al. 2002; Lowell & Shulman, 2005; Mogensen et al. 2007; Boushel et al. 2007; Phielix et al. 2008; de Feyter et al. 2008a,b; Holloszy, 2009; Holloway et al. 2010; Lenaers et al. 2010; Hoeks & Schrauwen, 2012).
Of potential importance, mitochondrial function has been traditionally assessed as a capacity measurement as maximal ADP concentrations have been utilized to promote mitochondrial respiration (Boushel et al. 2007; Mogensen et al. 2007; Phielix et al. 2008). However, these previous examinations of mitochondrial function may not reflect the in vivo situation as under most physiological conditions ATP turnover and subsequent ADP provision is thought to limit respiration (Wilson, 1994). Therefore, from a physiological standpoint, it may be more appropriate to examine mitochondrial function in the presence of submaximal concentrations of ADP, although this has yet to be considered within the context of skeletal muscle insulin resistance.
In addition to mitochondrial dysfunction, a causal role of mitochondrial reactive oxygen species (ROS) production has been proposed to explain the progression of insulin resistance and T2DM in animals and humans (Brownlee, 2001; Houstis et al. 2006; Anderson et al. 2009). In support of this concept, mitochondrial ROS generation is elevated in the over-fed and insulin-resistant states, and attenuating mitochondrial ROS emission using mitochondrial-targeted antioxidant approaches prevents diet-induced insulin resistance (Anderson et al. 2009; Boden et al. 2012). Therefore, numerous studies (Houstis et al. 2006; Chen et al. 2008; Anderson et al. 2009; Hoehn et al. 2009; Lee et al. 2010; Boden et al. 2012) have suggested that by decreasing mitochondrial-derived ROS, insulin sensitivity can be maintained or improved. However, a relationship between mitochondrial ROS and insulin resistance is not universally found, indicating there is still some disparity (Paglialunga et al. 2012).
Importantly, the supply of ADP to mitochondria decreases the propensity for ROS emission (Korshunov et al. 1997). Therefore, the ability of the mitochondria to transport ADP into the mitochondrial matrix to subsequently decrease ROS emission may have a role in the protection of insulin sensitivity. In this regard, adenine nucleotide translocase (ANT) has an obligatory role in counter-transporting ADP and ATP across the inner mitochondrial membrane (Klingenberg, 2008). Skeletal muscle expresses two primary ANT isoforms, ANT1 and ANT2, but ANT2 possesses a higher transport efficiency and has a higher Vmax (Dorner et al. 2006). The transport of ADP is also the governing factor for oxidative phosphorylation (Pfaff et al. 1965), indicating that any alteration in ADP transport can directly impact mitochondrial function (Dorner et al. 2006). Therefore, ADP transport may sit as a nexus between mitochondrial function/dysfunction and mitochondrial ROS production. Intriguingly, the polyphenolic compound resveratrol has been previously shown to improve the oxidative status of the muscle (Chen et al. 2011) concomitantly with improving insulin sensitivity (Baur et al. 2006) and may increase ANT expression (Seymour et al. 2010; Rimbaud et al. 2011).
Therefore, we investigated the potential link between submaximal ADP-stimulated respiration rates, ROS generation and skeletal muscle insulin sensitivity in a widely used model of T2DM, the ZDF rat. It is hypothesized that submaximal ADP-stimulated respiration will be repressed in the ZDF rat and that resveratrol will recover this decrement back to lean control values.
Methods
Animals
Male ZDF rats (Charles River, Wilmington, MA, USA) were housed in individual cages, with a reverse 12:12 h light–dark cycle, and were provided with food and water ad libitum. Lean control (LC) rats (n= 13) were fed a stock diet. Twenty-six ZDF (Zucker diabetic fatty) rats (n= 13 each group) were randomly assigned to either a stock diet (ZDF) or a stock diet supplemented with resveratrol (Cayman Chemicals, Ann Harbor, MI, USA; 200 mg kg−1 body weight similar to previous publications (Lagouge et al. 2006)) for 6 weeks (ZDF+RESV). Previous work has shown that ZDF rats are insulin resistant at 6 weeks and become type II diabetic by 12 weeks, so we attempted to delay the onset of skeletal muscle insulin resistance by treating with resveratrol from 5 weeks until 11 weeks of age (Suh et al. 2005). Soleus (for glucose uptake) and red gastrocnemius (all other experimental procedures) muscles were removed under isoflurane. Originally, this study was planned with n= 8 for all experiments. However, upon completing the first set of experiments additional animals (n= 5) were acquired to determine a link between decreased ADP transport and mitochondrial ROS emission (i.e. ROS in the presence of ADP (Fig. 6B)). Additionally, ADP titrations (see Fig. 3) and maximal ROS (see Fig. 6A) measurements were repeated as these are key findings and we aimed to ensure repeatability (n= 13). This study was approved by the University of Guelph Animal Care Committee, and conforms to the guide for the care and use of laboratory animals published by the US National Institutes of Health.
Figure 6. Mitochondrial content and adenine nucleotide translocase isoform content.
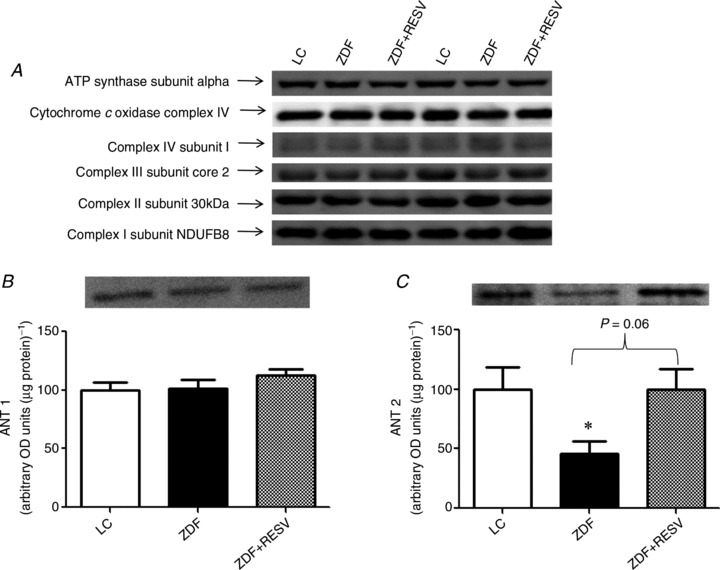
A, muscle homogenates from lean control (LC), ZDF control rats (ZDF) and ZDF rats supplemented with resveratrol (ZDF+RESV) were analysed for protein content of mitochondrial markers (30 μg loaded): complex I subunit NDUFB8 (Mitosciences), complex II subunit 30 kDa (Mitosciences), complex III subunit core 2 (Mitosciences), complex IV subunit I (Mitosciences), ATP synthase subunit alpha (Mitosciences). No significant differences were found. Two representative blots are shown. B, there were no differences in ANT1 content between groups (20 μg protein loaded). C, ZDF rats showed a decrease in ANT2 expression and supplementation with resveratrol increased ANT2 expression up to LC values (40 μg protein loaded). Values represent means ± SEM; n= 8. *Significantly (P < 0.05) different from LC.
Figure 3. Pyruvate respiratory kinetics are not different between groups.
A, Pyruvate respiration rates across all concentrations examined were not different between groups. B and C, apparent glutamate Km (B) and Vmax (C) were also not significantly different. Values represent means ± SEM; n= 8.
Measurement of circulating variables
Fasting blood glucose (Freestyle lite, Abbott Laboratories, St-Laurent, QC, Canada), insulin (ELISA: Millipore, Billerica, MA, USA), free fatty acids (FFAs; colorimetric assay: Wako Diagnostics, Richmond, VA, USA) and triglycerides (colorimetric assay: Sigma-Aldrich, Oakville, ON, Canada) we all measured from commercially available kits.
Basal and insulin-stimulated glucose transport
Glucose transport was measured as previously described (Ritchie et al. 2011). Briefly, pregassed (95% O2/5% CO2) Medium 199 containing 0.1% bovine serum albumin (BSA) was warmed to 30°C and used as a base for all glucose uptake buffers. Insulin (10 mU ml−1) (Humulin R; Eli Lilly, Toronto, Ontario, Canada) was added to all buffers for the insulin-stimulated condition. Excised soleus strips were placed in glass vials containing preincubation buffer for 30 min in the presence or absence of insulin (10 mU ml−1; maintained in all subsequent buffers). Soleus strips were then weighed and digested for 10 min in 1 ml of 1 m NaOH at 95°C. Muscle from each sample was sampled in duplicate and analysed by liquid scintillation counting, from which glucose transport was calculated.
Preparation of permeabilized muscle fibres
The preparation of permeabilized muscle fibre bundles (PmFBs) was adopted from Perry et al. (2011), as we have previously reported (Smith et al. 2011). Following dissection of red gastrocnemius, fibre bundles (∼2 mg) were separated in BIOPS buffer containing, CaK2-EGTA (2.77 mm), K2-EGTA (7.23 mm), Na2-ATP (5.77 mm), MgCl2.6H2O (6.56 mm), Na2-phosphocreatine (15 mm), imidazole (20 mm), dithiothreitol (0.5 mm) and MES (50 mm). Following separation, fibre bundles were placed in BIOPS containing 40 μg ml−1 saponin, agitated for 30 min and then fibres prepared for respiration were washed in respiration buffer (MIRO5) containing EGTA (0.5 mm), MgCl2.6H2O (3 mm), potassium lactobionate (60 mm), KH2PO4 (10 mm), Hepes (20 mm), sucrose (110 mm) and fatty acid-free BSA (1 g l−1). Fibres prepared for H2O2 emission were washed in Buffer Z containing K-MES (110 mm), KCl (30 mm), EGTA (1 mm), K2HPO4 (10 mm) and MgCl2.6H2O (10 mm). PmFBs were left in cold MIRO5 or Buffer Z until analysis.
Permeabilized muscle fibre respiration
Mitochondrial respiration was measured in PmFBs by high-resolution respirometry (Oroboros Oxygraph-2 k, Innsbruck, Austria) at 37°C and room air saturated oxygen tension in the presence of 25 μm blebbistatin to ensure PmFB relaxation (Perry et al. 2011). In the presence of 5 mm ADP and 2 mm malate, separate PmFBs from the same animal were used to determine complex I (pyruvate) and complex I+II (pyruvate+succinate) respiration rates. Separate PmFBs from the same animal in the presence of 5 mm ADP and 2 mm malate were then used to determine glutamate (0, 100, 175, 250, 500, 2000, 4000 μm) and pyruvate (0, 15, 30, 50, 75, 150, 500, 1000 μm) stimulated respiration across a range of substrate concentrations. ADP (0, 100, 175, 250, 500, 1000, 2000, 4000, 6000 μm)-stimulated respiration was determined in the presence of 10 mm pyruvate and 2 mm malate. To measure palmitoyl-coenzyme A (P-CoA)-supported respiration, MIRO5 + 5 mm ADP, 2 mm malate and 2 mm l-carnitine was used as the respiration medium. P-CoA (150 μm) was added to initiate respiration. To measure palmitate-supported respiration, MIRO5 + 1 mm ATP + 5 mm ADP, 2 mm malate, and 2 mm l-carnitine + 1 mm CoA was used as the respiration medium. Palmitate (250 μm) was added to initiate fatty acid-supported respiration. Exogenous cytochrome c (10 μm) was added at the end of all respiration experiments to ensure outer mitochondrial membrane integrity. The addition of cytochrome c did not significantly elevate the respiration rate in any experiment.
Permeabilized muscle fibre mitochondrial H2O2 emission
Measurement of mitochondrial H2O2 emission was similar to a previously described method (Anderson et al. 2009). Briefly, mitochondrial H2O2 emission was determined fluorometrically (Lumina, Thermo Scientific, Waltham, MA, USA) in a constantly stirring cuvette at 37°C (peltier controlled). PmFBs were placed in a cuvette containing Buffer Z supplemented with 25 μm blebbistatin, 40 U ml−1 CuZnSOD, 10 μm Amplex Red (Invitrogen, Carlsbad, CA, USA), 0.5 U ml−1 horseradish peroxidase with or without 100 μm ADP. Mitochondrial H2O2 emission was initiated by the addition of 10 mm succinate. The rate of H2O2 emission was calculated from the slope (fluorescence/min), after subtracting the background from a standard curve established with the same reaction conditions and normalized to freeze-dried muscle weight.
Glutathione measurements
GSH and GSSG measurements were determined as previously described (Anderson et al. 2009). Briefly, two muscle chips (in triplicate wells) were used to determine GSH and two separate muscle chips (in triplicate wells) were used to determine GSSG in the presence of the scavenger methyl-2-vinylpyridinium triflate (M2VP). Total GSH and GSSG were measured as per the manufacturer's instructions (Oxis International, Inc. Beverly Hills, CA, USA).
Western blotting
All samples were analysed for total protein (BCA protein assay), and samples were separated by electrophoresis on SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes as previously reported (Smith et al. 2011). Commercially available antibodies were used to detect OXPHOS cocktail (ab110413, Abcam, Mitosciences, Cambridge, MA, USA), ANT1 (ab110322, Abcam), ANT2 (ab118125, Abcam) and cytochrome c oxidase complex IV (A21347, Invitrogen).
Statistics
The apparent Km for glutamate, pyruvate and ADP was estimated on independent experiments utilizing Michaelis–Menten kinetics utilizing Prism software (GraphPad Software, Inc., La Jolla, CA, USA), while the maximal respiration (Vmax) was specified as the highest respiration value directly determined, as previously described (Perry et al. 2012). A one-way analysis of variance (ANOVA) with a Newman–Keuls post hoc analysis was utilized for all comparisons except for Fig. 1A where a Student's two-tailed t test was used. Statistical significance was accepted at P≤ 0.05.
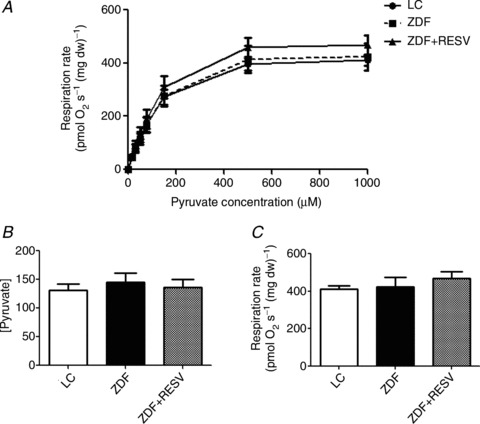
Figure 1. Depressed insulin-stimulated glucose uptake is not associated with decreased mitochondrial respiratory capacity.
A, soleus strips from control ZDF rats (ZDF) were unable to significantly promote glucose (3-O-methyl-glucose) uptake in response to 10 mU ml−1 insulin, and resveratrol treatment (ZDF+RESV) recovers this decrement. B, mitochondrial complex I and complex I+II supported respiratory capacity is not different between lean control (LC), ZDF and ZDF+RESV. Respiratory control ratios were also unchanged: LC = 7.59 ± 0.63, ZDF = 7.65 ± 0.63 and ZDF+RESV = 8.08 ± 0.27. Values represent means ± SEM; n= 8. *Significantly different (P < 0.05) from basal value. PM, pyruvate+malate; PMD, pyruvate+malate+ADP; PMDS, pyruvate+malate+ADP+succinate; PMDSc, pyruvate+malate+ADP+cytochrome c.
Results
Resveratrol recovers skeletal muscle insulin sensitivity in ZDF rats
The body weights of the ZDF and ZDF+RESV groups were significantly elevated (P < 0.05) compared to LC (Table 1). Importantly, fasting blood glucose and insulin concentrations were significantly increased in the ZDF group compared to LC. Resveratrol increased blood insulin concentrations and normalized blood glucose values. Circulating FFAs and triglyceride were also significantly elevated in the ZDF group, but these values were not altered with resveratrol supplementation (Table 1).
Table 1.
Basic characteristics
| Lean control | ZDF | ZDF+RESV | |
|---|---|---|---|
| Body weight (g) | 288 ± 9.0 | 384 ± 5.2* | 371 ± 7.5* |
| Blood glucose (mmol l-1) | 4.1 ± 0.8 | 11.0 ± 1.8*† | 5.4 ± 0.3 |
| Insulin (pmol l-1) | 257.7 ± 73.3 | 960.7 ± 118.4*† | 1536.1 ± 155.4* |
| FFA (mmol l-1) | 0.6 ± 0.05 | 0.9 ± 0.15* | 1.0 ± 0.15* |
| Triglycerides (mmol l-1) | 0.2 ± 0.02 | 4.8 ± 0.6* | 4.3 ± 0.6* |
Values represent means ± SEM; n = 8. *Significantly (P < 0.05) different from LC. †Significantly (P < 0.05) different from ZDF+RESV.
To investigate insulin-stimulated glucose uptake in skeletal muscle, we utilized an incubated soleus muscle preparation. Insulin increased glucose uptake in LC animals (∼40%), but did not stimulate glucose uptake in untreated ZDF rats (P > 0.05). In contrast, administration of resveratrol in ZDF rats increased insulin-stimulated glucose uptake ∼40%. As a result, insulin-stimulated glucose uptake was only repressed in the soleus muscle of untreated ZDF animals (Fig. 1A).
Submaximal ADP-stimulated respiration is impaired in ZDF rats
Skeletal muscle insulin sensitivity was not associated with alterations in the maximal capacity of the electron transport chain (complex I and complex I+II) as the LC, ZDF and ZDF+RESV groups displayed similar maximal rates (Fig. 1B). To examine mitochondrial respiratory characteristics, we determined respiration rates in the presence of various concentrations of glutamate or pyruvate, which supply reducing equivalents to complex I. Maximal respiration, the apparent Km and respiration rates at submaximal substrate concentrations for glutamate and pyruvate were not different between groups (Figs. 2 and 3), indicating that electron transport chain function was similar. In contrast, while the ADP apparent Km and Vmax values were not significantly different between groups (Fig. 4B and C) submaximal ADP-stimulated respiration rates (250, 500, 2000 μm ADP; Fig. 4A) in ZDF rats were significantly lower than in both LC and resveratrol-treated ZDF rats.
Figure 2. Glutamate respiratory kinetics are not different between groups.
A, Glutamate respiration rates across all concentrations examined were not different between groups. B and C, apparent glutamate Km (B) and Vmax (C) were also not significantly different. Values represent means ± SEM; n= 8.
Figure 4. Submaximal ADP-stimulated respiration is significantly lower in ZDF animals and resveratrol recovers this decrease.
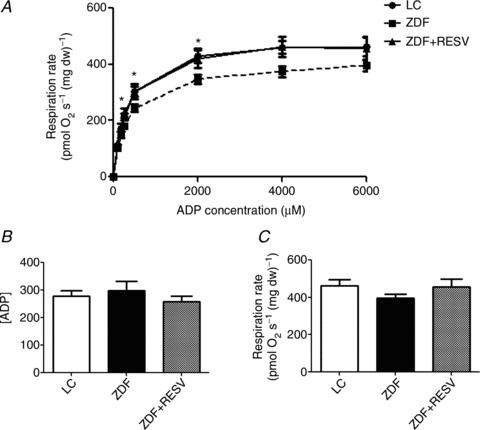
A, At ADP concentrations of 250, 500 and 2000 μm, ZDF respiration rates are significantly lower than both lean control (LC) and resveratrol-supplemented (ZDF+RESV) groups. B and C, apparent ADP Km (B) and Vmax (C) were not significantly different. Values represent means ± SEM; n= 13. *Significantly (P < 0.05) different from LC and ZDF+RESV.
These data are the first reporting submaximal ADP-stimulated respiration in a model of T2DM and suggest that mitochondrial dysfunction is present when assessed in this manner (Fig. 4), while electron transport capacity is not altered.
Lipid-supported respiration is elevated with resveratrol treatment
P-CoA- and palmitate-supported state III (5 mm ADP) respiration rates were not different between LC and ZDF (Fig. 5A and B). In contrast, state III P-CoA- (P= 0.08) and palmitate- (P < 0.05) supported respiration rates were higher in resveratrol-treated animals in the face of unchanged respiratory capacity (Fig. 5A and B).
Figure 5. State III fatty acid-supported respiration rates are elevated with resveratrol treatment.
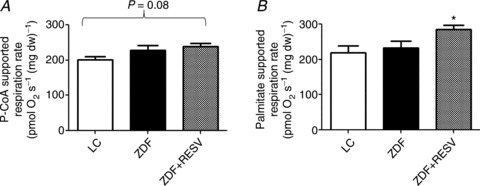
Resveratrol supplementation increased state III (5 mm ADP) palmitoyl-CoA (P-CoA, 150 μm) (A) and palmitate (250 μm) (B) supported respiration rates. Values represent means ± SEM; n= 8 (A), n= 5 (B). *Significantly (P < 0.05) different from LC.
Mitochondrial and ANT content
In ZDF rats treated with resveratrol there was no induction of mitochondrial biogenesis (representative blots shown in Fig. 6A) to account for the increased submaximal ADP-stimulated respiration observed in the current study. Considering submaximal ADP-stimulated respiration appears to be depressed in the ZDF rat and recovered with resveratrol supplementation, we next measured the protein expression of ANT1 and 2 to investigate if these differences in ADP-stimulated respiration were affiliated with the content of ADP transport proteins. ANT1 protein abundance was not different across the three groups (Fig. 6B). In contrast, ANT2 protein content was significantly reduced in the ZDF group compared to LC (−34%; Fig. 6C) and following resveratrol supplementation, ANT2 protein content was not different from LC values. The increase in ANT2 protein content may help account for the improved submaximal ADP-stimulated respiration observed with resveratrol supplementation.
Mitochondrial H2O2 emission and cellular redox state
We next investigated a potential mechanism of action to account for how submaximal ADP-stimulated respiration could be related to skeletal muscle insulin sensitivity. Mitochondrial-derived ROS have been linked to insulin resistance and the provision of ADP to mitochondria can decrease ROS production (Korshunov et al. 1997; Anderson et al. 2009). Therefore, we examined mitochondrial H2O2 emission rates (a marker of mitochondrial ROS production) in the presence and absence of a submaximal ADP concentration (100 μm). Between groups, the maximal capacity of mitochondria to emit H2O2 was not different (Fig. 7A). In contrast, in the presence of a submaximal ADP concentration, H2O2 emission rates were ∼34% higher in the ZDF group compared to LC (P= 0.06; Fig. 7B). Following treatment with resveratrol, H2O2 emission rates were ∼40% lower (P < 0.05) in the ZDF+RESV group compared to ZDF (Fig. 7B). These data suggest that resveratrol treatment may improve skeletal muscle insulin sensitivity by enhancing ADP transport, which results in a decrease in mitochondrial ROS production. To determine if these changes in mitochondrial H2O2 emission rates were associated with the cellular redox state, we measured the ratio of reduced glutathione to oxidized glutathione (GSH/GSSG ratio). The LC animals surprisingly had the lowest GSH/GSSG ratios, suggesting they had the greatest oxidative stress despite not being overfed (Table 2). It may be that ZDF animals have evolved compensatory adaptations to accommodate the chronic fuel oversupply. Nonetheless, resveratrol treatment increased the GSH/GSSG ratio, independent of altering total GSH content within the ZDF animal, suggesting that the skeletal muscle of the ZDF+RESV group was less oxidized than that of the ZDF group, supporting our functional PmFB data. Together, these data suggest a potential link between mitochondrial function and mitochondrial ROS production in the aetiology of skeletal muscle insulin resistance.
Figure 7. In ZDF rats, supplementation with resveratrol decreases the propensity for H2O2 emission in the presence of 100 μm ADP.
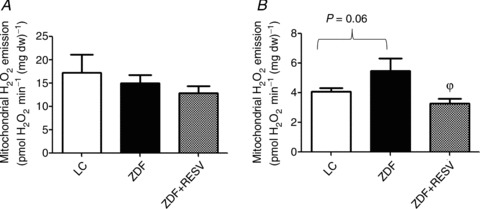
A, the maximal capacity of the mitochondria to emit H2O2 was not different between lean controls (LC), ZDF control (ZDF) and ZDF supplemented with resveratrol (ZDF+RESV). B, in the presence of 100 μm ADP, the capacity of the mitochondria to emit H2O2 was significantly lower in the ZDF+RESV group compared to ZDF. n= 13 (A) and n= 5 (B). All H2O2 emission measurements were done in duplicate. ϕSignificantly (P < 0.05) different from ZDF.
Table 2.
Cellular redox status
| GSH | GSSG | GSH/GSSG | |
|---|---|---|---|
| LC | 6.03 ± 0.44 | 0.15 ± 0.01 | 40.6 ± 2.51 |
| ZDF | 6.46 ± 0.53 | 0.11 ± 0.02 | 64.1 ± 7.95 |
| ZDF+RESV | 6.72 ± 0.62 | 0.08 ± 0.01 | 86.1 ± 13.3*φ |
ZDF rats supplemented with resveratrol (ZDF+RESV) had a higher reduced glutathione (GSH)/oxidized glutathione (GSSG) ratio compared to ZDF and LC. Values represent means ± SEM; n= 8. *Significantly (P < 0.05) different from LC. φSignificantly (P < 0.05) different from ZDF.
Discussion
In the current study we provide evidence for a potential link between mitochondrial function and ROS emission in the context of skeletal muscle insulin sensitivity. Specifically, we show that mitochondrial respiration at submaximal ADP concentrations is impaired in a model of T2DM, an observation that coincided with decreased ANT2 protein content and a decreased ability of ADP to attenuate mitochondrial ROS emission. We further show that resveratrol treatment in ZDF rats recovers skeletal muscle insulin sensitivity, submaximal ADP-stimulated respiration and ANT2 protein content in concert with improving ADP attenuation of mitochondrial ROS and cellular redox balance (GSH/GSSG ratio). Together, our data suggest a novel link between mitochondrial function, ROS emission and skeletal muscle insulin sensitivity, and places a new emphasis on ANT2 protein content as a potential regulator of insulin sensitivity.
Mitochondrial dysfunction in T2DM
There is considerable controversy surrounding the notion of mitochondrial dysfunction as a cause of insulin resistance (Kelley et al. 2002; Boushel et al. 2007; Mogensen et al. 2007; Phielix et al. 2008; de Feyter et al. 2008a,b; Holloszy, 2009; Holloway et al. 2010; Lenaers et al. 2010; Hoeks & Schrauwen, 2012), which may reflect differences in methodologies and experimental approaches. In the current study, there were no differences in maximal ADP-stimulated respiration, supporting three previous studies in ZDF rats that utilized in vivo phosphorous magnetic resonance spectroscopy (P-MRS) and ex vivo determinations of mitochondrial function (de Feyter et al. 2008a; Lenaers et al. 2010; Holloway et al. 2010). In contrast, titrating ADP revealed attenuated respiration rates in ZDF animals at submaximal ADP concentrations. Therefore, we suggest that mitochondrial dysfunction, as defined by a decrease in submaximal ADP-stimulated respiration, is associated with the progression of T2DM. While this method of ADP titration has not previously been utilized to study skeletal muscle mitochondrial function in insulin-resistant muscle, another research group has utilized titrations of various substrates in human muscle from type II diabetics (Larsen et al. 2011). In their study, Larsen et al. (2011) unexpectedly found that complex I and complex II sensitivity were increased in humans with T2DM. Within the context of mitochondrial ROS production, increased mitochondrial substrate sensitivity may be detrimental as proton motive force would be generated more rapidly per reducing equivalent supplied, which would be exacerbated in the presence of attenuated submaximal ADP transport. Clearly, future work should examine submaximal ADP-stimulated respiration in humans with T2DM to address this speculation.
Resveratrol and mitochondrial fatty acid-supported respiration
Following resveratrol supplementation, we observed an increase in maximal fatty acid-supported respiration (both P-CoA and palmitate) in the face of unchanged respiratory capacity. These data match previous work in resveratrol-supplemented humans examining mitochondrial bioenergetics utilizing PmFBs (Timmers et al. 2011). They observed that octanoylcarnitine-supported respiration and citrate synthase activity were elevated while respiratory capacity was unchanged in obese humans following 30 days of resveratrol supplementation (Timmers et al. 2011). Octanoylcarnitine bypasses carnitine palmitoyltransferase I (CPT-I) and therefore has direct access to the mitochondrial matrix where beta oxidation takes place (Noland et al. 2007). Considering that all fatty acid-supported (palmitate, P-CoA and octanoylcarnitine) respiration rates increase with resveratrol supplementation, resveratrol appears to influence fatty acid oxidation independent of CPT-I. Although CPT-I has traditionally been viewed as the primary rate-limiting step for mitochondrial fatty acid oxidation (Shepherd et al. 1966), an earlier study pointed out that tissue acylcarnitine levels are increased under conditions known to involve increased rates of fatty acid oxidation (Bremer & Norum, 1967). The increase in acylcarnitine concentration suggests that the rate-limiting step must be downstream of CPT-I because if CPT-I was rate-limiting there would be no excess substrate to undergo the carnitine acetyltransferase (CrAT) reaction to produce the acylcarnitines. Therefore, altered regulation of fatty acid oxidation downstream of CPT-I (potentially beta oxidation) is likely with resveratrol supplementation and has been previously observed (Chen et al. 2011). Classically, the regulation of beta oxidation has been attributed to the balance of substrates and products, although beta oxidation can also be regulated by the cellular redox state (Eaton, 2002), suggesting a possible mechanism by which resveratrol could promote fatty acid oxidation independent of CPT-I.
Proton leak and post-translational modifications of ANT
ANT has previously been suggested to account for 50–66% of basal proton leak and it has been speculated that an increase in ANT protein could lower ROS emission through the dissipation of proton motive force (Brand et al. 2005). However, in the current study, non ADP-stimulated respiration (state IV) was unchanged following resveratrol administration despite increased ANT2 protein content. These data suggest that ANT protein does not independently determine basal proton leak. To explain this discrepancy, we hypothesize that post-translational regulation of ANT may induce the uncoupling function of ANT similar to the mechanism surrounding the induction of UCP2- and UCP3-mediated uncoupling (Mailloux et al. 2011). Indeed, considering the critical function ANT has in regulating energy homeostasis, it is not surprising that numerous studies have observed extensive post-translational regulation of ANT function. Evidence now exists that ANT1 can be phosphorylated on tyrosine 194 and phosphorylation is associated with an increase in activity (Feng et al. 2008). Exposure to high oxygen tension or oxidative stress-induced glutathionylation result in decreased ADP transport into the mitochondrial matrix (Yan & Sohal, 1998; Queiroga et al. 2010). Additionally, long-chain fatty acyl-CoA moieties can directly suppress ANT function (Ho & Pande, 1974). While these data indicate that ANT function can be regulated, this does not diminish the physiological implications of increasing ANT2 protein in the current study. Instead, the improvements in cellular redox balance and rates of fatty acid-supported respiration following resveratrol supplementation probably further improve ANT function as resveratrol has been linked to reducing oxidative stress and increasing fatty acid metabolism (current study and others (Chen et al. 2011; Timmers et al. 2011)).
ANT content, oxidative stress and insulin resistance
Taking into account our finding that submaximal ADP-stimulated respiration is depressed in ZDF rats, we attempted to identify a mechanism by which ADP dynamics could be related to insulin resistance. Previous work has highlighted a link between skeletal muscle oxidative stress and insulin sensitivity, as mitochondrial-targeted antioxidant interventions and genetic approaches that over-express antioxidant enzymes within mitochondria protect against diet-induced skeletal muscle insulin resistance (Anderson et al. 2009; Boden et al. 2012). Our work extends these findings by demonstrating that improved ADP dynamics are associated with decreased H2O2 emission and improved cellular redox state, which are all associated with improved skeletal muscle insulin sensitivity. Our speculative interpretation is as follows: increasing ANT2 expression generates an environment whereby mitochondrial ROS emission is mitigated at a faster rate in the presence of low ADP concentrations. Considering ADP levels in skeletal muscle are low in the absence of exercise, an increase in ANT content and the resultant augmented ADP sensitivity would protect against ROS-induced insulin resistance (Brownlee, 2001; Houstis et al. 2006; Anderson et al. 2009). To further support our hypothesis that ADP dynamics are linked to ROS production and therefore insulin resistance, previous work in a liver cell line has shown that over-expression of ANT2 decreases oxidative stress and enhances mitochondrial function (Kim et al. 2012). Additionally, mitochondria isolated from ANT1-deficient mice produce greater amounts of ROS, resulting in damaged mtDNA and cellular dysfunction (Graham et al. 1997), while in cardiac tissue, over-expression of ANT1 protects against the development of diabetes-induced cardiomyopathy (Wang et al. 2009). Together, we speculate a mechanism whereby improved ADP dynamics decrease mitochondrial ROS emission and maintain cellular redox balance, resulting in enhanced insulin sensitivity. While ZDF animals share a number of similar traits with human T2DM, including hyperglycaemia, insulin resistance, hyperlipidaemia (Greene et al. 1994) and elevated acylcarnitines (Mihalik et al. 2010), we recognize that the ZDF animal model is not fully representative. This is apparent as the GSH/GSSG ratio is higher in ZDF animals than in LC animals while in insulin-resistant and type II diabetic humans a reduction in this ratio has been reported (Anderson et al. 2009). Previous studies showing a protection of insulin sensitivity by mitigating mitochondrial ROS production have utilized acute (6 h; Hoehn et al. 2009) or short-term (4–6 weeks; Houstis et al. 2006; Anderson et al. 2009) antioxidant interventions. However, a recent study has divorced the relationship between the oxidative state of the muscle and insulin sensitivity when treating with a mitochondrial-targeted antioxidant chronically (16 weeks; Paglialunga et al. 2012). Therefore, our work, in combination with others (Houstis et al. 2006; Anderson et al. 2009; Hoehn et al. 2009) may suggest that ROS is important as an early (4–6 weeks) sensor of fuel oversupply to induce insulin resistance, however this mechanism becomes disconnected from insulin sensitivity by 16 weeks (Paglialunga et al. 2012). Nonetheless, resveratrol improved the redox state within the ZDF animal concomitantly with improved skeletal muscle glucose uptake. Therefore, it will be important to discern whether our current observations regarding ADP dynamics in ZDF animals extend to human type II diabetic individuals.
Conclusion
In summary, our data suggest that mitochondrial dysfunction is present in skeletal muscle insulin resistance when assessed at submaximal ADP concentrations and that ADP dynamics may influence skeletal muscle insulin sensitivity through alterations in the propensity for mitochondrial ROS emission. We found that submaximal ADP-stimulated respiration is impaired in ZDF animals and resveratrol supplementation improved submaximal ADP-stimulated respiration concomitantly with enhancing insulin sensitivity. The favourable effects of resveratrol may be mediated through an improved ability of ADP to attenuate mitochondrial ROS emission.
Acknowledgments
None declared.
Glossary
Abbreviations
- ANT
adenine nucleotide translocase
- CPT-I
carnitine palmitoyltransferase I
- FFA
free fatty acid
- LC
lean control
- P-CoA
palmitoyl-coenzyme A
- PmFB
permeabilzed muscle fibre bundle
- ROS
reactive oxygen species
- T2DM
type 2 diabetes mellitus
- ZDF
Zucker diabetic fatty
Additional information
Competing interests
None declared.
Author contributions
B.K.S., C.G.R.P., D.C.W. and G.P.H. designed experiments, performed experiments, analysed and interpreted data and wrote the manuscript. E.A.F.H., I.R.R. and M.S.B. performed experiments and edited the manuscript. P.D.N. and J.C.S. interpreted data and edited the manuscript.
Funding
This work was funded by The Natural Sciences and Engineering Research Council of Canada (NSERC; G.P.H. and J.C.S.) and CIHR (D.C.W.) and infrastructure was purchased with the assistance of the Canadian Foundation for Innovation (G.P.H., J.C.S. and D.C.W.) as well as the Ontario Research Fund (G.P.H., J.C.S. and D.C.W.). B.K.S. is supported by an NSERC graduate scholarship and D.C.W. is a Canada Research Chair. Funding from Carleton University (J.C.S.) is acknowledged. Additional support was provided by grants from the United States Public Health Service: [R01 DK073488] and [DK074825] (P.D.N.).
References
- Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, III, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119:573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le CD, Shaw RJ, Navas P, Puigserver P, Ingram DK, de CR, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden MJ, Brandon AE, Tid-Ang JD, Preston E, Wilks D, Stuart E, Cleasby ME, Turner N, Cooney GJ, Kraegen EW. Overexpression of manganese superoxide dismutase ameliorates high-fat diet-induced insulin resistance in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2012;303:E798–805. doi: 10.1152/ajpendo.00577.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsoe R, Dela F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia. 2007;50:790–796. doi: 10.1007/s00125-007-0594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD, Pakay JL, Ocloo A, Kokoszka J, Wallace DC, Brookes PS, Cornwall EJ. The basal proton conductance of mitochondria depends on adenine nucleotide translocase content. Biochem J. 2005;392:353–362. doi: 10.1042/BJ20050890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer J, Norum KR. The mechanism of substrate inhibition of palmityl coenzyme A: carnitine palmityltransferase by palmityl coenzyme A. J Biol Chem. 1967;242:1744–1748. [PubMed] [Google Scholar]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Chen L, Na R, Gu M, Salmon AB, Liu Y, Liang H, Qi W, Van RH, Richardson A, Ran Q. Reduction of mitochondrial H2O2 by overexpressing peroxiredoxin 3 improves glucose tolerance in mice. Aging Cell. 2008;7:866–878. doi: 10.1111/j.1474-9726.2008.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL, Zhang HH, Zheng J, Hu X, Kong W, Hu D, Wang SX, Zhang P. Resveratrol attenuates high-fat diet-induced insulin resistance by influencing skeletal muscle lipid transport and subsarcolemmal mitochondrial β-oxidation. Metabolism. 2011;60:1598–1609. doi: 10.1016/j.metabol.2011.04.002. [DOI] [PubMed] [Google Scholar]
- de Feyter HM, Lenaers E, Houten SM, Schrauwen P, Hesselink MK, Wanders RJ, Nicolay K, Prompers JJ. Increased intramyocellular lipid content but normal skeletal muscle mitochondrial oxidative capacity throughout the pathogenesis of type 2 diabetes. FASEB J. 2008a;22:3947–3955. doi: 10.1096/fj.08-112318. [DOI] [PubMed] [Google Scholar]
- de Feyter HM, van den Broek NM, Praet SF, Nicolay K, van Loon LJ, Prompers JJ. Early or advanced stage type 2 diabetes is not accompanied by in vivo skeletal muscle mitochondrial dysfunction. Eur J Endocrinol. 2008b;158:643–653. doi: 10.1530/EJE-07-0756. [DOI] [PubMed] [Google Scholar]
- Dorner A, Giessen S, Gaub R, Grosse SH, Schwimmbeck PL, Hetzer R, Poller W, Schultheiss HP. An isoform shift in the cardiac adenine nucleotide translocase expression alters the kinetic properties of the carrier in dilated cardiomyopathy. Eur J Heart Fail. 2006;8:81–89. doi: 10.1016/j.ejheart.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Eaton S. Control of mitochondrial β-oxidation flux. Prog Lipid Res. 2002;41:197–239. doi: 10.1016/s0163-7827(01)00024-8. [DOI] [PubMed] [Google Scholar]
- Feng J, Zhu M, Schaub MC, Gehrig P, Roschitzki B, Lucchinetti E, Zaugg M. Phosphoproteome analysis of isoflurane-protected heart mitochondria: phosphorylation of adenine nucleotide translocator-1 on Tyr194 regulates mitochondrial function. Cardiovasc Res. 2008;80:20–29. doi: 10.1093/cvr/cvn161. [DOI] [PubMed] [Google Scholar]
- Graham BH, Waymire KG, Cottrell B, Trounce IA, MacGregor GR, Wallace DC. A mouse model for mitochondrial myopathy and cardiomyopathy resulting from a deficiency in the heart/muscle isoform of the adenine nucleotide translocator. Nat Genet. 1997;16:226–234. doi: 10.1038/ng0797-226. [DOI] [PubMed] [Google Scholar]
- Greene SF, Johnson PR, Eiffert KC, Greenwoodt MR, Stern JS. The male obese Wistar diabetic fatty rat is a new model of extreme insulin resistance. Obes Res. 1994;2:432–443. doi: 10.1002/j.1550-8528.1994.tb00090.x. [DOI] [PubMed] [Google Scholar]
- Ho CH, Pande SV. On the specificity of the inhibition of adenine nucleotide translocase by long chain acyl-coenzyme A esters. Biochim Biophys Acta. 1974;369:86–94. doi: 10.1016/0005-2760(74)90195-7. [DOI] [PubMed] [Google Scholar]
- Hoehn KL, Salmon AB, Hohnen-Behrens C, Turner N, Hoy AJ, Maghzal GJ, Stocker R, Van RH, Kraegen EW, Cooney GJ, Richardson AR, James DE. Insulin resistance is a cellular antioxidant defense mechanism. Proc Natl Acad Sci U S A. 2009;106:17787–17792. doi: 10.1073/pnas.0902380106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeks J, Schrauwen P. Muscle mitochondria and insulin resistance: a human perspective. Trends Endocrinol Metab. 2012;23:444–450. doi: 10.1016/j.tem.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Holloszy JO. Skeletal muscle “mitochondrial deficiency” does not mediate insulin resistance. Am J Clin Nutr. 2009;89:463S–466S. doi: 10.3945/ajcn.2008.26717C. [DOI] [PubMed] [Google Scholar]
- Holloway GP, Gurd BJ, Snook LA, Lally J, Bonen A. Compensatory increases in nuclear PGC1α protein are primarily associated with subsarcolemmal mitochondrial adaptations in ZDF rats. Diabetes. 2010;59:819–828. doi: 10.2337/db09-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- Kim HS, Je JH, Son TG, Park HR, Ji ST, Pokharel YR, Jeon HM, Kang KW, Kang HS, Chang SC, Kim HS, Chung HY, Lee J. The hepatoprotective effects of adenine nucleotide translocator-2 against aging and oxidative stress. Free Radic Res. 2012;46:21–29. doi: 10.3109/10715762.2011.636042. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. The ADP and ATP transport in mitochondria and its carrier. Biochim Biophys Acta. 2008;1778:1978–2021. doi: 10.1016/j.bbamem.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Larsen S, Stride N, Hey-Mogensen M, Hansen CN, Andersen JL, Madsbad S, Worm D, Helge JW, Dela F. Increased mitochondrial substrate sensitivity in skeletal muscle of patients with type 2 diabetes. Diabetologia. 2011;54:1427–1436. doi: 10.1007/s00125-011-2098-4. [DOI] [PubMed] [Google Scholar]
- Lee HY, Choi CS, Birkenfeld AL, Alves TC, Jornayvaz FR, Jurczak MJ, Zhang D, Woo DK, Shadel GS, Ladiges W, Rabinovitch PS, Santos JH, Petersen KF, Samuel VT, Shulman GI. Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell Metab. 2010;12:668–674. doi: 10.1016/j.cmet.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaers E, de Feyter HM, Hoeks J, Schrauwen P, Schaart G, Nabben M, Nicolay K, Prompers JJ, Hesselink MK. Adaptations in mitochondrial function parallel, but fail to rescue, the transition to severe hyperglycemia and hyperinsulinemia: a study in Zucker diabetic fatty rats. Obesity (Silver Spring) 2010;18:1100–1107. doi: 10.1038/oby.2009.372. [DOI] [PubMed] [Google Scholar]
- Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- Mailloux RJ, Seifert EL, Bouillaud F, Aguer C, Collins S, Harper ME. Glutathionylation acts as a control switch for uncoupling proteins UCP2 and UCP3. J Biol Chem. 2011;286:21865–21875. doi: 10.1074/jbc.M111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalik SJ, Goodpaster BH, Kelley DE, Chace DH, Vockley J, Toledo FG, DeLany JP. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity (Silver Spring) 2010;18:1695–1700. doi: 10.1038/oby.2009.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen M, Sahlin K, Fernstrom M, Glintborg D, Vind BF, Beck-Nielsen H, Hojlund K. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes. 2007;56:1592–1599. doi: 10.2337/db06-0981. [DOI] [PubMed] [Google Scholar]
- Noland RC, Woodlief TL, Whitfield BR, Manning SM, Evans JR, Dudek RW, Lust RM, Cortright RN. Peroxisomal-mitochondrial oxidation in a rodent model of obesity-associated insulin resistance. Am J Physiol Endocrinol Metab. 2007;293:E986–1001. doi: 10.1152/ajpendo.00399.2006. [DOI] [PubMed] [Google Scholar]
- Paglialunga S, van Bree B, Bosma M, Valdecantos MP, Mengual-Cladera E, Jorgensen JA, van Buerden D, den Hartog GJ, Ouwens DM, Briede JJ, Schrauwen P, Hoeks J. Targeting of mitochondrial reactive oxygen species production does not avert lipid-induced insulin resistance in muscle tissue from mice. Diabetologia. 2012;55:2759–2768. doi: 10.1007/s00125-012-2626-x. [DOI] [PubMed] [Google Scholar]
- Perry CG, Kane DA, Herbst EA, Mukai K, Lark DS, Wright DC, Heigenhauser GJ, Neufer PD, Spriet LL, Holloway GP. Mitochondrial creatine kinase activity and phosphate shuttling are acutely regulated by exercise in human skeletal muscle. J Physiol. 2012;590:5475–5486. doi: 10.1113/jphysiol.2012.234682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry CG, Kane DA, Lin CT, Kozy R, Cathey BL, Lark DS, Kane CL, Brophy PM, Gavin TP, Anderson EJ, Neufer PD. Inhibiting myosin-ATPase reveals dynamic range of mitochondrial respiratory control in skeletal muscle. Biochem J. 2011;437:215–222. doi: 10.1042/BJ20110366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff E, Klingenberg M, Heldt HW. Unspecific permeation and specific exchange of adenine nucleotides in liver mitochondria. Biochim Biophys Acta. 1965;104:312–315. doi: 10.1016/0304-4165(65)90258-8. [DOI] [PubMed] [Google Scholar]
- Phielix E, Schrauwen-Hinderling VB, Mensink M, Lenaers E, Meex R, Hoeks J, Kooi ME, Moonen-Kornips E, Sels JP, Hesselink MK, Schrauwen P. Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes. 2008;57:2943–2949. doi: 10.2337/db08-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queiroga CS, Almeida AS, Martel C, Brenner C, Alves PM, Vieira HL. Glutathionylation of adenine nucleotide translocase induced by carbon monoxide prevents mitochondrial membrane permeabilization and apoptosis. J Biol Chem. 2010;285:17077–17088. doi: 10.1074/jbc.M109.065052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimbaud S, Ruiz M, Piquereau J, Mateo P, Fortin D, Veksler V, Garnier A, Ventura-Clapier R. Resveratrol improves survival, hemodynamics and energetics in a rat model of hypertension leading to heart failure. PLoS One. 2011;6:e26391. doi: 10.1371/journal.pone.0026391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie IR, Gulli RA, Stefanyk LE, Harasim E, Chabowski A, Dyck DJ. Restoration of skeletal muscle leptin response does not precede the exercise-induced recovery of insulin-stimulated glucose uptake in high-fat-fed rats. Am J Physiol Regul Integr Comp Physiol. 2011;300:R492–500. doi: 10.1152/ajpregu.00602.2010. [DOI] [PubMed] [Google Scholar]
- Seymour EM, Bennink MR, Watts SW, Bolling SF. Whole grape intake impacts cardiac peroxisome proliferator-activated receptor and nuclear factor κB activity and cytokine expression in rats with diastolic dysfunction. Hypertension. 2010;55:1179–1185. doi: 10.1161/HYPERTENSIONAHA.109.149393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd D, Yates DW, Garland PB. The rate-limiting step in the oxidation of palmitate or palmitoyl-coenzyme A by rat-liver mitochondria. Biochem J. 1966;98:3C–4C. doi: 10.1042/bj0980003c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BK, Jain SS, Rimbaud S, Dam A, Quadrilatero J, Ventura-Clapier R, Bonen A, Holloway GP. FAT/CD36 is located on the outer mitochondrial membrane, upstream of long-chain acyl-CoA synthetase, and regulates palmitate oxidation. Biochem J. 2011;437:125–134. doi: 10.1042/BJ20101861. [DOI] [PubMed] [Google Scholar]
- Suh YH, Kim Y, Bang JH, Choi KS, Lee JW, Kim WH, Oh TJ, An S, Jung MH. Analysis of gene expression profiles in insulin-sensitive tissues from pre-diabetic and diabetic Zucker diabetic fatty rats. J Mol Endocrinol. 2005;34:299–315. doi: 10.1677/jme.1.01679. [DOI] [PubMed] [Google Scholar]
- Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen-Kornips E, Hesselink MK, Kunz I, Schrauwen-Hinderling VB, Blaak EE, Auwerx J, Schrauwen P. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ebermann L, Sterner-Kock A, Wika S, Schultheiss HP, Dorner A, Walther T. Myocardial overexpression of adenine nucleotide translocase 1 ameliorates diabetic cardiomyopathy in mice. Exp Physiol. 2009;94:220–227. doi: 10.1113/expphysiol.2008.044800. [DOI] [PubMed] [Google Scholar]
- Wilson DF. Factors affecting the rate and energetics of mitochondrial oxidative phosphorylation. Med Sci Sports Exerc. 1994;26:37–43. [PubMed] [Google Scholar]
- Yan LJ, Sohal RS. Mitochondrial adenine nucleotide translocase is modified oxidatively during aging. Proc Natl Acad Sci U S A. 1998;95:12896–12901. doi: 10.1073/pnas.95.22.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]


