Abstract
Recent studies have suggested that centrally generated motor commands contribute to the perception of position and movement at the wrist, but not at the elbow. Because the wrist and elbow experiments used different methods, this study was designed to resolve the discrepancy. Two methods were used to test both the elbow and wrist (20 subjects each). For the wrist, subjects sat with their right arm strapped to a device that restricted movement to the wrist. Before each test, voluntary contraction of wrist flexor or extensor muscles controlled for muscle spindle thixotropy. After relaxation, the wrist was moved to a test angle. Position was indicated either with a pointer, or by matching with the contralateral wrist, under two conditions: when the reference wrist was relaxed or when its muscles were contracted isometrically (30% maximum). The elbow experiment used the same design to measure position sense in the passive elbow and with elbow muscles contracting (30% maximum). At the wrist when using a pointer, muscle contraction altered significantly the perceived wrist angle in the direction of contraction by 7 deg [3 deg, 12 deg] (mean [95% confidence interval]) with a flexor contraction and 8 deg [4 deg, 12 deg] with an extensor contraction. Similarly, in the wrist matching task, there was a change of 13 deg [9 deg, 16 deg] with a flexor contraction and 4 deg [1 deg, 8 deg] with an extensor contraction. In contrast, contraction of elbow flexors or extensors did not alter significantly the perceived position of the elbow, compared with rest. The contribution of central commands to position sense differs between the elbow and the wrist.
Key points
Knowing the position of our limbs is critical for accurate movement. Central motor command signals generated by the brain contribute to position sense at the human wrist, but this could not be demonstrated at the elbow.
We tested whether this represents a fundamental difference between the two joints or whether it reflects the two different methods used to measure position sense.
For both measurement methods, contraction of wrist muscles led to illusions that the wrist is displaced. No such illusions were detected at the elbow during muscle contraction.
Thus, the contribution of centrally generated command signals to position sense differs between joints. Any contribution at the elbow joint is small and new methods will be needed to reveal it.
Introduction
We are constantly aware of the position of our body in space because proprioceptors in our muscles and skin provide information about the position and movement of our limbs, as well as the forces involved in moving them. This information is not only important for self-awareness in our surroundings, it is also needed to control movements accurately. The key role played by proprioceptors in motor control is demonstrated in rare cases when proprioceptive afferents from muscle, joint and skin are lost and normal movement control is no longer possible (e.g. Cole, 1995; Hermsdorfer et al. 2008).
Although the question of which signals contribute to the sense of joint position and movement has been debated many times (e.g. McCloskey, 1978, 1981; Gandevia, 1996), the general view over the last 30–40 years has been that the sense is derived from information provided by peripheral receptors. A key study by Goodwin et al. (1972) showed that muscle spindles were important contributors to limb position sense. Skin stretch receptors also contribute (Edin & Johansson, 1995; Collins et al. 2005). Joint receptors are unlikely to be important, except as limit detectors of movement (Burgess & Clark, 1969). As well as peripheral signals, it has been proposed that central signals associated with motor commands can provide positional information. This concept dates back to von Helmholtz (1867), but remained controversial (e.g. McCloskey & Torda, 1975). By the mid-20th century the view emerged that central commands did not contribute directly to position sense and acted only to provide ‘corollary discharges’ (Sperry, 1950) or ‘efference copies’ (von Holst, 1954), signals that were used to distinguish between self-generated afferent activity arising from motor commands and activity generated by sources outside the body (for a review, see Proske & Gandevia, 2012).
In the last decade the role of centrally generated motor commands in limb position sense has been reassessed. It was believed that centrally generated signals contributed to position sense at the elbow. Supporting evidence came from experiments in which arm muscles were exercised to fatigue (Walsh et al. 2004, 2006; Allen & Proske, 2006). After elbow flexor force had fallen by 30%, in a forearm matching task, subjects felt the position of their fatigued arm to be more extended than it really was. At the time it was believed that the fatigued arm felt heavier and the extra effort required to support it against the force of gravity provided an additional position signal to generate the observed position errors. This explanation was subsequently shown not to be correct (Allen et al. 2007, 2010).
In order to determine whether an effort signal could contribute at other joints, a different type of experiment was performed at the wrist. Here, the subject's forearm, wrist and hand were paralysed and anaesthetised using an ischaemic pressure block. Subjects reported illusory changes in hand position (Gandevia et al. 2006) and movement when asked to make voluntary efforts with their paralysed wrist muscles (Walsh et al. 2010). Illusory changes in wrist angle were still present when the subject's arm was paralysed, but with intact sensory nerves, and comparable effects occurred at finger joints (Smith et al. 2009). These studies support the view that central motor command signals can contribute directly to limb position sense.
Other recent studies of position sense at the elbow in subjects with intact peripheral nerves have been unable to confirm this result. A study at the elbow under gravity-neutral conditions showed that, if the contraction history of elbow muscles was controlled, no effects of muscle contraction on position sense were detected (Ansems et al. 2006). The authors proposed that previous reports of effects of muscle contraction on forearm position sense in the vertical plane (see Winter et al. 2005) were not caused by a central command signal, but the result of the contraction altering spindle responses.
Skeletal muscle, including the intrafusal muscle fibres of spindles, has a property called thixotropy, a passive stiffness that depends on the past history of movement (Lakie et al. 1984). The biomechanical basis of thixotropy was provided by Hill (1968), who described the presence of long-term, stable bonds between actin and myosin in resting muscle. These bonds form, and if the muscle is subsequently stretched, they resist the stretch with stiffness and passive tension rises. If the stretch is large enough, the bonds detach and re-form at the longer length. When the muscle is subsequently shortened, the compressive forces acting on the bonds are insufficient to detach them, and so the muscle fibres are prevented from shortening and may fall slack. A slack fibre is one whose length is greater than the distance between the fibre's points of attachment (Proske et al. 1993). In other words, during lengthening and shortening movements, thixotropy assigns to muscle a hysteresis-like behaviour and, possibly, muscle may even become slack when shortened.
If a muscle is not deliberately conditioned, its thixotropic state remains unknown. For position sense at the elbow, if elbow muscles have not been conditioned, it is likely that, in the period immediately before the measurement, the arm would have been moved about without any accompanying contraction, leading to the development of slack in muscle fibres. Slack in muscle spindles lowers their firing rate (Morgan et al. 1984) and, for position sense at the elbow, this alters the perceived joint angle. In experiments by Winter et al. (2005), the elbow flexor contraction required to support the loaded arm against gravity would have removed any pre-existing slack, leading to an increase in muscle spindle firing rate. The resulting perceived change in elbow angle was mistakenly attributed to a central command signal.
The realisation that muscle thixotropy could alter the patterns of activity coming from muscle spindles was important for the study of human position sense (Gregory et al. 1988). At the elbow, when thixotropic effects had been carefully taken into account, there was no evidence for an influence from the sense of effort on forearm position sense (Allen et al. 2007, 2010). Despite this, evidence from the paralysed and anaesthetised wrist, as well as from subsequent experiments on the intact wrist, has shown that, even when muscle contraction history was controlled, contraction of wrist muscles still biased position sense (Walsh et al. 2009). That is, there was clear evidence for centrally generated signals contributing to position sense at the wrist.
We were therefore confronted by two different results, at two different joints, relating to a fundamental aspect of proprioception. It is important to resolve this issue, because it promises to advance our understanding of the role of centrally generated motor commands in proprioception. The question becomes: is limb position signalled not only by peripheral receptors, but by positional information arising from central command signals during voluntary contractions? Could it be that the reported differences between the wrist and elbow are simply caused by contraction history effects? Or, alternatively, is there a fundamental difference between proprioception at the two joints? In the present study, the two groups responsible for the differing outcomes collaborated to resolve these issues. Position sense was measured at both joints, in two ways, to take into account differences in measurement techniques used by each group. In one series, arm or wrist position was indicated by alignment of a pointer with the perceived position of the limb; in the other, the perceived position was indicated by placement of the other hand or arm in a matching task.
Methods
A total of 40 healthy subjects (15 men), aged 21–40 years, participated in the study. Twenty (nine men) participated in Experiment 1 and 20 (six men) participated in Experiment 2. All subjects gave informed written consent and this study was approved by the Human Research Ethics Committee at the University of New South Wales and the Human Ethics Committee at Monash University. The experimental procedures complied with the Declaration of Helsinki. The experimental protocol was fully explained to the subjects, but they were not aware of the experimental hypothesis. The authors were not subjects in the study.
Experiment 1 – the wrist
The experiments used two methods of measuring position sense, a pointing task and a matching task. These two methods were chosen in order to precisely replicate the conditions of measurements made previously at the elbow (Ansems et al. 2006; Allen et al. 2010) and at the wrist (Gandevia et al. 2006; Smith et al. 2009; Walsh et al. 2010). Each task was performed on a different day, with half the subjects performing the pointing task first. For both tasks, the subject's right arm was strapped to a table and the hand was held, with fingers straight, in a manipulandum that restricted movement to flexion and extension of the wrist (Fig. 1A).
Figure 1. The experimental setup.
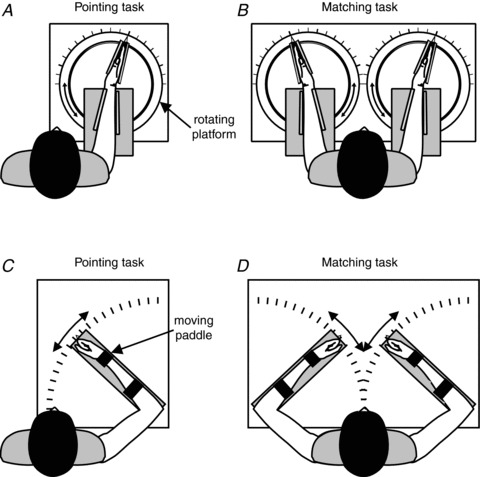
For wrist tasks A and B, the subject sat at the table with the right forearm strapped to the table and the hand clamped in a manipulandum that was connected to a rotating platform. This restricted movement to flexion and extension of the wrist joint. For the pointing task A, the subject's right hand was covered with a second table that included a pointer with an axis co-linear to the right wrist joint. The subject moved the pointer with the left hand to indicate the perceived position of the right wrist. The rotating platform could be locked to allow the subject to make voluntary isometric contractions. The matching task B differed from the pointing task in that the subject's left forearm and hand were restrained in the same way as the right forearm and hand. Instead of using a pointer with the left hand, the subject matched the perceived position of the right wrist by moving the left wrist. The subject's arms were concealed from view by a second table. The setup for the elbow tasks C and D was similar to that for the wrist tasks, except that the subject's arm (or arms for the matching task) was strapped to paddles that restricted movement to flexion and extension of the elbow joint. The pointer for the pointing task had an axis co-linear with the elbow joint. As for the wrist, the right arm was covered for the pointing task and both arms were covered for the matching task.
Pointing task
In the session using the pointing task, the subject's right arm was covered with a 6 mm sheet of medium-density fibre board and the subject had a pointer with an axis co-linear with the right wrist joint. The pointer was operated with the left hand to indicate the perceived angle of the right wrist. At the start of the session, the subject performed maximum voluntary contractions (MVCs) with both the right wrist extensor muscles and the right wrist flexor muscles. Two MVCs were performed with each of these muscle groups, and the higher value was taken as the maximum and used to calculate the target voluntary contractions. All wrist MVCs were performed with the wrist held out straight from the forearm. Electromyographic (EMG) activity was monitored in the wrist flexors and extensors of the right arm using silver–silver chloride surface electrodes (band-pass filtered 16–1000 Hz) and a CED 1902 amplifier (Cambridge Electronic Design, Cambridge, UK). EMG signals were used to ensure that the subject remained relaxed when required. The position of the pointer was measured using a potentiometer that was coupled to the shaft of the pointer. The potentiometer output was sampled at 100 Hz using a CED Power 1401 (Cambridge Electronic Design). When calibrated, the signal-to-noise ratio allowed for the accurate measurement of pointer angles as small as 0.2 deg.
Each experimental trial began with the subject performing a conditioning contraction with either the wrist flexor or extensor muscles. These contractions controlled the effect of muscle thixotropy on muscle spindle discharges and put the proprioceptive input from wrist muscles in a defined state (for a review, see Proske et al. 1993). These conditioning contractions (50% MVC) were performed with the muscle at a short length. So, if the wrist flexors were conditioned, the contraction was performed with the wrist fully flexed, and the conditioning contraction of the wrist extensors was performed with the wrist fully extended. Conditioning contractions were of 5 s duration and then the subject was told to relax. The trial did not continue until at least 10 s later and when the EMG activity in wrist muscles had subsided. With the subject relaxed, the experimenter turned the rotating platform to which the hand had been strapped (Fig. 1) to a test position. Then, in half the trials, the subject was told to ‘show me where your wrist is’, and used the left hand to move the pointer to indicate the position of the right wrist. In the other half of the trials, the subject generated an isometric contraction at the test position with the same muscle group that had performed the conditioning contraction. This contraction was 30% of maximum and the subject was shown the required force with a cursor on an oscilloscope screen. The subject was required to hold the target force, and then the experimenter said ‘show me where your wrist is’, and the subject used the pointer to indicate the position of the wrist whilst it was contracting. Once the wrist position had been signalled, the subject was told to relax and the trial was over.
Two test angles were used, −15 deg and 15 deg, where 0 deg represented a straight wrist with the hand in line with the forearm. We used the convention that angles in the direction of flexion were negative and angles in the direction of extension were positive. Therefore, −15 deg meant that the hand had been flexed by 15 deg from the position at which it had been aligned with the forearm. Similarly, +15 deg meant that the hand had been extended by 15 deg. There were two types of muscle contraction, flexor or extensor, and there were rest trials and contraction trials. This gave eight different trials which were repeated five times in random order.
Matching task
This was similar to the pointing task. The main difference was that both arms were strapped to the table and both hands were held by a manipulandum that restricted movement to flexion and extension at the wrist (Fig. 1B). Both arms were covered and, instead of using a pointer to indicate the position of the right wrist, the subject matched its position by placement of the left wrist. The position of the left wrist was measured with a potentiometer in a similar way to the pointer used in the pointing task. This potentiometer was coupled to the shaft of the rotating platform that was holding the left wrist. Its setup, calibration and accuracy were the same as for the pointer. The experimental trials were performed as for the pointing task, except that the 50% of maximum conditioning contraction was performed with both arms at the same time. So, if the subject was instructed to contract the wrist flexors to 50% MVC, both wrist flexors were contracted together for 5 s. After at least 10 s, when the subject had relaxed and the EMG activity was silent, the right wrist was moved to the test position. If it was a rest trial, the subject was then told to ‘match’, that is, indicate the perceived position of the right wrist by placement of the left wrist in a matching position. In the contraction trials, the subject performed a 30% MVC at the test angle with wrist flexors or extensors. Once the target force had been reached, the subject moved the left, non-contracting wrist to match the position of the right wrist, whilst continuing to contract the right wrist muscles. The subject indicated verbally when the match had been made and was then told to relax and the trial was over. As for the pointing task, there were two test angles (−15 deg and 15o), two types of muscle contraction (flexion or extension) and two force levels (rest or contract) resulting in eight different trials. Each was presented five times in random order.
Experiment 2 – the elbow
The elbow experiment followed the same format as the wrist experiment. There were two sessions performed by each subject, the pointing task and the matching task. Each task was performed on a different day with half of the subjects doing the pointing task first. The key difference from the first experiment was the test joint. In Experiment 2 the arm was strapped to a manipulandum which restricted movement of the arm, in the horizontal plane, to flexion or extension at the elbow joint. (Fig. 1C, D).
Pointing task
Here, the right arm was strapped to the manipulandum (Fig. 1C) and then covered with a sheet of 6 mm medium-density fibreboard. The subject was provided with a pointer that was hinged at a position co-linear with the elbow joint of the right arm, and used the left hand to move this pointer to indicate the elbow angle. The rest of the experiment, including the form of muscle conditioning, was the same as for the pointing task in Experiment 1, except that the contractions were carried out by elbow flexors and extensors. So, to condition elbow flexors, the arm was fully flexed (elbow angle <30 deg, with 180 deg being a straight arm) and the subject was asked to contract the arm in the direction of flexion. To condition the extensors, the arm was extended (elbow angle >150 deg) and the subject was asked to contract the arm in the direction of extension. EMG activity was monitored from the elbow flexors and extensors to ensure that the subject complied with the subsequent instruction to relax. Conditioning contractions were 50% of maximum and the test contractions were 30% of maximum. As for the wrist, there were eight trial types. They were repeated five times and presented in a random order.
Matching task
As for the pointing task, this mimicked the matching task from Experiment 1. Both of the subject's arms were strapped to a manipulandum and then covered (Fig. 1D). The position of the right elbow was indicated by matching its position using the left elbow. A potentiometer was not used to measure the left elbow angle. Instead, a calibrated protractor was printed on the table under the left forearm. A pointer from the manipulandum that held the left arm indicated the left elbow angle on this protractor. This method allowed elbow angles of 0.25 deg to be measured accurately. In all other respects the experiment was the same as for the wrist.
Data analysis
Data for single subjects (Fig. 2) and individual trials (Fig. 3) are presented raw with no processing or transformation. ‘Error’ data (Figs 4 and 5) were calculated by subtraction of the reference angle from the indicator angle for each trial to produce a difference, or error value. In the pointing task the indicator angle was the angle at which the subject placed the pointer and the reference angle was the angle of the right joint, positioned by the experimenter. For the matching task the indicator angle was the angle of the matching joint (left limb) that the subject moved to complete the matching task. The reference angle was the angle of the right joint that the experimenter had set at the start of the trial.
Figure 2. Data from a single subject for the pointing task at the wrist joint.
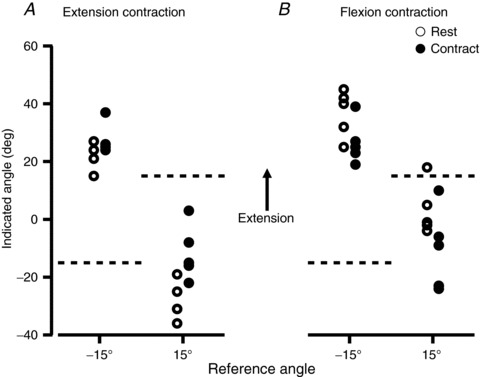
Each point shows an individual trial. Subjects were tested at two reference wrist angles, −15 deg and +15 deg, with 0 deg defined as the angle at which the wrist was straight from the forearm. We used the convention that angles into flexion were assigned negative values and angles into extension positive values. Therefore, a test angle of −15 deg meant that the reference wrist was flexed by 15 deg. The reference angle is shown at the bottom of the figure and as a dashed line on the ordinate. Open circles represent ‘Rest’ trials, where the position of the relaxed hand was indicated. Filled circles are ‘Contract’ trials, where the wrist flexors or extensors were contracting isometrically (30% maximum) whilst the wrist position was being indicated. A, values for which, before the measurement, wrist extensor muscles had been conditioned with a contraction. B, values for which wrist flexors had been conditioned. In both A and B, the muscle group that was used for conditioning was also used to generate the isometric contraction.
Figure 3. Group data from 20 subjects for the pointing task at the wrist joint.
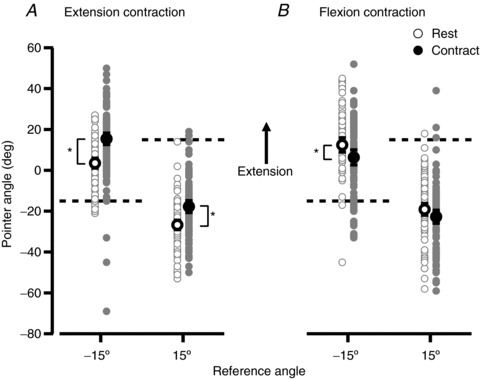
Grey points show individual measurements and black points show the mean (± 95% confidence interval). Open circles show data for the ‘Rest’ condition, where the position of the relaxed hand was indicated. Filled circles show data for ‘Contract’ trials, where the hand was generating an isometric contraction (30% maximum) whilst its position was being indicated. Test angles of −15 deg and +15 deg were used and are indicated by the dashed lines. A, data from trials in which the wrist extensors were conditioned at the start of the trial and the Contract trial used an extensor contraction. B, data from trials in which the wrist flexors were conditioned at the start of the trial and the Contract trial used a flexor contraction. *indicates a pair of means that are significantly different from each other (P < 0.05).
Figure 4. Mean perceived wrist angles for the group, expressed as an error.
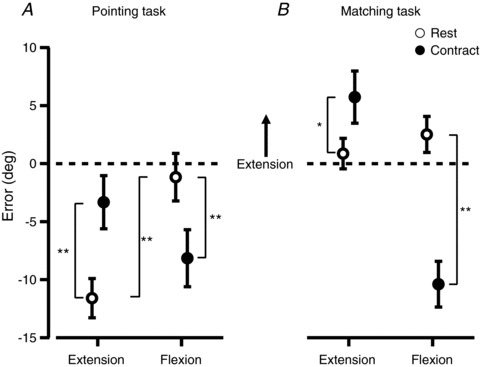
Errors were calculated by subtracting the reference angle from the pointer angle, and data from the two test angles have been pooled. All data points show the mean ± 95% confidence interval. A, results when subjects indicated the angle of their right wrist by moving a pointer with the left hand. Open circles show the ‘Rest’ condition and filled circles show the ‘Contract’ condition where a 30% of maximum isometric contraction was performed whilst the wrist angle was indicated. Extension and flexion labels show whether a wrist extension or flexion contraction was performed in the contract conditions. The same muscle group performed a conditioning contraction at the start of every trial. B, results when subjects indicated the angle of the right wrist by matching its position with their left wrist. *indicates a significant difference with P < 0.05. **indicates a significant difference with P < 0.001.
Figure 5. Mean perceived elbow angles for the group, expressed as an error.
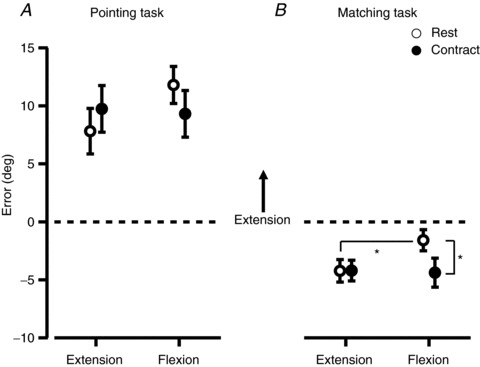
Errors were calculated by subtracting the reference angle from the pointer angle, and data from the two test angles have been pooled. All data points show the mean ± 95% confidence interval. A, results when subjects indicated the angle of their right wrist by moving a pointer with the left hand. Open circles show the ‘Rest’ condition and filled circles show the ‘Contract’ condition where a 30% of maximum isometric contraction was performed whilst the wrist angle was indicated. Extension and flexion labels show whether a wrist extension or flexion contraction was performed in the contract conditions. The same muscle group performed a conditioning contraction at the start of every trial. B, results when subjects indicated the angle of the right wrist by matching its position with their left elbow. *indicates a significant difference with P < 0.05.
Statistical testing of the error data used a two-way repeated-measures ANOVA. Within-subject factors were the contraction direction (flexion vs. extension) and contraction (rest vs. contract). Comparison of variances (Fig. 6) was performed using Levene's test. All statistical testing was performed with α= 0.05.
Figure 6. Comparison of standard deviations (SDs) of pointing task and matching task.
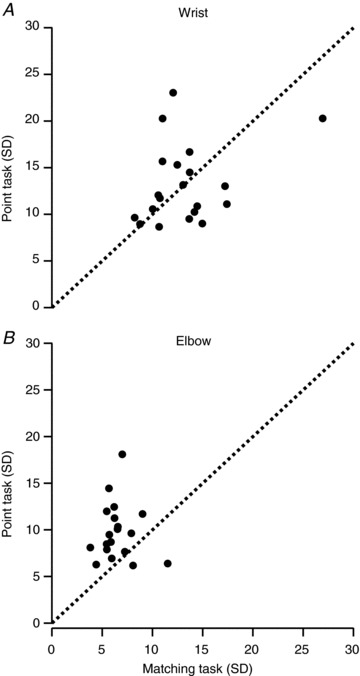
A plot of the SD of the mean pointing error versus the SD of the mean matching error for each subject for measurements made at the wrist (A) and elbow (B). Each point represents the data from one subject, pooled across muscle groups (flexion and extension) and conditions (rest and contract). The dotted line is the line of equality.
Results
Experiment 1 – the wrist
Pointing task
Figure 2 shows raw data from one subject for the pointing task at the wrist. Figure 2A shows the pointer angle when the wrist extensor muscles had been conditioned at the start of the trial and Fig. 2B shows data when the wrist flexors had been conditioned. Two test angles were used, +15 deg and −15 deg, where the hand's position was extended by 15 deg or flexed by 15 deg, respectively, with 0 deg representing the position at which the wrist was in line with the forearm. Wrist angles in the direction of extension from the zero position were assigned a positive value and wrist angles into flexion were given a negative value. The subject in Fig. 2 showed a trend consistent with an effect of muscle contraction on the perceived wrist position. For example, for wrist extensor conditioning (Fig. 2A), when the subject made an extensor contraction whilst indicating wrist angle, the wrist was perceived as more extended than when the wrist muscles were relaxed. This was true for both test angles. Trends in the opposite direction occurred for wrist flexor contractions. Here, the position of the wrist was perceived as more flexed (Fig. 2B). The pooled data from 20 subjects (Fig. 3) showed the same trends. For extensor contractions at a wrist angle of −15 deg, the perceived position of the wrist changed from 3.5 deg [1.1 deg, 5.9 deg] ([95% confidence interval]) in the direction of extension to 15.4 deg [12.6 deg, 18.2 deg]. At 15 deg, the perceived position shifted, again in the direction of extension, from −26.7 deg [−29.0 deg, 24.4 deg] to −17.7 deg [−20.6 deg, 14.8 deg]. There was a significant difference between the perceived wrist position during wrist extensor contractions when compared to rest, for both test angles (P < 0.05). For flexion contractions, at −15 deg the value shifted from 12.4 deg [8.9 deg, 15.9 deg] in the direction of flexion to 6.4 deg [2.7 deg, 10.1 deg], and at 15 deg it shifted in the direction of flexion from −19.1 deg [16.2 deg, 22.0 deg] to −22.7 deg [−25.9 deg, −19.5 deg]. Here, the shift in position was significant only for the test angle of −15 deg, and not for +15 deg. There was considerable scatter of values between subjects. To reduce this variance and to make the effect of muscle contraction clearer, we subtracted the reference angle from the pointer angle for each trial, to produce a pointing error. Errors from both test angles were then pooled.
Figure 4A shows the mean errors pooled across all subjects. The conditioning contractions performed at the start of each trial had a significant effect on the perceived wrist angle (P < 0.001). This effect was expected and is consistent with the thixotropic properties of muscle spindles (see Introduction). For rest trials, in which the wrist flexors had been conditioned, the indicated wrist angle was 10.4 deg [6.7 deg, 14.2 deg] more extended than rest trials in which wrist extensors had been conditioned (Fig. 4A).
When subjects performed an isometric wrist flexion, they indicated a wrist angle that was, on average, 7.0 deg [2.5 deg, 11.5 deg] more flexed than at rest (P < 0.001). During contraction with the wrist extensors, subjects indicated that their wrist was, on average, 8.3 deg [4.3 deg, 12.2 deg] more extended than at rest (Fig. 4A). That is, muscle contraction produced a perception that the wrist was displaced in the same direction as the voluntary contraction.
Matching task
In contrast with the pointing task, we found no significant effect of the conditioning contractions on position errors in the relaxed wrist during the matching task (Fig. 4B). However, muscle contraction again had a significant effect on the perceived wrist angle for both wrist flexor and extensor contractions. For flexor contractions subjects indicated, on average, that their wrist was perceived as 12.9 deg [9.4 deg, 16.4 deg] more flexed during the contraction than when at rest (P < 0.001). For extensor contractions, on average, the wrist was perceived as 4.9 deg [1.3 deg, 8.4 deg] more extended than at rest. The direction of the errors was consistent with that seen during the pointing task. In the pointing task the size of the effect of the flexion and extension contractions was similar, but in the matching task the size of the effect of the flexion contraction was twice as great as that for the extension contraction.
Experiment 2 – the elbow
Pointing task
Figure 5A shows the pooled data for this task. We found no significant effect of the conditioning contractions on the perceived elbow angle during the pointing task. Contraction of the elbow flexor or extensor muscles produced no significant change in perceived elbow angle (flexors relaxed, 11.8 deg [10.2 deg, 13.4 deg]; flexors contracting, 9.3 deg [7.3 deg, 11.3 deg]; extensors relaxed, 7.8 deg [5.8 deg, 9.8 deg]; extensors contracting, 9.7 deg [7.7 deg, 11.8 deg]). However, there were small, non-significant, trends in the same direction as seen at the wrist. Overall, there was no significant effect of voluntary contractions on position sense.
Matching task
In the matching task we found a small, but significant, effect of conditioning contractions on the perceived elbow angle (Fig. 5B). The direction of this error was consistent with that seen previously under similar experimental conditions (Ansems et al. 2006). This pattern was opposite to that seen in Experiment 1, in which the pointing task showed an effect, but the matching task did not. Flexion contractions produced a small, but significant, change in the perceived elbow angle (2.8 deg [0.6 deg, 5.0 deg]) when compared to rest. The direction was the same as for the wrist, with the elbow being perceived as more flexed during the flexion contraction. We found no significant effect of extension contractions on the perceived elbow angle (extensors relaxed, −4.2 deg [−5.2 deg, −3.2 deg]; extensors contracting, −4.2 deg [−5.1 deg, −3.3 deg]).
Variability in matching performance
The confidence intervals for the elbow were greater in the pointing task than in the matching task (Fig. 5). A similar, but weaker, trend was apparent at the wrist (Fig. 4). The errors in Figs 4 and 5 are the position errors (constant errors). To assess differences in matching performance by subjects using the two methods of signalling joint position, the standard deviation (SD) of the pointing task for each subject was plotted against that for the matching task (Fig. 6). If measurement consistency in the two tasks had been the same, values would lie scattered evenly about the line of equality. This is roughly the case for the wrist, with only a couple more points above the line than below. However, at the elbow, there are many more points above than below the line. The pooled SDs of the data show a similar picture. At the wrist the group SD for pointing was 15.7 deg and for matching was 14.7 deg, but at the elbow the pointing SD of 13.6 deg was almost double the matching SD of 7.4 deg. Statistical analysis showed that for both the wrist (P < 0.002) and the elbow (P < 0.0001) there was a significant difference between variance for pointing versus matching. When each subject's pointing and matching variances were compared, at the wrist there were significant differences in the values for two subjects, whereas at the elbow there were significant differences for 13 subjects. Thus, indicating limb position with a pointer was less consistent than using the other limb for matching, particularly at the elbow.
Furthermore, the group SD for elbow matching (7.4 deg) is also half that of the group SD for wrist matching (14.7 deg; P < 0.0001), whereas the two pointing group SDs are significantly different (P < 0.005), but similar in size (wrist, 15.7 deg; elbow, 13.6 deg). Individual subject SDs are less revealing here, with none showing a difference between wrist and elbow for matching, but only two showing a significant difference for pointing. To sum up, although consistency in pointing performance was similar at the two joints, matching performance was more consistent at the elbow.
Discussion
Comparing the wrist with the elbow
We investigated the effect of muscle contraction on the sense of joint position at the wrist and the elbow. The main aim was to try to reconcile reported differences in claims about a role of motor commands in limb position sense at the wrist (Gandevia et al. 2006; Smith et al. 2009) and elbow (Ansems et al. 2006; Allen et al. 2010). The question was whether there was a systematic difference between joints or whether the evidence for centrally generated signals of joint position was dependent on the method used to show it. Previously, position sense has often been measured using a two-limb matching task (e.g. Goodwin et al. 1972). More recently, Gandevia et al. (2006) and Smith et al. (2009) measured position sense at the wrist by asking subjects to indicate the position of the unseen reference hand by moving a pointer with their other hand. This was seen as a cleaner measurement of the perceived wrist angle because it did not involve any potentially confounding effects from the other limb. The experiments at the elbow all used a two-arm matching task, and so it was necessary to use both of these methods to settle the discrepancy between the joints. There are reasons why position sense may perform differently with these two tasks and these are discussed below.
Our results show that there is a systematic difference between the elbow and wrist in the contribution of centrally generated command signals to position sense, regardless of the method used to reveal it. We found a clear contribution of central command signals to joint position sense at the wrist, but were unable to detect a similar contribution at the elbow. A further difference between the two joints was a larger difference in performance variability at the elbow for pointing versus matching, compared with performance at the wrist.
The present study confirms the findings of Smith et al. (2009) and extends them to conditions in which wrist muscles and their muscle spindles were placed in a fully defined state. Smith et al. (2009) measured the effects of isometric efforts in both the intact and paralysed wrist. The position of the wrist was indicated with a pointer. Before paralysis, an isometric effort led to a significant shift in the perceived position of the wrist in the direction of the voluntary effort. After wrist muscles had been paralysed (without blocking afferent nerves), the same effort produced an even larger shift. Thus, this study demonstrated that positional signals generated by isometric efforts persist during paralysis, in the absence of any muscle activity. That is, they could not have been generated indirectly by afferents responding to the mechanical stimulus from the contraction.
This finding is important, because one potential explanation for the presence of effort-related position signals at the wrist is that mechanical changes in the contracting muscle are responsible. When the wrist is placed in an extended posture, the extensor muscles are shortened and the flexor muscles are stretched. If the extensors then undergo an isometric contraction, this may be associated with some internal shortening in that muscle, as the contracting muscle fibres stretch the tendons. Muscle spindles would be shortened as well, signalling a shorter muscle, that is, a more extended wrist. However, such an explanation is unlikely, given that position signals generated by central commands are present in paralysed muscle (Smith et al. 2009).
In the present study, position errors were dependent on the form of conditioning, flexion or extension, on whether or not wrist muscles were contracting, and on the method of indicating limb position (Fig. 4). Both at the wrist and elbow, the fact that errors were distributed differently, depending on whether a pointing or matching task was used, leads to the conclusion that the two methods are not measuring exactly the same thing. What might be some of the differences?
Indicating limb position using a two-limb matching task
In locating one arm or hand relative to the other, we routinely rely on the proprioceptive signals coming from both arms (e.g. Lackner, 1984; Lackner & Taulieb, 1984; White & Proske, 2009; Izumizaki et al. 2010). At the wrist, a two-hand matching task improves performance over pointing, but only a little. However, at the elbow, matching performance is much more precise than pointing. It seems likely that the proprioceptive information provided by the indicator hand during matching at the wrist is just as comprehensive as that provided by the indicator elbow. In the simplest interpretation of the matching process, the brain is likely to listen to proprioceptive afferent signals from the two arms and, when their difference is at a minimum, the arms are assumed to be aligned.
Our current hypothesis for the difference in matching performance at the wrist and elbow is that the result observed at the wrist is likely to be typical for matching performance at other joints. At the elbow, however, we postulate the recruitment of additional neural pathways to achieve the observed accuracy. The reason for the need for extra accuracy in aligning the forearms is the importance of the posture for everyday activities. It is used for any task in which we work with both hands in front of us. The close cooperation of the two arms in indicating limb position has recently been used to propose that the two arms can sometimes be considered to act as a single instrument in the execution of movements (Izumizaki et al. 2010). These sorts of propositions remind us of the evolution of humans as tool makers, where the position of the two hands relative to one another is critical. There is evidence that for position sense at the arm the important parameter is the limb end-point position (Bosco et al. 2000), that is, the position of the hand (Fuentes & Bastian, 2010). Therefore, it is likely that the parameter signalled in the present experiments on the forearm was the position of the hand, not the angle at the elbow (Soechting, 1982; Gooey et al. 2000). In addition, performance at the elbow may include a component of evolutionary origin. When our ancestors moved by quadrupedal locomotion, inter-limb coordination, particularly at large joints, would have been an important requirement. Perhaps, present-day performance continues to be influenced by neural pathways laid down a long time ago.
In the present experiments all joint displacements were in the horizontal plane to minimise any effects of gravity. This meant that forearm position sense was measured using a posture that was not quite typical. Position sense at the elbow is most accurate when measured in the vertical plane, with the arms in front of the body (Gooey et al. 2000) and with the palmar surfaces of the hands facing each other. This is the position we adopt during skilled manipulations with both hands. Therefore, it is likely that differences between pointing and matching at the elbow would have been even greater if measurements had been made in the vertical plane.
An additional finding in the present study is that the SDs of position errors for the matching task were systematically smaller than for the pointing task (Fig. 6). In other words, subjects performed more consistently when they used both arms or both hands than when they used a pointer, and this trend was greater at the elbow than at the wrist. Furthermore, pointing performance at the elbow and wrist was similar, whereas matching performance was very different between the two joints.
Indicating limb position with a pointer
At the elbow, why might performance with a pointer be less consistent than in a two-arm matching task? When, in the absence of vision, we are asked to indicate the location in space of an arm, we presumably use proprioceptive signals coming from that arm and refer them to a central representation of the body (e.g. Gallagher, 2005). The map, which is likely to be constantly changing (Walsh et al. 2010), has presumably been established, based on visual, haptic and proprioceptive information. When the subject aligns the pointer with the felt position of their hidden hand or elbow, they are able to see much of their body, including the hand used to control the pointer, providing visual confirmation of the location of different body parts on the map and therefore reliability of the map. Our data suggest that the precision with which the position of an unseen limb can be indicated with a pointer is similar for the elbow and wrist, but at the elbow this is inherently less precise than a comparison of signals in a two-limb matching task. It would be interesting to determine whether this finding can be generalised to other body parts.
Limb position sense during a contraction
In an earlier study, Ansems et al. (2006) measured position sense at the elbow, in the relaxed state and whilst arm muscles supported loads of up to 25% MVC. Position sense was measured using a forearm matching task, in the horizontal plane. Provided that arm muscles had been appropriately conditioned, small, not statistically significant, position errors were generated when the subject supported a load. This result has been confirmed in the present study (Fig. 5). For the elbow, the differences in position errors, with and without isometric efforts, were small: 2–3 deg for the pointing task and 0–3 deg for the matching task. By comparison, the differences at the wrist were 7–8 deg for the pointing task and 5–13 deg for matching. Therefore, centrally generated command signals do not seem to manifest themselves at the elbow in the same way as they do at the wrist.
On reflection, it is remarkable that, when a blindfolded subject supports a 25% MVC weight with one arm, position sense, as measured in a two-arm matching task, is no less accurate than when the arm is not supporting any weight. The current view is that spindles are the principal proprioceptors, with a contribution from skin (Proske & Gandevia, 2012). During a voluntary contraction there is fusimotor co-activation (e.g. Vallbo, 1971, 1974; Burke et al. 1978), and so the profile of spindle discharges in the arm supporting the load would differ from that in a relaxed arm. What might be the underlying neural processes that allow the achievement of a near-identical outcome in position sense? It is conceivable that, whilst a centrally generated position signal may not operate directly at the elbow, as it does at the wrist, a corollary of the motor discharge is still used in calculating the position signal. It was suggested by Allen et al. (2007) that in arm matching tasks whilst the arm was supporting a load, the motor corollary was used to calculate the reafferent component of the total spindle signal, and this reafference was subtracted to derive any remaining exafferent signal. Reafference refers to the afferent signals generated by one's own actions and exafference refers to afferent signals caused by purely external, environmental influences. A mechanism and the associated neural pathways have been proposed by Proske and Gandevia (2012). When the arm is relaxed, any forward signal (Wolpert et al. 1995; see also Walsh et al. 2011) will be zero. Therefore, the exafferent signal can fully express itself in position sense. As soon as the arm muscles contract, the command signal provides access to forward models. When the strength of the contraction increases, the command signal increases as well, to alter the anticipated level of feedback. In a stereotypical task, such as determining the arm position, forward models would be expected to be available for different limb positions under different loads.
These considerations can be applied to the present experiments at the forearm. They raise the question: why does an isometric effort directly generate position signals at the wrist, but not at the elbow? Distal muscles, especially in the hand, are smaller and used in finer motor tasks than more proximal muscles. Thus, any additional signal about the position of the hand would be useful. Furthermore, smaller muscles will have a higher motor command to force ratio and a motor command signal may be more useful here than in larger, proximal muscles. It could also be that the distal muscles make more use of the additional information from central commands because these muscles tend to cross more joints, at times making muscle spindle information potentially ambiguous (Sturnieks et al. 2007; see also Goodwin et al. 1972). This argument cannot be used for wrist muscles versus elbow muscles as both cross a similar number of joints. However, the long finger flexors and extensors cross many joints and thus would benefit from an additional signal. Finally, there may again be an evolutionary consideration. During quadrupedal locomotion the forelimbs will routinely have to bear the weight of the body. Achieving inter-limb coordination in the face of a constantly changing load would be difficult if, at the same time, load-related position signals were coming from elbow muscles.
Conclusions
We have shown that centrally generated command signals contribute to joint position sense at the wrist under conditions in which the muscles are placed in a defined thixotropic state. However, similar experiments carried out at the elbow did not reveal an effect of isometric efforts on position sense. This does not mean that motor command signals are not used at the elbow; rather, their processing is likely to be different. What this means more broadly for proprioception is uncertain and, as a first step, it will require a survey of different joints. What the present experiments have revealed is an unexpected difference in the processing of central and peripheral proprioceptive information at two adjacent joints. They have therefore broadened our understanding of proprioceptive mechanisms and, as most movements involve muscle contraction, this work has widespread implications.
Acknowledgments
We are grateful to Dr Nobuhiro Hagura for his comments on the manuscript.
Glossary
Abbreviations
- EMG
electromyographic
- MVC
maximum voluntary contraction
Additional information
Competing interests
None.
Author contributions
Each author contributed to all aspects of the study. Wrist experiments were performed at Neuroscience Research Australia (formerly Prince of Wales Medical Research Institute) in Sydney, NSW, Australia. Elbow experiments were performed at both Monash University Department of Physiology and Neuroscience Research Australia.
Funding
This work was supported by the National Health and Medical Research Council (of Australia).
References
- Allen TJ, Ansems GE, Proske U. Effects of muscle conditioning on position sense at the human forearm during loading or fatigue of elbow flexors and the role of the sense of effort. J Physiol. 2007;580:423–434. doi: 10.1113/jphysiol.2006.125161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TJ, Leung M, Proske U. The effect of fatigue from exercise on human limb position sense. J Physiol. 2010;588:1369–1377. doi: 10.1113/jphysiol.2010.187732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TJ, Proske U. Effect of muscle fatigue on the sense of limb position and movement. Exp Brain Res. 2006;170:30–38. doi: 10.1007/s00221-005-0174-z. [DOI] [PubMed] [Google Scholar]
- Ansems GE, Allen TJ, Proske U. Position sense at the human forearm in the horizontal plane during loading and vibration of elbow muscles. J Physiol. 2006;576:445–455. doi: 10.1113/jphysiol.2006.115097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco G, Poppele RE, Eian J. Reference frames for spinal proprioception: limb end-point based or joint-level based. J Neurophysiol. 2000;83:2931–2945. doi: 10.1152/jn.2000.83.5.2931. [DOI] [PubMed] [Google Scholar]
- Burgess P, Clark F. Characteristics of knee joint receptors in the cat. J Physiol. 1969;203:317–335. doi: 10.1113/jphysiol.1969.sp008866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Hagbarth KE, Lofstedt L. Muscle spindle activity in man during shortening and lengthening contractions. J Physiol. 1978;277:131–142. doi: 10.1113/jphysiol.1978.sp012265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. Pride and a Daily Marathon. London: Gerald Duckworth & Co; 1995. [Google Scholar]
- Collins DF, Refshauge KM, Todd G, Gandevia SC. Cutaneous receptors contribute to kinesthesia at the index finger, elbow, and knee. J Neurophysiol. 2005;94:1699–1706. doi: 10.1152/jn.00191.2005. [DOI] [PubMed] [Google Scholar]
- Edin BB, Johansson N. Skin strain patterns provide kinesthetic information to the human central nervous system. J Physiol. 1995;487:243–251. doi: 10.1113/jphysiol.1995.sp020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes CT, Bastian AJ. Where is your arm? Variations in proprioception across space and tasks. J Neurophysiol. 2010;103:164–171. doi: 10.1152/jn.00494.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S. How the Body Shapes the Mind. New York: Oxford University Press; 2005. [Google Scholar]
- Gandevia SC. Kinesthesia: roles for afferent signals and motor command. In: Rowell L, Shepherd J, editors. Handbook on Integration of Motor, Circulatory, Respiratory and Metabolic Control during Exercise. Bethesda, MD: American Physiological Society; 1996. pp. 128–172. [Google Scholar]
- Gandevia SC, Smith JL, Crawford M, Proske U, Taylor JL. Motor commands contribute to human position sense. J Physiol. 2006;571:703–710. doi: 10.1113/jphysiol.2005.103093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Matthews PBC. The contribution of muscle afferents to kinæsthesia shown by vibration induced illusion of movement and by the effects of paralysing joint afferents. Brain. 1972;95:705–748. doi: 10.1093/brain/95.4.705. [DOI] [PubMed] [Google Scholar]
- Gooey K, Bradfield O, Talbot J, Morgan D, Proske U. Effects of body orientation, load and vibration on sensing position and movement at the human elbow joint. Exp Brain Res. 2000;133:340–348. doi: 10.1007/s002210000380. [DOI] [PubMed] [Google Scholar]
- Gregory JE, Morgan DL, Proske U. Aftereffects in the responses of cat muscle spindles and errors of limb position sense in man. J Neurophysiol. 1988;59:1220–1230. doi: 10.1152/jn.1988.59.4.1220. [DOI] [PubMed] [Google Scholar]
- von Helmholtz H. Helmholtz's Treatise on Physiological Optics, vol. 3. Menasha, WI: Optical Society of America; 1867. [Google Scholar]
- Hermsdorfer J, Elias Z, Cole JD, Quaney BM, Nowak DA. Preserved and impaired aspects of feed-forward grip force control after chronic somatosensory deafferentation. Neurorehabil Neural Repair. 2008;22:374–384. doi: 10.1177/1545968307311103. [DOI] [PubMed] [Google Scholar]
- Hill DK. Tension due to interaction between the sliding filaments in resting striated muscle. The effect of stimulation. J. Physiol. 1968;199:637–684. doi: 10.1113/jphysiol.1968.sp008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Holst E. Relations between the central nervous system and the peripheral organs. Br J Anim Behav. 1954;2:89–94. [Google Scholar]
- Izumizaki M, Tsuge M, Akai L, Proske U, Homma I. The illusion of changed position and movement from vibrating one arm is altered by vision or movement of the other arm. J Physiol. 2010;588:2789–2800. doi: 10.1113/jphysiol.2010.192336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner JR. Some influences of tonic vibration reflexes on the position sense of the contralateral limb. Exp Neurol. 1984;85:107–113. doi: 10.1016/0014-4886(84)90165-1. [DOI] [PubMed] [Google Scholar]
- Lackner JR, Taulieb AB. Influence of vision on vibration-induced illusions of limb movement. Exp Neurol. 1984;85:97–106. doi: 10.1016/0014-4886(84)90164-x. [DOI] [PubMed] [Google Scholar]
- Lakie M, Walsh EG, Wright GW. Resonance at the wrist demonstrated by the use of a torque motor: an instrumental analysis of muscle tone in man. J Physiol. 1984;353:265–285. doi: 10.1113/jphysiol.1984.sp015335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey DI. Kinaesthetic sensibility. Physiol Rev. 1978;58:763–820. doi: 10.1152/physrev.1978.58.4.763. [DOI] [PubMed] [Google Scholar]
- McCloskey DI. Corollary discharges: motor commands and perception. In: Brookhart J, Mountcastle V, Brooks V, Geiger S, editors. Handbook of Sensory Physiology. Bethesda, MD: American Physiological Society; 1981. pp. 1415–1447. [Google Scholar]
- McCloskey DI, Torda TA. Corollary motor discharges and kinaesthesia. Brain Res. 1975;100:467–470. doi: 10.1016/0006-8993(75)90503-x. [DOI] [PubMed] [Google Scholar]
- Morgan DL, Prochazka A, Proske U. The after-effects of stretch and fusimotor stimulation on the responses of primary endings of cat muscle spindles. J Physiol. 1984;356:465–477. doi: 10.1113/jphysiol.1984.sp015477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U, Gandevia SC. The proprioceptive senses: their roles in signalling body shape, body position and movement and muscle force. Physiol Rev. 2012;92:1651–1697. doi: 10.1152/physrev.00048.2011. [DOI] [PubMed] [Google Scholar]
- Proske U, Morgan DL, Gregory JE. Thixotropy in skeletal muscle and in muscle spindles: A review. Prog Neurobiol. 1993;41:705–721. doi: 10.1016/0301-0082(93)90032-n. [DOI] [PubMed] [Google Scholar]
- Smith JL, Crawford M, Proske U, Taylor JL, Gandevia SC. Signals of motor command bias joint position sense in the presence of feedback from proprioceptors. J Appl Physiol. 2009;106:950–958. doi: 10.1152/japplphysiol.91365.2008. [DOI] [PubMed] [Google Scholar]
- Soechting JF. Does position sense at the elbow reflect a sense of elbow joint angle or one of limb orientation. Brain Res. 1982;248:392–395. doi: 10.1016/0006-8993(82)90601-1. [DOI] [PubMed] [Google Scholar]
- Sperry RW. Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol. 1950;43:482–489. doi: 10.1037/h0055479. [DOI] [PubMed] [Google Scholar]
- Sturnieks DL, Wright JR, Fitzpatrick RC. Detection of simultaneous movement at two human arm joints. J Physiol. 2007;585:833–842. doi: 10.1113/jphysiol.2007.139089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo ÅB. Muscle spindle response at the onset of isometric voluntary contractions in man. Time difference between fusimotor and skeletomotor effects. J Physiol. 1971;218:405–431. doi: 10.1113/jphysiol.1971.sp009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo ÅB. Human muscle spindle discharge during isometric voluntary contractions. Amplitude relations between spindle frequency and torque. Acta Physiol Scand. 1974;90:319–336. doi: 10.1111/j.1748-1716.1974.tb05594.x. [DOI] [PubMed] [Google Scholar]
- Walsh LD, Allen TJ, Gandevia SC, Proske U. The effect of eccentric exercise on position sense at the human forearm in different postures. J Appl Physiol. 2006;100:1109–1116. doi: 10.1152/japplphysiol.01303.2005. [DOI] [PubMed] [Google Scholar]
- Walsh LD, Gandevia SC, Taylor JL. Illusory movements of a phantom hand grade with the duration and magnitude of motor commands. J Physiol. 2010;588:1269–1280. doi: 10.1113/jphysiol.2009.183038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh LD, Hesse CW, Morgan DL, Proske U. Human forearm position sense after fatigue of elbow flexor muscles. J Physiol. 2004;558:705–715. doi: 10.1113/jphysiol.2004.062703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh LD, Smith JL, Gandevia SC, Taylor JL. The combined effect of muscle contraction history and motor commands on human position sense. Exp Brain Res. 2009;195:603–610. doi: 10.1007/s00221-009-1832-3. [DOI] [PubMed] [Google Scholar]
- Walsh LD, Taylor JL, Gandevia SC. Overestimation of force during matching of externally generated forces. J Physiol. 2011;589:547–557. doi: 10.1113/jphysiol.2010.198689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White O, Proske U. Illusions of forearm displacement during vibration of elbow muscles in humans. Exp Brain Res. 2009;192:113–120. doi: 10.1007/s00221-008-1561-z. [DOI] [PubMed] [Google Scholar]
- Winter JA, Allen TJ, Proske U. Muscle spindle signals combine with the sense of effort to indicate limb position. J Physiol. 2005;568:1035–1046. doi: 10.1113/jphysiol.2005.092619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert D, Ghahramani Z, Jordan M. An internal model for sensorimotor integration. Science. 1995;269:1880–1882. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]


