Abstract
Objective
To retrospectively evaluate the association of idiopathic inflammatory myopathy (IIM) and malignancy in patients seen at 1 academic center over a 23-year period.
Methods
Patients were identified using the International Classification of Diseases, 9th edition (ICD-9) codes and diagnoses, then confirmed by chart review. Population cancer statistics obtained from the US Centers for Disease Control for Vermont and New Hampshire were used for comparison.
Results
Chart review confirmed IIM in 198 of 483 patients initially identified by ICD-9 codes. Within 5 years of diagnosis with IIM, malignancy developed in 32 patients (16.2%), 24 of whom (75%) had dermatomyositis (DM). Malignancy and DM developed within 1 year in 75%. The cancer risk associated with DM was much greater than the risk associated with other IIM. The most frequent tumor types were breast, lung, pancreas, and colon. DM patients with cancer were more frequently male and ≥ 45 years of age than those without cancer. There were no cases of interstitial lung disease among patients with cancer and any form of IIM. The incidence of cancer was increased in patients with DM compared to age- and sex-matched population controls, both over a 5-year interval surrounding the diagnosis of DM and over the lifetime interval following diagnosis.
Conclusion
The risk of cancer in IIM is concentrated among patients with DM. The association between DM and cancer was enhanced by its temporal relationship (< 1 year) in 87.5% of these cases. Patients with malignancy-associated DM were more frequently male and over age 45 and less likely to have interstitial lung disease.
Key Indexing Terms: Dermatomyositis, Polymyositis, Malignancy
The idiopathic inflammatory myopathies (IIM) are a heterogeneous group of diseases. Since 1975, this group has included polymyositis (PM), dermatomyositis (DM), amyopathic DM, myositis associated with connective tissue disease (M-CTD), and pediatric/juvenile DM (JDM)1. Inclusion body myositis (IBM) and amyopathic DM have subsequently been classified as IIM2,3. The first report of an association between IIM and malignancy was credited to Stertz, who published this observation in 19164. Since then, various authors have demonstrated malignancy risk in both DM and PM5-17. This relationship has also been evaluated in amyopathic DM, IBM, and JDM7,18-21. In this study, we determined the relationship between cancer and IIM by conducting a retrospective review of 198 patients treated during the past 23 years at a single institution that functions both as a regional hospital and as a tertiary medical center.
Materials and Methods
Following approval by the Committee for the Protection of Human Subjects at Dartmouth Medical School, we conducted a retrospective review of all patients who were treated for IIM at Dartmouth-Hitchcock Medical Center (DHMC) between January 1985 and April 2008. DHMC serves as both a regional and tertiary care hospital serving New Hampshire and Vermont. The patient population treated at DHMC is drawn almost entirely from these 2 states and is largely of European descent, particularly English and French-Canadian. Patients were identified according to the International Classification of Diseases, 9th Edition (ICD-9) codes for DM (710.3) and PM (710.4), which were submitted for hospital admissions, clinic visits, and pathology reports. The presence of malignancy was determined by concurrent ICD-9 searches for cancer. Each patient's chart was reviewed to confirm the accuracy of IIM and cancer diagnoses. To be considered cancer-related, the neoplasm had to be diagnosed 2 years prior to or 3 years following the identification of IIM14. Nonmelanomatous skin cancers were excluded from the analysis. This study was approved by the hospital's institutional review board.
The criteria proposed by Bohan and Peter were used to identify IIM1. These included proximal muscle weakness, elevated serum concentrations of muscle enzymes, the presence of characteristic cutaneous lesions (DM/amyopathic DM/JDM), and findings on tissue biopsy and electromyography (EMG). Magnetic resonance imaging (MRI) was also used to support a diagnosis in instances where biopsy and EMG were either nondiagnostic or not performed. Patients were classified as having DM, PM, amyopathic DM, IBM, JDM, and overlap syndromes or M-CTD. Amyopathic DM was characterized by a rash (Gottron's papules, shawl sign, heliotrope rash), no evidence of proximal muscle weakness, and no elevation of muscle enzymes for 2 years from the time of initial diagnosis. Patients who were younger than 18 years at the time of diagnosis were placed in the JDM group. Adult patients with a second rheumatologic disease were assigned to the overlap group.
Laboratory test results were reviewed from the time of diagnosis with IIM, including antinuclear antibodies (ANA), anti-double-stranded DNA antibodies (dsDNA), extractable nuclear antigens (ENA), myositis-specific and myositis-associated antibodies, rheumatoid factor (RF), anticardiolipin antibodies (ACA), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), creatine phosphokinase (CPK), aldolase, and lactate dehydrogenase (LDH). The presence of interstitial lung disease (ILD) was determined by pulmonary function tests and computed tomography studies. The tumor type, histological variety, and temporal relationship to the diagnosis of IIM were determined in cases of malignancy. In patients who developed IIM in association with recurrent or metastatic cancer, the temporal relationship was determined with reference to the initial diagnosis of malignant disease. The length of followup time was measured for each patient.
Cancer statistics obtained from the US Centers for Disease Control (CDC) WONDER online database for the states of Vermont and New Hampshire from 1999 to 2002 were used to calculate standardized incidence ratios (SIR) using counts of cancer and observation times for the IIM patients22. Two separate approaches to the analysis were used. First, we compared the incidence of cancer over the 5-year period spanning 2 years preceding and 3 years following IIM relative to the expected rate of cancer diagnosis in an age- and sex-matched population over a 5-year span. Second, we compared the lifetime incidence of cancer following the diagnosis of IIM to population cancer statistics. Those patients who developed cancer before IIM were excluded from this analysis. Population cancer data were used to determine the expected cancer incidence in an age- and sex-matched population over the followup time spanned by our study. In the case of 18 study patients, the followup time was known to be less than 1 year, but the exact duration was not available; these patients were given an estimated followup time of 0.5 years. A chi-squared approximation to the Poisson distribution was performed to provide 95% confidence intervals (CI) for the SIR23. Odds ratios (OR) were calculated to analyze factors affecting cancer incidence within IIM patients. In instances where cells contained a value of 0, an estimated OR was calculated using a substituted value of 0.5. A logistic-regression model was created to assess adjusted OR for the development of cancer in DM for gender, age, and CPK elevation.
Results
Confirmation and classification of IIM
On the basis of ICD-9 codes for PM and DM, 483 patients were identified. Chart review was performed on each patient to confirm the diagnosis of IIM. Over half the patients (58.9%) failed to meet criteria for a diagnosis of IIM based on available data and were excluded from further analysis, a rate of exclusion similar to that of previous studies that have utilized chart review13. These excluded patients typically exhibited ≤ 1 of the criteria for IIM. Thus we confirmed the diagnosis of IIM in 198/483 patients (41.1%). Of these 198 patients with IIM, 61 (30.8%) were classified as DM, 63 (31.8%) as PM, 23 (11.6%) as amyopathic DM, 22 (11.1%) as M-CTD, 11 (5.6%) as IBM, and 18 (9.1%) as JDM. Among the cases of DM and PM, 27.4% were “definite” by Bohan and Peter criteria1, 50.8% were “probable,” and 21.8% were “possible.” MRI and skin biopsy were also used to support diagnoses in 27 and 58 IIM patients, respectively.
Increased association of DM with malignancy
We used the criteria that a malignancy was associated with IIM if it was diagnosed within 2 years prior to or 3 years following the diagnosis of IIM14. Using these criteria, an association with malignancy was met by 32 (16.2%) of the 198 patients with IIM. Of these, an associated cancer was more frequently represented in the DM category, with 24 of 32 patients (75%). The remaining 8 cases of cancer associated with IIM were distributed among PM (3 cases, 9.4%) and amyopathic DM (3 cases, 9.4%), with overlap and IBM having 1 case each (3.1%). There were no malignancies in the pediatric population.
The risk of cancer associated with DM was much greater than that with other adult IIM (OR 10.46, 95% CI 4.34–25.21). DM was associated more frequently with malignancy than was PM alone (OR 12.97, 95% CI 3.65–46.13). Not surprisingly, the lifetime cancer risk was much greater in DM (35 cancers in 61 patients), compared with adult PM (8 cancers in 63 patients), amyopathic DM (5 cancers in 23 patients), IBM (4 cancers in 11 patients), and overlap syndromes (3 cancers in 22 patients).
While a clear temporal relationship between cancer and DM is seen (Figure 1), the occurrence of cancer in the non-DM population is dispersed over time (Figure 2). Among the cases of cancer associated with DM, 21 occurred within 2 years preceding or following the diagnosis of DM (87.5%), while 16 (75%) occurred less than 1 year before or after the diagnosis. The remaining 3 cancers were diagnosed within 3 years. In 7 cases (29.2%), the diagnosis of malignancy preceded the diagnosis of DM by > 6 months. The diagnosis of DM preceded malignancy by > 6 months in 3 cases (12.5%). In 14 patients (58.3%), the 2 diagnoses were made within 6 months. Seven of the malignancies (29.2%) were noted to have been identified by screening measures undertaken as part of the investigation for newly diagnosed DM, including computed tomography imaging, endoscopy, and serum tumor markers. The tumors identified as having occurred in temporal association with DM are listed in Table 1. Of the 24 patients with DM temporally associated with malignancy, 2 experienced clinical recurrences of DM 7 and 8 years later in connection with the diagnosis of an entirely different cancer (Table 1). Two other patients were noted to demonstrate improvement in their DM symptoms following resection of associated tumors.
Figure 1.
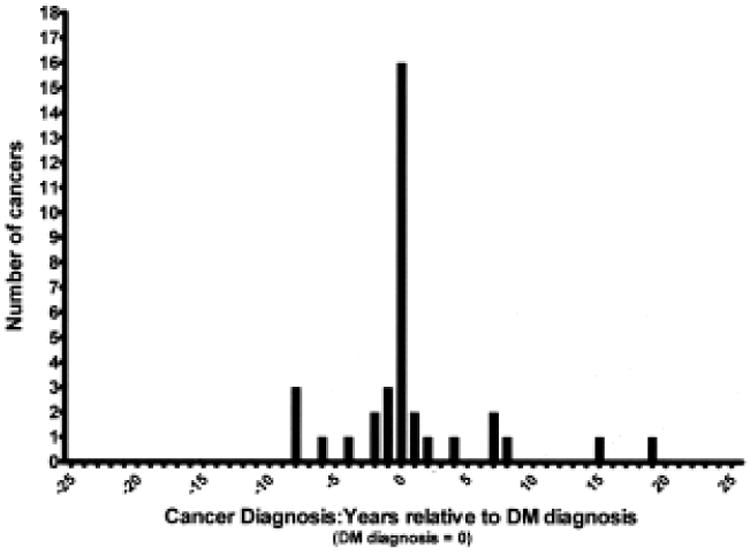
Chart review revealed a clear temporal relationship between cancer diagnosis and dermatomyositis (DM) diagnosis.
Figure 2.
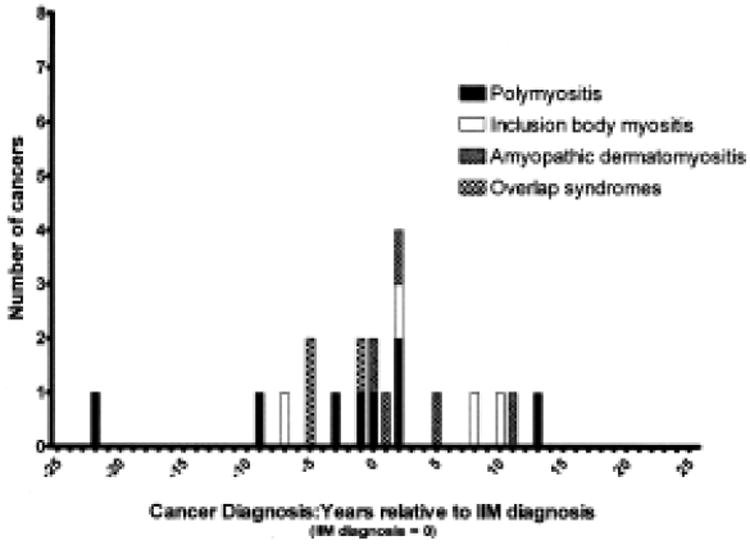
The occurrence of cancer in patients with non-dermatomyositis idiopathic inflammatory myopathy (IIM) is dispersed over time.
Table 1.
Tumors found in association with dermatomyositis (DM).
| Primary Organ | No. | Histology |
|---|---|---|
| Breast | 6 | Carcinoma (6) |
| Bladder | 1 | Squamous (1) |
| Cervix | 1* | Squamous (1) |
| Colon | 3 | Adenocarcinoma (3), including 1 rectal |
| Endometrium | 1 | Adenocarcinoma (1) |
| Larynx | 1** | Squamous (1) |
| Lung | 5 | Small-cell (3), squamous (1), adenocarcinoma (1) |
| Ovary | 2 | Adenocarcinoma (1), cystadenocarcinoma (1) |
| Pancreas | 3 | Adenocarcinoma (2), neuroendocrine (1) |
| Parotid | 1 | Mixed (1) |
| Prostate | 1 | Adenocarcinoma (1) |
| Unknown | 1 | Adenocarcinoma (1) |
Patient initially developed DM with cervical cancer. DM returned in association with a mixed parotid tumor 7 years later.
Patient initially developed DM with squamous cell cancer of the piriform sinus. DM returned in association with adenocarcinoma of the lung 8 years later.
Age and sex in DM and cancer-associated DM
Table 2 shows the age and gender distribution of patients with IIM. Patients with DM were predominantly female (75.4%). In contrast, patients with cancer-associated DM were more frequently male than those without cancer (33.3% vs 18.9%). The average age at the time of diagnosis with DM was 56.7 years. Patients with cancer-associated DM were more frequently ≥ 45 years of age than those without cancer (95.8% vs 73%; OR 8.52, 95% CI 1.01–71.7). DM patients did not differ significantly in age from those with PM or amyopathic DM. Patients with overlap syndrome were younger than DM patients (48.7 vs 56.7 years; p = 0.04). The average age of JDM patients at time of diagnosis was 9.2 years.
Table 2.
Characteristics of patients with idiopathic inflammatory myopathy.
| Diagnosis | Patients, n | Age, yrs, mean + SD | % Male |
|---|---|---|---|
| Dermatomyositis (DM) | 61 | 56.7 ± 15.0 | 24.6 |
| With cancer | 24 | 60.1 ±11.4 | 33.3 |
| Without cancer | 37 | 54.6 ±16.7 | 18.9 |
| Polymyositis | 63 | 55.7 ± 14.3 | 31.8 |
| With cancer | 3 | 72.0 ±8.7 | 100.0 |
| Without cancer | 60 | 54.9 ± 14.0 | 28.3 |
| Amyopathic DM | 23 | 54.4 ±12.7 | 30.0 |
| With cancer | 3 | 50.0 ±2.6 | 33.3 |
| Without cancer | 20 | 55.1 ± 13.5 | 30.0 |
| Overlap syndromes | 22 | 48.7 ±17.1 | 27.3 |
| With cancer | 1 | 83 | 0.0 |
| Without cancer | 21 | 47.0 ± 15.7 | 28.6 |
| Inclusion body myositis | 11 | 64.7 ±6.2 | 72.7 |
| With cancer | 1 | 64 | 0.0 |
| Without cancer | 10 | 64.8 ±6.5 | 80.0 |
| Juvenile dermatomyositis | 18 | 9.2 ±6.5 | 27.8 |
| With cancer | 0 | NA | NA |
| Without cancer | 18 | 9.2 ±5.6 | 27.8 |
Laboratory testing
ANA testing was performed in 73.8% of patients with DM. A positive ANA was found in 52.9% of patients with cancer-associated DM, and in 71.4% of patients with DM without cancer. The rate of ANA testing did not differ between these groups. There was no difference in the results of ENA testing between DM patients with and those without neoplasm. Myositis panel testing was performed in 6 patients with cancer-associated DM and 8 DM patients without cancer; these tests were positive in 0 and 3 patients, respectively. The mean or median CPK elevation was greater in cancer-associated DM, but this was not statistically significant. Similarly, more patients with cancer-associated DM had an elevated CPK at the time of diagnosis (16/20, 80%), but again this difference was not significant compared to DM without cancer (22/35, 62.9%). There was no difference in the frequency of abnormal results for RF, Jo-1 antigen, anti-dsDNA, ACA, ESR, CRP, LDH, aldolase, or muscle biopsy among DM patients with and those without cancer (some data not shown). Table 3 shows the results of univariate analysis comparison between DM patients with and without cancer. A multivariate logistic regression failed to show any relationship between male sex, age at DM diagnosis, and CPK elevation with the presence of cancer (data not shown).
Table 3. Potential risk factors for cancer among patients with dermatomyositis.
| Variables | No. with Cancer/Total Assessed (%) | No. without Cancer/Total Assessed (%) | OR | 95% CI | p |
|---|---|---|---|---|---|
| Age ≥ 45 yrs | 23/24 (95.8) | 27/37 (73.0) | 8.52 | (1.01, 71.7) | 0.038 |
| Male | 8/24 (33.3) | 7/37 (18.9) | 2.14 | (0.66, 6.99) | 0.21 |
| Positive biopsy | 4/5 (80.0) | 12/18 (66.7) | 2.00 | (0.18, 22.07) | 1 |
| ILD | 0/24 (0) | 6/37 (16.2) | 0.11* | (0.006, 2.02)* | 0.14* |
| Elevated CPK | 16/20 (80.0) | 22/35 (62.9) | 2.36 | (0.65, 8.61) | 0.19 |
| ESR ≥ 25 mm/h | 12/16 (75.0) | 15/27 (55.6) | 2.40 | (0.61, 9.38) | 0.21 |
| Positive ANA | 9/17 (52.9) | 20/28 (71.4) | 0.45 | (0.13, 1.58) | 0.21 |
| Positive ENA | 0/8 (0) | 2/18 (11.1) | 0.50* | (0.02, 12.43)* | 0.67* |
| Positive myositis panel | 0/6 (0) | 3/8 (37.5) | 0.14* | (0.005, 3.48)* | 0.23* |
| Positive RF | 0/10 (0) | 2/15 (13.3) | 0.33* | (0.01, 8.03)* | 0.49* |
| Positive dsDNA | 0/6 (0) | 0/12 (0) | NA | NA | |
| Positive Jo-1 | 0/11 (0) | 0/26 (0) | NA | NA |
0.5 added for cells containing zero. ILD: interstitial lung disease; CPK: creatine phosphokinase; ESR: erythrocyte sedimentation rate; ANA: antinuclear antibodies; ENA: extractable nuclear antigens; RF: rheumatoid factor; dsDNA: anti-double-stranded DNA antibodies.
Interstitial lung disease
We showed a relationship between ILD and cancer-associated IIM. There were no cases of ILD in the 24 patients with DM and an associated malignancy, while 6/37 DM patients without cancer had ILD (Table 3). Looking at all patients with IIM, ILD was found in 27 patients; 6 with DM (22.2%), 16 with PM (59.3%), 1 with amyopathic DM (3.7%), and 4 with overlap syndromes (14.8%). There were no cases of ILD among the IBM or pediatric patients. There were no cases of ILD among patients with cancer in any of the IIM groups (estimated OR 0.07, 95% CI 0.004–1.18).
Standardized incidence ratios
The CDC WONDER online database22 contains the cancer incidence statistics for the United States between 1999 and 2002. We utilized the information from this database for Vermont and New Hampshire to calculate the SIR for cancer in all IIM patients relative to these geographical controls over 2 time periods. First, we examined the 5-year window incorporating the 2 years before and 3 years following the diagnosis with IIM (Table 4). Within this timeframe, we found a significant elevation in cancer incidence in both men (SIR 3.19, 95% CI 1.65–5.58) and women (SIR 3.68, 95% CI 2.25–5.68) with IIM. This increased risk was concentrated exclusively in the patients with DM, where a greater than 7-fold increase in SIR was seen for both women and men.
Table 4.
Observed and expected numbers of cancers diagnosed within 2 years preceding and 3 years following diagnosis of idiopathic inflammatory myopathy, with standardized incidence ratio (SIR).
| Diagnosis | Gender | Observed | Expected | SIR (95% CI) | p, 2-tailed |
|---|---|---|---|---|---|
| DM | Female | 16 | 2.15 | 7.44 (4.25, 12.09) | < 0.0001 |
| DM | Male | 8 | 1.01 | 7.89 (3.41, 15.55) | < 0.0001 |
| PM | Female | 0 | 1.77 | 0 (0, 2.09) | 0.34 |
| PM | Male | 3 | 1.64 | 1.82 (0.38, 5.33) | 0.46 |
| ADM | Female | 2 | 0.72 | 2.76 (0.33, 9.98) | 0.33 |
| ADM | Male | 1 | 0.24 | 4.19 (0.11, 23.35) | 0.42 |
| IBM | Female | 1 | 0.22 | 4.46 (0.11, 24.88) | 0.40 |
| IBM | Male | 0 | 0.72 | 0 (0, 5.09) | 0.97 |
| JDM | Female | 0 | 0.01 | 0 (0, 446.8) | 1.00 |
| JDM | Male | 0 | 0.01 | 0 (0, 685.02) | 1.00 |
| MCTD | Female | 1 | 0.56 | 1.79 (0.05, 9.97) | 0.86 |
| MCTD | Male | 0 | 0.13 | 0 (0, 27.81) | 1.00 |
| All | Female | 20 | 5.43 | 3.68 (2.25, 5.68) | < 0.0001 |
| All | Male | 12 | 3.76 | 3.19 (1.65, 5.58) | < 0.01 |
DM: dermatomyositis; PM: polymyositis; ADM: amyopathic DM; IBM: inclusion body myositis; JDM: juvenile DM; MCTD: myositis associated with connective tissue disease; All: summed data for all IIM diagnoses.
Second, we examined the lifetime risk of cancer following the diagnosis of IIM to determine whether this association with cancer was maintained over time. In contrast to the window surrounding the diagnosis of IIM, the elevation in lifetime cancer incidence in IIM and DM was more modest. A statistically significant increase was seen in female patients with IIM (SIR 2.17, 95% CI 1.31–3.39) and female patients with DM (SIR 5.57, 95% CI 2.97–9.53). In contrast, increases in cancer incidence in male IIM and DM patients were not found to be statistically significant. The remaining IIM diagnoses demonstrated less precise estimates of SIR, which did not yield statistically significant elevations in cancer incidence in either sex (Table 5).
Table 5.
Observed and expected numbers of cancers diagnosed during lifetime following diagnosis of idiopathic inflammatory myopathy, with standardized incidence ratio (SIR).
| Diagnosis | Gender | Observed | Expected | SIR (95% CI) | p, 2-tailed |
|---|---|---|---|---|---|
| DM | Female | 13 | 2.33 | 5.57 (2.97, 9.53) | < 0.0001 |
| DM | Male | 4 | 1.17 | 3.43 (0.94, 8.79) | 0.06 |
| PM | Female | 1 | 2.9 | 0.34 (0.01, 1.92) | 0.43 |
| PM | Male | 2 | 1.42 | 1.41 (0.17, 5.1) | 0.83 |
| ADM | Female | 3 | 1.34 | 2.24 (0.46, 6.56) | 0.30 |
| ADM | Male | 2 | 0.46 | 4.36 (0.53, 15.76) | 0.16 |
| IBM | Female | 2 | 0.56 | 3.59 (0.44, 12.98) | 0.22 |
| IBM | Male | 1 | 1.46 | 0.68 (0.02, 3.81) | 1.00 |
| JDM | Female | 0 | 0.04 | 0 (0, 102.43) | 1.00 |
| JDM | Male | 0 | 0.01 | 0 (0, 386.81) | 1.00 |
| MCTD | Female | 0 | 1.58 | (0, 2.33) | 0.41 |
| MCTD | Male | 0 | 0.16 | 0 (0, 23.74) | 1.00 |
| All | Female | 19 | 8.75 | 2.17 (1.31, 3.39) | < 0.01 |
| All | Male | 9 | 4.67 | 1.93 (0.88, 3.66) | 0.10 |
DM: dermatomyositis; PM: polymyositis; ADM: amyopathic DM; IBM: inclusion body myositis; JDM: juvenile DM; MCTD: myositis associated with connective tissue disease; All: summed data for all IIM diagnoses.
Discussion
The majority of published studies report an association between DM and malignancy7-15,17,24-28. Some studies have recorded the relationship within 1 year of diagnosis26, while others have ranged from 5 years to over a lifetime14,27. We used 5 years (2 years prior and 3 following IIM)14 for our initial assessment, and found that 24 of 61 DM patients (39.3%) were diagnosed with a malignancy in this interval. Most of these cancers (16/24, 75%) occurred within 1 year prior to or following the diagnosis of DM, confirming other reports8,10,13,14,17,26,27. This proximity of cancer to IIM raises the question of whether increased surveillance at time of DM diagnosis accounts for the observed increase in malignancy. However, an increased risk of malignancy in the time following the diagnosis of DM has been noted even when the initial year of followup is excluded7,9,12. In our series, the types of cancer seen with DM were all solid tumors; no cases of lymphoma were seen.
Using geographical controls permitted by the CDC WONDER database, we found that the cancer incidence rate in the window surrounding the diagnosis of IIM was increased more than 7-fold for both men and women and was limited to those with DM. In contrast, when patients with IIM who presented with malignancy were excluded and subsequent “lifetime” occurrences of cancer were analyzed, we found a significantly increased risk of malignancy in women with DM (SIR 5.57, 95% CI 2.97–9.53), and an elevation that approached significance in men with DM (SIR 3.43, 95% CI 0.94–8.79). There was no significant association with cancer and other types of IIM, although the potential for such an association was not entirely excluded by our data.
A potential weakness of our study is the possible existence of referral bias, which has been invoked in the past to question the authenticity of the association between IIM and malignancy5. The majority of dermatologic and rheumatologic care in the surrounding regions of Vermont and New Hampshire occurred at this institution over the duration of the study. However, given the absence of public registries of either patients with cancer or patients with inflammatory myopathy in this region, we cannot exclude the possibility of referral bias. In addition, our study represented a single-institution review of a rare disease that yielded 198 study subjects.
Our results parallel those found by Airio, et al, who observed that the relative risk of cancer was significantly elevated (SIR 6.5, 95% CI 3.9–10) among patients with DM but not patients with PM in a population-based study of IIM in Finland13. The similarity in our results despite the difference in our patients (single institution vs national population) suggests that our findings are not confounded by referral bias or unique demographic factors. Indeed, the similarity of our findings despite different ethnic groups (Finnish vs a US population of European descent) is notable.
While several large studies have reported a significant relationship between PM and cancer7-12, we did not observe this, nor was there a temporal relationship between the few observed cancers and the onset of PM. Callen, et al first demonstrated a stronger association between cancer and DM than with PM15, and others have published similar findings13,14,16,17. The accuracy of diagnostic criteria for PM may contribute to this variability29,30. We utilized both ICD-9 codes and chart review from office visits and hospital admissions. This is in contrast to previous studies, which examined hospital admissions using ICD codes or chart review to confirm diagnoses based on combined clinical and diagnostic data8-11,14,18,27,28,31-33. Had we relied on ICD codes without chart review, we would have found that 24.8% of patients with DM and 12.6% of patients with PM were diagnosed with a malignancy over the specified 5-year interval.
This result emphasizes the importance of accurate diagnosis in precisely defining the cancer risk in IIM. Each patient included in this study was individually reviewed by 1 or more of the authors to ensure diagnostic accuracy. We found that only 198 of 483 (41%) patients identified on the basis of ICD-9 codes met criteria for inclusion in our study. While this exclusion rate seems high, it is similar to the rate of exclusion reported when both ICD codes and individual chart review were employed by Airio, et al13. In addition to observing a similar rate of exclusion on the basis of chart review, these authors observed a comparable association of cancer with DM but not PM. Diagnoses encountered among excluded patients included diseases that are sometimes confused with IIM, such as polymyalgia rheumatica, fibromyalgia, and vasculitis. The majority of miscoded patients' charts, however, included no mention of a suspected myopathy.
We attempted to identify factors that are predictive of the presence of cancer in patients with IIM (Table 3). Patients with malignancy tended to be older and more frequently male. Studies have shown an increased risk of malignancy with increased age8,11,14,17,25,31,34 and male sex14,17,25,31. In contrast to other studies5,14, we did not find a significantly higher CPK among patients with DM without cancer. The lone risk factor for malignancy of statistical significance by univariate analysis was age ≥ 45 years (OR 8.52, 95% CI 1.01–71.7). Multivariate analysis of sex, age, and CPK elevation failed to demonstrate any significant association with cancer risk. We found that ILD did not occur in any patients with cancer, in support of previous studies that have demonstrated a decreased risk of cancer in patients with ILD14,31.
Our study found a clear linkage between DM and malignancy. This was not seen with other forms of IIM. Of 61 patients with DM, 24 (39.3%) developed a tumor in association with their myositis diagnosis. In the DM population, a strong temporal relationship was seen, with 16/24 (75%) occurring within 1 year of the diagnosis of DM. There was less statistical evidence to suggest an increased risk of malignancy in patients with PM or other types of IIM. In the DM population, a relationship was observed between cancer and age, male sex, and the absence of ILD. Along with the lack of an observed relationship of cancer with either PM or amyopathic DM, our data indicate that a unique relationship between the tumor and the host immune system account for the concurrent targeting of muscle and skin, resulting in the specific characteristics of DM. Further, the manifestations of this autoimmune process in cancer-associated DM are highly specific, yielding classical skin findings but apparent sparing of the lung.
References
- 1.Bohan A, Peter JB. Polymyositis and dermatomyositis (parts 1 and 2) N Engl J Med. 1975;292:344–7. 403–7. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter S, Karpati G, Heller I, Eisen A. Inclusion body myositis: a distinct variety of idiopathic inflammatory myopathy. Neurology. 1978;28:8–17. doi: 10.1212/wnl.28.1.8. [DOI] [PubMed] [Google Scholar]
- 3.Euwer RL, Sontheimer RD. Amyopathic dermatomyositis: a review. J Invest Dermatol. 1993;100:124S–127S. doi: 10.1111/1523-1747.ep12356896. [DOI] [PubMed] [Google Scholar]
- 4.Stertz G. Polymyositis. Berl Klin Wochenschr. 1916;53:489. [Google Scholar]
- 5.Lakhanpal S, Bunch TW, Ilstrup DM, Melton LJ., 3rd Polymyositis-dermatomyositis and malignant lesions: does an association exist? Mayo Clin Proc. 1986;61:645–53. doi: 10.1016/s0025-6196(12)62030-8. [DOI] [PubMed] [Google Scholar]
- 6.Lyon MG, Bloch DA, Hollak B, Fries JF. Predisposing factors in polymyositis-dermatomyositis: results of a nationwide survey. J Rheumatol. 1989;16:1218–24. [Google Scholar]
- 7.Buchbinder R, Forbes A, Hall S, Dennett X, Giles G. Incidence of malignant disease in biopsy-proven inflammatory myopathy. A population-based cohort study. Ann Intern Med. 2001;134:1087–95. doi: 10.7326/0003-4819-134-12-200106190-00008. [DOI] [PubMed] [Google Scholar]
- 8.Chow WH, Gridley G, Mellemkjaer L, McLaughlin JK, Olsen JH, Fraumeni JF., Jr Cancer risk following polymyositis and dermatomyositis: a nationwide cohort study in Denmark. Cancer Causes Control. 1995;6:9–13. doi: 10.1007/BF00051675. [DOI] [PubMed] [Google Scholar]
- 9.Hill CL, Zhang Y, Sigurgeirsson B, Pukkala E, Mellemkjaer L, Airio A, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96–100. doi: 10.1016/S0140-6736(00)03540-6. [DOI] [PubMed] [Google Scholar]
- 10.Sigurgeirsson B, Lindelöf B, Edhag O, Allander E. Risk of cancer in patients with dermatomyositis or polymyositis. A population-based study. N Engl J Med. 1992;326:363–7. doi: 10.1056/NEJM199202063260602. [DOI] [PubMed] [Google Scholar]
- 11.Stockton D, Doherty VR, Brewster DH. Risk of cancer in patients with dermatomyositis or polymyositis, and follow-up implications: a Scottish population-based cohort study. Br J Cancer. 2001;85:41–5. doi: 10.1054/bjoc.2001.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zantos D, Zhang Y, Felson D. The overall and temporal association of cancer with polymyositis and dermatomyositis. J Rheumatol. 1994;21:1855–9. [PubMed] [Google Scholar]
- 13.Airio A, Pukkala E, Isomäki H. Elevated cancer incidence in patients with dermatomyositis: a population based study. J Rheumatol. 1995;22:1300–3. [PubMed] [Google Scholar]
- 14.Andras C, Ponyi A, Constantin T, Csiki Z, Szekanecz E, Szodoray P, et al. Dermatomyositis and polymyositis associated with malignancy: a 21-year retrospective study. J Rheumatol. 2008;35:438–44. [PubMed] [Google Scholar]
- 15.Callen JP, Hyla JF, Bole GG, Jr, Kay DR. The relationship of dermatomyositis and polymyositis to internal malignancy. Arch Dermatol. 1980;116:295–8. [PubMed] [Google Scholar]
- 16.Holden DJ, Brownell AK, Fritzler MJ. Clinical and serologic features of patients with polymyositis or dermatomyositis. Can Med Assoc J. 1985;132:649–53. [PMC free article] [PubMed] [Google Scholar]
- 17.Wakata N, Kurihara T, Saito E, Kinoshita M. Polymyositis and dermatomyositis associated with malignancy: a 30-year retrospective study. Int J Dermatol. 2002;41:729–34. doi: 10.1046/j.1365-4362.2002.01648.x. [DOI] [PubMed] [Google Scholar]
- 18.Ang P, Sugeng MW, Chua SH. Classical and amyopathic dermatomyositis seen at the National Skin Centre of Singapore: a 3-year retrospective review of their clinical characteristics and association with malignancy. Ann Acad Med Singapore. 2000;29:219–23. [PubMed] [Google Scholar]
- 19.Fung WK, Chan HL, Lam WM. Amyopathic dermatomyositis in Hong Kong — association with nasopharyngeal carcinoma. Int J Dermatol. 1998;37:659–63. doi: 10.1046/j.1365-4362.1998.00453.x. [DOI] [PubMed] [Google Scholar]
- 20.Sherry DD, Haas JE, Milstein JM. Childhood polymyositis as a paraneoplastic phenomenon. Pediatr Neurol. 1993;9:155–6. doi: 10.1016/0887-8994(93)90055-h. [DOI] [PubMed] [Google Scholar]
- 21.Whitmore SE, Watson R, Rosenshein NB, Provost TT. Dermatomyositis sine myositis: association with malignancy. J Rheumatol. 1996;23:101–5. [PubMed] [Google Scholar]
- 22.Cancer Registry Public Information Data: 1999–2002, WONDER On-line Database. [Accessed September 24, 2009];US Department of Health and Human Services, National Program of Cancer Registries, Centers for Disease Control and Prevention. 2005 Nov; Available at: http://wonder.cdc.gov/cancer-v2002.html.
- 23.Sahai H, Khurshid A. Confidence intervals for the mean of a Poisson distribution: a review. Biometrical J. 1993;35:857–67. [Google Scholar]
- 24.Barnes BE, Mawr B. Dermatomyositis and malignancy. A review of the literature. Ann Intern Med. 1976;84:68–76. doi: 10.7326/0003-4819-84-1-68. [DOI] [PubMed] [Google Scholar]
- 25.Basset-Seguin N, Roujeau JC, Gherardi R, Guillaume JC, Revuz J, Touraine R. Prognostic factors and predictive signs of malignancy in adult. A study of 32 cases. Arch Dermatol. 1990;126:633–7. [PubMed] [Google Scholar]
- 26.Bonnetblanc JM, Bernard P, Fayol J. Dermatomyositis and malignancy. A multicenter cooperative study. Dermatologica. 1990;180:212–6. [PubMed] [Google Scholar]
- 27.Manchul LA, Jin A, Pritchard KI, Tenenbaum J, Boyd NF, Lee P, et al. The frequency of malignant neoplasms in patients with polymyositis-dermatomyositis. A controlled study. Arch Intern Med. 1985;145:1835–9. [PubMed] [Google Scholar]
- 28.Maoz CR, Langevitz P, Livneh A, Blumstein Z, Sadeh M, Bank I, et al. High incidence of malignancies in patients with dermatomyositis and polymyositis: an 11-year analysis. Semin Arthritis Rheum. 1998;27:319–24. doi: 10.1016/s0049-0172(98)80052-8. [DOI] [PubMed] [Google Scholar]
- 29.van der Meulen MF, Bronner IM, Hoogendijk JE, Burger H, van Venrooij WJ, Voskuyl AE, et al. Polymyositis: an overdiagnosed entity. Neurology. 2003;61:316–21. doi: 10.1212/wnl.61.3.316. [DOI] [PubMed] [Google Scholar]
- 30.Amato AA, Griggs RC. Unicorns, dragons, polymyositis, and other mythological beasts. Neurology. 2003;61:288–9. doi: 10.1212/wnl.61.3.288. [DOI] [PubMed] [Google Scholar]
- 31.Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case-control study. Br J Dermatol. 2001;144:825–31. doi: 10.1046/j.1365-2133.2001.04140.x. [DOI] [PubMed] [Google Scholar]
- 32.Leow YH, Goh CL. Malignancy in adult dermatomyositis. Int J Dermatol. 1997;36:904–7. doi: 10.1046/j.1365-4362.1997.00190.x. [DOI] [PubMed] [Google Scholar]
- 33.Oddis CV, Conte CG, Steen VD, Medsger TA., Jr Incidence of polymyositis-dermatomyositis: a 20-year study of hospital diagnosed cases in Allegheny County, PA 1963-1982. J Rheumatol. 1990;17:1329–34. [PubMed] [Google Scholar]
- 34.Chinoy H, Fertig N, Oddis CV, Ollier WE, Cooper RG. The diagnostic utility of myositis autoantibody testing for predicting the risk of cancer-associated myositis. Ann Rheum Dis. 2007;66:1345–9. doi: 10.1136/ard.2006.068502. [DOI] [PMC free article] [PubMed] [Google Scholar]


