Supplemental Digital Content is available in the text.
Key Words: acute lymphoblastic leukemia, osteonecrosis, corticosteroid, dexamethasone
Abstract
Steroid-induced osteonecrosis (ON) is a challenging complication encountered during modern chemotherapy for childhood acute lymphoblastic leukemia (ALL). We retrospectively assessed the incidence of ON and its risk factors in a total of 1095 patients enrolled in 3 consecutive Japanese Children’s Cancer and Leukemia Study Group ALL studies (ALL941 [1994 to 2000], n=464; ALL2000 [2000 to 2004], n=305; and ALL2004 [2004 to 2010], n=326). ON was diagnosed in 16 patients, of whom 15 were symptomatic. The cumulative incidence of ON was 0.76% in ALL941, 0.35% in ALL2000, and 3.6% in ALL2004. The incidence of ON in ALL941/2000, in which only prednisolone was administered as a steroid, was significantly lower than that in ALL2004, in which dexamethasone was used as a partial substitute for prednisolone (P<0.01). In ALL2004, sex and age were significantly correlated with the incidence of ON (1.3% in boys vs. 6.7% in girls, P=0.0132; 0.42% for age <10 y vs. 15.6% for age ≥10 y, P<0.0001), suggesting that girls aged 10 years and above are at a greater risk of ON onset. These results indicate that the risk of ON should be considered when administering dexamethasone as part of ALL protocol treatment in girls aged 10 years and above.
Recent advances in treatment strategies for childhood acute lymphoblastic leukemia (ALL) have improved the overall survival rate by 80% to 90%.1–3 Enhanced chemotherapeutic agents, refined risk classification criteria, and improved supportive care have contributed to these high cure rates, but significant toxicity remains a major risk factor that causes long-term morbidity and decreased quality of life. Osteonecrosis (ON) has been increasingly documented in pediatric ALL and presents a challenging complication during modern chemotherapy.4–7 ON can result in joint dysfunction and subsequent impairments in activities of daily living among long-term survivors.8,9 Well-known risk factors for ON include age above 10 years, female sex, and use of dexamethasone (DEX).5,7 Although the precise pathophysiology of ON remains unknown, corticosteroid administration has been shown to induce ischemia, upregulate apoptosis of osteoblasts and osteocytes, and prolong osteoclast lifespans.10
Most previous studies regarding ON in children with ALL have been limited to European and North American study groups, as there is little data concerning Japanese or Asian patients. Therefore, the aim of the present study was to assess the incidence, risk factors, and morbidity of corticosteroid-induced ON in ALL studies conducted by the Japanese Childhood Cancer and Leukemia Study Group (JCCLSG). We retrospectively analyzed the data of 1095 patients enrolled in 3 consecutive ALL studies (ALL941, ALL2000, and ALL2004) conducted by the JCCLSG. Prednisolone (PSL) was used as the primary corticosteroid in all studies, with DEX acting as a partial substitute for PSL in ALL2004. ON patients were practically identified by symptoms and ON was confirmed with imaging studies in all patients.
MATERIALS AND METHODS
Patients and Treatment
ALL941, ALL2000, and ALL2004 were conducted between 1994 and 2000, 2000 and 2004, and 2004 and 2010, respectively. The therapies on these studies were risk adjusted but not randomized. Patients enrolled in ALL941 and ALL2000 were aged 1 to 15 years, whereas those enrolled in ALL2004 were aged 1 to 18 years. All participants were newly diagnosed with B-precursor ALL or T-cell ALL, and those with a mature B-cell phenotype and Philadelphia chromosome-positive ALL were excluded. All studies were conducted across 18 hospitals that were members of the JCCLSG. The study protocol was approved by the institutional review board of each study, and written informed consent was provided by the patients or their legal guardians before treatment.
The treatment protocols adopted in ALL941 and ALL2000 were reported previously.11,12 The patients were stratified according to leukocyte count and age at the time of diagnosis into standard-risk (SR), high-risk (HR), and high-high-risk (HHR) groups. The ALL941 and ALL2000 study protocols were almost identical except for the addition of doxorubicin administration to patients with a leukocyte count <10,000/µL and age below 5 years in ALL2000. Treatment schedules and adopted drugs are briefly described in the supplement (see Supplemental Digital Content 1, Table 1,http://links.lww.com/JPHO/A55).
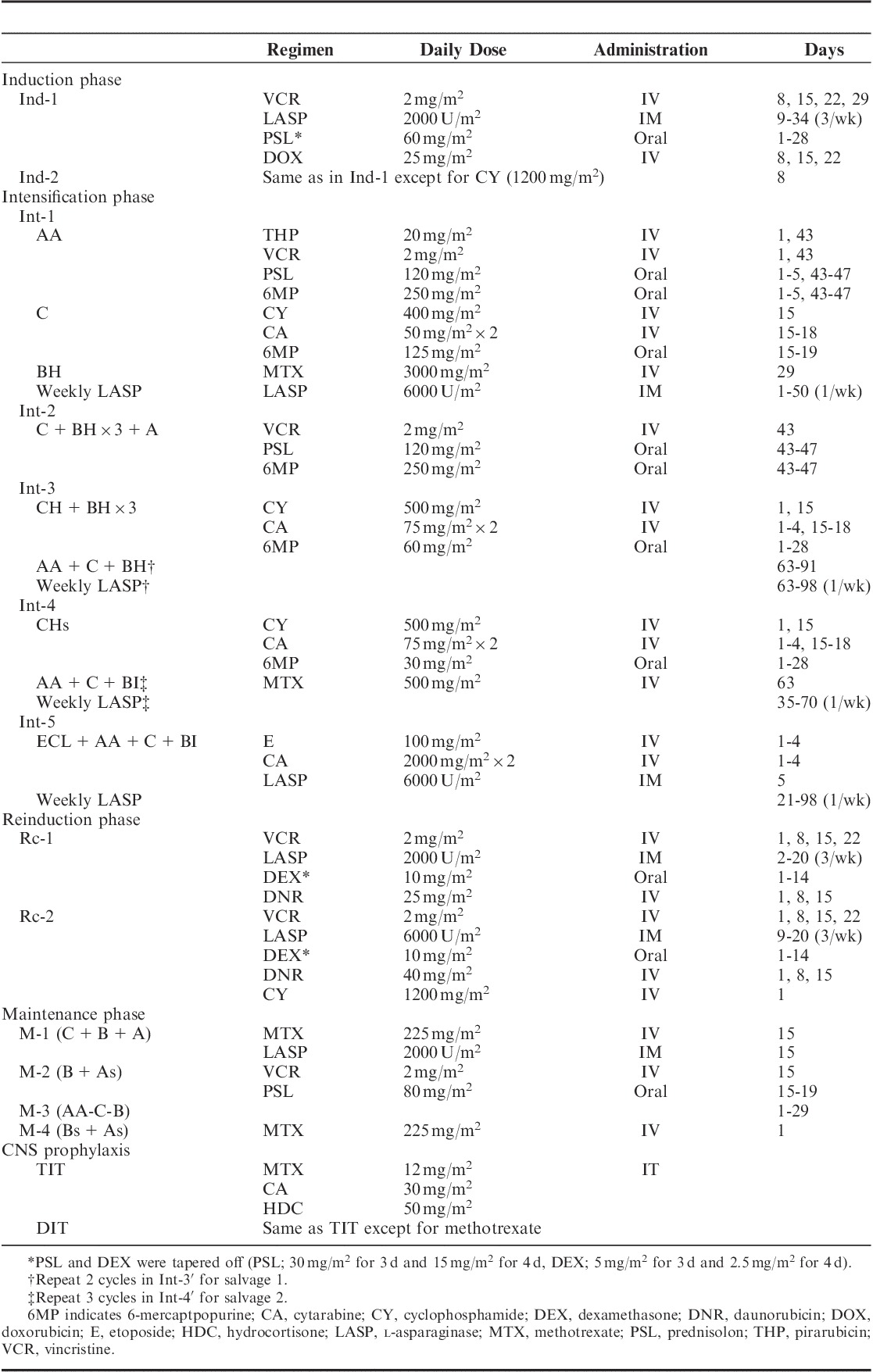
The ALL2004 treatment protocols are described in Figure 1 and Table 1. Previous risk classification criteria were modified according to the National Cancer Institute criteria,13 resulting in a shift from HR to SR in 6- to 9-year-old patients with leukocyte counts of 5000 to 10,000 cells/µL. After a 7-day PSL regimen, induction therapy in the SR and HR groups was almost identical to that in previous studies.11,12 In the HHR group, cyclophosphamide was added on day 8. After achieving complete remission, all risk groups received the same intensification therapy (Int-1). At week 15, SR patients with MRD levels <10−3 received further intensification therapy (Int-2) that was followed by maintenance therapy (M-1 and M-2) until week 110. In the HR and HHR groups, patients with MRD levels <10−3 at week 15 received 2 cycles of reinduction/intensification therapy (Rc1/Int-2 and Rc1/Int-3 in HR, Rc2/Int-4 and Rc2/Int-5 in HHR group) that was followed by the same maintenance therapy (M-3 and M-4) until week 165. Patients with MRD levels ≥10−3 at week 12 in the SR and HR/HHR groups were assigned to salvage arms 1 and 2, respectively. In the salvage regimen, patients received intensification therapy comprising 2 cycles of Rc-2/etopside+cytarabine+l-asparaginase. For CNS prophylaxis, SR and HR patients received extended TIT injections beginning from day 1. When IT was combined with a high dose of methotrexate, only cytarabine and hydrocortisone were injected (double intrathecal [DIT] injection). TIT injections were repeated every 6 weeks in the first year, every 8 weeks in the second year, and every 12 weeks in the third year. The HHR group patients included in salvage arm 2 received 18 Gy of CRT in addition to 6 and 7 doses of TIT injections until week 22 and 32 of therapy, respectively.
FIGURE 1.
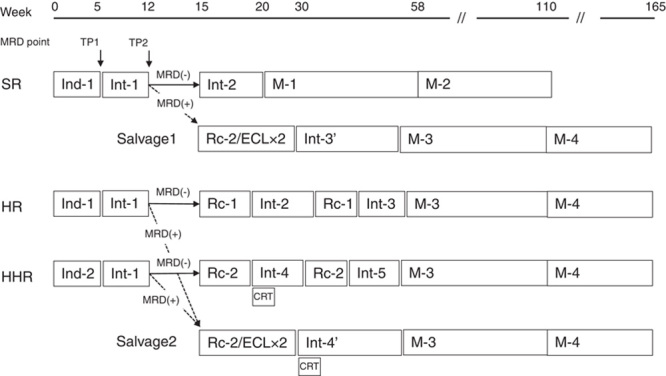
Treatment framework and minimal residual disease (MRD) stratification in the ALL2004 study. Patients with MRD levels ≥10−3 at week 12 received salvage therapy (dotted arrows), whereas the remainder continued to receive the initial risk-adapted therapy (solid arrows). Treatment schedules are shown in Table 1. CRT indicates cranial radiotherapy; HR, high-risk group; HHR, high-high-risk group; SR, standard-risk group.
TABLE 1.
Drug Dosage and Schedule for ALL2004
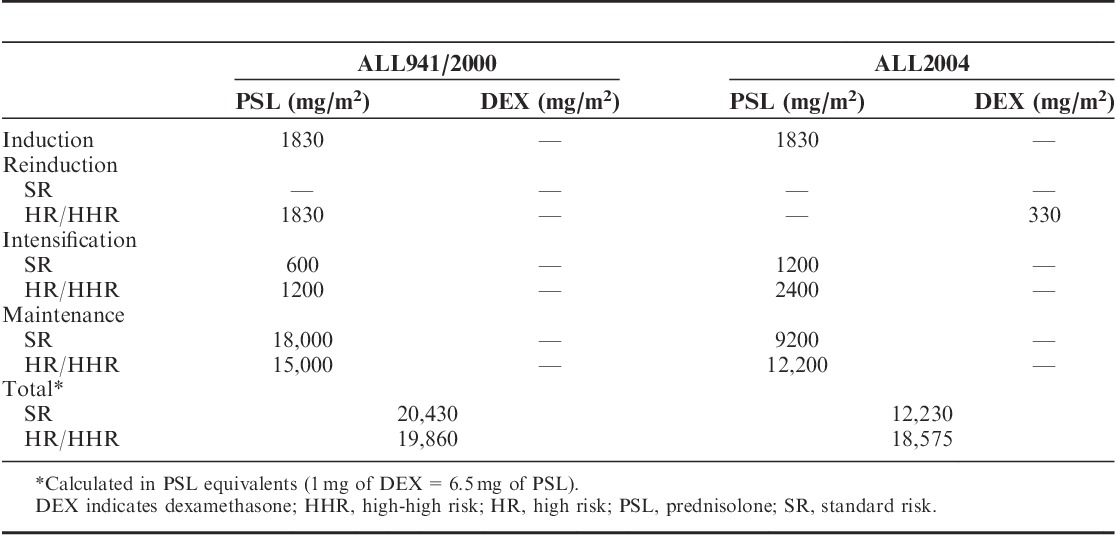
Cumulative doses of the corticosteroids administered in ALL941/2000 and ALL2004 are listed in Table 2.
TABLE 2.
Cumulative Dose of Corticosteroid in Trials ALL941/2000 and ALL2004
Identification of ON Patients
Because the significance of this therapy-related toxicity had not been fully appreciated until the early 2000s, case report form in the 3 studies did not request data regarding ON. Thus, cases with ON were collected by the questionnaire specified for ON to the investigators of JCCLSG. Most of the ON patients were identified based on clinical symptoms such as bone pain and further confirmed with diagnostic imaging studies (x-ray/magnetic resonance imaging [MRI]) by the each institutional radiologists, except one who was asymptomatic and diagnosed by imaging studies at the discretion of primary physician.
Statistical Analysis
Differences in the categorical variables of patient characteristics were analyzed using the χ2 test. The cumulative incidence of ON during the study period was estimated using Kaplan-Meier analysis. The median follow-up periods were 147.4, 100.3, and 43.6 months for patients enrolled in ALL941, ALL2000, and ALL2004, respectively (median follow-up period, 78.7 mo for all patients). Differences in cumulative incidence between patient subsets were tested using the log-rank test.
RESULTS
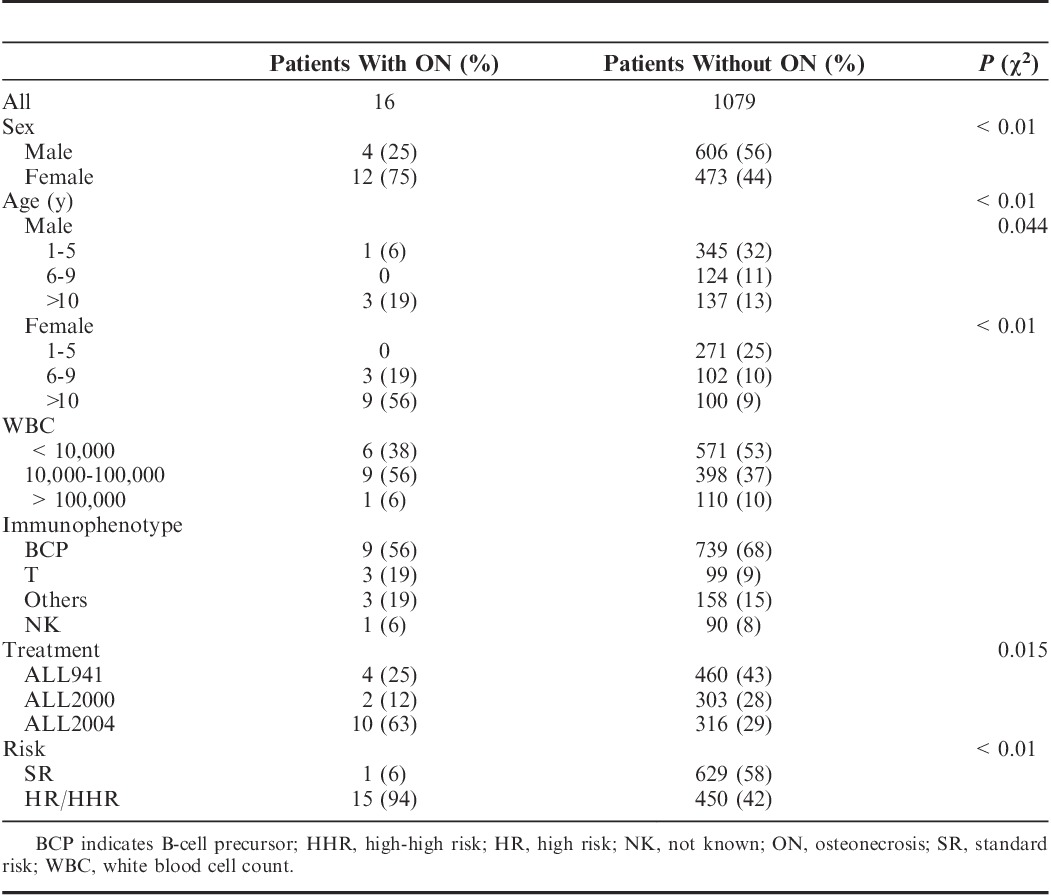
A total of 1095 patients were enrolled in the present study (ALL941, n=464; ALL2000, n=305; and ALL2004, n=326). Sixteen of 1095 patients developed ON during or after treatment, 4 (0.86%), 2 (0.65%), and 10 (3.1%) were in ALL941, ALL2000, and ALL2004, respectively. In patients with ON, the median age at diagnosis of ALL was 11.5 years (range, 5 to 16 y) and the male to female ratio was 1:3. When patients were evaluated by risk classification, only 1 patient in the SR group and 15 patients in the HR/HHR groups developed ON. Comparisons of the characteristics of patients with and without ON are presented in Table 3, which shows a predominance of females aged 10 years and above, treatment with ALL2004, and high risk (P<0.01) in patients with ON. Notably, 9 of the 12 female and 3 of the 4 male patients with ON were aged 10 years and above, the latter was marginally significant (P=0.044). ON was diagnosed at median treatment weeks 56.5 (range, 32 to 264) and 66 (range, 37 to 120) in ALL941/2000 and ALL2004, respectively. The median cumulative corticosteroid doses at the time of ON onset were as follows: PSL, 5700 mg/m2 (range, 3480 to 13,880 mg/m2) in ALL941/2000 and PSL, 6030 mg/m2 (range, 3480 to 13,800 mg/m2) and DEX, 330 mg/m2 (range, 240 to 330 mg/m2) in ALL2004. As described in Table 2, SR patients in ALL2004 originally did not receive DEX, and despite the cumulative dose of PSL far exceeded the median doses for patients with ON at onset, none of them eventually developed ON. To obtain total PSL equivalents, DEX was multiplied by a conversion factor of 6.514; therefore, a relatively higher steroid dose was used in ALL2004 compared with that used in ALL941/2000.
TABLE 3.
Comparison of Patient Characteristics Between With and Without ON
The cumulative 5-year incidence of ON was 0.76% (SE, 0.43%), 0.35% (SE, 0.35%), and 3.6% (SE, 1.1%) in ALL941, ALL2000, and ALL2004, respectively (Fig. 2), with a significant difference between ALL2004 and ALL941/2000 (P<0.01). To assess the contribution of sex and age to ON incidence in patients receiving DEX-containing protocols, the cumulative incidence of ON was estimated in ALL2004 (Figs. 3A, B). Both sex and age were significantly associated with the 5-year ON incidence rate (P<0.01), whereas female sex and age 10 years and above were HR factors for ON. The cumulative 5-year incidence of ON for girls over 10 years of age was 25.6% (SE, 8.4%), which was extremely higher than the rest of patients in ALL2004 (P<0.0001) (Fig. 3C).
FIGURE 2.
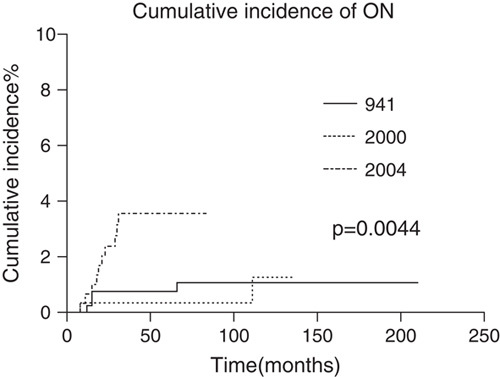
The cumulative incidence of osteonecrosis (ON) in the 3 Japanese Childhood Cancer and Leukemia Study Group studies on acute lymphoblastic leukemia (ALL). ALL941: 0.76%, SE, 0.43%; ALL2000: 0.35%, SE, 0.35%; ALL2004: 3.6%, SE, 1.1%.
FIGURE 3.
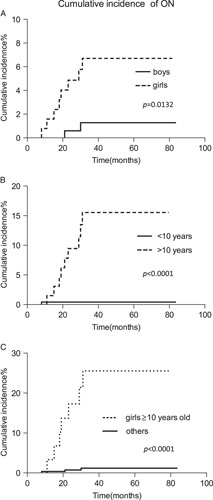
The cumulative incidence of osteonecrosis (ON) in ALL2004 according to sex (a), age (b), and combined (c). A, Boys (n=2/190): 1.3%, SE, 0.9%; girls (n=8/136): 6.7%, SE, 2.3%. B, Age below 10 years (n=1/249): 0.42%, SE, 0.42%; age 10 years and above (n=9/77): 15.6%, SE, 4.8%. C, Girls 10 years and above (n=7/33): 25.6%, SE, 8.42%; others (n=3/293): 1.19%, SE, 0.68%.
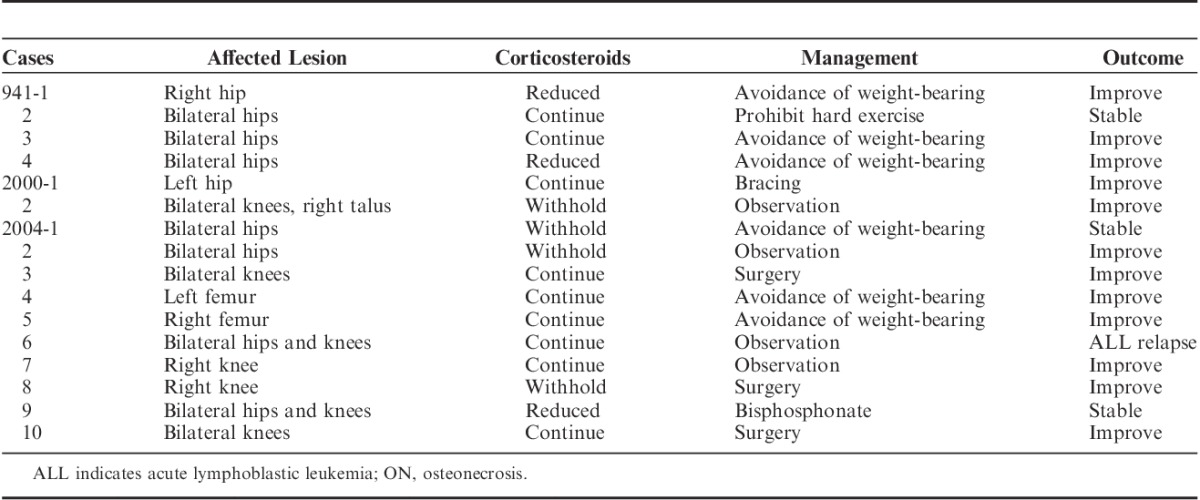
The characteristics of the 16 patients who eventually developed ON are listed in Table 4. All patients showed typical imaging findings on MRI except 1 (case 941-3) who underwent only x-ray that showed bilateral flattened femoral head. The most commonly affected joints and bones were the hip joint (44%), the knee joint (25%), and the femur (13%). Three patients (19%) exhibited multiple lesions. Nine (56%) continued to receive the planned steroid therapy despite the diagnosis of ON, whereas the doses were decreased or withdrawn in 7 (44%). ON management varied for each patient depending on the physician discretion. Most patients (75%) received supportive care only and were advised to avoid lifting heavy weights (grade 2 according to Common Terminology Criteria for Adverse Event version 4.0). Three patients (19%) underwent surgical intervention (grade 3) and 1 was treated with oral bisphosphonates (grade 2). With the median follow-up times of 33 months (range, 4 to 194), the clinical outcomes of ON were as follows: 12 with amelioration of ON and 3 with stable disease, except 1 who suffered a relapse of leukemia.
TABLE 4.
Affected Joints and Clinical Course of Patients With ON
DISCUSSION
In the 3 most recent JCCLSG ALL studies, we found that a significant number of patients developed ON during or after treatment. ALL2004 study was conducted to evaluate the efficacy of DEX usage as a corticosteroid in the context of intensification of reinduction phase, comparing with the preceding 2 studies wherein PSL was the only corticosteroid adopted. This strategy also enabled us to compare the DEX toxicity with that of PSL. The results clearly demonstrated the higher incidence of ON in ALL2004, indicating DEX exposure was the risk for ON in ALL chemotherapy.
The overall incidence of ON was 1.5% (16/1095), which was comparable with that in a previous study by the Japan Association of Childhood Leukemia Study (JACLS) (2.4%, Hiroki H, Yasushi I, Teruaki H, Makoto Y, Megumi O, Tooru K, Shinichiro N, Junichi H, Keizo H, Keiko Y, and Tatsutoshi N; unpublished data). In studies from Europe and the United states, the ON incidence was highly variable (1% to 2% up to 9%) and dependent on patient characteristics and treatment intensity.5–7 Furthermore, the detection methods of ON have significantly affected the incidence. Recent report from St Jude Total XV study showed that 17.6% of patients had the symptomatic ON, whereas the asymptomatic ON was detected in >50% of patients by the prospective screening with MRI test.15 With regard to the effects of race, the incidence of ON is reportedly higher in whites than in patients of African descent.7 Although it remains unclear whether the Asian race is related to an increased risk of ON, our results showed that the incidence of ON in Japanese children seemed to be comparable with that in European and American children. However, it should be taken into account the limitation of the present assessment: the possible missing of asymptomatic cases and the diagnosis partly depending on physician’s discretion.
In this study, female sex, age 10 years and above, and the use of DEX as a corticosteroid were significant risk factors for ON. Of the 33 female patients aged over 10 years who received DEX, 7 developed ON (cumulative incidence, 25.6%). This was the extremely higher incidence of ON comparing with the rest of patients. Although females were found to be at a higher risk of developing ON in the Children’s Cancer Study Group (CCG) and Italian studies,5,7 there was no such correlation in studies performed in the UK and Germany and at the Dana Farber Cancer Institute (Boston, MA).6,16,17 In addition, a Japanese study conducted by the JACLS failed to show a significant female predominance (male to female ratio, 7:9). Therefore, the effects of sex on ON pathogenesis remain unclear.
A significant contribution of age to ON onset has been robustly documented by most retrospective and prospective studies.5–7,9,16,18–21 Among children aged 10 years and above, those aged 16 to 20 years were at the highest risk of ON. The eligible patient age was 1 to 15 years in ALL941/2000 and 1 to 18 years in ALL2004; therefore, we may have underestimated the incidence of ON. Further monitoring is necessary when ALL treatment protocols designed for children are extended to adolescence and young adulthood.
The potential effect of DEX on ALL is 6.5 times that of PSL, resulting in an increase in the use of DEX for ALL treatment. Because DEX is more toxic to bone tissues,14,22 a higher incidence of ON has been a major concern in the design of treatment protocols. In ALL2004, DEX was incorporated only in the reinduction phase because an increased incidence of ON and mortality was reported with the use of DEX in the induction phase.23 Nonetheless, our data revealed a higher cumulative incidence of ON associated with DEX administration; this finding was comparable with the results of the Dana Farber Consortium study DFCI 00-01, wherein DEX was used in postremission intensification therapy and/or in the maintenance phase.24 Although the total corticosteroid dose (analyzed as PSL equivalents) at therapy completion were slightly lower in ALL2004 than in ALL941/2000 (Table 2), ON was most frequent in patients who had received only DEX in the HR group in ALL2004. These results suggest that DEX administration at any dose (as PSL equivalents) and in any treatment phase affects the incidence of ON. A recent report from the CCG found that DEX administration could influence the risk of ON21 and that alternate-week DEX administration during delayed intensification therapy decreased ON incidence compared with continuous DEX. In our ALL2004 protocol, DEX was administered continuously for 2 weeks, and it would have been beneficial to modify the DEX schedule from continuous administration to alternate-week administration.
Recently, biological and genetical basis for ON development has been extensively investigated. Children’s Oncology Group tested 12 polymorphisms of candidate genes and identified children with PAI-1 GA/AA genotypes were significantly associated with ON.25 Another study from St Jude Children’s Research Hospital showed polymorphisms of ACP1 were associated with risk of symptomatic ON as well as with lower serum albumin and higher cholesterol levels.15 These results suggest that some patients are prone to develop ON and individualized therapy should be needed in the future ALL studies.
In the present report, cases with ON were retrospectively collected by the questionnaire, and most of the ON patients were identified by symptoms and confirmed with imaging studies (x-ray/MRI) without central review. Despite such limitations, the clinical features of all 16 ON patients in our study were virtually comparable with those of patients in previous studies.6,7,16 Weight-bearing joints were commonly affected, whereas asymptomatic lesions might have been overlooked.15 Once ON is confirmed, the physician must decide whether steroids should be withheld or continued, considering that no consensus guideline is available thus far. Most of our patients were prescribed a planned dose of steroids without compromising functional outcomes after ON development. We believe that it may not be necessary to withhold steroids at the risk of leukemia relapse.
Bisphosphonates, which are structurally similar to pyrophosphates, inhibit osteoclast activity and bone turnover, thus exerting beneficial effects on bone mineralization.26 Alendronate, a third-generation bisphosphonate, is reportedly effective in the prevention of femoral head collapse in ON patients.27 Wiernikowski et al28 showed that alendronate-induced changes in bone mineral metabolism/homeostasis benefited bone mineralization in children with ALL or non-Hodgkin lymphoma with steroid-induced osteopenia. Another bisphosphonate, pamidronate, was shown to be effective in the management of pain and motor function recovery in symptomatic ON occurring in children with ALL.29 In the present study, alendronate was administered to 1 patient with symptomatic ON of the bilateral hip and knee joints; this resulted in no further deterioration of functional outcome and no treatment-induced side effects. However, further studies are required to clarify the potential benefits of concomitant bisphosphonate and steroid use for ON treatment.
In summary, the overall incidence of ON was 1.5% in the JCCLSG ALL studies, which was comparable with that reported in previous studies conducted in the Unites States and Europe. The known risk factors of age above 10 years, female sex, and DEX use were all significantly associated with an increase in the cumulative incidence of ON. In our future studies, we are intending to routinely screen for ON development with MRI test, especially those incorporating DEX in the treatment protocol. Although an ON management regimen remains to be established, steroids should not be withheld at the risk of ALL relapse.
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.jpho-online.com.
The authors declare no conflict of interest.
Reprints: Nobuyuki Hyakuna, MD, Center of Bone Marrow Transplantation, Hospital of University of the Ryukyus, 207 Uehara, Nishihara-cho, Okinawa 903-0215, Japan (e-mail: hyakunan@med.u-ryukyu.ac.jp).
Contributor Information
Collaborators: for The Japanese Childhood Cancer and Leukemia Study Group (JCCLSG)
REFERENCES
- 1.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation.N Engl J Med. 2009;360:2730–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia from 1990-2005: a report from the Children’s Oncology Group.J Clin Oncol. 2012;30:1663–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moricke A, Zimmermann M, Reiter A, et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000.Leukemia. 2010;2:265–284 [DOI] [PubMed] [Google Scholar]
- 4.te Winkel ML, Pieters R, Hop WC, et al. Prospective study on incidence, risk factors, and long-term outcome of osteonecrosis in pediatric acute lymphoblastic leukemia.J Clin Oncol. 2011;29:4143–4150 [DOI] [PubMed] [Google Scholar]
- 5.Arico M, Boccalatte MF, Silvestri D, et al. Osteonecrosis: an emerging complication of intensive chemotherapy for childhood acute lymphoblastic leukemia.Haematologica. 2003;88:747–753 [PubMed] [Google Scholar]
- 6.Burger B, Beier R, Zimmermann M, et al. Osteonecrosis: a treatment related toxicity in childhood acute lymphoblastic leukemia (ALL)—experiences from study ALL-BFM 95.Pediatr Blood Cancer. 2005;44:220–225 [DOI] [PubMed] [Google Scholar]
- 7.Mattano LA, Jr, Sather HN, Trigg ME, et al. Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: a report from the Children’s Cancer Group.J Clin Oncol. 2000;18:3262–3272 [DOI] [PubMed] [Google Scholar]
- 8.Sala A, Mattano LA, Jr, Barr RD.Osteonecrosis in children and adolescents with cancer: an adverse effect of systemic therapy.Eur J Cancer. 2007;43:683–689 [DOI] [PubMed] [Google Scholar]
- 9.Kadan-Lottick NS, Dinu I, Wasilewski-Masker K, et al. Osteonecrosis in adult survivors of childhood cancer: a report from the childhood cancer survivor study.J Clin Oncol. 2008;26:3038–3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerachian MA, Séguin C, Harvey EJ.Glucocorticoids in osteonecrosis of the femoral head: a new understanding of the mechanisms of action.J Steroid Biochem Mol Biol. 2009;114:121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe A, Katano N, Kikuta A, et al. Strategy of cumulative dose reduction of drugs with late effects: Children’s Cancer and Leukemia Study Group of Japan (CCLSG), CCLSG ALL941 protocol study.Blood. 2003;102:223a.(Abstr 783)12649143 [Google Scholar]
- 12.Yamaji K, Okamoto T, Yokota S, et al. Minimal residual disease-based augmented therapy in childhood acute lymphoblastic leukemia: a report from the Japanese Childhood Cancer and Leukemia Study Group.Pediatr Blood Cancer. 2010;55:1287–1295 [DOI] [PubMed] [Google Scholar]
- 13.Smith M, Arthur D, Comitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia.J Clin Oncol. 1996;14:18–24 [DOI] [PubMed] [Google Scholar]
- 14.Ito C, Evans WE, McNinch L, et al. Comparative cytotoxicity of dexamethasone and prednisolone in childhood acute lymphoblastic leukemia.J Clin Oncol. 1996;14:2370–2376 [DOI] [PubMed] [Google Scholar]
- 15.Kawedia JD, Kaste SC, Pei D, et al. Pharmacokinetic, pharmacodynamic, and pharmacogenetic determinants of osteonecrosis in children with acute lymphoblastic leukemia.Blood. 2011;117:2340–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vora A, Wade R, Mitchell C, et al. Incidence and outcome of osteonecrosis in children and young adults with acute lymphoblastic leukaemia treated on the medical research council UK Study ALL 2003.Blood (ASH Annual Meeting Abstracts). 2008;112:910 [Google Scholar]
- 17.Strauss AJ, Su JT, Dalton VM, et al. Bony morbidity in children treated for acute lymphoblastic leukemia.J Clin Oncol. 2001;19:3066–3072 [DOI] [PubMed] [Google Scholar]
- 18.Bomelburg T, von Lengerke HJ, Ritter J.Incidence of aseptic osteonecrosis following the therapy of childhood leukemia.Haematol Blood Transfus. 1990;33:577–579 [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro RC, Fletcher BD, Kennedy W, et al. Magnetic resonance imaging detection of avascular necrosis of the bone in children receiving intensive prednisone therapy for acute lymphoblastic leukemia or non-Hodgkin lymphoma.Leukemia. 2001;15:891–897 [DOI] [PubMed] [Google Scholar]
- 20.Relling MV, Yang W, Das S, et al. Pharmacogenetic risk factors for osteonecrosis of the hip among children with leukemia.J Clin Oncol. 2004;22:3930–3936 [DOI] [PubMed] [Google Scholar]
- 21.Mattano LA, Jr, Devidas M, Nachman JB, et al. Effect of alternate-week versus continuous dexamethasone scheduling on the risk of osteonecrosis in paediatric patients with acute lymphoblastic leukaemia: results from the CCG-1961 randomised cohort study.Lancet Oncol. 2012;13:906–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bostrom BC, Sensel MR, Sather HN, et al. Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: a report from the Children’s Cancer Group.Blood. 2003;101:3809–3817 [DOI] [PubMed] [Google Scholar]
- 23.Hurwitz CA, Silverman LB, Schorin MA, et al. Substituting dexamethasone for prednisone complicates remission induction in children with acute lymphoblastic leukemia.Cancer. 2000;88:1964–1969 [PubMed] [Google Scholar]
- 24.Vrooman LM, Neuberg DS, Stevenson KE, et al. Dexamethasone and individualized asparaginase dosing are each associated with superior event free survival in childhood acute lymphoblastic leukemia: results from DFCI-ALL consortium protocol 00-01.Blood (ASH Annual Meeting Abstracts). 2009;114:321 [Google Scholar]
- 25.French D, Hamilton LH, Mattano LA, Jr, et al. A PAI-1 (SERPINE1) polymorphism predicts osteonecrosis in children with acute lymphoblastic leukemia: a report from the Children’s Oncology Group.Blood. 2008;111:4496–4499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodan GA, Fleisch HA.Bisphosphonates: mechanisms of action.J Clin Invest. 1996;97:2692–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai KA, Shen WJ, Yang CY, et al. The use of alendronate to prevent early collapse of the femoral head in patients with nontraumatic osteonecrosis. A randomized clinical study.J Bone Joint Surg Am. 2005;87:2155–2159 [DOI] [PubMed] [Google Scholar]
- 28.Wiernikowski JT, Barr RD, Webber C, et al. Alendronate for steroid-induced osteopenia in children with acute lymphoblastic leukaemia or non-Hodgkin’s lymphoma: results of a pilot study.J Oncol Pharm Pract. 2005;11:51–56 [DOI] [PubMed] [Google Scholar]
- 29.Leblicq C, Laverdière C, Décarie JC, et al. Effectiveness of pamidronate as treatment of symptomatic osteonecrosis occurring in children treated for acute lymphoblastic leukemia.Pediatr Blood Cancer. 2013;60:741–741 [DOI] [PubMed] [Google Scholar]


