Abstract
A systematic review of studies in humans was conducted to update evidence on the association between the amount of sugars intake and dental caries and on the effect of restricting sugars intake to < 10% and < 5% energy (E) on caries to inform the updating of World Health Organization guidelines on sugars consumption. Data sources included MEDLINE, EMBASE, Cochrane Database, Cochrane Central Register of Controlled Trials, Latin American and Caribbean Health Sciences, China National Knowledge Infrastructure, Wanfang, and South African Department of Health. Eligible studies reported the absolute amount of sugars and dental caries, measured as prevalence, incidence, or severity. The review was conducted and reported in accordance with the PRISMA statement, and the evidence was assessed according to GRADE Working Group guidelines. From 5,990 papers identified, 55 studies were eligible – 3 intervention, 8 cohort, 20 population, and 24 cross-sectional. Data variability limited meta-analysis. Of the studies, 42 out of 50 of those in children and 5 out of 5 in adults reported at least one positive association between sugars and caries. There is evidence of moderate quality showing that caries is lower when free-sugars intake is < 10% E. With the < 5% E cut-off, a significant relationship was observed, but the evidence was judged to be of very low quality. The findings are relevant to minimizing caries risk throughout the life course.
Keywords: nutrition policy, oral health, sucrose, carbohydrates, adult, child
Introduction
Historically, numerous independent expert and consensus reports have concluded that sugars are the most important dietary factor in the development of dental caries (Sheiham, 2001; WHO/FAO, 2003). However, recommendations have not yet been developed through systematic review of the evidence.
In 2010, the World Health Organization (WHO) launched a Guideline Development Process defining a protocol for the process of revising and issuing dietary recommendations for populations (WHO, 2010). To update the recommendations for sugars through this process, WHO commissioned a systematic literature review. The objectives were to systematically review all available published data relating to the amount of sugars consumption and levels of dental caries and to report the findings for both adults and children. The WHO guideline development group formulated questions relating to the effects of sugars on dental caries (Table 1). These questions pertained to whether increasing or decreasing the amount of sugars intake affected measures of dental caries and whether the evidence supports a threshold for intake.
Table 1.
Questions Posed by the WHO Nutrition Guidance Expert Advisory Group – Subgroup on Diet and Health, to Develop Recommendations Regarding Sugars Intakes
| Question | |
|---|---|
| 1 | What is the effect on dental caries of a reduction in free sugars intake in adults? |
| 2 | What is the effect on dental caries of a reduction in free sugars intake in children? |
| 3 | What is the effect on dental caries of an increase in free sugars intake in adults? |
| 4 | What is the effect on dental caries of an increase in free sugars intake in children? |
| 5 | What is the effect on dental caries of restricting sugars intake to below 10% energy to reduce risk of dental caries in adults? |
| 6 | What is the effect on dental caries of restricting sugars intake to below 10% energy to reduce risk of dental caries in children? |
Methods
Guided by the WHO Guideline development process, a systematic review was conducted and reported according to the PRISMA statement (www.prisma-statement.org).
All appropriate randomized controlled trials (RCTs) and intervention and observational studies, published since 1950, were sought. Reviews were included if they contained a new analysis of existing data.
Participants were healthy humans (without acute illness, but those overweight or with hypertension or diabetes could be included) in developing, transitional, or industrialized countries. All age groups were included. No language restrictions were used.
Studies were included if they reported any intervention intended to alter sugars intake in one arm of the study compared with diet with a different sugars content in another study arm, and also included information on dental caries, change in caries, or comparisons of higher vs. lower caries as an outcome, with a timescale of at least one year. Observational studies were included if they reported absolute sugars or change in sugars intake and also included information on dental caries (as defined above). All timescales were included.
Sugars were defined as any of: total sugars, free sugars, added sugars, sucrose, non-milk extrinsic (NME) sugars, expressed as g or kg /day or /yr or as percentage E. This review, therefore, aimed to identify the evidence for total sugars (and any component thereof) on dental caries. Studies were also included if they reported a per capita population sugar intake. Studies that reported solely on frequency of sugars intake were excluded.
Dental caries outcomes included were: caries prevalence, incidence and/or severity, measured as DMF Index, DMFT, dmft, DMFS, dmfs, deft, dft, or comparisons between caries and no caries or higher caries vs. lower caries. The protocol is available in Supplemental Material 1 (WHO NUGAG Sub-Group on Diet and Health; available online at http://jdr.sagepub.com/supplemental).
Pooling of studies was conducted with RevMan software (version 5, Cochrane Collaboration) and random effects analysis.
Search Strategy
MEDLINE, EMBASE, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, LILACS (Latin American and Caribbean Health Sciences), CNKI (China National Knowledge Infrastructure), Wanfang (China), and the South African Department of Health databases were searched from 1950 through November 2011. Details of the search strategies are in Supplemental Material 2 (Search Strategies). The archives at the WHO Collaborating Centre for Nutrition and Oral Health at Newcastle University and reference lists of reviews were searched and experts contacted for further relevant papers. Abstracts and unpublished studies were not included.
Study Selection
Search hits (titles and/or abstracts) were initially screened by one person to exclude studies clearly outside the scope of the review. The remaining search hits were subjected to independent duplicate assessment of inclusion. Included studies were subjected to duplicate data extraction. Differences between reviewers’ results were resolved by discussion. Studies were grouped by type: intervention, cohort, population, or cross-sectional. Details of the individual studies included are presented in Supplemental Material 3 (Details of Included Studies).
Assessment of Quality of Evidence
The Grading of Recommendations Assessment Development and Evaluation (GRADE) system (GRADE Working Group, 2004) was used to assess the quality of the body of evidence in relation to each of the review questions. The GRADE system rates the overall quality of evidence by taking into consideration factors including: design limitations, consistency of results across the available studies, precision of results, directness and likelihood of publication bias, magnitude of effect, evidence of a dose response, strength of association, and the direction of plausible biases. The quality of the evidence was categorized as high, moderate, low, or very low. GRADE assessment (see Supplemental Material 4; Grade Profile Tables) was carried out to assess the entirety of the body of evidence by the authors and was further refined with the guidance by the NUGAG Subgroup on Diet and Health.
Results
The Fig. presents a flow diagram for the searches and identification of included studies. From all databases combined, 5,990 search hits were found. After initial screening, 284 potentially relevant papers were identified and further screened to exclude papers that clearly did not include an absolute measure of sugars intake. The remaining 116 full papers were assessed for inclusion/exclusion by two reviewers. Sixty-five papers were included (from 55 studies) and 43 (42 studies) were excluded (summarized in Supplemental Material 5; Characteristics of Studies Excluded).
Figure.
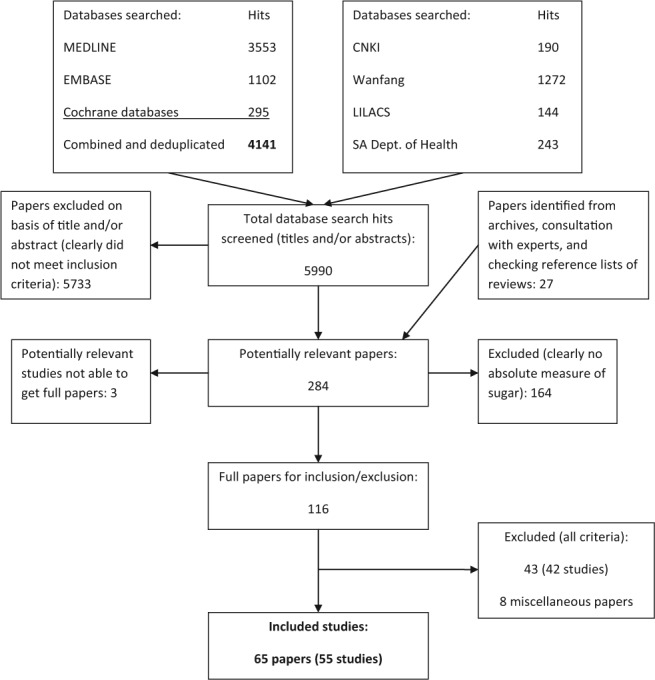
Flow chart of searches.
The majority of studies identified were conducted in children, while only 4 studies were of adults. One study examined both children and adults. No RCTs were identified. Of the included studies, 3 (4 papers) were intervention, 8 (12 papers) were prospective cohort, 20 (25 papers) were population, and 24 (25 papers) were cross-sectional. Tables 2 and 3 summarize the included studies. References cited are for the first publication from the corresponding cited study. A full list of references from each study is provided in Supplemental Material 3.
Table 2.
Summary of Included Studies¥ Conducted in Adults: Characteristics and Exposure/Outcome Relationship [positive (+), neutral (0), or negative (−)]
| Study Type | Study | Country | Sugars | Sugars Measure | Dental Outcome | + / 0 / − † | n | Age* (yrs) |
|---|---|---|---|---|---|---|---|---|
| Non-randomized intervention | ||||||||
| Scheinin et al., 1976 | Finland | Sucrose | Food diary | DMFS | + | 125 | 27.2 mean | |
| Gustafsson et al., 1954 | Sweden | Sucrose | Supplied food | DMFT | + | 663 | 31.9 mean | |
| Population | ||||||||
|
Fisher, 1968;
Holloway et al., 1968 |
Tristan da
Cunha, UK |
Sucrose | National data | DMFT, % teeth with caries | +/+/+ | 219 | 20-29
30-39 40-49 |
|
| Cross-sectional | ||||||||
| Newbrun et al., 1980 | USA &
Switzerland |
Sucrose | 7-day food diary | DMFT,
DMFS, oral hygiene status |
+ | 31 | 29.1 mean | |
| Papas et al., 1995 | USA | Sucrose | 3-day food diary | + | 141 | 47-83 | ||
References cited are for the first publication from the corresponding cited study. Where studies are associated with more than one paper, a full list of references from each is provided in Supplemental Material 3.
‘+’ indicates a positive and significant relationship between sugars and dental caries, ‘0’ indicates no significant relationship, and ‘-’ indicates a negative and significant relationship. If results were reported for separate cohorts within the same study, all relationships are listed and separated by a forward slash (‘/’).
Ages are listed as reported. If age range was reported, the lower and upper limits are listed by a dash (‘–‘). If a mean was reported that is indicated, or if more than one age cohort was reported, the ages of each cohort are listed, separated by a forward slash (‘/’).
Table 3.
Summary of Included Studies¥ Conducted in Children: Characteristics and Exposure/Outcome Relationship [positive (+), neutral (0), or negative (-)]
| Study | Country | Sugars | Sugars Measure | Dental Outcome | +/0/- † | n | Age* | Comment |
|---|---|---|---|---|---|---|---|---|
| Non-randomized intervention studies | ||||||||
| King et al., 1955 | UK | Sucrose | Weighed intake | % teeth with caries | -/0/+ | 392 London
203 Sheffield 225 Liverpool |
2-5 | |
| Longitudinal cohort studies | ||||||||
| Battellino et al., 1997 | Argentina | Sucrose | FFQ | dmft,
dmfs |
+ | 820 | 4 | |
| Rodrigues et al., 1999 | Brazil | Added sugars | Weighed intake | Caries increment | + | 510 | 3 | |
| Mackeown et al., 2000 | South Africa | Added sugars | FFQ | dmfs, caries prevalence | 0 | 259 | 1 | |
| Karjalainen et al., 2001 | Finland | Sucrose | Food diary | dmft caries incidence | + | 135 | 3 | |
| Stecksen-Blicks and Gustafsson, 1986 | Sweden | Sucrose | 7-day food diary | dmfs
DMFT caries increment |
0/+ | 88 (8 yrs) /
97 (13 yrs) |
8/
13 |
|
| Ruottinen et al., 2004 | Finland | Sucrose | Food diary | DMFT | + | 66 | 0.6 | |
| Rugg-Gunn et al., 1984 | England | Total sugars | Food diary | DMFT
DMFS DFS |
+ | 405 | 11.5 | |
| Burt et al., 1988 | USA | Total sugars | 24-hour recall | DMFS | + | 499 | 10-15 | |
| Population studies | ||||||||
| Schulerud, 1950 | Norway | Sucrose | National data | % teeth with caries | + | 3-12 | ||
| Okuya, 1960 | Japan | Sucrose | National data | Caries incidence | + | 8,399 | 6-13 | |
| Fisher, 1968 | UK, Tristan da Cunha | Sucrose | National data | dmft,
% teeth with caries |
+/+ | 219 | 1-5/
13-19/ |
|
| Toverud, 1957 | Denmark
Norway Finland |
Sucrose | National data | Caries incidence | +/+/+ | 6 Denmark
7 Norway 7 Finland |
||
| Takahashi, 1959 | Japan | Sucrose | National data | Caries incidence | + | 7,894 | 6-11 | |
| Koike, 1959 | Japan | Sucrose | National data | Caries incidence | + | 10,553 | 6-11 | |
| Shimamura, 1974 | Japan | Sucrose | National data | Caries incidence | + | ? | 1st grade
4th grade |
|
| Sreebny, 1982 | World-wide | Sucrose | FAO data | dmft
DMFT |
0/+ | 5-6/
12 |
23 countries for 5-6 year olds /47 countries for 12 year olds | |
| Künzel and Fischer, 1997 | Germany | Sucrose | Not clear | DMFT | +/+ | >200,000 | 6-10/
11-15 |
|
| Birkhed et al., 1989 | Sweden | Sucrose | National data | % teeth with caries
% population with caries |
+/+ | 7/
7-16 |
% populationcaries-free reported, but results inverted for summary table | |
| Downer, 1999 | UK | Sucrose | National data | dmft
DMFT |
+/+ | 5/
12 |
||
| Jamel et al., 2004 | Iraq | Sucrose | National data | DMFT, % population with caries | +/+/+
& +/+/+ |
6-7/
11-12/ 14-15 |
% population caries-free reported, but results inverted for summary table | |
| Buttner, 1971 | World-wide | Sucrose | National data | DMFT | + | 11-12 | 18 countries | |
| Marthaler, 1990 | World-wide | Sucrose | National data | DMFT | + | 12 | 12 countries | |
| Sivaneswaren and Barnard, 1993 | Australia | Sucrose | National data | DMFT | + | 12 | ||
| Woodward and Walker, 1994 | World-wide | Sucrose | National data | DMFT | + | 12 | 90 countries | |
| Miyazaki and Morimoto, 1996 | Japan | Sucrose | National data | DMFT | + | 12 | ||
| Ruxton et al., 1999 | World-wide | Sucrose | National data | DMFT | 0 | 12 | 67 countries. Same data as Sreenby; Woodward & Walker | |
| Diehnelt and Kiyak, 2001 | World-wide | Sucrose | National data | DMFT | + | 12 | 29 countries | |
| Downer et al., 2008 | Europe | sucrose | FAO data | DMFT | - | 12 | 29 countries | |
| Cross-sectional studies | ||||||||
| Richardson et al., 1978 | South Africa | Sucrose | FFQ | % caries prevalence | + | 1,154 | 1-6 | |
| Cleaton-Jones et al., 1984a | South Africa | Sucrose | Question-naire | % children with caries | + | 1-6 | ||
| Marques and Messer, 1992 | USA | Total sugars | 7-day food diary | % children with caries | 0 | 628 | 4 | p = .08 |
| Moynihan and Holt, 1996 | UK | NMES | 4-day weighed food diary | % children with caries | + | 1,658 | 1.5-4.5 | |
| Akyüz et al., 1996 | Turkey | Not clear if sucrose or total sugars | 3-day estimated food diary | dft and DMFT, % caries free, with >4 caries compared | + | 120 | 6-11 | Oral hygiene also associated with caries |
| Gibson and Williams, 1999 | UK | NMES | 4-day weighed food diary | Presence of caries | 0/+ | 1,450 (2 groups of higher/lower oral hygiene) | 1.5-2.5 | Sugar important in those with poor oral hygiene |
| Leite, 1999 | Brazil | Not clear if sucrose or total sugars | 4-day weighed food diary | dmft | + | 52 | 4 | |
| MacKeown et al., 2000 | South Africa | Added sugars | FFQ | dmft | + | 1,639 | 1 | |
| Marshall et al., 2007 | USA | NMES | Food diary | % children with caries | 0 | 634 | 4.5-6.9 | Sugar intake was high in all children |
| Lottes and Henderson, 1979 | USA | Sucrose | 7-day food diary | dmft | + | 107 | 2-11 | |
| Kleemola-Kujala and Rasanen, 1979 | Finland | Sucrose | 24-hour recall | dmfs | 0/+/0 | 167 ( 5 yrs)
186 (9 yrs) 191 (13 yrs) |
5/9/13 | All had an intake < 10% E |
| Cleaton-Jones et al., 1984b | South Africa | Sucrose | Questionnaire | dmft | 0 | 715 | 5 | |
| Yabao et al., 2005 | Philippines | Sucrose | FFQ | DMFT | + | 6-12 | Quality of data questionable | |
| Martinsson, 1972 | Sweden | Sucrose | 24-hour recall | DFS | + boys
/0 girls |
307 (boys & girls) | 14 | All girls consumed> 10% E from sugars |
| Retief et al., 1975 | South Africa | Sucrose | Questionnaire | DMFT | + | 16-17 | ||
| Walker et al., 1981 | South Africa | Not clear if sucrose or total sugars | FFQ | DMFT | + | 2,642 | 16-18 | |
| Steyn et al., 1987 | South Africa | Sucrose | 24-hour recall | DMFT
DMFS |
+/0 | 843 | 12 | Many comparisons and results varied (either + or 0) by ethnic group but discussion on fluoride differences absent |
| Larsson et al., 1992 | Sweden | Sucrose | 5-day food diary | DMFS | 0 | 199 | 15 | |
| Beighton et al., 1996 | England | Total sugars | 3-day food diary | DMFT
. DFMS |
0 | 405 | 11-12 | All groups consumed very high intakes |
| Arnadottir et al., 1998 | Iceland | Total sugars | questionnaire | Approximal caries | + | 150 | 14 mean | |
| MacIntyre and du Plessis, 2006 | South Africa | Added sugars | 24-hour recall | DMFT | +/+ | 50/50 | 10/15 | |
| Masson et al., 2010 | Scotland | NMES | FFQ | Dental health status | + | 2,800 | 3-17 | OR reported |
References cited are for the first publication from the corresponding cited study. Where studies are associated with more than one paper, a full list of references from each is provided in Supplemental Material 3.
‘+’ indicates a positive and significant relationship between sugars and dental caries, ‘0’ indicates no significant relationship, and ‘-’ indicates a negative and significant relationship. If results were reported for separate cohorts within the same study, all relationships are listed and separated by a forward slash (‘/’).
Ages are listed as reported. If age range was reported, the lower and upper limits are listed by a dash (‘–‘); if a mean was reported, that is indicated; if more than one age cohort was reported, the ages of each cohort are listed, separated by a forward slash (‘/’).
Assessment of Data for Meta-analysis and Dose-response
There was considerable variability in the data-reporting formats, e.g., the range of outcomes (e.g., DMFS, dmfs, DMFT, dmft, % caries, caries incidence), the types of outcomes (e.g., continuous data, odds ratios, number with caries, % with caries, caries prevalence), the ages of studied populations, variations in study length, the range and variety of interventions (for intervention studies), the time points at which outcomes were measured, the years over which the studies were conducted (from 1950 to present), and differences in, or lack of information on, fluoride exposure. There was variation in the terminology used for reporting sugars; however, the reviewed studies largely related to sugars intake which, using current terminology, would be described as ‘free sugars’ (WHO/FAO, 2003).
Moreover, some studies examined higher and lower tertiles of sugars intake and the effect on dental caries. Other studies took higher and lower quartiles of dental caries and then examined the sugars intake of those groups. As a result, the data were not in any consistent format to provide sufficient data to allow pooling within each study type. However, some crude analyses were conducted by pooling data across study types (Appendix). Dose-response data, where available, are described in Supplemental Material 3.
Since this systematic review did not identify any RCTs, and the non-randomized intervention studies had serious design flaws, the GRADE profile was generated from the cohort studies.
Effect of Reducing or Increasing Sugars
The analysis included 8 cohort studies (Table 4). None was excluded on the basis of quality. Six studies reported free sugars or components of free sugars, and 2 reported total sugars. Six out of 8 studies accounted for fluoride exposure.
Table 4.
Summary of Longitudinal Cohort Studies¥ Used in the GRADE Assessment
| Cohort Study | Dentition | Evidence |
|---|---|---|
| * Rugg-Gunn et al., 1984 | Permanent | 405 English children aged 11-12 yrs at baseline.
A low statistically significant correlation between DMFS increment and amount of sugars intake was found. Regression of DMFS increment on amount of sugars indicated that there was an average increase of 1.28 DMFS over 2 yrs with each rise of 100 g of sugars intake. Highest total sugar intake (163 g, of which 122 g added, i.e., > 10% E) developed 0.9 DMFS per yr more than lowest total sugars intake (78 g/day, of which 46 g added < 10% E). Those in the lower sugars group developed 3.2 DMFS over 2 yrs. |
| * Stecksen-Blicks and Gustafsson, 1986 | Permanent | 88 13-year-old and 83 8-year-old Swedish children.
Those aged 13 yrs in the lower caries group consumed ~ 9.8% E as sucrose (52.4 g/day) compared with ~14.5% E (76.9 g) in the high-caries group. Those in the lower-caries group developed 0-2 cavities. Difference in mean sugars intake of high- vs. low-caries groups in children aged 8 did not reach significance. Discriminate analysis showed meals per day and sugars intake correctly classified 61% of 8-year-olds as high/low caries. |
| Battellino et al., 1997 | Primary | 820 Argentine children aged 4 yrs at baseline. A relationship between lower socio-economic class and higher caries was found.
The correlation between the amount of sugars consumed and dmft was r = +0.4. Mean sugars intake of all groups was > 10% E. |
| Burt et al., 1988 | Permanent | 499 1- to 15-year-old children in the USA. A higher % E from sugars increased the probability of caries on all tooth surfaces. A high sugars intake (g/day) was associated with total caries increment. Each additional 5 g sugars was associated with a 1% increase in the probability of developing caries.
Comparing high- and low-sugars consumers, a small, non-significant difference in pit and fissure caries was found. A 3-fold increase in approximal caries, bordering on significance, was found (p = .06). Those in the highest quartile of sugars intake had a relative risk of 1.22 of developing caries; this increased to 1.8 for approximal caries. Both high- and ‘low’-sugars groups had sugars intake >10% energy. |
| * Rodrigues et al., 1999 | Primary | Participants were 2.99 times more likely to have higher caries when free sugar was 16% E (53.3 g) compared with < 10% E (~7.5% E) (22.9 g). At the lower level of sugars intake (7.5% E, 22.9 g/day), 1 dmfs/yr developed. |
| MacKeown et al., 2000 | Primary | 259 South African children aged 1 yr at baseline.
Change in caries incidence and prevalence between 1 and 5 yrs was not significantly associated with added sugars intake. Prevalence of caries increased from 1.5% at age 1 (when sugars intake ~6% E) to 62.2% at age 5 (when sugars intake >14.4% E). |
| * Karjalainen et al., 2001 | Primary | 135 Finnish children aged 3 yrs at baseline. Sucrose intake in those who developed caries was >10% E. Sugars intake in those who remained free of cavities (WHO criteria) was < 9% E. |
| * Ruottinen et al., 2004 | Primary and permanent | Children in Finland studied from 7 mos to 10 yrs. Comparison of highest and lowest 5 percentiles.
DMFT was 0.5 when sucrose was < 10% E (~8% E) and was 1.4 when sucrose intake was >10% E. The mean dmft/DMFT was 3.9 when sucrose was >10% E and was 1.9 when sucrose intake was <10% E. The mean dmft was 2.7 when sucrose intake was >10% E. and it was 1.1 when sucrose intake was < 10 E. |
Studies providing data that enabled comparisons of levels of dental caries development between levels of free sugars consumption above and below 10% of energy intake†, highlighted (*).
For adults and older children, 10% E equates to approximately 55 g/day; for children aged 7-10 yrs, this equates to approximately 50 g per day, for children aged 4-6, 44 g/day, and, for 1- to 3-year-olds, to approximately 32 g/day based on the UK Estimated Average Requirement for energy intake for each group (Department of Health, 1991).
In the studies that reported total sugars (Rugg-Gunn et al., 1984; Burt et al., 1988), free sugars made up a sizable proportion of total sugars, the proportion of free sugars increasing as total sugars intake increased.
References cited are for the first publication from the corresponding cited study. Where studies are associated with more than one paper, a full list of references from each is provided in Supplemental Material 3.
A consistent association was detected: 7 out of 8 studies reported higher dental caries with higher sugars intake. For the analysis relating dental caries in adults, data were not downgraded for indirectness, although all cohort studies were conducted in children. This was because the etiology of dental caries is the same in children and adults and because dental caries is a progressive disease, tracking from childhood to adulthood. Although no cohort studies in adults were identified, analysis of data from all identified studies of adults of other study design detected a statistically significant positive relationship between sugars and levels of caries.
Although data from the cohort studies were not suitable for pooling, there was evidence of a large effect for the individual cohort studies. Rodrigues et al. (1999) reported an OR of 2.75; Rugg-Gunn et al. (1984) reported regression of DMFS increment on amount of sugars, which indicated that there was an average increase of 1.28 DMFS over 2 years with each rise of 100 g/day of sugars; Ruottinen et al. (2004) reported DMFT in a high-sugars-consumption group of 1.4 compared with 0.5 in a low-sugars-consumption group; and Burt et al. (1988) reported that each additional 5 g of sugars intake was associated with a 1% increase in the probability of developing caries. Data from other studies did not report an effect size for caries. The study by Battellino et al. (1997) supports a large effect but is likely to be overestimated due to confounding by socio-economic status. From all studies available that presented data in a suitable format for pooling (for all study designs, see Appendix), comparisons of higher and lower sugars intake were SMD for DMFT 0.82 (95% CI 0.67, 0.97), and when measured as caries prevalence, the risk ratio was 7.15 (95% CI 2.82, 8.14). Overall, the quality of evidence, assessed by the GRADE process, categorized the evidence for an effect on dental caries of increasing or decreasing sugars intake as ‘moderate’ for both children and adults. The GRADE summary tables are in the Supplemental Material 4.
Population studies support the dose-response effect, with 18 out of 20 showing a positive, one a neutral, and one a negative association (Tables 2, 3) between sugars intake and dental caries. Nine population studies provided evidence of positive correlations between sugars intake and caries levels [Schulerud, 1950 (Supplemental Material 3); Koike, 1959; Takahashi, 1959; Buttner, 1971; Sreebny, 1982; Woodward and Walker, 1994 (developing countries only); Miyazaki and Morimoto, 1996; Diehnelt and Kiyak, 2001 (developing countries only); Downer, 1999] (details in Supplemental Material 3).
Effect of Restricting Free Sugars Intake to < 10% E
Five of the 8 cohort studies identified enabled us to compare dental caries development when sugars consumption was equivalent to a level < 10% E or > 10% E (Rugg-Gunn et al., 1984; Stecksen-Blicks and Gustafsson, 1986; Rodrigues et al., 1999; Karjalainen et al., 2001; Ruottinen et al., 2004) (summarized in Table 4). All studies accounted for fluoride exposure. A consistent association between sugars intake and caries was detected. The results from all studies found higher caries with sugars intake > 10% E compared with < 10% E. There was no evidence of indirectness although all 5 cohort studies were conducted in children, for the reasons already described. The quality of evidence as assessed by the GRADE process was classified as ‘moderate’ for both children and adults. The GRADE summary tables are in Supplemental Material 4.
It was not possible to combine the data from the 5 cohort studies to produce a forest plot, because of the aforementioned variability in data reporting. However, by combining data from all study types that presented data in a similar format, crude ‘forest plots’ were derived to provide a visual representation of the combined data, presented in the Appendix.
Analysis of data from the identified population studies supports the cohort data. Nine of 10 population studies that enabled a comparison showed lower caries (< DMFT 3) when sugars intake was < 15 to 20 kg/person/yr (< 40-55 g/day < 10% E) compared with higher intakes [Schulerud, 1950; Toverud, 1957; Koike, 1959; Takahashi, 1959; Fisher, 1968; Shimamura, 1974; Sreebny, 1982; Woodward and Walker, 1994; Miyazaki and Morimoto, 1996; Jamel et al., 2004], and one study showed DMFT < 4 when sugars intake was < 20 kg/person/yr (Buttner, 1971).
Effect of Restricting Free Sugars Intake to < 5% E
Three national population surveys of Japanese children (Table 5) were identified that enabled dental caries to be compared when annual per capita sugars < 10 kg/person/yr (~5% E) vs. >10 kg but < 18.25 kg/yr (~10% E). These showed lower caries progression when per capita sugar was < 10 kg/yr. The study first reported by Takahashi (1959) found a log-linear relationship between dental caries increment and sugar intakes between 0.2 kg and 5-7.5 kg/person/yr in first permanent molars erupted for 7-8 yrs (r = +0.8). Koike (1959) also found a log-linear relationship between sugar consumption and annual dental caries incidence between a sugar intake of 0.1 and 15 kg/person/yr. The correlation between sugar and dental caries was r = +0.8 in the lower first permanent molars and r = +0.6 for the upper first permanent molars. Okuya (1960) showed that dental caries decreased (but not to zero) when sugar availability decreased from 15 kg to < 10 kg/person/yr. The correlation between sugar intake and dental caries was +0.7 in second permanent molars.
Table 5.
Population-based National Studies¥ that Provided Data Facilitating Comparisons of Levels of Dental Caries Development between Levels of Free Sugars Consumption above and below 10 kg/person/yr (~5% energy intake)
| Population Study | Dentition | Evidence |
|---|---|---|
| Takahashi, 1959 (Tokyo, Japan) | First permanent molars | Sugars availability before WWII was 15 kg/person/yr and decreased to 0.2 kg (0.1% E) in 1946.
Log-linear relationship between sugars and caries increment between 0.2 kg and 5-7.5 kg/person/yr in teeth erupted for 7-8 yrs, r = 0.8. |
| Okuya, 1960 (Tokyo, Japan) | Second permanent molars | Sugars decreased from 15 kg/person/yr to <10 kg/person/yr. Dental caries decreased but not to zero. Correlation between sugars and caries in second molars was 0.7. |
| Koike, 1959 (Kyoto, Japan) | Lower and upper first permanent molars | Sugars intake decreased from 15 kg/person/yr to < 10 kg/person/yr. Dental caries decreased but not to zero. Mean correlation between sugars availability and annual caries incidence rate was 0.8 in the lower first permanent molars and 0.6 for the upper first permanent molars.
Straight-line log-linear relationship between sugars and annual caries incidence between 0.1 and 15 kg/person/yr. |
References cited are for the first publication from the corresponding cited study. Where studies are associated with more than one paper, a full list of references from each is provided in Supplemental Material 3.
A GRADE profile table was generated from these studies (Supplemental Material 4). Because of the problems inherent in using per capita sugars consumption data, the quality of evidence was downgraded for risk of bias. There was no evidence of inconsistency: The results from all of the 3 studies found higher caries with higher sugars intake. Data were not downgraded for indirectness, although all were conducted in permanent dentition of children, for the reasons already described. The studies were undertaken in populations with low fluoride exposure, and thus show indirectness in extrapolation to populations with good fluoride exposure. The GRADE quality assessment process characterized the quality of this evidence as Very Low (see Supplemental Material 4). Only 3 worldwide ecological studies enabled average DMFT of 12-year-olds to be compared when annual per capita sugars availability was < 10 kg vs. > 10 kg - < 20 kg. Of these, 1 showed lower average DMFT in children consuming the lower level of sugars.
Discussion
This in-depth systematic literature review has identified largely consistent evidence supporting a relationship between the amount of sugars intake and the development of dental caries across age groups. Moreover, based on the objective criteria defined in the GRADE process, the evidence has been classified as of moderate quality. The review process has also shown evidence of moderate quality to support limiting intake of free sugars to < 10% E and, moreover, has provided some evidence, albeit classified as of very low quality, to indicate the benefit of limiting free sugars intake to < 5% E. Although meta-analysis was limited, analysis of the data indicated a large effect size for the impact of sugars intake on dental caries [e.g., SMD for DMFT 0.82 (CI 0.67-0.97)]. An important strength in this review is the consistency of the data, despite methodological weaknesses in many studies, the long time period over which the studies were conducted, and the variability in the study approaches taken.
Effect of Fluoride
Many studies support the protective role of fluoride as the most important factor that has led to a decline in dental caries in Northern Europe, North America, and Australia; nonetheless, dental caries persists in these countries (especially in adults). However, it has been suggested that fluoride retards the development of cavitation lesions to a later age (Sheiham, 2001). Evidence from a number of identified studies shows that, despite the protection offered by fluoride, the relationship between sugars and dental caries remains (Rugg-Gunn et al., 1984; Burt et al., 1988; Marthaler, 1990; Holt, 1991; Sivaneswaran and Barnard, 1993; Künzel and Fischer, 1997; Arnadottir et al., 1998; Leite, 1999; Rodrigues et al., 1999; Ruottinen et al., 2004; Masson et al., 2010). Moreover, many parts of the world do not benefit from fluoride exposure. All of the cohort studies used in the GRADE analysis comparing free sugars intake > 10% E and < 10% E considered fluoride exposure. The national survey data used in the GRADE analysis comparing sugars availability < 5% E and > 5% E were obtained from non-fluoridated populations. However, this does not necessarily mean that fluoridated populations would not potentially benefit from this lower level of sugars, because, as discussed, the impact of sugars on dental caries in populations exposed to fluoride may manifest at a later age.
The purpose of this review was to provide evidence to assist WHO to establish recommendations for levels of dietary sugars intake based on a quantifiable amount. For this reason, the evidence relating to amount, and not frequency, of sugars was assessed.
GRADE Quality Assessment
Criteria from GRADE for assessing the strength of the evidence recognizes, in the absence of RCTs, evidence from other study types as important. In the absence of RCTs, the GRADE profiles (Supplemental Material 4) were based on the identified cohort studies. In the absence of RCTs with which to conduct funnel plots and limited possibility to combine data, publication bias was difficult to assess. Of the 8 cohort studies included in the GRADE analysis, only 2 declared source of funding, both from national agencies. The GRADE assessment relating to the impact of increasing/decreasing sugars on caries risk was based on all identified cohort studies. The GRADE assessment of the evidence comparing caries levels at free sugars intake above and below levels equivalent to 10% E was based on the 5 cohort studies that provided data enabling this comparison to be made. The data used in the GRADE assessment, though based solely on cohort studies, are compatible with and supported by data from other studies: Evidence from population studies showed lower dental caries when sugars intake was < 10% E; however, sugars intake was estimated from per capita sugars intake/availability assessed at a national level, and confounders such as fluoride exposure are not accounted for. Since population studies will incur a higher risk of bias, they provide lower quality evidence.
The recommendation of the 2002 Joint WHO/FAO Expert Consultation on ‘Diet, Nutrition and the Prevention of Chronic Diseases’ (WHO/FAO 2003), that free sugars should provide no more than 10% E, was largely based on data from population studies. The present GRADE analysis of data from cohort studies further strengthens the evidence showing lower caries when free sugars intake is < 10% E.
Limiting Free Sugars Intake to < 5% E
In the most recent review by WHO on the impact of sugars on dental caries (WHO/FAO, 2003), consistent evidence from population studies was found to show that when free sugars availability/intake was < 15 to 20 kg/person/yr (< 10% E), the average DMFT of 12-year-old children was < 3 (the WHO oral health goal for 2000). However, by modern-day standards, this is not considered to be ‘low’. Moreover, in most studies, dental caries was diagnosed at the cavitation level – a late stage in the disease process. The pathological process of dental caries begins well before cavitation. Although, for the purposes of worldwide epidemiological surveys, assessing dental caries at the cavitation level remains the norm, in many industrialized countries, surveys use scoring systems that diagnose caries at the pre-cavitation stage as well as the cavitation stage [e.g., International Caries Detection and Assessment System (ICDAS)]. Pre-cavitation damage may occur at levels of sugars intake below that associated with low/no cavities.
The studies included in the GRADE analysis of the effect of limiting free sugars intake to < 10% E showed that dental caries levels were lower when sugars intake is < 10% E compared with > 10% E. However, even the groups with free sugars intake < 10% E had some caries (in 1/5 studies, children were free of cavities). Likewise, the population studies showed dental caries levels in children aged 12 yrs to be lower (< DMFT 3), but not absent, when sugars intake is < 15-20 kg/person/yr.
Most dental surveys have looked at children (often aged 12 yrs) in whom the permanent teeth are relatively newly erupted. Dental caries is a progressive disease – being free of cavities at age 12 yrs does not mean being caries-free for life. Most dental caries is now occurring in adults. Analysis of data shows that dental caries in childhood progresses to adulthood. Analysis of trajectories in caries trends indicates that even when DMFT of children is < 3, a high incidence of dental caries occurs in adults: Analysis of data from the Dunedin Longitudinal Study shows that children with DMFS < 3 go on to develop DMFS 15 by the age of 32 yrs (Broadbent et al., 2008). The National Chinese Dental Surveys showed that DMFT increased from 4.3 at age 20 yrs to 6.5 by 29 yrs, and from 7.6 at age 30 yrs to 11.3 at age 39 yrs (when sugar availability was < 10 kg/person/yr). High dental caries incidence occurs in adult populations even in the presence of fluoride: An annual caries increment of 1 carious surface/person has been reported in older adults in the USA (Griffin et al., 2005). It is incorrect to assume that low dental caries at age 12 yrs is indicative of good dental health in a population throughout the life course. Even small reductions in risk of dental caries in childhood are significant to later life.
Epidemiological evidence of children and adults with low sugars intake is sparse, and only 3 national population studies were found (Koike, 1959; Takahashi, 1959; Okuya, 1960). All showed that reducing sugars to < 10 kg/person/yr (~ 5% E) decreased dental caries compared with when sugars intakes were > 15 kg/person/yr. Therefore, although a sugars intake below 15 to 20 kg/person/yr (or < 10% E) will reduce risk of dental caries, it is not correct to say that it will prevent caries activity in all age groups across the life course. The ecological studies used in the GRADE analysis provide evidence, albeit classified as of very low quality, to show lower risk of dental caries with sugars intake < 5% E compared with > 5- < 10% energy. Analysis of these data, in addition to evidence showing the progressive nature of dental caries and the lifelong impact of sugars on dental health, suggests further benefits from limiting free sugars intake to < 5% E.
Research Requirements
There are few data on the impact on caries of reducing sugars through dietary interventions and health education. Research into feasible approaches to reduce free-sugars intake and the impact on dental caries, through well-designed controlled intervention studies in different age groups, in areas with and without exposure to fluoride is needed.
There are few data on the relationship between sugars and dental caries in adults and older people. Since the burden of disease moves to adulthood, it is important that the relationship between diet and dental caries be investigated in these groups. Because of the continuing trend to retain teeth into later life, coupled with the increase in the older population globally, research into sugars intake in relationship to development of caries, and the impact of caries-preventive strategies, in these older age groups is needed.
This systematic review has highlighted the diversity of reporting sugars intake. There is a need to develop feasible standardized methods to assess free sugars intake to facilitate surveillance across populations.
Conclusions
This in-depth systematic review shows consistent evidence of moderate quality supporting a relationship between the amount of sugars consumed and dental caries development.
There is evidence of moderate quality to show that dental caries is lower when free-sugars intake is < 10% E.
Dental caries progresses with age, and the effects of sugars on the dentition are lifelong. Even low levels of caries in childhood are of significance to levels of caries throughout the life-course. Analysis of the data suggests that there may be benefit in limiting sugars to < 5% E to minimize the risk of dental caries throughout the life course.
Acknowledgments
The authors thank the WHO NUGAG Subgroup on Diet and Health for their contributions to this work, and Prof. Jim Mann and Dr. Lisa Te Morenga, University of Otago, for their assistance with the GRADE summary tables.
Footnotes
The research was funded by Newcastle University’s Centre for Oral Health Research.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Akyüz S, Pince S, Garibagaoglu M. (1996). Nutrient intake and dental health in school children. J Marmara Univ Dent Fac 2:535-539 [PubMed] [Google Scholar]
- Arnadottir IB, Rozier RG, Saemundsson SR, Sigurjons H, Holbrook WP. (1998). Approximal caries and sugar consumption in Icelandic teenagers. Community Dent Oral Epidemiol 26:115-121 [DOI] [PubMed] [Google Scholar]
- Battellino LJ, Cornejo LS, Dorronsoro de Cattoni ST, Luna Maldonado de Yankilevich ER, Calamari SE, Azcura AI, et al. (1997). Oral health status evaluation of pre-school children: longitudinal epidemiologic study (1993-1994), Cordoba, Argentina. Rev Saude Publica 31:272-281 [article in Spanish]. [DOI] [PubMed] [Google Scholar]
- Beighton D, Adamson A, Rugg-Gunn A. (1996). Associations between dietary intake, dental caries experience and salivary bacterial levels in 12-year-old English schoolchildren. Arch Oral Biol 41:271-280 [DOI] [PubMed] [Google Scholar]
- Birkhed D, Sundin B, Westin SI. (1989). Per capita consumption of sugar-containing products and dental caries in Sweden from 1960 to 1985. Community Dent Oral Epidemiol 17:41-43 [DOI] [PubMed] [Google Scholar]
- Broadbent JM, Thomson WM, Poulton R. (2008). Trajectory patterns of dental caries experience in the permaent dentition to the fourth decade of life. J Dent Res 87:69-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt BA, Eklund SA, Morgan KJ, Larkin FE, Guire KE, Brown LO, et al. (1988). The effects of sugars intake and frequency of ingestion on dental caries increment in a three-year longitudinal study. J Dent Res 67:1422-1429 [DOI] [PubMed] [Google Scholar]
- Buttner W. (1971). Zuckeraufnahme und karies. In: Grundfragen der Ernahrungswissenschaft. Cremer HD, editor. Freiburg im Breisgau: Rombach [Google Scholar]
- Cleaton-Jones P, Richardson BD, Winter GB, Sinwel RE, Rantsho JM, Jodaikin A. (1984a). Dental caries and sucrose intake in five South African preschool groups. Community Dent Oral Epidemiol 12:381-385 [DOI] [PubMed] [Google Scholar]
- Cleaton-Jones P, Richardson BD, Sinwel R, Rantsho J, Granath L. (1984b). Dental caries, sucrose intake and oral hygiene in 5-year-old South African Indian children. Caries Res 18:472-477 [DOI] [PubMed] [Google Scholar]
- Diehnelt DE, Kiyak HA. (2001). Socioeconomic factors that affect international caries levels. Community Dent Oral Epidemiol 29:226-233 [DOI] [PubMed] [Google Scholar]
- Downer MC. (1999). Caries experience and sucrose availability: an analysis of the relationship in the United Kingdom over fifty years. Community Dent Health 16:18-21 [PubMed] [Google Scholar]
- Downer MC, Drugan CS, Blinkhorn AS. (2008). Correlates of dental caries in 12-year-old children in Europe: a cross-sectional analysis. Community Dent Health 25:70-78 [PubMed] [Google Scholar]
- Fisher FJ. (1968). A field survey of dental caries, periodontal disease and enamel defects in Tristan da Cunha. Br Dent J 125:447-453 [PubMed] [Google Scholar]
- Gibson S, Williams S. (1999). Dental caries in pre-school children: associations with social class, toothbrushing habit and consumption of sugars and sugar-containing foods. Further analysis of data from the National Diet and Nutrition Survey of children aged 1.5-4.5 years. Caries Res 33:101-113 [DOI] [PubMed] [Google Scholar]
- GRADE Working Group: Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. (2004). Grading quality of evidence and strength of recommendations. BMJ 328:1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin SO, Griffin PM, Swann JL, Zlobin N. (2005). New coronal caries in older adults: implications for prevention. J Dent Res 84:715-720 [DOI] [PubMed] [Google Scholar]
- Gustafsson BE, Quensel CE, Lanke LS, Lundquist C, Grahnén H, Bonow BE, et al. (1954). The Vipeholm dental caries study. The effect of different levels of carbohydrate intake on caries activity in 436 individuals observed for 5 years. Acta Odontol Scand 11:232-264 [DOI] [PubMed] [Google Scholar]
- Holloway PJ, James PMC, Slack GL. (1968). Dental disease in Tristan da Cunha. British Dent J 115:19-25 [Google Scholar]
- Holt RD. (1991). Foods and drinks at four daily time intervals in a group of young children. Br Dent J 170:137-143 [DOI] [PubMed] [Google Scholar]
- Jamel H, Plasschaert A, Sheiham A, Jamel H, Plasschaert A, Sheiham A. (2004). Dental caries experience and availability of sugars in Iraqi children before and after the United Nations sanctions. Int Dent J 54:21-25 [DOI] [PubMed] [Google Scholar]
- Karjalainen S, Söderling E, Sewon L, Lapinleimu H, Simell O. (2001). A prospective study on sucrose consumption, visible plaque and caries in children from 3 to 6 years of age. Community Dent Oral Epidemiol 29:136-142 [DOI] [PubMed] [Google Scholar]
- King JD, Mellanby M, Stones HH, Green HN. (1955). The effect of sugar supplements on dental caries in children. Spec Rep Ser Med Res Counc (G B) 288:1-55 [PubMed] [Google Scholar]
- Kleemola-Kujala E, Rasanen L. (1979). Dietary pattern of Finnish children with low high caries experience. Community Dent Oral Epidemiol 7:199-205 [DOI] [PubMed] [Google Scholar]
- Koike H. (1959). Studies on caries incidence in the first molar in relation with amount of sugar on primary school children in Kyoto. J Okayama Med Soc 72:407 [Google Scholar]
- Künzel W, Fischer T. (1997). Rise and fall of caries prevalence in German towns with different F concentrations in drinking water. Caries Res. 31:166-173 [DOI] [PubMed] [Google Scholar]
- Larsson B, Johansson I, Ericson T. (1992). Prevalence of caries in adolescents in relation to diet. Community Dent Oral Epidemiol 20:133-137 [DOI] [PubMed] [Google Scholar]
- Leite TA. (1999). Dental caries and sugar consumption in a group of public nursery school children. Rev Odontol Univ São Paulo 13:13-18 [Google Scholar]
- Lottes MT, Henderson HZ. (1979). The relation of sucrose consumption to dental caries in a sample of Indianapolis children. J Indiana Dent Assoc 58:22-25 [PubMed] [Google Scholar]
- MacIntyre UE, du Plessis JB. (2006). Dietary intakes and caries experience in children in Limpopo Province, South Africa. S Afr Dent J 61:058-063 [PubMed] [Google Scholar]
- MacKeown JM, Cleaton-Jones PE, Edwards AW. (2000). Energy and macronutrient intake in relation to dental caries incidence in urban black South African preschool children in 1991 and 1995: the Birth-to-Ten study. Public Health Nutr 3:313-319 [DOI] [PubMed] [Google Scholar]
- Marques AP, Messer LB. (1992). Nutrient intake and dental caries in the primary dentition. Pediatr Dent 14:314-321 [PubMed] [Google Scholar]
- Marshall TA, Eichenberger-Gilmore JM, Larson MA, Warren JJ, Levy SM. (2007). Comparison of the intakes of sugars by young children with and without dental caries experience. J Am Dent Assoc 138:39-46 [DOI] [PubMed] [Google Scholar]
- Marthaler TM. (1990). Changes in the prevalence of dental caries: how much can be attributed to changes in diet? Caries Res 24(Suppl 1):3-15 [DOI] [PubMed] [Google Scholar]
- Martinsson T. (1972). Socio-economic investigation of school children with high and low caries frequency. 3. A dietary study based on information given by the children. Odontol Revy 23:93-113 [PubMed] [Google Scholar]
- Masson LF, Blackburn A, Sheehy C, Craig LC, MacDiarmid JI, Holmes BA, et al. (2010). Sugar intake and dental decay: results from a national survey of children in Scotland. Br J Nutr:1-10 [DOI] [PubMed] [Google Scholar]
- Miyazaki H, Morimoto M. (1996). Changes in caries prevalence in Japan. Eur J Oral Sci 104(Pt 2):452-458 [DOI] [PubMed] [Google Scholar]
- Moynihan PJ, Holt RD. (1996). The national diet and nutrition survey of 1.5 to 4.5 year old children: summary of the findings of the dental survey. Br Dent J 181:328-332 [DOI] [PubMed] [Google Scholar]
- Newbrun E, Hoover C, Mettraux G, Graf H. (1980). Comparison of dietary habits and dental health of subjects with hereditary fructose intolerance and control subjects. J Am Dent Assoc 101:619-626 [DOI] [PubMed] [Google Scholar]
- Okuya Y. (1960). The epidemiological study of the relation between caries incidence and sugar consumption on the second molar. Shikwa Gakuho 60:1120-1134 [Google Scholar]
- Papas AS, Joshi A, Palmer CA, Giunta JL, Dwyer JT. (1995). Relationship of diet to root caries. Am J Clin Nutr 61:423S-429S; [DOI] [PubMed] [Google Scholar]
- Retief DH, Cleaton-Jones PE, Walker AR. (1975). Dental caries and sugar intake in South African pupils of 16 to 17 years in four ethnic groups. Br Dent J 138:463-469 [DOI] [PubMed] [Google Scholar]
- Richardson BD, Cleaton-Jones P, McInnes PM, Rantsho JM, Pieters L. (1978). Total sucrose intake and dental caries in black and white South African children of 1-6 years. Part II: dental caries and sucrose intake. J Dent Assoc S Afr 33:539-544 [PubMed] [Google Scholar]
- Rodrigues CS, Watt RG, Sheiham A. (1999). Effects of dietary guidelines on sugar intake and dental caries in 3-year-olds attending nurseries in Brazil. Health Promot Int 14:329-335 [Google Scholar]
- Rugg-Gunn AJ, Hackett AF, Appleton DR, Jenkins GN, Eastoe JE. (1984). Relationship between dietary habits and caries increment assessed over two years in 405 English adolescent school children. Arch Oral Biol 29:983-992 [DOI] [PubMed] [Google Scholar]
- Ruottinen S, Karjalainen S, Pienihakkinen K, Lagström H, Niinikoski H, Salminen M, et al. (2004). Sucrose intake since infancy and dental health in 10-year-old children. Caries Res 38:142-148 [DOI] [PubMed] [Google Scholar]
- Ruxton CH, Garceau FJ, Cottrell RC. (1999). Guidelines for sugar consumption in Europe: is a quantitative approach justified? Eur J Clin Nutr 53:503-513 [DOI] [PubMed] [Google Scholar]
- Scheinin A, Mäkinen KK, Ylitalo K. (1976). Turku sugar studies. V. Final report on the effect of sucrose, fructose and xylitol diets on the caries incidence in man. Acta Odontol Scand 34:179-216 [DOI] [PubMed] [Google Scholar]
- Schulerud A. (1950). Dental caries and nutrition during wartime in Norway. Oslo, Norway: Fabritius and Sonners Trykkeri [Google Scholar]
- Sheiham A. (2001). Dietary effects on dental diseases. Public Health Nutr 4:569-591 [DOI] [PubMed] [Google Scholar]
- Shimamura S. (1974). [Incidence of caries in permanent teeth during a period of 20 kg annual sugar consumption per person in Japan]. Koku Eisei Gakkai Zasshi 24:228-234 [article in Japanese]. [PubMed] [Google Scholar]
- Sivaneswaran S, Barnard PD. (1993). Changes in the pattern of sugar (sucrose) consumption in Australia 1958-1988. Community Dent Health 10:353-363 [PubMed] [Google Scholar]
- Sreebny LM. (1982). Sugar availability, sugar consumption and dental caries. Community Dent Oral Epidemiol 10:1-7 [DOI] [PubMed] [Google Scholar]
- Stecksen-Blicks C, Gustafsson L. (1986). Impact of oral hygiene and use of fluorides on caries increment in children during one year. Community Dent Oral Epidemiol 14:185-189 [DOI] [PubMed] [Google Scholar]
- Steyn NP, Albertse EC, van Wyk Kotze TJ, van Wyk CW, van Eck M. (1987). Sucrose consumption and dental caries in twelve-year-old children of all ethnic groups residing in Cape Town. J Dent Assoc S Afr 42:43-49 [PubMed] [Google Scholar]
- Takahashi K. (1959). Statistical study on caries incidence in the first molar in relation with amount of sugar consumption. Jpn J Oral Hyg 9:136 [Google Scholar]
- Toverud G. (1957). The influence of war and post-war conditions on the teeth of Norwegian schoolchildren. Milbank Mem Fund Q 25:373-459 [PubMed] [Google Scholar]
- Walker AR, Dison E, Duvenhage A, Walker BF, Friedlander I, Aucamp V. (1981). Dental caries in South African black and white high school pupils in relation to sugar intake and snack habits. Community Dent Oral Epidemiol 9:37-43 [DOI] [PubMed] [Google Scholar]
- WHO (2010). Handbook for Guideline Development. URL accessed on 9/24/2013 at: www.who.int/hiv/topics/mtct/grc_handbook_mar2010_1.pdf
- WHO/FAO (2003). Diet nutrition and the prevention of chronic diseases. Technical Report Series 916. Geneva, Switzerland: WHO; [PubMed] [Google Scholar]
- Woodward M, Walker AR. (1994). Sugar consumption and dental caries: evidence from 90 countries. Br Dent J 176:297-302 [DOI] [PubMed] [Google Scholar]
- Yabao RN, Duante CA, Velandria FV, Lucas M, Kassu A, Nakamori M, et al. (2005). Prevalence of dental caries and sugar consumption among 6-12-y-old schoolchildren in La Trinidad, Benguet, Philippines. Eur J Clin Nutr 59:1429-1438 [DOI] [PubMed] [Google Scholar]


