Abstract
We previously demonstrated that topical application of fibroblast growth factor (FGF)-2 enhanced periodontal tissue regeneration. Although angiogenesis is a crucial event for tissue regeneration, the mechanism(s) by which topically applied FGF-2 induces angiogenesis in periodontal tissues has not been fully clarified. In this study, we investigated whether FGF-2 could induce vascular endothelial growth factor (VEGF)-A expression in periodontal ligament (PDL) cells and whether cell-to-cell interactions between PDL cells and endothelial cells could stimulate angiogenesis. FGF-2 induced VEGF-A secretion from MPDL22 cells (mouse periodontal ligament cell line) in a dose-dependent manner. Transwell and wound-healing assays revealed that co-stimulation with FGF-2 plus VEGF-A synergistically stimulated the migration of MPDL22 cells. Interestingly, co-culture of MPDL22 cells with bEnd5 cells (mouse endothelial cell line) also stimulated VEGF-A production from MPDL22 cells and tube formation by bEnd5 cells. Furthermore, time-lapse analysis revealed that MPDL22 cells migrated close to the tube-forming bEnd5 cells, mimicking pericytes. Thus, FGF-2 induces VEGF-A expression in PDL cells and induces angiogenesis in combination with VEGF-A. Cell-to-cell interactions with PDL cells also facilitate angiogenesis.
Keywords: growth factors, cell communication, endothelial cells, tissue regeneration, cell migration, pericytes
Introduction
Several lines of evidence have revealed that topical application of recombinant cytokines can induce regeneration of periodontal tissues. We previously demonstrated that human recombinant basic fibroblast growth factor (FGF-2) induced significant periodontal tissue regeneration, accompanied by alveolar bone and cementum formation in beagle dogs and non-human primates (Murakami et al., 1999, 2003; Takayama et al., 2001). Furthermore, we conducted randomized controlled clinical trials to evaluate the safety and efficacy of FGF-2 application and showed that 0.3% FGF-2 exhibited maximal efficacy in inducing periodontal regeneration (Kitamura et al., 2008, 2011).
FGF-2 stimulates various cellular functions (Folkman and Shing, 1992). In vitro studies have revealed that FGF-2 induced potent proliferative responses and cell migration and regulated extracellular matrix production by periodontal ligament (PDL) cells, which are critical cellular events during the process of wound healing and regeneration of periodontal tissues (Takayama et al., 1997, 2008; Shimabukuro et al., 2011). Another important mechanism by which FGF-2 stimulates periodontal tissue regeneration is angiogenesis, a process of new blood vessel formation in various physiological and pathological conditions such as embryonic development, tumor growth, wound repair, and inflammation.
Although FGF-2 can directly stimulate angiogenesis, many other factors and molecules are also involved in this biological event. In particular, vascular endothelial growth factor (VEGF)-A, a pro-angiogenic factor initially identified as an endothelial cell (EC)-specific growth factor, induces vascular permeability and angiogenesis (Ferrara et al., 2003). In addition, direct cell-to-cell communication is also important to orchestrate wound healing and tissue regeneration (Grellier et al., 2009a). However, little is known regarding the cell-to-cell interactions involved in periodontal regeneration induced by FGF-2.
In this study, we focused on the possible involvement of VEGF-A in FGF-2-induced angiogenesis at sites of periodontal tissue defects. Furthermore, we examined the significance of cell-to-cell interactions between PDL cells and EC. During angiogenesis, we demonstrated that FGF-2 increased the expression of VEGF-A, and that FGF-2 and VEGF-A synergistically promoted the migration of PDL cells and tube formation of EC. We further demonstrated that: (a) cell-to-cell interactions between PDL cells and EC stimulated tube formation of EC and (b) PDL cells have a phenotype similar to that of pericytes in co-culture conditions. The present study suggests that topically applied FGF-2 stimulates angiogenesis at sites of periodontal tissue defects, both directly and indirectly, via VEGF-A produced by FGF-2-stimulated PDL cells. In addition, cell-to-cell interactions of EC with PDL cells support tube formation, probably by functioning as pericytes.
Materials & Methods
Materials
Human recombinant FGF-2 was kindly provided by Kaken Pharmaceutical Co., Ltd. (Tokyo, Japan). Mouse recombinant VEGF-A (VEGF164) and rabbit anti-mouse VEGF-A polyclonal antibodies (Ab) were purchased from R&D Systems (Minneapolis, MN, USA) and PeproTech Inc. (Rocky Hill, NJ, USA), respectively.
Cell Culture
We established a mouse PDL cloned cell line, MPDL22 cells, and maintained it as previously described (Yamada et al., 2007). Mouse gingival fibroblasts (MGF) were established as previously described (Iwayama et al., 2012). A mouse endothelial cell line, bEnd5 cells, was obtained from DS Pharma (Osaka, Japan) and cultured as previously described (Röhnelt et al., 1997).
RT-PCR and Real-time PCR
RT-PCR and Real-Time PCR were performed as previously described (Yanagita et al., 2008). The primers used for RT-PCR were as follows: GAPDH (glyceraldehyde-3- phosphate dehydrogenase), 5′-AGGTTGTCTCCTGCGACTTC-3′, 5′-CTTGC TCAGTGTCCTTGCTG-3′; and VEGF, 5′-CCTCCGAAACC ATGAACTTTCTGCTC-3′, 5′-CAGCCTGGCTCACCGCCTT GGCTT-3′(Saint-Geniez et al., 2009). PCR products were digitized and analyzed by means of the WinRoof software program (Mitani Corporation, Fukui, Japan). The real-time primer sequences were as follows: GAPDH, 5′-TGTGTCCGTCGTG GATCTGA-3′, 5′-TTGCTGTTGAAGTCGCAGGAG-3′; and NG2 (chondroitin sulfate proteoglycan) (Ozerdem et al., 2002), 5′-GCAGGCAAACGAAGACACTTGA-3′, 5′-TGAAGCTGC CACGGATAGGAG-3′. We analyzed dissociation curves to ensure the amplification of a single PCR product. The amount of cDNA was calculated from the standard curve for each sample. The relative expression is shown after normalization to GAPDH gene expression. The detailed method for RT-PCR and Real-time PCR is described in the online Appendix.
Measurement of VEGF in Culture Supernatants
Cells were seeded at a density of 5 × 105 and cultured for 24 hrs, and then stimulated with the indicated doses of FGF-2. After 48 hrs, the supernatants were collected. In some experiments, co-cultures were set up in a transwell in which 2 different cell types were physically separated and allowed to interact only through culture medium (co-culture without cell-to-cell interaction). MPDL22 (1 × 105) cells were cultured in the upper chamber, and 4 × 105 bEnd5 cells were cultured in the lower chamber. In the case of co-culture with cell-to-cell interactions, both cell types were cultured in the lower chamber. VEGF levels in supernatants were determined with the Mouse VEGF Quantikine ELISA kit (R&D). Absorbance (OD 450/650 nm) was measured by a microplate reader, Model 680 (BioRad, Hercules, CA, USA).
Cell Migration Assay
We performed 2 migration assays to determine the effects of FGF-2 and/or VEGF on cell motility. The first migration assay was performed with a Chemicon QCM™ 96-well migration assay kit (Chemicon Intl. Inc., Temecula, CA, USA), according to the manufacturer’s instructions. Migrating cells subsequently underwent lysis and were detected by CyQuant GR dye (Invitrogen Corp., Carlsbad, CA, USA). The intensity of fluorescence was measured by a fluorescence plate reader (Thermo Electron, Vantaa, Finland) with a 485-/538-nm filter set.
Another migration assay was conducted with the CytoSelect™ 24-well Wound Healing Assay kit (Cellbiolabs Inc., San Diego, CA, USA) according to the manufacturer’s instructions. Images were captured by microscopy (Nikon, Tokyo, Japan). The ratio of cells migrating into the cell-free space was determined with the WinRoof software program (Mitani Corporation).
MPDL22 and bEnd5 Culture in 3D Culture
Matrigel™ (BD Biosciences, San Jose, CA, USA) thawed on ice was added to μ-Slide Angiogenesis (Nippon Genetics, Tokyo, Japan). After gelation, bEnd5 cells, MPDL22 cells, or both were plated onto Matrigel with FGF-2, VEGF-A, or both, and incubated. After 24 hrs, images were captured. The ratio of bEnd5 cells:MPDL22 cells in all co-culture systems was 4:1, similar to that in capillaries. FGF-2 and VEGF-A were added to the culture medium at concentrations of 5 ng/mL (FGF-2) and 6.25 ng/mL or 25 ng/mL (VEGF-A), respectively. In some experiments, cells were pre-treated with 0.1 µg/mL of rabbit anti-mouse VEGF polyclonal Ab.
Confocal Microscopy
First, bEnd5 cells were stained with red fluorescent dye PKH26 (Sigma, St. Louis, MO, USA) and MPDL22 cells with green fluorescent dye PKH67 (Sigma) according to the manufacturer’s procedure. After being stained, bEnd5 and MPDL22 cells were co-cultured in Matrigel for 12 hrs. Images were captured by confocal microscopy (LSM510, Carl Zeiss Co., Ltd., Thornwood, NY, USA).
Time-lapse Microscopy
bEnd5 stained with CellTracker™ Orange CMTMR (Invitrogen) were co-cultured with MPDL22 stained with CellTracker™ Green CMFDA (Invitrogen) (bEnd5:MPDL22 ratio = 4:1) on glass-bottomed dishes (Matsunami Co., Osaka, Japan) with culture medium containing 2% Matrigel. Fluorescence images were captured with a Nikon BioStation IM (Nikon Instruments, Tokyo, Japan).
Flow Cytometric Analysis
We used flow cytometry to detect the expression of NG2, a pericyte marker, in MPDL22 cells. After FGF-2 and/or VEGF-A stimulation, MPDL22 cells were dispersed with cell dissociation buffer (Sigma) and collected. Cells were incubated with anti-NG2 chondroitin sulfate proteoglycan (Millipore, Billerica, MA, USA), then washed with phosphate-buffered saline (PBS). Cells were then stained with Alexa Fluor 488-conjugated rabbit IgG Ab (Invitrogen) as a secondary Ab. Flow cytometric analysis was performed by FACSCalibur (BD Biosciences).
Statistical Analysis
Data are summarized as the mean ± SD. Statistical analysis of results was performed by Student’s t test or analysis of variance (ANOVA), followed by Dunnett’s multiple comparison test. P values < .05 were considered significant.
Results
FGF-2 Up-regulated the Expression of VEGF-A in MPDL22
To examine the effects of FGF-2 on the expression of VEGF-A and its receptors, we stimulated mouse PDL (MPDL22) cells with various doses of FGF-2. Stimulation with FGF-2 treatment increased mRNA expression of VEGF-A and secretion of VEGF in a dose-dependent manner (Figs. 1A, 1B). The mouse VEGF-A gene was alternatively transcribed in at least 3 forms, VEGF120, VEGF164, and VEGF188, and expression of all isoforms was up-regulated. Similar results were found when human PDL cells were used (data not shown). VEGF-A production from FGF-2-stimulated MGF was also observed, although the amount was lower compared with MPDL22 cells (Fig. 1C).
Figure 1.

FGF-2 induced VEGF expression of MPDL22 cells in a dose-dependent manner. (A) MPDL22 cells were stimulated with FGF-2 at indicated doses for 24 hrs. Semi-quantitative RT-PCR verified VEGF-A expression in MPDL22. Expression levels of VEGF-A (164) were normalized to expression of GAPDH. Results of 1 representative experiment out of 3 separate experiments are shown. (B) MPDL22 cells were stimulated with FGF-2 at indicated doses for 48 hrs. The level of VEGF-A in the supernatant was determined by ELISA. Results of 1 representative experiment out of 8 separate experiments are shown. The data represent the means ± SD of triplicate assays.*p < .05, vs. 0 ng/mL FGF-2. (C) FGF-2-stimulated MGF produced VEGF-A. MPDL22 cells and MGF cells were stimulated with 10 ng/mL FGF-2 for 48 hrs, and the level of VEGF-A in the supernatant was determined by ELISA. Results of 1 representative experiment out of 3 separate experiments are shown. The data represent the means ± SD of triplicate assays. *p < .05, vs. 0 ng/mL FGF-2.
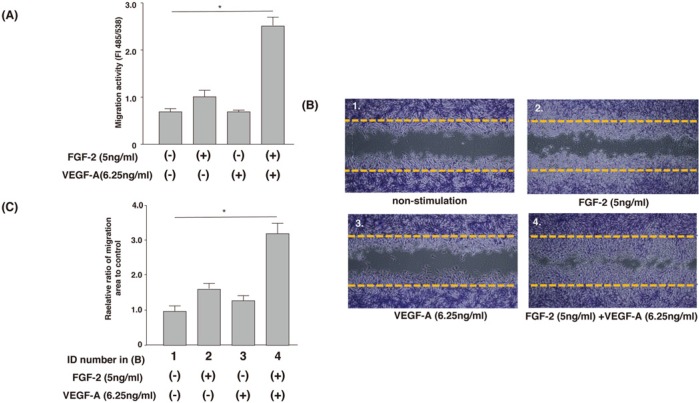
Co-stimulation of FGF-2 and VEGF Synergistically Increased the Migratory Activity of MPDL22 Cells
We performed cell migration assays using transwells to investigate the effects of FGF-2-VEGF-A co-stimulation on the migratory activity of MPDL22 cells. The use of 5 ng/mL FGF-2 or 6.25 ng/mL VEGF-A had no effect on the migratory activity of MPDL22 cells. Even when the VEGF-A concentration was increased to 50 ng/mL, the migratory activity of MPDL22 cells was not influenced (data not shown). Interestingly, co-stimulation of FGF-2 plus VEGF-A significantly enhanced the migratory activity. Cell migration was also evaluated by wound-healing assay. Cell-free spaces were created according to the manufacturer’s instruction, and cell migration was followed for 12 hrs in the presence or absence of FGF-2 and/or VEGF-A. As shown in Figs. 2B and 2C, co-stimulation of FGF-2 plus VEGF-A promoted cell migration into the cell-free spaces, similar to that observed in the transwell assay (Fig. 2A). Treatment of MPDL22 cells with 2.5 μg/mL mitomycin C (Kyowa Hakko, Osaka, Japan) did not affect the migratory activity induced by FGF-2 and/or VEGF.
Figure 2.
Co-stimulation of FGF-2 and VEGF-A synergistically induced the migration of MPDL22 cells. (A) Transwell assay. MPDL22 cells were added to the upper chamber of an inserted membrane filter and stimulated with or without FGF-2 (5 ng/mL)/VEGF-A (6.25 ng/mL) for 12 hrs. Migrating cells underwent lysis and were detected by CyQuant GR dye. The intensity of fluorescence was measured with a fluorescence plate reader. Results of 1 representative experiment out of 5 separate experiments are shown. The data represent the means ± SD of triplicate assays. *p < .05, vs. non-stimulation. (B) Wound-healing assay. Dotted lines represent the margins of cell monolayers at the start of culture. Results of 1 representative experiment out of 4 separate experiments are shown. (C) The ratio of cells migrating into cell-free spaces in (B) was determined with the WinRoof software program. The relative expression of migration was standardized against the migration areas without stimulation, which was arbitrarily designated a value of 1.0. Results of 1 representative experiment out of 4 separate experiments are shown.
Co-stimulation of FGF-2 and VEGF-A Stimulated Tube Formation
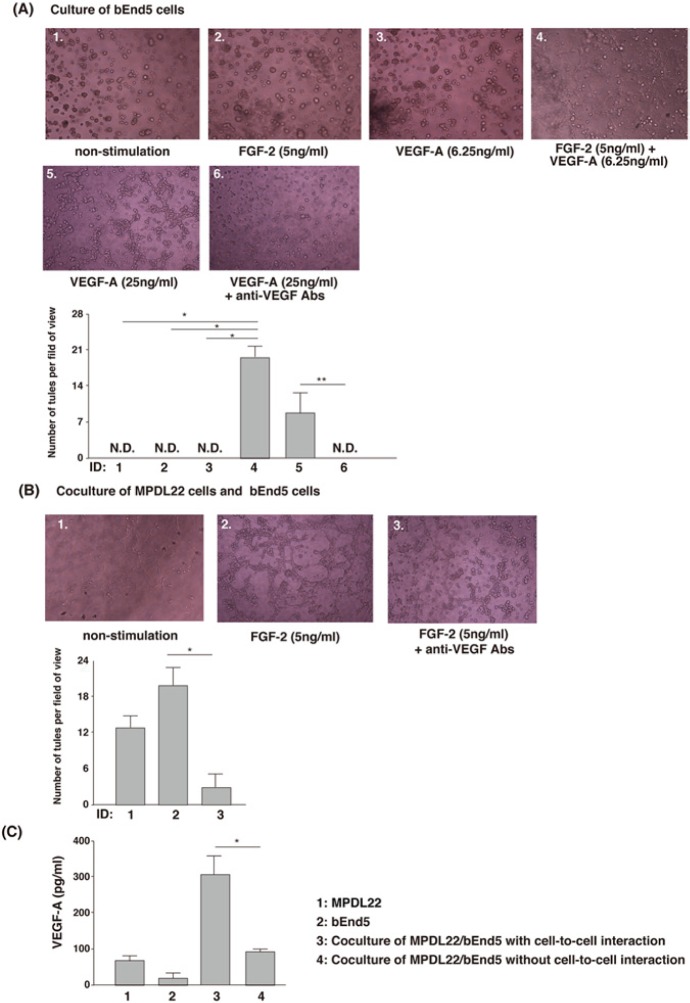
To address the effects of co-stimulation of FGF-2 and VEGF-A on angiogenesis during FGF-2-induced periodontal tissue regeneration, we investigated the morphological changes of bEnd5 cells after the stimulation. Although neither 5 ng/mL FGF-2 nor 6.25 ng/mL VEGF-A induced tube formation (Fig. 3A, ID 1-3), interestingly, co-stimulation with 5 ng/mL FGF-2 and 6.25 ng/mL VEGF-A synergistically promoted tube formation of bEnd5 cells (Fig. 3A, ID 4). However, 25 ng/mL VEGF-A alone induced tube formation, and anti-VEGF-A treatment abrogated tube formation (Fig. 3A, ID 5,6).
Figure 3.
FGF-2 and/or VEGF-A stimulation and the presence of MPDL22 cells up-regulated tube formation of bEnd5 cells. (A) Co-stimulation of FGF-2 and VEGF-A induced tube-like structures of bEnd5 cells at 12 hrs. Tube formation: Results of 1 representative experiment out of 3 separate experiments are shown. Number of tubes: Data represent the mean ± SD of 5 separate experiments. *p < .05, vs. non-stimulation, FGF-2, or VEGF-A alone. **p < .05, vs. anti-VEGF-A treatment. (B) Co-cultures with bEnd5 and MPDL22 with FGF-2 stimulation promoted tube formation at 12 hrs, and treatment with anti-VEGF neutralizing Ab abrogated tube formation of bEnd5 induced by co-culture with MPDL22 and FGF-2 stimulation. Tube formation: Results of 1 representative experiment of 3 separate experiments are shown. Number of tubes: Data represent the mean ± SD of 5 separate experiments. *p < .05, vs. anti-VEGF-A treatment. (C) Cell-to-cell interactions between MPDL22 and bEnd5 promoted VEGF production. Supernatant from 48-hour MPDL22 cells or bEnd5 cells culture, or from MPDL22/bEnd5 co-culture, was collected. The level of VEGF-A in the supernatant was determined by ELISA. Results of 1 representative experiment of 3 separate experiments are shown. The data represent the mean ± SD of triplicate assays. *p < .05, vs. co-culture of MPDL22/ bEnd5 cells without cell-to-cell interaction.
Co-culture with MPDL22 Cells Enhanced Tube Formation of bEnd5 Cells
Cell-to-cell interactions play crucial roles in homeostasis and wound-healing processes. In in vitro models used for the investigation of periodontal tissue regeneration, it is important to examine the effects of cell-to-cell interactions between PDL cells and other types of cells in periodontal tissues. We first investigated the morphological changes induced by co-culture of bEnd5 cells with MPDL22 cells. As shown in Fig. 3B, ID 1, tube formation was observed when bEnd5 cells were co-cultured with MPDL22 cells. These morphological changes were further enhanced in the presence of FGF-2 (Fig. 3B, ID 2). Intriguingly, treatment with anti-VEGF neutralizing Ab suppressed tube formation induced by the co-culture plus FGF-2 stimulation (Fig. 3B, ID 3). Furthermore, cell-to-cell interactions between MPDL22 cells and bEnd5 cells increased VEGF-A production (Fig. 3C). In contrast, co-culture of MPDL22 cells plus bEnd5 cells without cell-to-cell contact in transwell did not stimulate VEGF-A secretion (Fig. 3C). These results suggested that cell-to-cell interactions between MPDL22 cells and bEnd5 cells in the presence of FGF-2 stimulated tube formation, and that VEGF-A has an important role for enhanced tube formation.
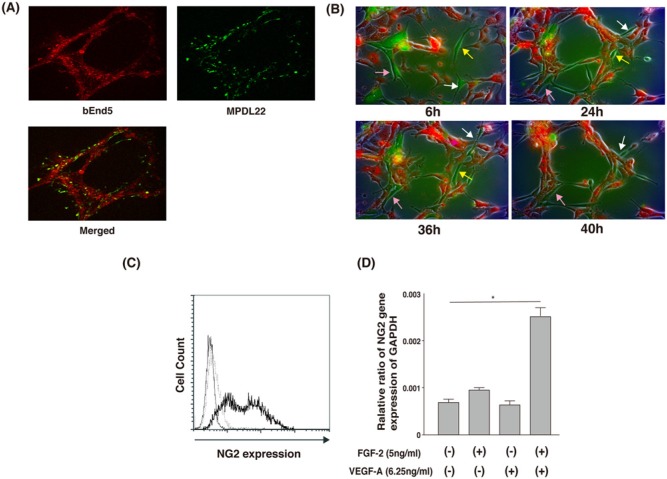
MPDL22 Cells Migrate to Tube-like Structures of Co-cultured bEnd5 Cells
To assess the positional relationship of MPDL22 cells and bEnd5 cells during tube formation, we examined the localization of PKH67-labeled MPDL22 cells (green) co-cultured with PKH26-labeled bEnd5 cells (red) by confocal microscopy. PKH67-labeled MPDL22 cells were located beside the tube-like structures of bEnd5 cells (Fig. 4A). Furthermore, time-lapse microscopy revealed that MPDL22 cells were recruited to the tube formation of bEnd5 cells, such as pericytes, under the dynamic nature of tube formation (Fig. 4B).
Figure 4.
MPDL22 possess characteristics similar to those of pericytes. (A) PKH26-labeled bEnd5 co-cultured with PHK67-labeled MPDL22 (bEnd5:MPDL22 ratio = 4:1) on a gel matrix. Images from red fluorescence, green fluorescence, and merged image are shown. MPDL22 localized around the network formed by bEnd5. (B) Time-lapse imaging of tube formation in co-culture of bEnd5 with MPDL22 cells for 6, 24, 36, and 40 hrs. CellTracker™ Orange CMTMR were co-cultured with CellTracker™ Green CMFDA (bEnd5:MPDL22 ratio = 4:1). Three different colored arrows show the position of identical MPDL22 at indicated times. (C) NG2 expression in MPDL22 was analyzed by flow cytometry. The thick line represents NG2, and the dashed line represents isotype control Ab staining. The thin line represents non-staining. Results of 1 representative experiment out of 3 separate experiments are shown. (D) NG2 expression in MPDL22 stimulated with FGF-2/VEGF-A was analyzed by real-time PCR. Co-stimulation of FGF-2 and VEGF-A enhanced NG2 expression in MPDL22. Results of 1 representative experiment of 3 separate experiments are shown. The data represent the mean ± SD of triplicate assays. *p < .05, vs. non-stimulation.
MPDL22 Cells Express the Pericyte Marker NG2
Confocal microscopic and time-lapse observation suggested that MPDL22 cells might be characteristic of pericytes. Thus, we examined the expression of NG2, a suggested pericyte marker (Ozerdem et al., 2002), on MPDL22 cells. As shown in Fig. 4C, MPDL22 cells expressed NG2, and NG2 expression in MPDL22 cells was enhanced by co-stimulation with FGF-2 and VEGF-A (Fig. 4D).
Discussion
FGF-2 is produced from various cell types and is a key cytokine/growth factor that promotes wound healing and tissue regeneration. FGF-2 has already been used for the treatment of intractable skin ulcers (Murakami, 2011). In addition, Kawaguchi et al. (2010), reported that the local application of FGF-2 in a human trial accelerated healing of fresh fractures. A recent study showed that FGF-2 and VEGF modulated proliferation and osteogenic differentiation of human periodontal ligament stem cells (Lee et al., 2012). In the present study, we focused on the possible involvement of PDL cells in angiogenesis at sites of FGF-2-induced periodontal regeneration. We first demonstrated that FGF-2-stimulated MPDL22 cells produced VEGF-A. This suggested that topically applied FGF-2 and FGF-2-induced VEGF-A synergistically stimulated angiogenesis during the course of periodontal regeneration. We also examined the co-stimulation effects of FGF-2 plus VEGF-A on cellular functions of MPDL22 cells and found that FGF-2 and VEGF-A synergistically promoted the migratory activity of MPDL22 cells. We previously reported that the phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathway was responsible for FGF-2-mediated cell migration of PDL cells (Shimabukuro et al., 2011). Signal pathways for cell migration via the FGF-receptor and VEGF-receptor are common and include the PI3K/Rac and p38/MAPK pathways (Cross and Claesson-Welsh, 2001; Olsson et al., 2006). In our preliminary experiment, we observed that the synergistic effects of FGF-2 and VEGF-A in cell migration assays were inhibited by PI3K inhibitor (wortmannin) or by p38 inhibitor (SB203580). Therefore, we speculate that co-stimulation with FGF-2 and VEGF-A synergistically activated these signaling pathways.
Although 5 ng/mL FGF-2 or 6.25 ng/mL VEGF-A had little effect on tube formation when used singly, co-stimulation of bEnd5 cells with FGF-2 and VEGF-A of the same concentration markedly promoted tube formation. Interestingly, the presence of MPDL22 cells further stimulated the morphological changes of bEnd5. The promotion of tube formation induced by co-stimulation of FGF-2 and VEGF-A was abrogated by anti-VEGF-A neutralizing Ab, suggesting that VEGF-A was important for FGF-2-induced angiogenesis or processes of periodontal regeneration at the sites where FGF-2 was applied. Indeed, FGF-2 and VEGF were previously shown to promote angiogenesis synergistically in vivo through enhancement of PDGF-B/PDGFRβ signaling (Kano et al., 2005). Since we observed that PDGFRβ was expressed at high levels on MPDL22 cells (data not shown), we speculated that, in this study, PDGF-B/PDGFRβ signaling may be involved in the effect of co-stimulation of FGF-2 and VEGF-A. In addition, cell-to-cell communication between PDL cells (MPDL22) and EC (bEnd5) induced VEGF-A secretion. Interestingly, it was reported that co-culture of EC and osteoblasts/osteoprogenitors induced VEGF expression (Stahl et al., 2004; Clarkin et al., 2008; Grellier et al., 2009b). Sekine et al. (2008) reported that EC co-cultured with cardiomyocytes enhanced VEGF, FGF-2, and hepatocyte growth factor, although EC co-cultured with skin fibroblasts did not increase these 3 angiogenic factors. This suggests that specific interactions between cells were necessary for the induction of angiogenic factors. Therefore, interactions between PDL cells and EC are important at the site of periodontal regeneration.
Confocal and time-lapse microscopy analysis revealed that MPDL22 cells migrated close to the tube-forming bEnd5-like pericytes. Pericytes are embedded within the vascular basement membrane of blood vessels and interact with EC to regulate development and support stabilization and maturation of vessels (Armulik et al., 2011). To date, pericyte-specific markers have not been fully identified (Armulik et al., 2011). Interestingly, MPDL22 cells expressed NG2 and PDGFRβ (data not shown), which are also detected on pericytes at high frequency. Moreover, NG2 expression was up-regulated by FGF-2 and VEGF-A co-stimulation. These results suggested that MPDL22 cells may have characteristics similar to those of pericytes and are consistent with a recent report by Iwasaki et al. (2012), who demonstrated that human periodontal ligament stem cells possessed pericyte-like characteristics. Cell-to-cell interactions between MPDL22 cells and bEnd5 cells stimulated VEGF-A production, suggesting the differential role of PDL cells during angiogenesis. Since a mouse PDL cloned cell line (MPDL22 cells) was used in this study, it is unlikely that MPDL22 cells differentiated into pericytes. However, periodontal-tissue-derived mesenchymal stem cells (Seo et al., 2004) may directly differentiate into pericytes in vivo.
In our previous study, histological observation in beagle dogs demonstrated the augmentation of angiogenesis qualitatively at FGF-2-applied sites (Murakami, 2011). Angiogenesis is a process fundamental to wound healing and tissue remodeling, because newly formed blood vessels provide nutrition and oxygen to tissues (Folkman and Shing, 1992). Here we showed that the direct effect of FGF-2 and the secondary effect of FGF-2-induced VEGF-A on vessel formation were induced at sites where FGF-2 was topically applied.
The present study demonstrated that FGF-2 induced VEGF-A production from PDL cells and that co-stimulation with FGF-2/VEGF-A promoted tube formation of EC. Direct contact between PDL cells and EC also stimulated angiogenesis, probably via the increased production of VEGF-A. We hypothesized that cell-to-cell interaction via junctional molecules such as gap or adherens junctions may be involved in the production of VEGF-A. These results suggested that FGF-2 and FGF-2-induced VEGF-A, as well as cell-to-cell interactions between PDL cells and EC, create a suitable environment for periodontal regeneration by orchestrating angiogenesis at FGF-2-applied sites.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This study was supported by Grants-in-Aid for Scientific Research numbers 23249086, 23390452, 23390478, 23593057, and 24890125, by Grants-in Aid from the Ministry of Health, Labour and Welfare (Japan), and by research funding from Kaken Pharmaceutical Co. Ltd.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this study.
References
- Armulik A, Genové G, Betsholtz C. (2011). Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21:193-215 [DOI] [PubMed] [Google Scholar]
- Clarkin CE, Emery RJ, Pitsillides AA, Wheeler-Jones CP. (2008). Evaluation of VEGF-mediated signaling in primary human cells reveals a paracrine action for VEGF in osteoblast-mediated crosstalk to endothelial cells. J Cell Physiol 214:537-544 [DOI] [PubMed] [Google Scholar]
- Cross MJ, Claesson-Welsh L. (2001). FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci 22:201-207 [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. (2003). The biology of VEGF and its receptors. Nat Med 9:669-676 [DOI] [PubMed] [Google Scholar]
- Folkman J, Shing Y. (1992). Angiogenesis. J Biol Chem 267:10931-10934 [PubMed] [Google Scholar]
- Grellier M, Bordenave L, Amédée J. (2009a). Cell-to-cell communication between osteogenic and endothelial lineages: implications for tissue engineering. Trends Biotechnol 27:562-571 [DOI] [PubMed] [Google Scholar]
- Grellier M, Ferreira-Tojais N, Bourget C, Bareille R, Guillemot F, Amédée J. (2009b). Role of vascular endothelial growth factor in the communication between human osteoprogenitors and endothelial cells. J Cell Biochem 106:390-398 [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Komaki M, Yokoyama N, Tanaka Y, Taki A, Kimura Y, et al. (2012). Periodontal ligament stem cells possess the characteristics of pericytes. J Periodontol 84:1425-1433 [DOI] [PubMed] [Google Scholar]
- Iwayama T, Yanagita M, Mori K, Sawada K, Ozasa M, Kubota M, et al. (2012). Adiponectin regulates functions of gingival fibroblasts and periodontal ligament cells. J Periodontal Res 47:563-571 [DOI] [PubMed] [Google Scholar]
- Kano MR, Morishita Y, Iwata C, Iwasaka S, Watabe T, Ouchi Y, et al. (2005). VEGF-A and FGF-2 synergistically promote neoangiogenesis through enhancement of endogenous PDGF-B-PDGFRbeta signaling. J Cell Sci 118(Pt 16):3759-3768 [DOI] [PubMed] [Google Scholar]
- Kawaguchi H, Oka H, Jingushi S, Izumi T, Fukunaga M, Sato K, et al. (2010). A local application of recombinant human fibroblast growth factor 2 for tibial shaft fractures: a randomized, placebo-controlled trial. J Bone Miner Res 25:2735-2743 [DOI] [PubMed] [Google Scholar]
- Kitamura M, Nakashima K, Kowashi Y, Fujii T, Shimauchi H, Sasano T, et al. (2008). Periodontal tissue regeneration using fibroblast growth factor-2: randomized controlled phase II clinical trial. PLoS One 3:e2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura M, Akamatsu M, Machigashira M, Hara Y, Sakagami R, Hirofuji T, et al. (2011). FGF-2 stimulates periodontal regeneration: results of a multi-center randomized clinical trial. J Dent Res 90:35-40 [DOI] [PubMed] [Google Scholar]
- Lee JH, Um S, Jang JH, Seo BM. (2012). Effects of VEGF and FGF-2 on proliferation and differentiation of human periodontal ligament stem cells. Cell Tissue Res 348:475-484 [DOI] [PubMed] [Google Scholar]
- Murakami S. (2011). Periodontal tissue regeneration by signaling molecule(s): what role does basic fibroblast growth factor (FGF-2) have in periodontal therapy? Periodontol 2000 56: 188-208 [DOI] [PubMed] [Google Scholar]
- Murakami S, Takayama S, Ikezawa K, Shimabukuro Y, Kitamura M, Nozaki T, et al. (1999). Regeneration of periodontal tissues by basic fibroblast growth factor. J Periodontal Res 34:425-430 [DOI] [PubMed] [Google Scholar]
- Murakami S, Takayama S, Kitamura M, Shimabukuro Y, Yanagi K, Ikezawa K, et al. (2003). Recombinant human basic fibroblast growth factor (bFGF) stimulates periodontal regeneration in class II furcation defects created in beagle dogs. J Periodontal Res 38:97-103 [DOI] [PubMed] [Google Scholar]
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. (2006). VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol 7:359-371 [DOI] [PubMed] [Google Scholar]
- Ozerdem U, Monosov E, Stallcup WB. (2002). NG2 proteoglycan expression by pericytes in pathological microvasculature. Microvasc Res 63:129-134 [DOI] [PubMed] [Google Scholar]
- Röhnelt RK, Hoch G, Reiss Y, Engelhardt B. (1997). Immunosurveillance modelled in vitro: naive and memory T cells spontaneously migrate across unstimulated microvascular endothelium. Int Immunol 9:435-450 [DOI] [PubMed] [Google Scholar]
- Saint-Geniez M, Kurihara T, D’Amore PA. (2009). Role of cell and matrix-bound VEGF isoforms in lens development. Invest Ophthalmol Vis Sci 50:311-321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine H, Shimizu T, Hobo K, Sekiya S, Yang J, Yamato M, et al. (2008). Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts. Circulation 118(14 Suppl):145S-152S [DOI] [PubMed] [Google Scholar]
- Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. (2004). Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364:149-155 [DOI] [PubMed] [Google Scholar]
- Shimabukuro Y, Terashima H, Takedachi M, Maeda K, Nakamura T, Sawada K, et al. (2011). Fibroblast growth factor-2 stimulates directed migration of periodontal ligament cells via PI3K/AKT signaling and CD44/hyaluronan interaction. J Cell Physiol 226:809-821 [DOI] [PubMed] [Google Scholar]
- Stahl A, Wenger A, Weber H, Stark GB, Augustin HG, Finkenzeller G. (2004). Bi-directional cell contact-dependent regulation of gene expression between endothelial cells and osteoblasts in a three-dimensional spheroidal coculture model. Biochem Biophys Res Commun 322:684-692 [DOI] [PubMed] [Google Scholar]
- Takayama S, Murakami S, Miki Y, Ikezawa K, Tasaka S, Terashima A, et al. (1997). Effects of basic fibroblast growth factor on human periodontal ligament cells. J Periodontal Res 32:667-675 [DOI] [PubMed] [Google Scholar]
- Takayama S, Murakami S, Shimabukuro Y, Kitamura M, Okada H. (2001). Periodontal regeneration by FGF-2 (bFGF) in primate models. J Dent Res 80:2075-2079 [DOI] [PubMed] [Google Scholar]
- Terashima Y, Shimabukuro Y, Terashima H, Ozasa M, Terakura M, Ikezawa K, et al. (2008). Fibroblast growth factor-2 regulates expression of osteopontin in periodontal ligament cells. J Cell Physiol 216:640-650 [DOI] [PubMed] [Google Scholar]
- Yamada S, Tomoeda M, Ozawa Y, Yoneda S, Terashima Y, Ikezawa K, et al. (2007). PLAP-1/asporin, a novel negative regulator of periodontal ligament mineralization. J Biol Chem 282:23070-23080 [DOI] [PubMed] [Google Scholar]
- Yanagita M, Kashiwagi Y, Kobayashi R, Tomoeda M, Shimabukuro Y, Murakami S. (2008). Nicotine inhibits mineralization of human dental pulp cells. J Endod 34:1061-1065 [DOI] [PubMed] [Google Scholar]