Abstract
Background:
The A1chieve, a multicentric (28 countries), 24-week, non-interventional study evaluated the safety and effectiveness of insulin detemir, biphasic insulin aspart and insulin aspart in people with T2DM (n = 66,726) in routine clinical care across four continents.
Materials and Methods:
Data was collected at baseline, at 12 weeks and at 24 weeks. This short communication presents the results for patients enrolled from Kerala, India.
Results:
A total of 1732 patients were enrolled in the study. Four different insulin analogue regimens were used in the study. Patients had started on or were switched to biphasic insulin aspart (n = 1203), insulin detemir (n = 212), insulin aspart (n = 312), basal insulin plus insulin aspart (n = 1) and other insulin combinations (n = 1). At baseline glycaemic control was poor for both insulin naïve (mean HbA1c: 10.0%) and insulin user (mean HbA1c: 8.3%) groups. After 24 weeks of treatment, both the groups showed improvement in HbA1c (insulin naïve: −2.4%, insulin users: −0.5%). SADRs including major hypoglycaemic events or episodes did not occur in any of the study patients.
Conclusion:
Starting or switching to insulin analogues was associated with improvement in glycaemic control with a low rate of hypoglycaemia.
Keywords: A1chieve study, insulin analogues, Kerala, type 2 diabetes mellitus
INTRODUCTION
62.4 million Indians were reported to have type 2 diabetes mellitus (T2DM) putting India on the forefront of diabetic epidemic across globe.[1,2] Fear of hypoglycaemia and gain in body weight act as barriers for initiation of insulin therapy.[3] Modern insulin analogues are a convenient new approach or tool to glycaemic control, associated with low number of hypoglycaemia and favourable weight change.[4] A1chieve, a multinational, 24-week, non-interventional study, assessed the safety and effectiveness of insulin analogues in people with T2DM (n = 66,726) in routine clinical care.[5] This short communication presents the results for patients enrolled from Kerala, India.
MATERIALS AND METHODS
Please refer to editorial titled: The A1chieve study: Mapping the Ibn Battuta trail.
RESULTS
A total of 1732 patients were enrolled in the study. The patient characteristics for the entire cohort divided as insulin-naïve and insulin users is shown in the Table 1. Glycaemic control at baseline was poor in this population. The majority of patients (69.5%) started on or switched to biphasic insulin aspart. Other groups were insulin detemir (n = 212), insulin aspart (n = 312), basal insulin plus insulin aspart (n = 1) and other insulin combinations (n = 1). After 24 weeks of treatment overall hypoglycaemic events reduced to nil for both insulin naïve (0.2 events/patient-year at baseline) and insulin user (2.0 events/patient-year at baseline) groups. The hypoglycaemia incidence in insulin naive group at 24 weeks was lower than that observed in insulin users at baseline. SADRs including major hypoglycaemic events did not occur in any of the study patients. Blood pressure decreased whereas overall lipid profile and quality of life improved at week 24 in the total cohort [Table 2 and 3].
Table 1.
Overall demographic data

Table 2.
Overall safety data
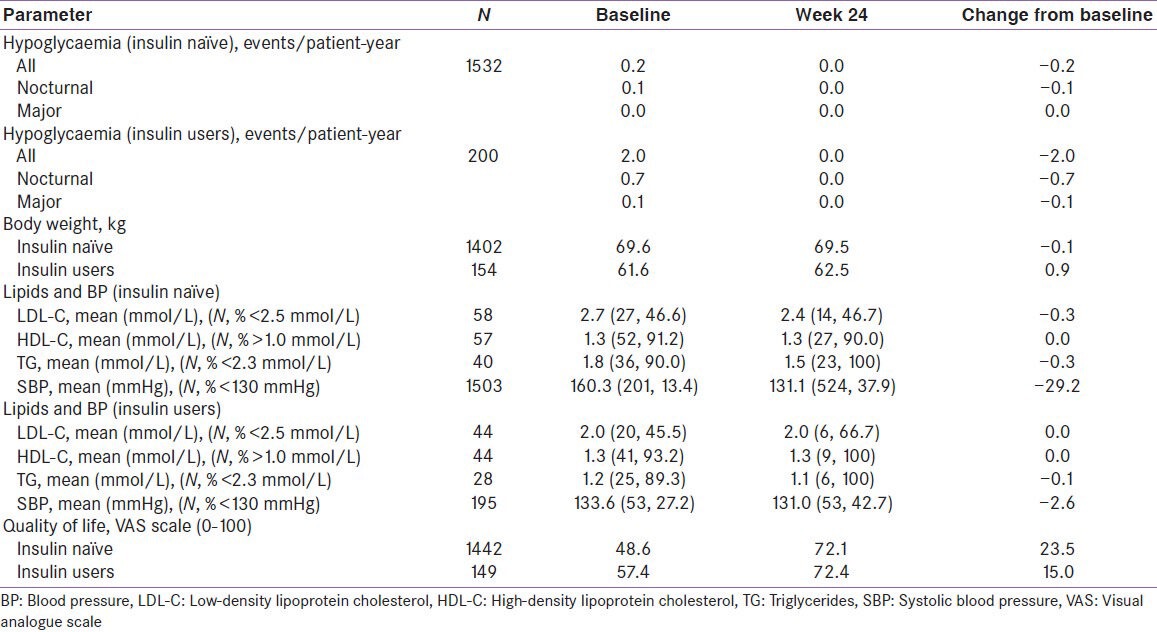
Table 3.
Insulin dose

All parameters of glycaemic control improved from baseline to study end in the total cohort [Table 4].
Table 4.
Overall efficacy data

Biphasic insulin aspart ± OGLD
Of the total cohort, 1203 patients started on biphasic insulin aspart ± OGLD, of which 1060 (88.1%) were insulin naïve and 143 (11.9%) were insulin users. After 24 weeks of starting or switching to biphasic insulin aspart, hypoglycaemic events reduced from 0.1 events/patient-year to nil in insulin naïve group and from 1.5 events/patient-year to zero events in insulin users. Body weight decreased and quality of life improved at the end of the study [Table 5 and 6].
Table 5.
Biphasic insulin aspart±oral glucose-lowering drug safety data

Table 6.
Insulin dose

All parameters of glycaemic control improved from baseline to study end in those who started on or were switched to biphasic insulin aspart for both insulin naïve and insulin user groups [Table 7].
Table 7.
Biphasic insulin aspart±oral glucose-lowering drug efficacy data

Basal + insulin aspart ± OGLD
Of the total cohort, only one patient who started on basal + insulin aspart ± OGLD group was insulin naive. After 24 weeks of starting or switching to insulin aspart, hypoglycaemic events reduced from 13.0 events/patient-year to 0.0 events/patient-year. Body weight decreased and quality of life improved after 24 weeks of treatment. All parameters of glycaemic control improved from baseline to study end in this patient.
Insulin detemir ± OGLD
Of the total cohort, 212 patients started on insulin detemir ± OGLD, of which 188 (88.7%) were insulin naïve and 24 (11.3%) were insulin users. After 24 weeks of starting or switching to insulin detemir, hypoglycaemic events reduced to nil for both insulin naïve (0.1 events/patient-year at baseline) and insulin user (3.3 events/patient-year at baseline) groups. An improvement in quality of life was observed at 24 weeks [Table 8 and 9].
Table 8.
Insulin detemir±oral glucose-lowering drug safety data

Table 9.
Insulin dose

All parameters of glycaemic control improved from baseline to study end in those who started on or were switched to insulin detemir ± OGLDs for both insulin-naïve and insulin user groups [Table 10].
Table 10.
Insulin detemir±oral glucose-lowering drug efficacy data

Insulin aspart ± OGLD
Of the total cohort, 312 patients who started on insulin aspart ± OGLD, of which 279 (89.4%) were insulin naïve and 33 (10.6%) were insulin users. After 24 weeks of treatment starting or switching to insulin aspart, hypoglycaemic events reduced from 0.6 events/patient-year to nil in insulin naïve group and from and from 3.2 events/patient-year to zero events in insulin users. A slight decrease in body weight was noted for insulin naïve group. Quality of life improved at the end of the study [Table 11 and 12].
Table 11.
Insulin aspart±oral glucose-lowering drug safety data

Table 12.
Insulin dose

All parameters of glycaemic control improved from baseline to study end in those who started on or were switched to insulin aspart ± OGLDs for both insulin naïve and insulin user groups [Table 13].
Table 13.
Insulin aspart±oral glucose-lowering drug efficacy data

CONCLUSION
Our study reports improved glycaemic control (HbA1c, FPG and PPPG) and quality of life following 24 weeks of treatment with any of the insulin analogues (Biphasic insulin aspart; basal + insulin aspart; insulin detemir; insulin aspart) with or without OGLD. Overall, a small reduction in weight was observed for insulin naïve group while body weight increased for insulin users. SADRs including major hypoglycaemic events or episodes did not occur in any of the study patients. Though the findings are limited by number of patients, still the trend indicates that insulin analogues can be considered effective and possess a safe profile for treating type 2 diabetes in Kerala, India.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Shetty P. Public health: India's diabetes time bomb. Nature. 2012;485:S14–6. doi: 10.1038/485s14a. [DOI] [PubMed] [Google Scholar]
- 3.Korytkowski M. When oral agents fail: Practical barriers to starting insulin. Int J Obes Relat Metab Disord. 2002;26(Suppl 3):S18–24. doi: 10.1038/sj.ijo.0802173. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch IB. Insulin analogues. N Engl J Med. 2005;352:174–83. doi: 10.1056/NEJMra040832. [DOI] [PubMed] [Google Scholar]
- 5.Shah SN, Litwak L, Haddad J, Chakkarwar PN, Hajjaji I. The A 1 chieve study: A 60 000-person, global, prospective, observational study of basal, meal-time, and biphasic insulin analogs in daily clinical practice. Diabetes Res Clin Pract. 2010;88(Suppl 1):S11–6. doi: 10.1016/S0168-8227(10)70003-6. [DOI] [PubMed] [Google Scholar]


