Abstract
Should we believe that osteoclasts are only involved in bone resorption? What about their contribution to bone formation? In this article I will review evidence that bone formation can be regulated by osteoclasts. Why is this? Likely because in the physiologic condition of bone remodeling, bone resorption and formation are balanced, and there is no better way to control this equilibrium than through a concerted action between the two cell types. Although the influence of osteoblasts on osteoclastic bone resorption is well documented and consolidated over time, what osteoclasts do to regulate osteoblast activity is still matter of intense investigation. The original hypothesis that all is in the osteoblast-seeking factors stored in the bone matrix, released and activated during bone resorption, is now being challenged by several studies, suggesting that osteoclasts are also capable of producing ‘clastokines' that regulate osteoblast performance. Indeed, several of them have been demonstrated to orchestrate osteoclast–osteoblast activities. However, we are probably still at the dawn of a new era, and future work will tell us whether any of these clastokines can be exploited to stimulate bone formation and rebalance bone remodeling in skeletal diseases.
Introduction
When Rodan and Martin1 drafted their pivotal hypothesis that osteoblasts had a role in the hormonal control of bone resorption, and Mundy2 proposed that transforming growth factor-β (TGFβ) released from bone matrix during bone resorption recruited and activated osteoblasts, I had the fortune to be a young emerging scientist. Those were exciting times, with new ideas looming on the horizon of bone research. After many years the scenario has changed, but Rodan and Martin1 and Mundy2 were right, and their hypotheses have been largely confirmed by experimental evidence. In this article, I will retrace the old and recent literature, first briefly addressing the osteoblast-to-osteoclast interactions, and then describing in more detail what is new in the field of the reverse situation: how osteoclasts control bone formation. Finally, I will discuss some controversial issues arguing the biological and clinical significance of the cross-talk between the two cell types.
How osteoblasts affect osteoclast activity
In the 1980s, bone cell biotechnology became a potent tool to understand deeply the cellular and molecular mechanisms of bone remodeling and the pathways that govern the orchestrated activities of bone cells within the so-called basic multicellular unit.3 Before that time, there were scanty data, but they were strong enough to allow Rodan and Martin1 to draft one of the most exciting theories, whereby osteoblasts were the key cells responding to osteoclast-activating hormones. They revolutionized the field, stating that parathyroid hormone (PTH) does not affect osteoclasts directly but through the osteoblast lineage.1 In 1982, Silve et al.4 had investigated the distribution and cellular localization of the PTH receptor in chick embryo calvarial bones. By autoradiography they showed that radio-labeled receptors were concentrated over osteoblasts and progenitor cells. No labeling was observed in osteoclasts, whereas the positive cells belonging to the osteoblast lineage showed radiolabeled receptors at the cell surface and in intracellular vesicles. PTH was already known as a pro-osteoclastic hormone, but what Silve's discovery4 and Rodan and Martin's theory1 told us was that an indirect mechanism recruited osteoblasts to respond to PTH, presumably by the induction of osteoclastogenic factors.5 It has to be noted, however, that an early report showed that PTH injections in chickens induced histologically appreciable osteoclast formation within 30 min.6 This could rely on a direct effect of PTH on osteoclast precursors,7 albeit in a peculiar context such as that of the medullary bone of laying hens, which is subjected to rapid resorption/deposition cycles to supply calcium for the daily egg shell formation.
We now know that among the PTH-induced osteoblast factors there is receptor activator of nuclear factor-κB transcription factor ligand (RANKL),8 the most potent osteoclastogenic cytokine, whose ratio with its decoy receptor, osteprotegerin (OPG), is enhanced by stimulation of the PTH receptor.9 Osteoblasts are not the only source of RANKL, and it could be argued that in a physiologic context this coupling activity does not make any biological sense because of the inconsistency of the need to stimulate bone resorption while bone formation is ongoing. However, as a matter of fact, after discovery of this first hormone-induced osteoblast-dependent osteoclast stimulation pathway, a plethora of other osteoblast molecules have been identified to enhance bone resorption.10 This occurrence culminated in the biotechnological approach to induce osteoclast differentiation in vitro by simply coculturing osteoclast precursors with osteoblasts in the presence of osteoblast-seeking factors such as PTH, vitamin D3 or prostaglandin E2 (PGE2).11 Indeed, the PTH and PGE2 pathways were shown to converge on cAMP through their respective cell surface receptors,12,13 whereas vitamin D3 was clarified to induce the expression of pro-osteoclastogenic cytokines, especially RANKL, through the activation of its cytosolic receptor.14,15 Of note, vitamin D3 is also known to target osteoclasts directly, although with a less clear significance compared with osteoblasts.
Osteoblasts are great producers of pro-osteoclastogenic cytokines, including RANKL, macrophage-colony stimulating factor, interleukin (IL)-1β, IL-6, IL-11, leukemia inhibitory factor, oncostatin M and others,16 altogether representing a combination of factors triggering osteoclastogenesis both in physiologic and in pathologic conditions.17 Nevertheless, osteoblasts also produce antiosteoclastogenic factors, such as OPG, granulocyte–macrophage-colony-stimulating factor, IL-3, IL-12 and IL-18, indicating that their pro-osteoclastogenic function is continuously being controlled and balanced for the sake of proper bone remodeling. Imbalance of bone remodeling could end up with increased bone resorption over bone formation, leading to bone loss diseases, or with excess bone formation over resorption, leading to high bone mass syndromes.18
Many of the discoveries described above have important clinical implications, both for diagnosis (i.e. altered pro-osteoclastogenic gene structure in Paget's disease of bone and osteopetrosis; cytokine imbalance in primary and secondary osteoporosis),19 and treatment (anti-RANKL therapy in osteoporosis and osteolytic bone metastases; targeted anti-inflammatory therapy by monoclonal antibodies or specific inhibitors).20 Therefore, what has been done so far in the understanding of osteoblast-to-osteoclast communication has been instrumental not only for the pure progress of science but also for the benefit of patients suffering from bone diseases.
The reverse situation: how osteoclasts affect osteoblast activity
In 1989 Oreffo et al.21 showed that latent TGFβ is activated into TGFβ by isolated osteoclasts. Latent TGFβ was known to be stored in the bone matrix and, once activated, to potently stimulate bone formation. On the basis of this evidence, Mundy2 proposed that TGFβ could represent one of the key coupling factors stimulating bone formation at the previous sites of bone resorption. Indeed, the bone matrix stores a plethora of growth factors22 that are released and activated by osteoclasts, further contributing, along with TGFβ, to the coupling of bone resorption and formation. Among them, bone morphogenetic proteins (BMPs), which share with TGFβ the intracellular SMAD/mitogen-activated protein kinase signaling pathway, have important roles in bone formation and tissue repair,23 so they are considered ground-breaking and versatile therapeutic agents in orthopedics and dentistry.
This line of evidence has averted scientists from considering the osteoclasts themselves as sources of secreted coupling factors with anabolic effect on bone formation.24 Indeed, this turned out to be the case when studies on osteopetrotic osteoclasts evidenced that, at least in certain forms of the disease, bone formation is not impaired, but could even be enhanced.
Osteopetrosis is a disorder of impaired osteoclast activity or lack of osteoclast differentiation.25 In the first case it is characterized by normal osteoclast formation, which could also be enhanced owing to the hyperparathyroidism observed in patients.25 In the case of impairment of osteoclast differentiation, there is a defect in the signaling to osteoclastogenesis, especially the RANKL/RANK pathway.25 In 2006, our group observed that there was a correlation between osteoclast and osteoblast numbers in bone biopsies of osteopetrotic patients, whereas such a correlation was not found between osteoblast number and serum PTH levels,24 suggesting that osteoclast-to-osteoblast coupling was active also when bone resorption was impaired.26 Similar observations were made by Henriksen et al.27 and prompted the hypothesis that not only bone resorption but also osteoclast numbers are critical to stimulate bone formation.22 On the basis of this hypothesis, osteoclasts were thought to secrete clastokines28 that could work as osteoblast anabolic factors contributing to coupling activity at the previous resorption sites.
Clastokines and bone formation
So far, there are several clastokines of interest (Table 1). Surprisingly, the first osteoclast-secreted molecule to be considered as a coupling factor is the type 5 tartrate-resistant acid phosphatase (TRAcP) isoform that we know as a lysosomal enzyme and osteoclast marker.29 TRAcP is thought to be implicated in bone resorption with a mechanism that still needs full elucidation. It is converted into an ATPase by enzymatic cleavage by trypsin or cathepsin30 and among its substrates there is the TGFβ receptor-interacting protein (TRIP-1).31 How these events affect bone resorption and/or formation has not yet been elucidated. Deletion of TRAcP in mice causes skeletal deformities32 and a role in endochondral ossification of TRAcP has therefore been proposed, whereas transgenic mice overexpressing TRAcP, despite an expected decrease of trabecular bone volume, display enhanced bone formation.33 In this context, in vitro evidence showed the ability of secreted TRAcP to increase alkaline phosphatase activity in osteoblast cultures,29 confirming the findings in transgenic mice.33
Table 1. Clastokines involved in osteoclast-dependent bone formation.
| Clastokine | Acronym | Effect on bone formation | References |
|---|---|---|---|
| Tartrate-resistant acid phosphatase | TRAcP | Stimulation | 27–31 |
| Sphingosine 1-phosphate | S1P | Stimulation | 32–35 |
| Bone morphogenetic protein 6 | BMP6 | Stimulation | 36,37 |
| Int/wingless 10b | Wnt10b | Stimulation | 36,37 |
| Sclerostin | SOST | Inhibition | 36,37 |
| Platelet-derived growth factor BB | PDGF BB | Inhibition | 39–42 |
| Hepatocyte growth factor | HGF | Stimulation | 43,44 |
| Collagen triple helix repeat containing 1 | CTHRC1 | Stimulation | 45,46 |
An important and well-documented secreted clastokine is the sphingosine 1-phosphate (S1P). This is a biologically active lysophospholipid that is implicated in the blood–bone marrow trafficking of osteoclast precursors.34 Upon phosphorylation by sphingosine kinase 1 (SPHK1), it promotes the recruitment of osteoblast precursors at the site of previous resorption and enhances the survival of mature osteoblasts.35 Mesenchymal cells express two S1P receptors (S1PR1 and S1PR2) that trigger the Janus kinase/signal transducers and activators of transcription 3 and focal adhesion kinase/phosphoinositide 3-kinase (PI3K)/AKT pathways, likely to be associated with the induction of chemotaxis. S1P also activates the RhoA GTPase, but this pathway does not seem to contribute to cell migration.36 Therefore, S1P is certainly to be considered an important osteoclast-secreted mediator of bone formation with a strong coupling role on the two cell types. Unfortunately, so far there is no translational impact of this observation as Heilmann et al.37 showed that the treatment of mice receiving an osteotomy of the femur with the S1P analog FTY720 did not improve fracture healing over vehicle-treated mice. Furthermore, the biological effects of S1P on bone remodeling is likely to be the result of a combination of events in which the osteoclast precursors are also involved. In fact, S1PR1 and S1PR2 are expressed by osteoclast precursors as well but their functions seem to counteract each other. S1PR1 appears to direct positive chemotaxis of osteoclast precursors toward S1P, whereas S1PR2 mediates their S1P chemorepulsion.38 It is believed that this reciprocal S1P-dependent regulation of chemotaxis could induce a fine tuning of bone remodeling by affecting the positioning of bone cells in the correct localization.
Along with S1P, BMP6 and Wnt10b (wingless 10b), two important enhancers of bone formation, have been found to be released by osteoclasts.39 The anabolic role of BMP6 and Wnt10b are well known and clearly documented,40 but what is new in recent work is that they are part of the osteoclast-secreted ‘tool kit'. Interestingly, osteoclast Wnt10b, but not S1P and BMP6, is induced by TGFβ and the ability of osteoclasts to promote bone formation in response to TGFβ is blocked by the Wnt10b inhibitor DKK-1.40 TGFβ-dependent induction of Wnt10b in osteoclasts is mediated by the SMAD2/3 signaling while independent of the AKT/MEK (mitogen-activated protein kinase\ERK kinase) pathways. These observations potentiate the coupling role of TGFβ that is now believed to act on osteoblast precursors both directly and through the release of Wnt10b by osteoclasts. Osteoclasts have also been demonstrated to secrete sclerostin, another antagonist of the Wnt pathway that inhibits osteoblast activity.41 Interestingly, sclerostin secretion by osteoclasts from aged mice was found to be higher than that of osteoclasts from young mice, concomitant with a diminished ability of aged osteoclasts to stimulate mineralization.42 Taken together, these observations support the hypothesis of a full implication of the Wnt signaling and their inhibitors in the fine control of osteoclast-to-osteoblast cross-talk.
Another study has explored the role of the BMP receptor 1 (BMPR1)43 in the coupling between bone resorption and bone formation. Genetic manipulation has demonstrated that conditional deletion of this receptor in mature osteoclasts, using a Ctsk/Cre mouse model, results in an increase of bone formation. These data suggest that the BMPR1 signal-transduction pathway may be implicated in the negative regulation of osteoclast-induced bone formation, underlying the need of balanced stimulation/inhibition cycles of osteoblast activity to generate a dynamic and controlled cross-talk between the two cell types.
A new factor found to be implicated in osteoclast-induced regulation of bone formation is platelet-derived growth factor-BB (PDGF-BB), which exhibits complex activities on the osteoblast lineage. It stimulates the in vitro growth of osteoblast precursors, consequently inhibiting osteoblast differentiation.44,45 At the end of a resorption cycle, osteoclasts die by apoptosis and at this stage it is believed that the PDGF-BB anti-osteoblastic signal is removed, thus allowing bone formation to be implemented.46 Another study has demonstrated that mature osteoclasts in which PDGF-BB expression is downregulated by small interfering RNA are prevented from attracting osteoblasts, whereas downregulation of PDGF-BB in osteoblasts reduces their ability to respond to chemotactic factors produced by osteoclasts.47 Taken together, these diverse observations suggest that PDGF-BB have a multifaceted role in the coupling of bone resorption with bone formation and further work is still necessary to fully elucidate the cascade of events in which PDGF-BB is implicated.
Another osteoclast-secreted factor claimed to represent an inducer of bone formation is the hepatocyte growth factor (HGF), found several years ago to be expressed and secreted by osteoclasts and to enhance proliferation of cells of the osteoblast lineage.48 HGF signals human osteoblasts through the PI3K, AKT, c-Src and AP-1 pathways, leading to the induction of osteopontin, a SIBLING extracellular matrix protein family member. These events are mediated by the HGF canonical c-Met receptor, whose inhibition blocks all these signal-transduction pathways and subsequently reduces osteopontin production.49
The last osteoclast-secreted factor found to enhance bone formation is collagen triple helix repeat containing 1 (CTHRC1),50 a glycoprotein associated with the Wnt family signaling.51 CTHRC1 is a downstream target of BMP2, expressed in bone in vivo. Cthrc1-deleted mice have a low bone mass and low bone formation. In contrast, Cthrc1 transgenic mice show high bone mass and high bone formation.52 CTHRC1 was found to enhance chemotaxis and osteoblast differentiation51 as well as matrix mineralization, along with an increased expression of osteoblast-specific genes, such as alkaline phosphatase, collagen 1a1 and osteocalcin, and an accelerated osteoblast proliferation.53 CTHRC1 was observed to be secreted by mature osteoclasts, especially when actively resorbing dentin. It is strongly induced by hydroxyapatite with a mechanism triggered by high extracellular calcium and, to a lesser extent, by phosphate. In vivo, it is stimulated by treatment with RANKL, which, after a phase of bone resorption, promotes a recovery phase of bone formation. Such a recovery phase is not observed in Cthrc1-null mice, suggesting that CTHRC1 is relevant for the osteoclast-to-osteoblast coupling. Targeted deletion of Cthrc1 in mice totally suppresses CTHRC1 expression in the bone tissue. In contrast, there is no consequence upon deletion of Cthrc1 in osteoblasts, suggesting that osteoblasts are the target of CTHRC1 produced by osteoclasts but are not themselves involved in the synthesis of the cytokine. Cthrc1 expression in bone is blunted by aging and by antiresorptive therapy with alendronate, implying that bone resorption is critical for the CTHRC1-induced control of bone formation. Taken together, these observations support a role of CTHRC1 in the context of the cross-talk between osteoclasts and osteoblasts within the bone multicellular unit.
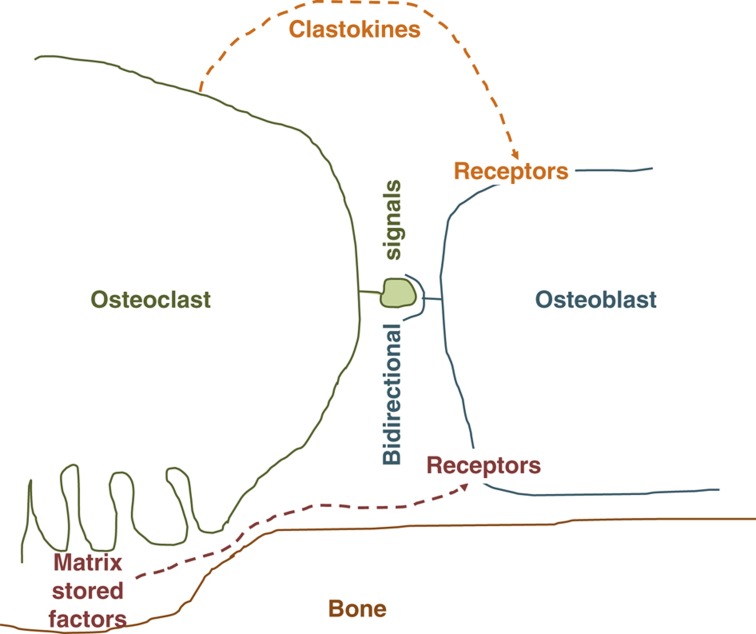
Bidirectional cell-to-cell signals
Another means for osteoclasts to communicate with osteoblasts and enhance bone formation is through the cell-to-cell contact caused by the interaction of the Eph receptor/ligand pathways.46 Osteoclasts express the EphrinB2 ligand, whereas osteoblast precursors display the Eph4 receptor.54 Although they are considered ligand and receptor, respectively, the underlying mechanism is known to induce bidirectional signals. The intracellular pathway associated with the ligand EphrinB2 causes the downregulation in osteoclasts of important transcription factors (c-Fos and NFATc1), which ends up with a suppression of osteoclast formation. At the same time, the intracellular pathway associated to the Eph4 receptor in preosteoblasts induces a reduction of RhoA and an enhancement of osteoblast differentiation.54 According to the Authors' theory, this bidirectional signaling could smooth the progress from the resorption and the formation phase in the bone remodeling cycle.46 The EphrinB2/Eph4 bidirectional signal seems to be counterbalanced by the EphrinA2/EphA2 pathway that regulates bone remodeling in the initiation phase.55 EphrinA2 and EphA2 are both expressed by the osteoclast lineage enhancing differentiation, whereas EphA2 expressed by osteoblasts precursors generate antiosteoblastogenic signals suppressing bone formation.55
Bidirectional signals are also associated with the semaphorins/plexins pathways.28,55 These pathways, initially identified as critical mechanisms of axon guidance, turned out to regulate many other cellular and tissue functions. In the bone, semaphorin 3A, produced by the osteoblast lineage, has been found to repress bone resorption while increasing bone formation.56 The antiosteoclast effect is due to the interaction of semaphorin 3A with the membrane protein neuropilin-1, which blocks RANKL-induced osteoclast differentiation, inhibiting RhoA and the immunoreceptor tyrosine-based activation motif (ITAM) signaling. In contrast, in osteoblasts this pathway activates the Wnt/β-catenin signal. The activity of semaphorin 3A is mediated by binding of the semaphorin 3A/neuropilin-1 complex to the transmembrane protein plexin-A, which in osteoclasts prevents the complex to bind the ITAM-associated immune receptors, whereas in osteoblasts it recruits FARP2, inducing the Rac-GTP/β-catenin signal. Interestingly, in osteoclasts semaphorins 6C and 6D antagonize semaphorin 3A by recruiting plexin-A and preventing its binding to neuropilin-1.56
Is the osteoclast–osteoblast cross-talk always required?
The concerted action of osteoclasts and osteoblasts is well documented in many ways, and experimental evidence clearly supports the existence of molecular mechanisms involved in their cross-talk. However, there are situations in which the two cells types are not so keen to cooperate and this occurs in both physiologic and pathologic conditions. For instance, during modeling, a process that ensures harmonic accrual and correct shaping of the bones in growing subjects, and that decline with age, bone resorption and formation are uncoupled,57 implying that in this context bone resorption is not essential for bone formation. Furthermore, when osteoclasts are reduced by denosumab or are pathologically absent due to RANK or RANKL gene mutations58,59 bone formation still occurs. These observations suggest that while osteoclasts are able to generate anabolic signals that enhance osteoblast activity, especially at the bone remodeling sites, at least modeling-based bone formation appears independent of osteoclasts. Clinical evidence shows that osteoblast anabolic therapy with teraparatide combined with the anti-RANKL antibody, denosumab, has additive effects on bone mineral density of osteoporotic patients, although denosumab markedly reduces osteoclast numbers.60 Pierroz et al.61 confirmed that PTH exerts its osteoblast anabolic effect even in mice treated with denosumab, or in RANKL-deficient mice that present with no osteoclast at all. Finally, it has been demonstrated that fracture healing is potentiated when the antiresorptive agent zoledronate is administered in combination with BMP-7. In fact, in osteotomized rats subjected to autograft, the callus volume was doubled in BMP-7-treated animals compared with controls, but it resulted fourfold higher when BMP-7 was administered in combination with zoledronate, also resulting in an improved strength compared with BMP-7 alone.62 These data indicate that there is no need of osteoclast bone resorption to stimulate bone formation in fracture repair. It is clear though that osteoblasts do not universally require stimulatory osteoclast-derived signals to perform bone formation, but probably there are narrow and specific conditions in which osteoclasts and osteoblasts rely on coregulatory pathways to accomplish their functions. Therefore, the questions remain (i) whether the cooperation between the two cell types, so well demonstrated in experimental conditions, has any relevant biological and/or clinical significance, and (ii) how osteoclasts and osteoblasts discriminate remodeling versus modeling, working as a team or independently of each other, respectively. Further work is therefore necessary to clarify these fundamental aspects of bone biology and understand whether we are dealing with a pathophysiologically relevant phenomenon.
Conclusions
The basic multicellular unit governing bone remodeling could be envisioned as a band that plays a complex music with rhythms and sonorities that alternate at specific times and sites. In order for the process to be conducted in a coordinated manner, cells interact via cross-talk and exchange of signals and information. Osteoclast and osteoblast lineages do so using a variety of mechanisms that orchestrate their activities in both directions. Although osteoblasts were long known to regulate osteoclast activity mainly by paracrine and cell-to-cell signals, osteoclasts were initially thought to be inert. New technologies and in-depth observations have first led to the identification of factors stored in the bone matrix that, liberated in the microenvironment and activated either enzymatically or by low pH, operated as chemotactic, proliferating or differentiating factors (Figure 1). Later on, it became clear that bone resorption was not the only osteoclast activity capable of stimulating bone formation, and clastokines were identified as paracrine anabolic inducers of osteoblasts (Figure 1). Most interestingly, bidirectional cell-to-cell contacts and paracrine signals have recently been described to cause simultaneous and opposite regulation of osteoclast–osteoblast activities in different phases of the bone remodeling cycle (Figure 1). It is very interesting to note that the osteoclast/osteoblast regulation is subjected to a tight balance between stimulations and inhibitions, leading to fine tuning of cellular activities that ensures the maintenance of a physiologic bone mass, especially in adulthood. Disruption of this balance causes bone diseases, with a prevalence of bone resorption over formation in osteopenic syndromes and of bone formation over resorption in high bone mass syndromes. What we should expect in the future from the improvement of the knowledge on the mechanisms whereby bone cells talk to each other is the discovery of agents that could be used for safe and efficient therapies to rebalance bone remodeling. The recent discovery that osteocytes too largely contribute to the regulation of bone remodeling on one hand makes the field even more complex and hardly decipherable, but on the other hand extends the possibilities that at least a few anabolic signals could be exploited for ground-breaking therapies in bone diseases. Without this prospective, the experimental evidence accumulated thus far on the cross-talk between osteoclasts and osteoblasts could remain a pure scientific exercise without any solid translational impact.
Figure 1.
Schematic representation of putative pathways involved in osteoclast-dependent bone formation.
Acknowledgments
I am indebted to Dr Rita Di Massimo for manuscript editing. The original work was supported by the Telethon Grant No. GGP06019.
Footnotes
The author declares no conflict of interest.
References
- Rodan GA, Martin TJ. Role of osteoblasts in hormonal control of bone resorption—a hypothesis. Calcif Tissue Int 1981;33:349–351. [DOI] [PubMed] [Google Scholar]
- Mundy GR. The effects of TGF-beta on bone. Ciba Found Symp 1991;157:137–143. [PubMed] [Google Scholar]
- Seeman E. Bone modeling and remodeling. Crit Rev Eukaryot Gene Expr 2009;19:219–233. [DOI] [PubMed] [Google Scholar]
- Silve CM, Hradek GT, Jones AL, Arnaud CD. Parathyroid hormone receptor in intact embryonic chicken bone: characterization and cellular localization. J Cell Biol 1982;94:379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev 1999;20:345–357. [DOI] [PubMed] [Google Scholar]
- Miller SC. Rapid activation of the medullary bone osteoclast cell surface by parathyroid hormone. J Cell Biol 1978;76:615–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teti A, Rizzoli R, Zambonin Zallone A. Parathyroid hormone binding to cultured avian osteoclasts. Biochem Biophys Res Commun 1991;174:1217–1222. [DOI] [PubMed] [Google Scholar]
- Takayanagi H. New developments in osteoimmunology. Nat Rev Rheumatol 2012;8:684–689. [DOI] [PubMed] [Google Scholar]
- Silva BC, Costa AG, Cusano NE, Kousteni S, Bilezikian JP. Catabolic and anabolic actions of parathyroid hormone on the skeleton. J Endocrinol Invest 2011;34:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh NC, Crotti TN, Goldring SR, Gravallese EM. Rheumatic diseases: the effects of inflammation on bone. Immunol Rev 2005;208:228–251. [DOI] [PubMed] [Google Scholar]
- Martin TJ. Paracrine regulation of osteoclast formation and activity: milestones in discovery. J Musculoskelet Neuronal Interact 2004;4:243–253. [PubMed] [Google Scholar]
- Friedlander G, Amiel C. Cellular mode of action of parathyroid hormone. Adv Nephrol Necker Hosp 1994;23:265–279. [PubMed] [Google Scholar]
- Blackwell KA, Raisz LG, Pilbeam CC. Prostaglandins in bone: bad cop, good cop? Trends Endocrinol Metab 2010;21:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg C, Campbell MJ. Vitamin D receptor signaling mechanisms: integrated actions of a well-defined transcription factor. Steroids 2013;78:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Kalinowski J, Jastrzebski S, Lorenzo JA. 1,25(OH)2 vitamin D3-stimulated osteoclast formation in spleen-osteoblast cocultures is mediated in part by enhanced IL-1 alpha and receptor activator of NF-kappa B ligand production in osteoblasts. J Immunol 2002;169:2374–2380. [DOI] [PubMed] [Google Scholar]
- Atkins GJ, Haynes DR, Geary SM, Loric M, Crotti TN, Findlay DM. Coordinated cytokine expression by stromal and hematopoietic cells during human osteoclast formation. Bone 2000;26:653–661. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL. Osteoclasts; culprits in inflammatory osteolysis. Arthritis Res Ther 2006;8:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh NC, Gravallese EM. Bone remodeling in rheumatic disease: a question of balance. Immunol Rev 2010;233:301–312. [DOI] [PubMed] [Google Scholar]
- Del Fattore A, Teti A. Osteoclast genetic diseases. In Plaseska-Karanfilska D (ed) Human Genetic Diseases. Skopje: InTech, 2011, pp 57–58. [Google Scholar]
- Roux S. New treatment targets in osteoporosis. Joint Bone Spine 2010;77:222–228. [DOI] [PubMed] [Google Scholar]
- Oreffo RO, Mundy GR, Seyedin SM, Bonewald LF. Activation of the bone-derived latent TGF beta complex by isolated osteoclasts. Biochem Biophys Res Commun 1989;158:817–823. [DOI] [PubMed] [Google Scholar]
- Mundy GR. Local control of bone formation by osteoblasts. Clin Orthop Relat Res 1995;313:19–26. [PubMed] [Google Scholar]
- Dean DB, Watson JT, Moed BR, Zhang Z. Role of bone morphogenetic proteins and their antagonists in healing of bone fracture. Front Biosci (Landmark Ed) 2009;14:2878–2888. [DOI] [PubMed] [Google Scholar]
- Karsdal MA, Martin TJ, Bollerslev J, Christiansen C, Henriksen K. Are nonresorbing osteoclasts sources of bone anabolic activity? J Bone Miner Res 2007;22:487–494. [DOI] [PubMed] [Google Scholar]
- Sobacchi C, Schulz A, Coxon FP, Villa A, Helfrich MH. Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat Rev Endocrinol 2013;9:522–536. [DOI] [PubMed] [Google Scholar]
- Del Fattore A, Peruzzi B, Rucci N, Recchia I, Cappariello A, Longo M et al. Clinical, genetic, and cellular analysis of 49 osteopetrotic patients: implications for diagnosis and treatment. J Med Genet 2006;43:315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen K, Andreassen KV, Thudium CS, Gudmann KN, Moscatelli I, Crüger-Hansen CE et al. A specific subtype of osteoclasts secretes factors inducing nodule formation by osteoblasts. Bone 2012;51:353–361. [DOI] [PubMed] [Google Scholar]
- Boyce BF. Advances in the regulation of osteoclasts and osteoclast functions. J Dent Res 2013;92:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Fattore A, Fornari R, Van Wesenbeeck L, de Freitas F, Timmermans JP, Peruzzi B et al. A new heterozygous mutation (R714C) of the osteopetrosis gene, pleckstrin homolog domain containing family M (with run domain) member 1 (PLEKHM1), impairs vesicular acidification and increases TRACP secretion in osteoclasts. J Bone Miner Res 2008;23:380–391. [DOI] [PubMed] [Google Scholar]
- Mitić N, Valizadeh M, Leung EW, de Jersey J, Hamilton S, Hume DA et al. Human tartrate-resistant acid phosphatase becomes an effective ATPase upon proteolytic activation. Arch Biochem Biophys 2005;439:154–164. [DOI] [PubMed] [Google Scholar]
- Sheu TJ, Schwarz EM, Martinez DA, O'Keefe RJ, Rosier RN, Zuscik MJ et al. Phage display technique identifies a novel regulator of cell differentiation. J Biol Chem 2003;278:438–443. [DOI] [PubMed] [Google Scholar]
- Hayman AR, Cox TM. Tartrate-resistant acid phosphatase knockout mice. J Bone Miner Res 2003;18:1905–1907. [DOI] [PubMed] [Google Scholar]
- Angel NZ, Walsh N, Forwood MR, Ostrowski MC, Cassady AI, Hume DA. Transgenic mice overexpressing tartrate-resistant acid phosphatase exhibit an increased rate of bone turnover. J Bone Miner Res 2000;15:103–110. [DOI] [PubMed] [Google Scholar]
- Ishii M, Egen JG, Klauschen F, Meier-Schellersheim M, Saeki Y, Vacher J et al. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature 2009;458:524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu J, Kim HJ, Chang EJ, Huang H, Banno Y, Kim HH. Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast–osteoblast coupling. EMBO J. 2006;25:5840–5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quint P, Ruan M, Pederson L, Kassem M, Westendorf JJ, Khosla S et al. Sphingosine 1-phosphate (S1P) receptors 1 and 2 coordinately induce mesenchymal cell migration through S1P activation of complementary kinase pathways. J Biol Chem 2013;288:5398–53406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmann A, Schinke T, Bindl R, Wehner T, Rapp A, Haffner-Luntzer M et al. Systemic treatment with the sphingosine-1-phosphate analog FTY720 does not improve fracture healing in mice. J Orthop Res 2013;31:1845–1850. [DOI] [PubMed] [Google Scholar]
- Ishii M, Kikuta J, Shimazu Y, Meier-Schellersheim M, Germain RN. Chemorepulsion by blood S1P regulates osteoclast precursor mobilization and bone remodeling in vivo. J Exp Med 2010;207:2793–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson L, Ruan M, Westendorf JJ, Khosla S, Oursler MJ. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci USA 2008;105:20764–20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota K, Quint P, Ruan M, Pederson L, Westendorf JJ, Khosla S et al. TGF-β induces Wnt10b in osteoclasts from female mice to enhance coupling to osteoblasts. Endocrinology 2013;154:3745–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusu N, Laurikkala J, Imanishi M, Usui H, Konishi M, Miyake A et al. Sclerostin is a novel secreted osteoclast-derived bone morphogenetic protein antagonist with unique ligand specificity. J Biol Chem 2003;278:24113–2417. [DOI] [PubMed] [Google Scholar]
- Ota K, Quint P, Ruan M, Pederson L, Westendorf JJ, Khosla S et al. Sclerostin is expressed in osteoclasts from aged mice and reduces osteoclast-mediated stimulation of mineralization. J Cell Biochem 2013;114:1901–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Murai J, Imai Y, Ikegami D, Kamiya N, Kato S et al. Conditional deletion of Bmpr1a in differentiated osteoclasts increases osteblastic bone formation, increasing volume of remodeling bone in mice. J Bone Miner Res 2011;26:2511–2522. [DOI] [PubMed] [Google Scholar]
- Kubota K, Sakikawa C, Katsumata M, Nakamura T, Wakabayashi K. Platelet-derived growth factor BB secreted from osteoclasts acts as an osteoblastogenesis inhibitory factor. J Bone Miner Res 2002;17:257–265. [DOI] [PubMed] [Google Scholar]
- O'Sullivan S, Naot D, Callon K, Porteous F, Horne A, Wattie D et al. Imatinib promotes osteoblast differentiation by inhibiting PDGFR signaling and inhibits osteoclastogenesis by both direct and stromal cell-dependent mechanisms. J Bone Miner Res 2007;22:1679–1689. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Irie N. Osteoblast–osteoclast communication. Arch Biochem Biophys 2008;473:201–209. [DOI] [PubMed] [Google Scholar]
- Sanchez-Fernandez MA, Gallois A, Riedl T, Jurdic P, Hoflack B. Osteoclasts control osteoblast chemotaxis via PDGF-BB/PDGF receptor beta signaling. PLoS One 2008;3:e3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grano M, Galimi F, Zambonin G, Colucci S, Cottone E, Zallone AZ et al. Hepatocyte gowth factor is a coupling factor for osteoclasts and osteoblasts in vitro. Proc Natl Acad Sci USA 1996;93:7644–7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HT, Tsou HK, Chang CH, Tang CH. Hepatocyte growth factor increases osteopontin expression in human osteoblasts through PI3K, Akt, c-Src, and AP-1 signaling pathway. PLoS One 2012;7:e38378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita S, Fumoto T, Matsuoka K, Park KA, Aburatani H, Kato S et al. Osteoclast-secreted CTHRC1 in the coupling of bone resorption to formation. J Clin Invest 2013;123:3914–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Nishimura O, Misaki K, Nishita M, Minami Y, Yonemura S et al. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev Cell 2008;15:23–36. [DOI] [PubMed] [Google Scholar]
- Kimura H, Kwan KM, Zhang Z, Deng JM, Darnay BG, Behringer RR et al. Cthrc1 is a positive regulator of osteoblastic bone formation. PLoS One 2008;3:e3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab 2006;4:111–121. [DOI] [PubMed] [Google Scholar]
- Irie N, Takada Y, Watanabe Y, Matsuzaki Y, Naruse C, Asano M et al. Bidirectional signaling through ephrinA2-EphA2benhances osteoclastogenesis and suppressed ostoblastogenesis. J Biol Chem 2009;284:14637–14644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Kumanogoh A. Semaphorins in bone development, homeostasis, and disease. Semin Cell Dev Biol 2013;24:163–171. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Nakashima T, Taniguchi M, Kodama T, Kumanogoh A, Takayanagi H. Osteoprotection by semaphorin 3A. Nature 2012;485:69–74. [DOI] [PubMed] [Google Scholar]
- Teti A. Bone development: overview of bone cells and signaling. Curr Osteoporos Rep 2011;4:264–273. [DOI] [PubMed] [Google Scholar]
- Guerrini MM, Sobacchi C, Cassani B, Abinun M, Kilic SS, Pangrazio A et al. Human osteoclast–poor osteopetrosis with hypogammaglobulinemia due to TNFRSF11A (RANK) mutations. Am J Hum Genet 2008;83:64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobacchi C, Frattini A, Guerrini MM, Abinun M, Pangrazio A, Susani L et al. Osteoclast–poor human osteopetrosis due to mutations in the gene encoding RANKL. Nat Genet 2007;39:960–962. [DOI] [PubMed] [Google Scholar]
- Tsai JN, Uihlein AV, Lee H, Kumbhani R, Siwila-Sackman E, McKay EA et al. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet 2013;382:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierroz DD, Bonnet N, Baldock PA, Ominsky MS, Stolina M, Kostenuik PJ et al. Are osteoclasts needed for the bone anabolic response to parathyroid hormone? A study of intermittent parathyroid hormone with denosumab or alendronate in knock-in mice expressing humanized RANKL. J Biol Chem 2010;285:28164–28173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosemark P, Isaksson H, McDonald MM, Little DG. Augmentation of autologous bone graft by a combination of bone morphogenic protein and bisphosphonate increased both callus volume and strength. Acta Orthop 2013;84:106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]