Abstract
Salt-sensitive hypertension leads to kidney injury. The Dahl salt-sensitive hypertensive rat (Dahl SS) is a model of salt-sensitive hypertension and progressive kidney injury. The current set of experimental studies evaluated the kidney protective potential of a novel epoxyecosatrienoic acid analog (EET-B) in Dahl SS hypertension. Dahl SS rats receiving high salt diet were treated with EET-B (10 mg/kg/d) or vehicle in drinking water for 14 days. Urine, plasma, and tissue samples were collected at the end of the treatment protocol to assess kidney injury, oxidative stress, inflammation, and endoplasmic reticulum stress. EET-B treatment in Dahl SS rats markedly reduced urinary albumin and nephrin excretion by 60–75% along with 30–60% reductions in glomerular injury, intra-tubular cast formation and kidney fibrosis without affecting blood pressure. In Dahl SS rats, EET-B treatment further caused marked reduction in oxidative stress with 25–30% decrease in kidney malondialdehyde content along with 42% increase of nitrate/nitrite and a 40% reduction of 8-isoprostane. EET-B treatment reduced urinary monocyte chemoattractant protein-1 by 50% along with a 40% reduction in macrophage infiltration in the kidney. Treatment with EET-B markedly reduced renal endoplasmic reticulum (ER) stress in Dahl SS rats with reduction in the kidney mRNA expressions and immunoreactivity of glucose regulatory protein 78 and C/EBP homologous protein. In summary, these experimental findings reveal that EET-B provides kidney protection in Dahl SS rats by reducing oxidative stress, inflammation and ER stress, and this protection was independent of reducing blood pressure.
Keywords: salt-sensitive hypertension, epoxygenase, glomerular injury, oxidative stress, inflammation, ER stress
INTRODUCTION
A substantial proportion of individuals with hypertension in industrialized countries consume high amounts of salt, and approximately 50% of individuals with essential hypertension are salt-sensitive, which is accompanied by progressive renal damage.1–4 The underlying mechanism of salt-sensitive hypertension and the associated renal injury is not yet clearly known, however, it is believed to be strongly associated with elevated oxidative stress and inflammation.5–7
A number of studies demonstrated that arachidonic acid metabolites of cytochrome P450 (CYP) epoxygenase, epoxyeicosatrienoic acids (EETs), possess anti-inflammatory and anti-oxidative activities providing strong organ protection in a number of pathologies including salt-sensitive hypertension.8–10 Inhibition of soluble epoxide hydrolase (sEH, Ephx2), which hydrolyzes EETs to their less biologically active dihydroxyeicosatrienoic acid metabolite, increases EETs bioavailability and provides kidney protection in a number of preclinical rodent models of human disease.11–13 These studies have clearly demonstrated that the kidney protection achieved by pharmacological inhibition of sEH or genetic disruption of Ephx2 is strongly associated with the anti-oxidative and anti-inflammatory effects of EETs.13,14
Due to promising biological actions of EETs in a number of preclinical disease models, interest in developing EET-based therapeutic strategies has grown enormously, and that inspired the development of sEH inhibitors. Since sEH metabolizes EETs to their less active diols, sEH inhibition, in principle, indirectly increases endogenous EET levels. But the major limitations of sEH inhibitors are that they result in a generalized increase in EETs and that their therapeutic effectiveness depends on CYP epoxygenase-mediated EET generation.9,10 This is an important limitation because renal and cardiovascular diseases are associated with impaired epoxygenase generation of EETs.8–10 Thus, if CYP epoxygenase mediated EET generation is impaired in a pathological condition then sEH inhibition will have a negligible effect to increase EET levels. It is further known that endogenously produced EETs are chemically and metabolically labile that could limit their therapeutic potential.15,16 As such, attempts have been made to develop EET analogs that possess EET-mimetic activity along with several key features important for stability and bioavailability.17,18 Several recently developed EET analogs have demonstrated a number of biological actions including organ protection.9,18 Although demonstrating great potential for organ protection, these previously developed EET analogs could not be administered orally.
In the present study, we have investigated the kidney protective effect of a novel orally active EET analog in a widely used model of salt-sensitive hypertension, the Dahl salt-sensitive (Dahl SS) rat. The Dahl SS rat has been widely used to delineate the molecular mechanisms underlying the development of salt-sensitive hypertension, and to evaluate the efficacy of pharmacologic interventions to lower blood pressure and prevent progressive kidney injury.19,20 In the current study, we determined that a novel orally active EET analog provides marked kidney protection in Dahl SS rats without affecting blood pressure. Experimental studies provide additional evidence that the kidney protective effects of the EET analog were associated with its anti-oxidative, anti-inflammatory and anti-endoplasmic reticulum (ER) stress properties.
MATERIALS AND METHODS
Materials
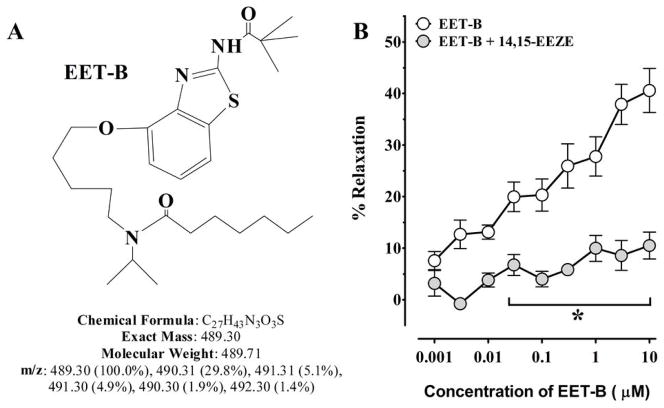
All chemicals were purchased from Sigma (Sigma-Aldrich, St. Louis, MO, USA) unless and otherwise mentioned. EET analog, EET-B [(N-(5-((2-acetamidobenzo[d]thiazol-4-yl)oxy) pentyl)-N-isopropylheptanamide)] was synthesized in the laboratory of J.R. Falck. The structure of EET-B provided in Fig 1A and the detail description of chemical synthesis process of EET-B has been described elsewhere.21 The EET antagonist, 14,15-EEZE [14,15-epoxyeicosa-5(Z)-enoic acid], was also synthesized in the laboratory of J.R. Falck.22,23
Figure 1.
Fig 1A–B. (A) Structure of EET-B. (B) Effect of 14,15-EEZE on EET-B induced relaxation in mesenteric resistance arteries. Values are mean± S.E.M., *p< 0.05. vs. EET-B alone.
Animals
The Medical College of Wisconsin Institutional Animal Care and Use Committee according to the National Institutes of Health Guidelines for care and use of laboratory animals approved all animal studies. Male C57BL/6 mice (20–35g) purchased from Charles River Laboratories (Wilmington, MA, USA) were used for vascular reactivity studies. Mice were housed in the Biomedical Resource Center at the Medical College of Wisconsin with a 12 hour light-dark cycle and free access to water and food. In order to determine the kidney protective and anti-hypertensive effect of EET analog in salt-sensitive hypertension, sets of 6–8 week-old male Dahl Salt sensitive or Dahl/SS (SS/JrHsdMcwiCrl) and SSBN13 rat (SS-Chr13BN/McwiCrl) were purchased from Charles River Laboratories. Rats were housed in the Biomedical Resource Center at the Medical College of Wisconsin with a 12 hour light-dark cycle and free access to water and all animals were given a high salt diet containing 8% NaCl starting on day 0 of the experimental protocol (Harlan Teklad, Madison, WI, USA). Experimental studies were divided into the following groups: Group 1 - SS13BN rats; Group2 - Dahl SS rats administered vehicle (0.05% ethanol and 0.1% PEG-400) in drinking water ad libitum for two weeks; Group 3 -Dahl SS rats administered EET analog EET-B at a dosage of 10mg/kg/d in drinking water ad libitum for two weeks. The dose of EET-B is selected from earlier studies with EET analogs carried out in our laboratory.24,25
Rats were trained for tail-cuff plethysmographic measurement of blood pressure for 5 days before the actual measurement of systolic blood pressure (SBP) on day 0, 7, and 14 of the treatment protocol. On day 0, 7 and 14, SBP was measured exactly at the same time of the day (9–10 am) in every occasion.
Vascular Reactivity
The vasodilator property of EET-B was determined in isolated mesenteric resistance arteries in the absence and presence of EET antagonist 14,15-EEZE. Please see the online supplement at http://hyper.ahajournals.org for the details of the vascular reactivity study.
Biochemical Analysis
At the end of the 14-day experimental period, urine, plasma and kidney tissue samples were collected for biochemical analysis. A number of renal injury markers along with markers of oxidative stress, inflammation and ER stress were studied. Please see the on-line supplement at http://hyper.ahajournals.org for the details of the biochemical analysis.
Kidney Histology and Immunohistochemical Analysis
Histology and immunohistochemical analysis were carried out using protocols provided by the manufacturer and our earlier published studies.19,20,25,26 Please see the on-line supplement at http://hyper.ahajournals.org for the details of the histological and immunohistochemical analysis.
Real-Time PCR Analyses
Real-Time PCR analysis was carried out to assess the mRNA expression of inflammatory (TGF-β1) and ER stress (GRP78 and CHOP) markers. Please see the on-line supplement at http://hyper.ahajournals.org for the details of the real-time PCR analysis.
Endothelial Cell Culture Studies
Human umbilical vein endothelial cells (HUVEC) were used to determine the anti-inflammatory activity of EET-B in the absence and presence of EET antagonist 14,15-EEZE. Please see the online supplement at http://hyper.ahajournals.org for the detailed methods.
Na+ Transport Studies
The effect of EET-B on sodium (Na+) transport was determined by studying its action on trans-epithelial Na+ current in immortalized mouse cortical collecting duct (mpkCCDc14) principal cells. Please see the on-line supplement at http://hyper.ahajournals.org for the detailed methods.
Statistical Analysis
Data are expressed as mean ± SE and were analyzed using one-way ANOVA followed by Tukey’s post-hoc test for multiple group comparisons using Prizm version 4.0 software by GraphPad Software Inc. (La Jolla, CA, USA). Statistical significance was assumed at p<0.05.
RESULTS
EET-B vasodilatory and anti-inflammatory actions are blocked by EET antagonism
Mesenteric resistance artery dilation to EET-B was assessed in the presence and absence of EET antagonist, 14,15-EEZE. Mesenteric resistance artery diameter was not different between experimental groups averaging 161±25 and 142±33 μm. EET-B resulted in a concentration dependent mesenteric resistance artery dilation reaching a maximum dilation of 42±2%. The EET antagonist, 14,15-EEZE abolished the EET-B mediated dilation of the mesenteric resistance (Fig 1B). Vasodilation to the nitric oxide donor, sodium nitroprusside (100μM) was similar in the absence (54±10%) or in the presence of 14,15-EEZE (59±11%).
In order to provide further evidence that EET-B is acting as an EET analog, we determined the anti-inflammatory activity of EET-B in cultured endothelial cells. EET-B significantly reduced the increase in endothelial cell monocyte chemoattractant protein-1 (MCP-1) levels evoked by TNF-α. The ability of EET-B to reduce endothelial cell MCP-1 levels was abolished by the presence of 14,15-EEZE (Fig S1).
EET-B treatment did not lower blood pressure in Dahl SS rats
Baseline systolic blood pressures (SBP) of Dahl SS (119±2 mmHg) and SSBN13 (112±2 mmHg) were similar at day 0. Dahl SS rats receiving high salt diet developed hypertension by day 7 with significantly higher SBP compared to SSBN13 rats (172±14 vs. 128±9 mmHg) and remained elevated on day14 (184±9 vs. 131±8 mmHg). EET-B treatment did not lower SBP in Dahl SS rats on day 7 (163±14 mmHg) or 14 day (183±4 mmHg). Urine volume averaged 70±10 in SSBN13, 61±8 in Dahl SS, and 60±5 mL/d in Dahl SS + EET-B on day 14. EET-B treatment did not alter sodium excretion in Dahl SS rats with urinary sodium excretion averaging 28±7 in SSBN13, 15±3 in Dahl SS, and 16±1 mmol/d in Dahl SS + EET-B groups. In relation to these findings, experiments were conducted to determine the effect of EET-B on sodium transport using isolated cortical collecting duct cells. EET-B did not significantly reduce the short-circuit current (Isc) in mpkCCDc14 principal cells, hence, indicating that EET-B did not alter trans-epithelial sodium transport (Fig S2).
EET-B treatment reduced kidney injury in Dahl SS rats
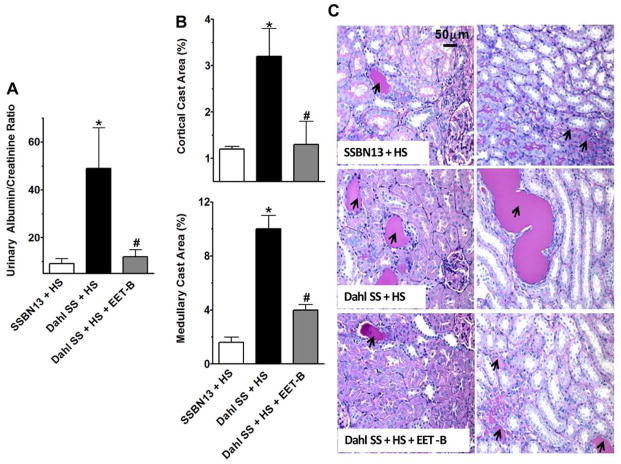
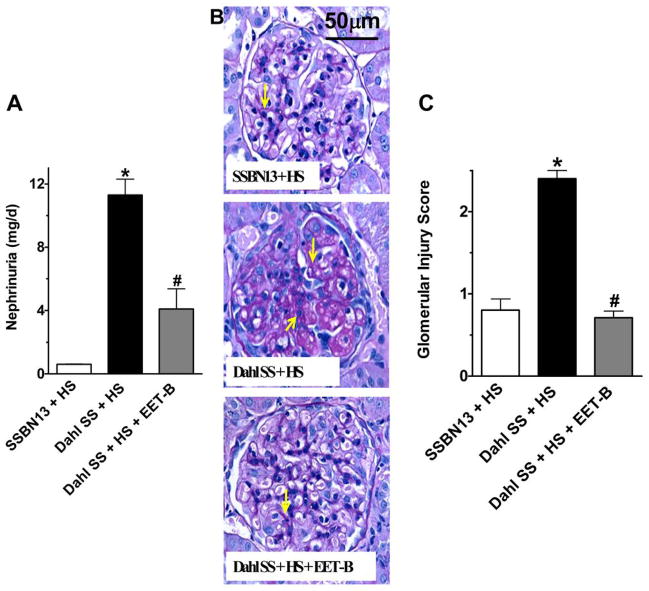
Dahl SS rats had marked kidney injury with 5-fold increase in urinary albumin to creatinine ratio (ACR) along with tubular injury manifested by vacuolation and desquamation of the renal epithelial cells along with intra-tubular proteinaceous cast formation involving 3 and 10% of the kidney cortical and medullary regions, respectively. EET-B treatment resulted in marked reductions of ACR and tubular cast formation in Dahl SS rats (Fig 2A–C). In Dahl SS rats, the kidney injury is also manifested by marked nephrinuria and the associated glomerular injury. Treatment with EET-B resulted in significant reductions of both nephrinuria and glomerular injury in Dahl SS rats (Fig 3A–C). The kidney protective effect of the EET analog EET-B was further evident with its effect on the plasma creatinine (PCr) and BUN levels. There was marked increase in PCr (3.0±0.6 vs. 1.5±0.2 mg/dL, p<0.05) and BUN (53.4±6.0 vs. 36.6±2.0 mg/dL, p<0.05) levels in Dahl SS rats compared to SSBN13 rats. EET-B treatment reduced both PCr (1.7±0.2 mg/dL) and BUN (32.0±2.0 mg/dL) levels in Dahl SS rats (p<0.05).
Figure 2.
Fig 2A–C. (A) Urinary albumin-creatinine ratio, (B) kidney cortical and medullary cast area in different experimental groups. (C) Representative photomicrographs of Periodic acid-Schiff (PAS) staining (200x) depicting tubular cast formation (black arrows) in the kidney cortical and medullary sections of rats from different experimental groups.*p<0.05 vs. SSBN13rats treated with vehicle; #p<0.05 vs. vehicle treated Dahl SS rats. Data expressed as mean ± SEM, n=6–7.
Figure 3.
Fig 3A–C. (A) Urinary excretion of nephrin, (B) representative photomicrographs of Periodic Acid-Schiff (PAS) staining (200x) depicting glomerular injury with mesangial expansion (yellow arrows) and other changes related to glomerular sclerosis in different experimental groups. (C) Semi-quantitative scoring of glomerular injury in rats from different experimental groups. *p<0.05 vs. SSBN13 rats treated with vehicle; #p<0.05 vs. vehicle treated Dahl SS rats. Data expressed as mean ± SEM, n=6–7.
EET-B treatment reduced renal inflammation in Dahl SS rats
Marked inflammation was observed in Dahl SS rats receiving high salt diet. In relation to this, Dahl SS rats demonstrate 70% greater urinary excretion of MCP-1 compared to SSBN13 rats. In parallel to elevated urinary MCP-1, Dahl SS rats also demonstrated 50–65% greater infiltration of macrophages/monocytes in the kidney of these rats compared to that in SSBN13 rats. Treatment with EET-B reduced inflammation in Dahl SS rats with marked reduction in urinary MCP-1 (50%) and infiltration of macrophages in the renal cortex (40%) and medulla (35%) of these rats (Fig 4A–D). In the present study, kidney T-lymphocyte infiltration was determined and there was a 30% increase in T-lymphocyte infiltration of T-lymphocytes in the kidney cortex of Dahl SS rats which was not significantly reduced by EET-B treatment (Fig S3).
Figure 4.
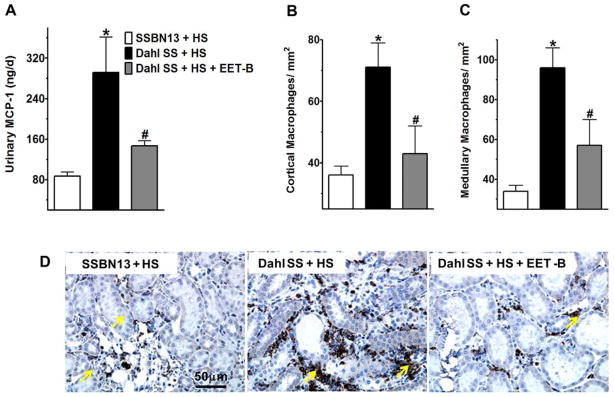
Fig 4A–D. Urinary excretion of monocyte chemoattractant protein-1 (MCP-1) (A), mean ED-1 or CD68 positive macrophage/monocyte counts in the kidney cortex (B) and medulla (C) of rats from different experimental groups. Representative photomicrographs of immunohistochemical staining depicting ED-1 or CD68 positive macrophage/monocyte (yellow arrows) in the kidney of rat from different experimental groups (D). *p<0.05 vs. SSBN13 rats treated with vehicle; #p<0.05 vs. vehicle treated Dahl SS rats. Data expressed as mean ± SEM, n=6–7.
EET-B treatment reduced renal and cardiac fibrosis in Dahl SS rats
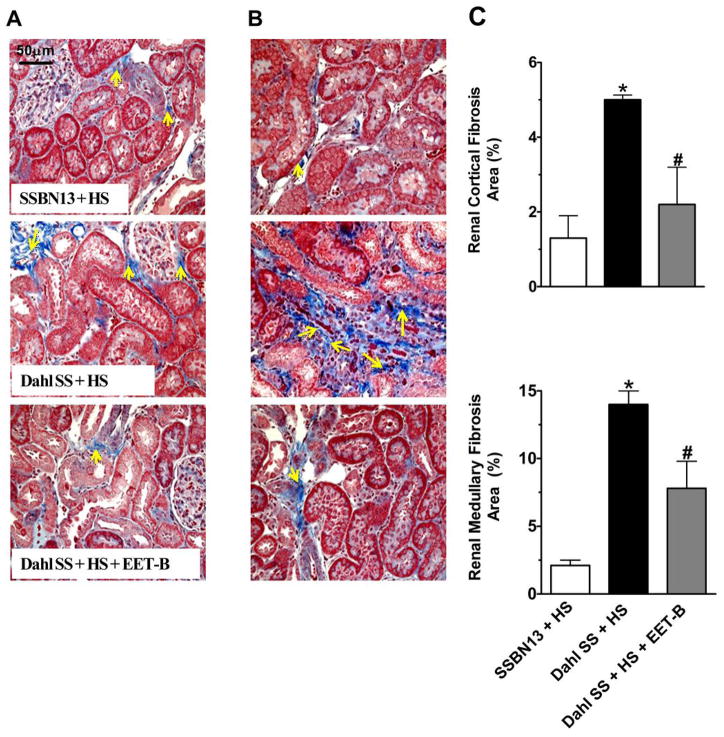
In this present study, we have also observed renal fibrosis in Dahl SS rats involving 5 and 14% of the kidney cortical and medullary regions, respectively. EET-B treatment reduced renal fibrosis by 40–50% in cortical and medullary regions of the kidney of Dahl SS rats (Fig 5A–C). In relation to renal fibrosis, we also observed marked elevation in the mRNA expression of tumour growth factor-β (TGF-β) in the kidney of Dahl SS rats, and EET-B treatment reduced this expression (Fig S4). In addition to renal fibrosis, Dahl SS rats had increased cardiac fibrosis compared to SSBN13 rats and EET-B treatment markedly attenuated this cardiac fibrosis in Dahl SS rats (Fig S5).
Figure 5.
Fig 5A–C. Representative photomicrographs of Masson’s-Trichrome staining of kidney cortical (A) and medullary (B) sections depicting fibrosis (yellow arrows) along with the calculated fibrotic area (%) in the renal cortical and medullary sections of rats from different experimental groups (C). *p<0.05 vs. SSBN13 rats treated with vehicle; #p<0.05 vs. vehicle treated Dahl SS rats. Data expressed as mean ± SEM, n=6–7.
EET-B treatment reduced renal oxidative stress in Dahl SS rats
Dahl SS rats receiving high salt diet exhibited marked oxidative stress with 40–60% elevated levels of malondialdehyde (MDA) in cortex and medulla of the kidney compared to that of SSBN13 rats. EET-B treatment attenuated oxidative stress in Dahl SS rats with marked reductions of renal cortical and medullary contents of MDA by 25 to 35% (Fig 6A–B). The anti-oxidative effects of EET-B in Dahl SS rats was further observed in terms of a significant 42% reduction in urinary 8-isoprostane excretion which was markedly elevated in Dahl SS rats compared to SSBN13 rats (Fig 6C). Dahl SS rats exhibited marked reduction in renal excretion of nitrate and nitrite, nitric oxide (NO) metabolites, compared to SSBN13 rats. EET-B treatment increased renal excretion of NO metabolites in Dahl SS rats by 40% and further indicated anti-oxidant effect of EET-B in Dahl SS rats (Fig 6D).
Figure 6.
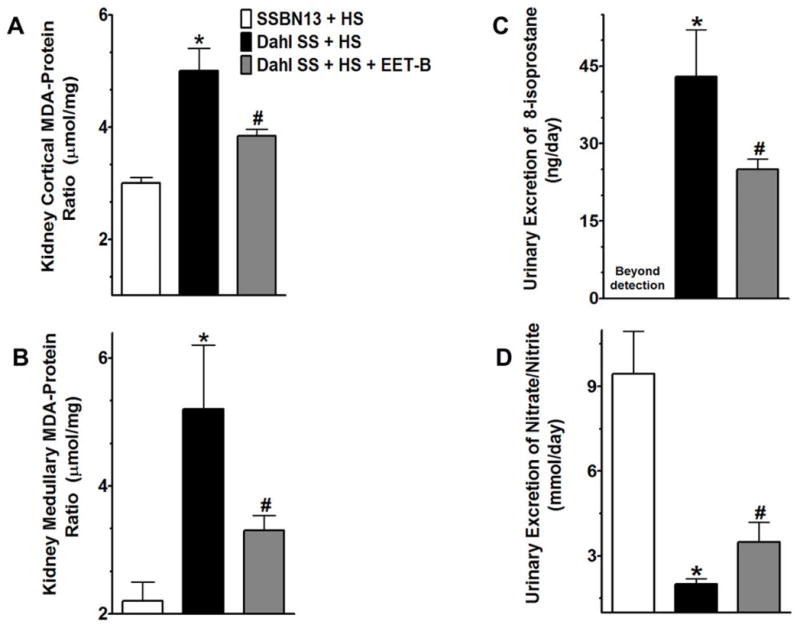
Fig 6A–D. Renal cortical (A) and medullary (B) contents of malondialdehyde (MDA) in rats of different experimental groups. Urinary excretion of 8-isoprostane (C) and nitrate/nitrite (D) in rats from different experimental groups. *p<0.05 vs. SSBN13 rats treated with vehicle; #p<0.05 vs. vehicle treated Dahl SS rats. Data expressed as mean ± SEM, n=6–7.
EET-B treatment reduced renal endoplasmic reticulum stress in Dahl SS rats
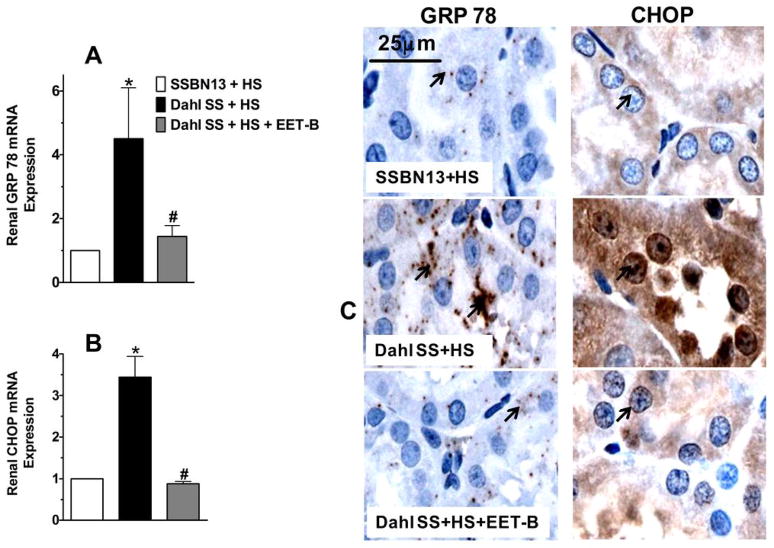
Apart from renal oxidative stress and inflammation, Dahl SS rats also exhibited marked endoplasmic reticulum (ER) stress with elevated mRNA expression of ER stress markers, GRP 78 and CHOP in the kidney. We found a 4.5-fold greater expression of GRP 78 and a 3.5-fold greater expression of CHOP mRNA in the kidney of Dahl SS rats compared to SSBN13 rats (Fig 7A–B). In line with these observations on the renal mRNA expression of GRP 78 and CHOP, we also found marked elevation in the immunoreactivity positive levels for GRP 78 and CHOP in the kidney of Dahl SS rats compared to SSBN13 rats (Fig 7C). Interestingly, treatment with EET-B markedly reduced renal ER stress in Dahl SS rats that was evident from a 70–72% reduction in the renal mRNA expressions of GRP 78 and CHOP along with reduced immunoreactivity of these ER stress markers in the kidney (Fig 7A–C).
Figure 7.
Fig 7A–C. Renal mRNA expression of endoplasmic reticulum stress markers glucose regulatory protein 78 or GRP78 (A) and C/EBP homologous protein or CHOP (B) in the kidney of rats from different experimental groups. Representative photomicrographs of immunohistochemical staining showing the expression and localization (black arrows) of GRP78 and CHOP in the kidney of rats from different experimental groups (C). *p<0.05 vs. SSBN13 rats treated with vehicle; #p<0.05 vs. vehicle treated Dahl SS rats. Data expressed as mean ± SEM, n=6–7.
DISCUSSION
There is strong evidence that EETs have the ability to protect organs by mechanisms involving anti-inflammatory and anti-oxidative activities. Indeed, it has been demonstrated that EETs provide organ protection in a number of pre-clinical rodent models of human diseases including hypertension, diabetes, and ischemic cardiac injury.9–11,25,27,28 One approach to target EET for combating diseases is the development of EET analogs that are designed to resist metabolism and have improved bioavailability.15,16 Recently, we developed a novel orally active EET analog, EET-B and determined its EET analog property. We clearly demonstrate that EET-B is an EET analog because the EET antagonist 14,15-EEZE abolished EET-B mediated mesenteric resistance artery vasodilatation and the anti-inflammatory activity of EET-B in cultured endothelial cells.
In several earlier studies, EET analogs have been demonstrated organ protection in a number of experimental disease models.16,25,27,28 It is known that salt-sensitive hypertension in human and experimental animal models have been associated with progressive kidney damage leading to end-stage renal disease caused by elevated inflammation and oxidative stress.20,29,30 Consequently, in the present study, we hypothesized that the novel orally active EET analog, EET-B, with its strong organ protective activities, will ameliorate salt-sensitive hypertension and the associated kidney injury. We utilized Dahl SS rats that serve as an excellent model of salt-sensitive hypertension and the associated kidney injury exhibiting many phenotypic characteristics common in human hypertension.31,32,33
We have demonstrated that EET-B provided strong kidney protection in salt-sensitive Dahl SS rats, evidenced by reductions of a number of important and relevant kidney injury markers. The renal protection is further evident from histopathological observations including markedly reduced tubular cast formation and fibrosis in the kidney. In relation to our findings in the present study, we have earlier demonstrated organ protective effects by pharmacological and genetic manipulation of EET metabolic pathway in different pathologies including hypertension. Pharmacological inhibition or genetic knockout of soluble epoxide hydrolase enzyme (sEHi and Ephx2 KO) that catalyzes the conversion of EETs to their diols have consistently been demonstrated to decrease kidney and heart damage associated with hypertension and other cardiovascular diseases.9,11,12,14 In particular, the kidney injury associated with hypertension is greatly attenuated by sEH inhibition in different experimental models of hypertension and diabetes.11–14 One limitation of sEH inhibitors are that they result in a generalized increase in EETs and their biological effects depend on the generation of EETs by CYP epoxygenase enzymes. It is further known that in pathological conditions, including that of Dahl SS rat, the endogenous EET generation can be low due to decreased levels or activities of CYP epoxygenase enzymes.10,34,35 The experimental findings of the present set of experiments provide evidence that an EET analog decreases kidney injury in Dahl SS salt-sensitive hypertension.
In the present study, we demonstrated that EET-B provided kidney protection in Dahl SS rats and that this protection was independent of any change in blood pressure and sodium excretion. Indeed, it is known that the Dahl SS rat is a genetic model of salt-dependent hypertension that exhibits impaired sodium excretion, the hallmark of salt-sensitive hypertension. It is also known that one mechanism by which EETs lower blood pressure is via a natriuretic effect.35 It is important to note that the structure of EET-B is designed to mimic 14,15-EET and 14,15-EET has been demonstrated to have variable effects on epithelial sodium transport which is pivotal for EETs natriuretic effect.36,37,38 In relation to this, in the present study we demonstrate that EET-B does not affect renal sodium excretion in the Dahl SS rat and does not inhibit sodium transport in isolated mpkCCDc14 cells. Thus despite the vasodilator, anti-oxidative, and anti-inflammatory effects of EET-B, the lack of an EET-B effect on sodium transport could explain the inability of EET-B to lower blood pressure in Dahl SS rat.
Similar to our findings in the present study, inhibitors of sEH, an important modulator of CYP epoxygenase pathway, also demonstrated blood pressure independent kidney protective actions. For example, experimental studies in type 2 diabetic rats receiving high-salt diet along with angiotensin II hypertension demonstrated that sEH inhibition could provide renal protection without lowering blood pressure.13 Therefore, it is highly plausible that the kidney protective effect of EET-B is related to properties other than effects on blood pressure. Indeed, our earlier experimental studies indicated that increased EET bioavailability by sEH inhibition possesses strong anti-inflammatory and anti-oxidative effects associated with its organ protective effects. For instance, pharmacological inhibition of sEH in hypertensive Goto-Kakizaki rats and disruption of sEH gene, Ephx2 in mice attenuated progression of kidney damage associated with hypertension and diabetes.13,14 These studies further demonstrated that the kidney protective effect of sEH inhibition by pharmacological and genetic manipulation was strongly associated with anti-oxidative and anti-inflammatory effects of EETs.12,13,14 With this background, it is possible that the observed EET-B mediated kidney protection in Dahl SS rats is associated with anti-oxidative and anti-inflammatory properties of this orally active novel EET analog.
Indeed, kidney injury in Dahl SS rat is strongly associated with inflammation and oxidative stress. A number of studies demonstrated enhanced oxidative stress with elevated production of vascular superoxide and increased plasma H2O2 concentration in hypertensive Dahl SS rats.27,39,40,41 It is widely demonstrated that the renal damage frequently observed in Dahl SS rat is related to increased oxidative stress involving reduced NO bioavailability and increased superoxide production in the kidney.39,40,41,42,43 A number of studies demonstrated that oxidative stress plays an important role in the development and progression of renal injury associated with excessive dietary salt ingestion.29,30,44 In line with these observations, we have observed marked oxidative stress with elevated kidney MDA levels, increased urinary excretion of 8-isoprostane, and reduced urinary excretion of nitrate/nitrite in Dahl SS rats. Interestingly, EET-B treatment reduced oxidative stress in these rats by reducing kidney MDA level, urinary excretion of 8-isoprostane, and by increasing urinary excretion of nitrate/nitrite. In support of our findings, in a recent study it was reported that up-regulation of epoxygenases and increased EETs increased the expression and activity of anti-oxidant enzyme superoxide dismutase during toxic insult, and consequently reduce oxidative stress.45 Another recent study demonstrated that renal NADPH oxidase and urinary MDA excretion were reduced in diabetic rats with genetically disrupted sEH system, and suggested the kidney protective role of EETs in diabetic renal pathology in relation to their anti-oxidant effect.14 Thus, the findings of the current study and previous studies provide convincing evidence that anti-oxidant actions in response to increasing EETs contribute to their organ protection.
Apart from anti-oxidative effect, the kidney protective potential of EETs are also associated with anti-inflammatory activity.13,14,45,46,47,48 These earlier reported findings in different pathologies indicated a strong possibility that the kidney protective effect of EET-B in Dahl SS rats could be due to a reduction in inflammation. As reported in earlier studies,47,48 we have, indeed, observed renal inflammation in Dahl SS rats. There was marked elevation in the urinary MCP-1, kidney macrophage/monocyte and T-lymphocyte infiltration in Dahl SS rat. EET-B treatment significantly decreased MCP-1 and kidney macrophage/monocyte but not T-lymphocyte infiltration in Dahl SS rats. Our observations support several earlier reports on the anti-inflammatory activity of EETs that has been implicated in protecting kidney in a number of pathologies. Increased bioavailability of EETs by genetic disruption of sEH reduces inflammation and provides kidney protection in streptozotocin-induced diabetes and in salt-sensitive hypertension.12,14 In the present study, we, therefore, clearly demonstrated that along with marked reduction in oxidative stress, an attenuation of renal inflammatory response contributed in the EET analog EET-B mediated kidney protection in Dahl SS rat.
Many reports indicated that ER stress and inflammation are linked and contribute to the pathophysiology of chronic inflammatory diseases like diabetes and hypertension. Indeed, evidence suggests that ER stress is a potential mediator of inflammation in cardiovascular diseases.49 In a recent study, ER stress was implicated in cardiac injury associated with angiotensin II hypertension in mice. Angiotensin II hypertension caused ER stress that was associated with cardiac hypertrophy and fibrosis in mice, and these pathological events were ameliorated by pharmacological inhibition of ER stress.50 Consequently in the present study we have investigated the involvement of ER stress and demonstrated marked elevation in renal mRNA expression of ER stress markers GRP78 and CHOP in Dahl SS rat. Interestingly, EET-B treatment markedly attenuated expression of these ER stress markers in the kidney of Dahl SS rats as evident from reduced mRNA expression and immunoreactivity positive for GRP 78 and CHOP. These observations indicate involvement of ER stress in the kidney injury of Dahl SS rat, and most importantly, indicated a novel mechanism by which EET could protect kidney in a pathological condition related to salt-sensitive hypertension.
PERSPECTIVE
We have clearly demonstrated the potential of a novel orally active EET analog in ameliorating renal damage in salt-sensitive hypertension. We provide substantial evidence that this novel EET analog ameliorated kidney injury by reducing renal inflammation, oxidative stress, and ER stress in Dahl SS salt-sensitive hypertension. Importantly, this study established the compelling potential of an EET-based therapeutics to treat progressive kidney injury in salt-sensitive hypertension. Moreover, with anti-oxidative, anti-inflammatory, and anti-ER stress, the novel orally active EET analog EET-B holds therapeutic promise for a number of cardiovascular pathologies that lead to end organ damage.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new?
This study demonstrated therapeutic promise of a novel orally active epoxyeicosatrienoic acid (EET) analog in hypertensive kidney diseases.
What is Relevant?
In this study we unveil kidney protective mechanism of a novel orally active EET analog in hypertensive kidney diseases.
We demonstrated that this EET analog provided kidney protection in salt-sensitive hypertension by reducing inflammation, oxidative stress and endoplasmic reticulum (ER) stress in the kidney.
Summary
Salt-sensitive hypertension leads to end-organ damage which is associated with elevated oxidative stress, inflammation and endoplasmic reticulum (ER) stress. We have demonstrated promising kidney protective effect of a novel EET analog in pre-clinical model of human salt-sensitive hypertension. We have clearly demonstrated that EET analog ameliorated kidney injury by reducing renal oxidative stress, inflammation and ER stress.
Acknowledgments
These studies were supported by NIH grant DK38226 and UL1RR031973 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources and the National Center for Advancing Translational Sciences to John D. Imig; Robert A. Welch Foundation (GL625910), and NIH grants GM31278 and DK38226 to John R. Falck; Postdoctoral Fellowship from the Midwest Affiliate of the American Heart Association to Md. Abdul Hye Khan. Excellent technical assistance provided by Jessica Myers, Praveena Narayanan, Katherine A. Walsh, Keng Lee, Breana Cummens, and Aye Mon is gratefully acknowledged.
Footnotes
Disclaimer: The manuscript and its contents are confidential, intended for journal review purposes only, and not to be further disclosed.
DISCLOSURE
None.
References
- 1.Kanbay M, Chen Y, Solak Y, Sanders PW. Mechanisms and consequences of salt sensitivity and dietary salt intake. Curr Opin Nephrol Hypertens. 2011;20:37–43. doi: 10.1097/MNH.0b013e32834122f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinberger MH. Pathogenesis of salt sensitivity of blood pressure. Curr Hypertens Rep. 2006;8:166–170. doi: 10.1007/s11906-006-0014-y. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger MH. Sodium and blood pressure 2003. Curr Opin Cardiol. 2004;19:353–356. doi: 10.1097/01.hco.0000127136.50978.db. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Iturbe B, Johnson RJ. The role of renal microvascular disease and interstitial inflammation in salt-sensitive hypertension. Hypertens Res. 2010;33:975–980. doi: 10.1038/hr.2010.148. [DOI] [PubMed] [Google Scholar]
- 5.Johnson RJ, Feig DI, Nakagawa T, Sanchez-Lozada LG, Rodriguez-Iturbe B. Pathogenesis of essential hypertension: historical paradigms and modern insights. J Hypertens. 2008;26:381–391. doi: 10.1097/HJH.0b013e3282f29876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franco M, Sanchez-Lozada LG, Bautista R, Johnson RJ, Rodriguez-Iturbe B. Pathophysiology of salt-sensitive hypertension: a new scope of an old problem. Blood Purif. 2008;26:45–48. doi: 10.1159/000110563. [DOI] [PubMed] [Google Scholar]
- 7.Vaziri ND, Rodríguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol. 2006;2:582–593. doi: 10.1038/ncpneph0283. [DOI] [PubMed] [Google Scholar]
- 8.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imig JD. Targeting epoxides for organ damage in hypertension. J Cardiovasc Pharmacol. 2010;56:329–335. doi: 10.1097/FJC.0b013e3181e96e0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imig JD. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol Rev. 2012;92:101–130. doi: 10.1152/physrev.00021.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imig JD, Zhao X, Zaharis CZ, Olearczyk JJ, Pollock DM, Newman JW, Kim IH, Watanabe T, Hammock BD. An orally active epoxide hydrolase inhibitor lowers blood pressure and provides renal protection in salt-sensitive hypertension. Hypertension. 2005;6:975–981. doi: 10.1161/01.HYP.0000176237.74820.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manhiani M, Quigley JE, Knight SF, Tasoobshirazi S, Moore T, Brands MW, Hammock BD, Imig JD. Soluble epoxide hydrolase gene deletion attenuates renal injury and inflammation with DOCA-salt hypertension. Am J Physiol Renal Physiol. 2009;297:F740–F748. doi: 10.1152/ajprenal.00098.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olearczyk JJ, Quigley JE, Mitchell BC, Yamamoto T, Kim IH, Newman JW, Luria A, Hammock BD, Imig JD. Administration of a substituted adamantyl urea inhibitor of soluble epoxide hydrolase protects the kidney from damage in hypertensive Goto-Kakizaki rats. Clin Sci (Lond) 2009;116:61–70. doi: 10.1042/CS20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elmarakby AA, Faulkner J, Al-Shabrawey M, Wang MH, Maddipati KR, Imig JD. Deletion of soluble epoxide hydrolase gene improves renal endothelial function and reduces renal inflammation and injury in streptozotocin-induced type 1 diabetes. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1307–R1317. doi: 10.1152/ajpregu.00759.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imig JD, Falck JR. US Patent #7,550,617 Compositions and methods for the treatment of renal and cardiovascular disease. 2009
- 16.Falck JR, Kodela R, Manne R, Atcha KR, Puli N, Dubasi N, Manthati VL, Capdevila JH, Yi XY, Goldman DH, Morisseau C, Hammock BD, Campbell WB. 14,15-epoxyeicosa-5,8,11-trienoic acid (14,15-EET) surrogates containing epoxide bioisosteres: influence upon vascular relaxation and soluble epoxide hydrolase inhibition. J Med Chem. 2009;52:5069–5075. doi: 10.1021/jm900634w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morisseau C, Hammock BD. Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol. 2005;45:311–333. doi: 10.1146/annurev.pharmtox.45.120403.095920. [DOI] [PubMed] [Google Scholar]
- 18.Batchu SN, Lee SB, Qadhi RS, Chaudhary KR, El-Sikhry H, Kodela R, Falck JR, Seubert JM. Cardioprotective effect of a dual acting epoxyeicosatrienoic acid analogue towards ischaemia reperfusion injury. Br J Pharmacol. 2011;162:897–907. doi: 10.1111/j.1476-5381.2010.01093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wadei HM, Textor SC. The role of the kidney in regulating arterial blood pressure. Nat Rev Nephrol. 2012;8:602–609. doi: 10.1038/nrneph.2012.191. [DOI] [PubMed] [Google Scholar]
- 20.Meng S, Cason GW, Gannon AWRL, Manning RD., Jr Oxidative stress in Dahl salt-sensitive hypertension. Hypertension. 2003;41:1346–1352. doi: 10.1161/01.HYP.0000070028.99408.E8. [DOI] [PubMed] [Google Scholar]
- 21.Imig JD, Campbell WB, Falck JR. International Patent Application # PCT/US2012/032090 Epoxyeicosatrienoic acid analogs and methods of making and using the same.
- 22.Gauthier KM, Deeter C, Krishna UM, Reddy YK, Bondlela M, Falck JR, Campbell WB. 14,15-Epoxyeicosa-5(Z)-enoic acid: a selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ Res. 2002;90:1028–1036. doi: 10.1161/01.res.0000018162.87285.f8. [DOI] [PubMed] [Google Scholar]
- 23.Gauthier KM, Jagadeesh SG, Falck JR, Campbell WB. 14,15-Epoxyeicosa-5(Z)-enoic-mSI: a 14,15- and 5,6-EET antagonist in bovine coronary arteries. Hypertension. 2003;42:555–561. doi: 10.1161/01.HYP.0000091265.94045.C7. [DOI] [PubMed] [Google Scholar]
- 24.Imig JD, Elmarakby A, Nithipatikom K, Wei S, Capdevila JH, Tuniki VR, Sangras B, Anjaiah S, Manthati VL, Sudarshan Reddy D, Falck JR. Development of epoxyeicosatrienoic acid analogs with in vivo anti-hypertensive actions. Front Physiol. 2010;1:157. doi: 10.3389/fphys.2010.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan MA, Liu J, Kumar G, Skapek SX, Falck JR, Imig JD. Novel orally active epoxyeicosatrienoic acid (EET) analogs attenuate cisplatin nephrotoxicity. FASEB J. 2013;27:2946–2956. doi: 10.1096/fj.12-218040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagasawa T, Hye Khan MA, Imig JD. Captopril attenuates hypertension and renal injury induced by the vascular endothelial growth factor inhibitor sorafenib. Clin Exp Pharmacol Physiol. 2012;39:454–461. doi: 10.1111/j.1440-1681.2012.05699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seubert JM, Zeldin DC, Nithipatikom K, Gross G. Role of epoxyeicosatrienoic acids protecting the myocardium following ischemia / reperfusion injury. Prostaglandins Other Lipid Mediat. 2007;82:50–59. doi: 10.1016/j.prostaglandins.2006.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gross GJ, Gauthier KM, Moore J, Falck JR, Hammock BD, Campbell WB, Nithipatikom K. Effects of the selective EET antagonist, 14,15-EEZE, on cardioprotection produced by exogenous or endogenous EETs in the canine heart. Am J Physiol Heart Circ Physiol. 2008;94:H2838–H2844. doi: 10.1152/ajpheart.00186.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian N, Thrasher KD, Gundy PD, Hughson MD, Manning RD., Jr Antioxidant treatment prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Hypertension. 2005;45:934–939. doi: 10.1161/01.HYP.0000160404.08866.5a. [DOI] [PubMed] [Google Scholar]
- 30.Tian N, Gu JW, Jordan S, Rose RA, Hughson MD, Manning RD., Jr Immune suppression prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol. 2007;292:H1018–H1025. doi: 10.1152/ajpheart.00487.2006. [DOI] [PubMed] [Google Scholar]
- 31.Campese VM. Salt sensitivity in hypertension. Hypertension. 1994;23:531–550. doi: 10.1161/01.hyp.23.4.531. [DOI] [PubMed] [Google Scholar]
- 32.Cowley AW, Jr, Roman RJ. The role of the kidney in hypertension. JAMA. 1996;275:1581–1589. [PubMed] [Google Scholar]
- 33.Shehata MF. Genetic and dietary salt contributors to insulin resistance in Dahl salt-sensitive (S) rats. Cardiovasc Diabetol. 2008;7:19. doi: 10.1186/1475-2840-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marino JP., Jr Soluble epoxide hydrolase, a target with multiple opportunities for cardiovascular drug discovery. Curr Top Med Chem. 2009;9:452–463. doi: 10.2174/156802609788340805. [DOI] [PubMed] [Google Scholar]
- 35.Carroll MA. Role of the adenosine (2A) receptor-epoxyeicosatrienoic acid pathway in the development of salt-sensitive hypertension. Prostaglandins Other Lipid Mediat. 2012;98:39–47. doi: 10.1016/j.prostaglandins.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pidkovka N, Rao R, Mei S, Gong Y, Harris RC, Wang WH, Capdevila JH. Epoxyeicosatrienoic acids (EETs) regulate epithelial sodium channel activity by extracellular signal-regulated kinase 1/2 (ERK1/2)-mediated phosphorylation. J Biol Chem. 2013;288:5223–5231. doi: 10.1074/jbc.M112.407981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun P, Antoun J, Lin DH, Yue P, Gotlinger KH, Capdevila J, Wang WH. Cyp2c44 epoxygenase is essential for preventing the renal sodium absorption during increasing dietary potassium intake. Hypertension. 2012;59:339–347. doi: 10.1161/HYPERTENSIONAHA.111.178475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei Y, Lin DH, Kemp R, Yaddanapudi GS, Nasjletti A, Falck JR, Wang WH. Arachidonic acid inhibits epithelial Na channel via cytochrome P450 (CYP) epoxygenase-dependent metabolic pathways. J Gen Physiol. 2004;124:719–727. doi: 10.1085/jgp.200409140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manning RD, Jr, Tian N, Meng S. Oxidative stress and antioxidant treatment in hypertension and the associated renal damage. Am J Nephrol. 2005;25:311–317. doi: 10.1159/000086411. [DOI] [PubMed] [Google Scholar]
- 40.Swei A, Lacy F, DeLano FA, Schmid-Schonbein GW. Oxidative stress in the Dahl hypertensive rat. Hypertension. 1997;30:1628–1633. doi: 10.1161/01.hyp.30.6.1628. [DOI] [PubMed] [Google Scholar]
- 41.Swei A, Lacy F, DeLano FA, Parks DA, Schmid-Schonbein GW. A mechanism of oxygen free radical production in the Dahl hypertensive rat. Microcirculation. 1999;6:179–187. [PubMed] [Google Scholar]
- 42.Hayakawa H, Coffee K, Raij L. Endothelial dysfunction and cardiorenal injury in experimental salt-sensitive hypertension: effects of antihypertensive therapy. Circulation. 1997;96:2407–2413. doi: 10.1161/01.cir.96.7.2407. [DOI] [PubMed] [Google Scholar]
- 43.Zhou MS, Schuman IH, Jaimes EA, Raij L. Renoprotection by statins is linked to a decrease in renal oxidative stress, TGF-beta, and fibronectin with concomitant increase in nitric oxide bioavailability. Am J Physiol Renal Physiol. 2008;295:F53–F59. doi: 10.1152/ajprenal.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kitiyakara C, Chabrashvili T, Chen Y, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. J Am Soc Nephrol. 2003;14:2775–2782. doi: 10.1097/01.asn.0000092145.90389.65. [DOI] [PubMed] [Google Scholar]
- 45.Liu L, Chen C, Gong W, Li Y, Edin ML, Zeldin DC, Wang DW. Epoxyeicosatrienoic acids attenuate reactive oxygen species level, mitochondrial dysfunction, caspase activation, and apoptosis in carcinoma cells treated with arsenic trioxide. J Pharmacol Exp Ther. 2011;339:451–463. doi: 10.1124/jpet.111.180505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y, Webb HK, Fukushima H, Micheli J, Markova S, Olson JL, Kroetz DL. Attenuation of cisplatin-induced renal injury by inhibition of soluble epoxide hydrolase involves nuclear factor κB signaling. J Pharmacol Exp Ther. 2012;341:725–734. doi: 10.1124/jpet.111.191247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tian N, Moore RS, Braddy S, Rose RA, Gu JW, Hughson MD, Manning RD., Jr Interactions between oxidative stress and inflammation in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol. 2007;293:H3388–H3395. doi: 10.1152/ajpheart.00981.2007. [DOI] [PubMed] [Google Scholar]
- 48.Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension. 2006;48:149–156. doi: 10.1161/01.HYP.0000228320.23697.29. [DOI] [PubMed] [Google Scholar]
- 49.Kolattukudy PE, Niu J. Inflammation, endoplasmic reticulum stress, autophagy, and the monocyte chemoattractant protein-1/CCR2 pathway. Circ Res. 2012;110:174–189. doi: 10.1161/CIRCRESAHA.111.243212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kassan M, Galán M, Partyka M, Saifudeen Z, Henrion D, Trebak M, Matrougui K. Endoplasmic reticulum stress is involved in cardiac damage and vascular endothelial dysfunction in hypertensive mice. Arterioscler Thromb Vasc Biol. 2012;32:1652–1661. doi: 10.1161/ATVBAHA.112.249318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.