Abstract
Introduction
PET imaging with Cu-ATSM for delineating hypoxia has provided valuable clinical information, but investigations in animal models of prostate cancer have shown some inconsistencies. As a defense mechanism in prostate cancer cells, the fatty acid synthesis pathway harnesses its oxidizing power for improving the redox balance despite conditions of extreme hypoxia, potentially altering Cu-ATSM hypoxia-selectivity.
Methods
Human prostate tumor cultured cell lines (PC-3, 22Rv1, LNCaP, and LAPC-4), were treated with an FAS inhibitor (C75, 100 μM) under anoxia. 64Cu-ATSM uptake into these treated cells, and non-treated anoxic cells, was then examined. Fatty acid synthase (FAS) expression level in each cell line was subsequently quantified by ELISA. An additional study was performed in PC-3 cells to examine the relationship between the restoration of 64Cu-ATSM hypoxia-selectivity and the concentration of C75 (100, 20, 4, or 0.8 μM) administered to the cells.
Results
Inhibition of fatty acid synthesis with C75 resulted in a significant increase in 64Cu-ATSM retention into prostate tumor cells in vitro under anoxia over 60 mins. Inhibition studies demonstrated higher uptake values of 20.9 ± 3.27, 103.0 ± 32.6, 144.2 ± 32.3, and 200.1 ± 79.3% at 15 mins over control values for LAPC-4, PC-3, LNCaP, and 22Rv1 cells, respectively. A correlation was seen (R2 = 0.911) with FAS expression plotted against % change in 64Cu-ATSM uptake with C75 treatment.
Conclusions
Although Cu-ATSM has clinical relevance in the PET imaging of hypoxia in many tumor types, its translation to the imaging of prostate cancer may be limited by the over-expression of FAS associated with prostatic malignancies.
Keywords: Fatty Acid Synthase, Cu-ATSM, PET, prostate cancer
Introduction
The onset of hypoxia in malignant tissues is fairly universal, though not homogeneous, and is associated and influenced by a myriad of complicated processes. It is thought that since tumor cells are metabolically more active and proliferative than normal cells, they drain the surrounding tissue and blood supply of oxygen faster than it is supplied [1-3]. Tumors with increased levels of hypoxia afford resistance to traditional radiation therapy, as well as reducing the effects of many chemotherapeutic agents [4-7]. Measuring tissue oxygenation is no trivial task. The most common method, the use of an Eppendorf O2 probe to measure oxygen tension, is effective but also quite invasive. Although the values obtained through this method have been predictive of metastatic potential and patient response to treatment [8-10], the technical difficulties of this technique make it generally undesirable.
In recent years, investigations into alternative, non-invasive methods for measuring pO2 have been pursued by numerous researchers [2]. The use of PET imaging in conjunction with radiolabeled molecules that undergo chemical changes in the presence or absence of oxygen has led to a few promising tracers. 18F-MISO (18F-fluormisonidazole) exploits a simple chemical cycle whereby it takes the place of oxygen as an electron acceptor and is reduced and trapped. In the presence of oxygen, the initially reduced compound returns to its original state by receiving an additional electron from oxygen in a futile cycle. Imaging with 18F-MISO has proved somewhat successful as a measure of oxygen levels, however the contrast between hypoxic and normoxic tissues is minimal (tumor to blood ratio > 1.2) [11-15]. 18F-FAZA (18F-fluoroazomycin arabinoside) has also been investigated as an alternative to 18F-MISO with faster background clearance while maintaining hypoxia selectivity [16, 17]. A recent pilot study showed promising results for clinical imaging in head and neck cancer patients [18].
Copper(II)-diacetyl-bis(N4-methylthiosemicarbazone), Cu-ATSM, labeled with a positron emitting isotope of copper (60Cu, 61Cu, 62Cu or 64Cu) has been shown, in vitro and in vivo, to be selective for hypoxic tissue [19-21]. The clinical use of Cu-ATSM for delineating hypoxic human tumors using PET has provided valuable information in cervical, lung and rectal cancers [22-25]. Although a rapid >4-fold increase in retention of Cu-ATSM in hypoxic over normoxic cells was observed with overall retention at around 90% in hypoxic compared to 25% in normoxic cells [20, 26], these investigations did not include the use of prostate tumor cell lines. Burgmann et al. reported diminished hypoxic selectivity of Cu-ATSM in vitro in a rat prostate cancer cell line, R3327-AT [27]. In their analysis of 6 different cell lines ratios of hypoxic or anoxic uptake of 64Cu-ATSM to normoxic uptake showed that the retention into the two prostate lines were consistently the lowest of the six lines examined. The same group also reported in vivo imaging studies comparing 64Cu-ATSM and 18F-MISO in two animal models of cancer [28]. In the FaDu human squamous cell carcinoma tumor model, the early (∼1-4 h) and late (∼19 h) 64Cu-ATSM images were similar and were in general accordance with 18F-MISO scans. However, their data did not support using Cu-ATSM as an indicator of hypoxia at early timepoints in the R3327-AT prostate model, with a negative correlation with 18F-MISO at 1 hour after administration. Therefore, although the use of Cu-ATSM has been validated in vitro and in vivo in multiple tumor models and clinically in various human cancers, there is a concern that its ability to delineate hypoxia in prostate tumors may be suspect.
Our recent work has demonstrated that there is extensive involvement of the fatty acid synthesis pathway in 1-11C-acetate uptake in prostate tumors, leading to a possible marker for fatty acid synthase (FAS) expression in vivo by PET [29]. FAS is a multifunctional enzymatic protein that catalyzes fatty acid biosynthesis [30] and FAS levels are associated with tumor aggressiveness in late-stage prostatic adenocarcinomas as well as a prognostic indicator for overall survival [31]. It has also been noted that the physiological significance of the fatty acid synthesis pathway in prostate cancers is in the harnessing of its oxidizing power for improving redox balance (i.e., lower NADH/NAD+ ratios) despite oxygen limiting (hypoxic) conditions [32]. Therefore, considering that the mechanism of Cu-ATSM hypoxia-selectivity is reliant on an hypoxia-induced intracellular chemical reduction of Cu(II) to Cu(I) but that the fatty acid synthesis pathway improves redox imbalance during hypoxia, we wanted to explore the relationships between the hypoxia-selectivity of Cu-ATSM and the expression levels of FAS in prostate tumor models.
Methods
All chemicals, unless otherwise stated, were purchased from Sigma-Aldrich Chemical Company, Inc (St. Louis, MO). All solutions were prepared using distilled, deionized water (>18 MΩ resistivity) by passing through a Milli-Q filtration system (Millipore Corp., Milford, MA). Radioactive samples were counted in a radioisotope calibrator (Capintec, Inc., Ramsey, NJ) for determination of mCi and an automated well scintillation Beckman 8000 gamma counter (Irvine, CA) for counts per minute. 64Cu was produced at Washington University School of Medicine on a CS-15 biomedical cyclotron using previously published methods [33] and 64Cu-ATSM was prepared as previously described [21]. Human prostate carcinoma tumor cell lines PC-3, LNCaP, and 22Rv1 were obtained from American Type Culture Collection (Manassas, VA) and LAPC-4 cells were a gift from Dr. Charles Sawyers at UCLA. Cells were maintained by serial passage in cell culture. LAPC-4 was shown in our laboratories to have relatively low FAS expression compared with the other prostate tumor cell lines and was therefore chosen as a ‘negative’ control.
In vitro 64Cu-ATSM uptake
A comparison of 64Cu-ATSM uptake under anoxic and normoxic conditions was made in three prostate cell lines – 22Rv1, LNCaP, and PC-3. The apparatus and methods used in the in vitro uptake study are based on those previously described in the literature [20]. Viability of the cells was measured with a hemocytometer utilizing trypan blue staining. Briefly, two three-neck flasks containing cells in suspension were immersed in a 37°C water bath by a rod connected to a stand rotating on an external orbital mixer. An anoxic (5% CO2, 95% N2) and normoxic (20% O2, 5% CO2, 95% N2) gas mixture were humidified and brought to 37°C before being passed continuously through each of the flasks. 30-50 mL of cell suspension (1 × 106 cells/mL) was added to each flask and given 1 hour to equilibrate to the environment. After equilibration, 100 μCi 64Cu-ATSM was added to each of the flasks in a small amount (∼5 μL) of ethanol. At 1, 5, 15, 30, 45, and 60 minutes, 200 μL of cell suspension was removed via pipet and placed in a 1.5 mL Eppendorf tube which was immediately centrifuged for 30 s at 3000 rpm to pellet the cells. 180 μL of the supernatant was then pipetted off and deposited in a separate tube. This was done in triplicate at each timepoint. All pellet and supernatant samples were counted on a gamma counter. Percent uptake was calculated as activity in the pellet (corrected for remaining supernatant) divided by the sum of the pellet and supernatant.
C75 treatment studies were performed in four prostate cell lines – 22Rv1, LNCaP, PC-3, and LAPC-4 following similar methods. In this case, both flasks were under anoxic conditions, while one flask received C75 for FAS inhibition. During the hour of equilibration, C75 was added to the cells (in 10 μL or less of DMSO) so that the final concentration in the flask was 100 μM, while the other flask received DMSO alone. At 1, 5, 15, 30, 45, and 60 minutes the same procedure of sample collection and analysis was performed.
FAS expression by ELISA
FAS expression of each tumor line was quantified using a FAS-detect™ ELISA kit (FASgen, Inc.) based on a two-site ELISA technique to quantitatively measure FAS in human serum. Cells were pelleted and lysed by addition of 1 mL of cell lysis buffer containing protease inhibitors and incubated on ice for 30 minutes. The cell debris was then repelleted and the final supernatant was analyzed. Results were compared to a standard curve of varying concentrations of FAS to determine the concentration in ng/mL. These values were normalized by protein concentration as determined by using a standard copper reduction/bicinchoninic acid assay (Pierce) with bovine serum albumin as the standard, resulting in a ratio with units of ng FAS/μg protein.
In vitro C75 dose response on 64Cu-ATSM uptake
These in vitro studies were performed using the same apparatus and methods as previously described in the initial in vitro experiments. In this study however, various amounts of C75 were added to the cells (in 10 μL or less of DMSO) so that the final concentration of C75 in each flask was 100, 20, 4, or 0.8 μM.
Results
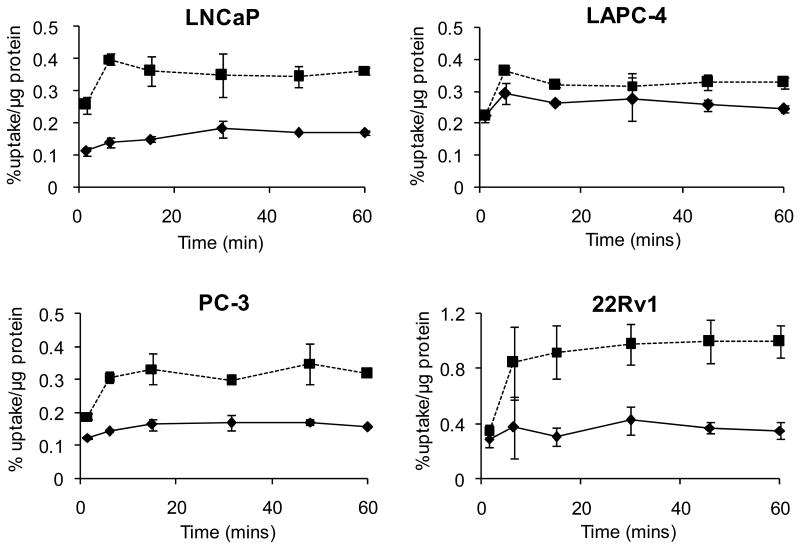
For each cell line, two three-neck flasks containing cells (35 mL) in suspension were placed under anoxic and normoxic conditions with or without pretreatment with C75 (100 μM). Uptake of 64Cu-ATSM was measured over time to determine if FAS inhibition (by C75) restored the cells' ability to trap Cu-ATSM under low oxygen conditions. Inhibition of FAS with C75 resulted in a dramatic increase in 64Cu-ATSM retention into prostate tumor cells in vitro under anoxic conditions (Fig. 1). The treated cells demonstrated higher uptake values at 15 minutes of 20.93 ± 3.271, 103.0 ± 32.57, 144.2 ± 32.28, and 200.1 ± 79.27 % over control values for LAPC-4, PC-3, LNCaP, and 22Rv1 cell lines, respectively. These values would later be correlated to FAS expression of each cell line.
Figure 1.
Uptake of 64Cu-ATSM into prostate tumor cells (in suspension) under anoxic conditions (5% CO2 + 95% N2) treated with C75 (dashed) and compared to control (solid). (n = 3 at each timepoint)
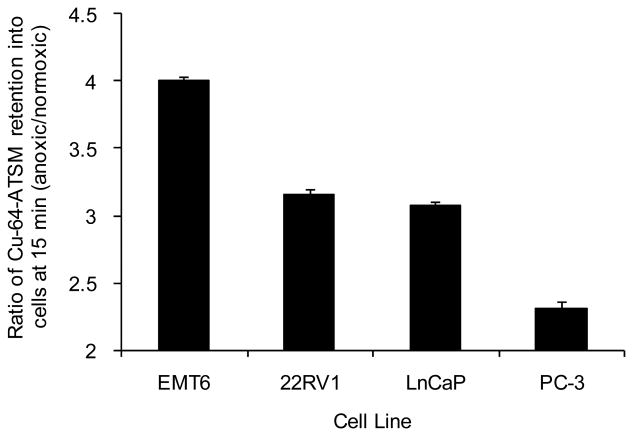
Ratios of anoxic to normoxic 64Cu-ATSM uptake demonstrate the selectivity of the tracer in a particular model, and so these were also compared over the 60 minute experiment (Fig. 2, 15 min data shown). The value obtained for the retention of 64Cu-ATSM into EMT-6 murine mammary carcinoma cells at 15 min is included for comparison [26]. The 15 minute time point was chosen since Cu-ATSM in these cells was shown to plateau at this point (Fig. 1). Similar kinetics were shown in Lewis et al., [26] and Dearling et al., 1998 [20]. Also given that this is entirely an in vitro study, the potential blood flow issues associated with in vivo studies was not felt to be a factor. It is also worth pointing out that in the human 60Cu-ATSM studies uptake in tumors [22, 23] resulted in high contrast levels between hypoxic and normoxic tissues by as little as 10-15 minutes post injection, and yielded clinically relevant information about tumor oxygenation that was predictive of tumor behavior and response to therapy. In this current study, values in all three of the cell lines examined under normoxic conditions, were lower than the EMT-6 ratios suggesting that visualization of solid tumors, derived from these prostate cancer cell lines may be difficult due to normoxic background retention in the surrounding tissue.
Figure 2.
Ratios of anoxic to normoxic 64Cu-ATSM uptake in prostate cancer cells lines at 15 mins demonstrating differing selectivity of the tracer in different models. The value obtained for the retention of 64Cu-ATSM into EMT-6 murine mammary carcinoma cells at 15 mins is included for comparison [26].
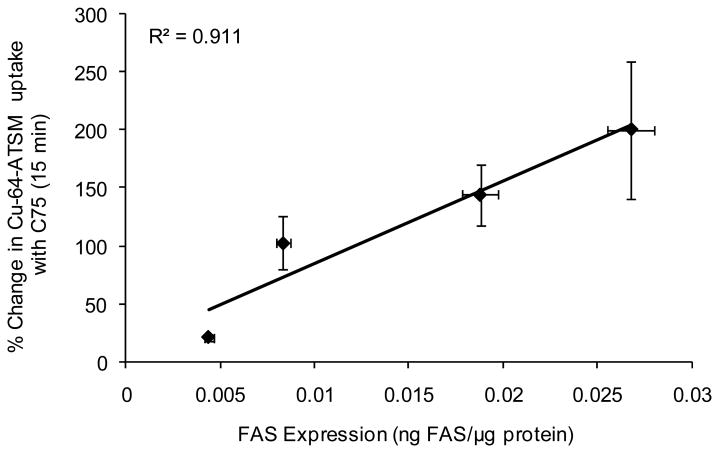
Samples of each cell line were analyzed to determine if relative amounts of FAS were related to the magnitude of Cu-ATSM retention at 15 min post-incubation. Quantification of FAS expression, by ELISA, resulted in values of 0.00438 ± 0.00024, 0.00832 ± 0.00038, 0.01877 ± 0.00092, and 0.02679 ± 0.00224 ng FAS/μg total protein for LAPC-4, PC-3, LNCaP, and 22Rv1, respectively. As expected, a correlation is seen (R2 = 0.911) with FAS expression plotted against the change in hypoxic retention resulting from inhibition of FAS (Fig. 3).
Figure 3.
Correlation of FAS expression as determined by ELISA to % change in 64Cu-ATSM uptake between C75 treated (100 μM) and control cells under anoxic conditions (5% CO2, 95% N2) at 15 min post-incubation. Points on the graph represent LAPC-4, PC-3, LNCaP, and 22Rv1 from left to right. (n = 3 at each timepoint)
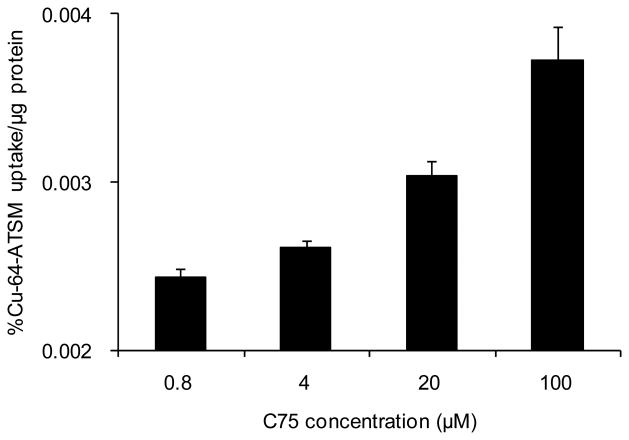
The dose response effect of C75 on the restoration of hypoxic retention of Cu-ATSM was measured in PC-3 cells utilizing the same cell suspension apparatus and anoxic gas mixture. During a 1-hour equilibration period, the four flasks were treated with varying concentrations of C75 (100, 20, 4, or 0.8 μM). Confirming the effect of FAS inhibition on 64Cu-ATSM uptake, the experiment showed increasing retention directly related to C75 concentration with values of 0.0037 ± 0.0002, 0.0030 ± 0.0001, 0.0026 ± 0.0001, and 0.0024 ± 0.0001 % uptake/μg protein for 100, 20, 4, and 0.8 μM respectively at 15 mins (Fig. 4).
Figure 4.
Dose response of 64Cu-ATSM uptake at 15 mins (under anoxic conditions) in PC-3 cells following FAS inhibition by C75. p = 0.0026 (100-20), p = 0.0002 (20-4).
Discussion
Low oxygen levels are a hallmark of malignant processes, leading to a cascade of oncogenic properties that support tumor growth and invasion [1-3, 34, 35]. Existing in this harsh environment, the cells go into survival mode and do not easily respond to DNA damaging chemotherapeutics or agents that promote apoptosis [6, 7]. In the absence of oxygen, the damaging radicals created by traditional radiation therapy recombine resulting in little damage to the target tissue [3-5]. Levels of tumor hypoxia have also been correlated to overall patient survival and tumor aggressiveness [1, 2, 36-38].
64Cu-ATSM [19], which targets hypoxia as opposed to metabolism, has already been confirmed as a clinically important PET agent in the prediction of prognosis in several cancers. It has been shown to be a useful clinical PET imaging tracer that can distinguish responders to conventional therapies from non-responders in patients with lung [23], cervical [22], rectal [24] and head and neck (data not published) cancer. It has also been suggested as a potential tool for radiation oncologists to tailor the radiation dose based on hypoxic regions, giving the resistant regions an increased dose [39].
Recently, Yuan et al, showed that the regional 64Cu-ATSM retention in two tumors types (R3230Ac and 9L) correlated closely with intrinsic markers for hypoxia, but in a third tumor type (FSA) a correlation was not observed [40]. The suggested reason for this low correlation between Cu-ATSM uptake and hypoxic distribution was the differing redox statuses of the tumor types. FSA tumors may have a lower-than-average redox potential with high concentrations of electron donors. If the speculated mechanism of Cu-ATSM uptake holds, then reduction, release and ultimate cellular trapping of Cu(I) would be caused by a decrease in the redox potential of the cell [19, 21, 27, 41, 42]. In the FSA tumor line, the lower-than-average redox potential caused reduction and trapping of 64Cu-ATSM in both hypoxic and normoxic areas. It was also suggested that the higher perfusion levels surrounding the hypoxic regions of the FSA tumor could contribute to the higher level of retention of Cu-ATSM in normoxic FSA cells. In vitro and in vivo experiments have also revealed inconsistencies in the hypoxic selectivity of Cu-ATSM in prostate cancer models [27, 28]. Although a very limited number of other tumor cell lines such as FSA have shown inconsistencies, prostate tumors clearly stand out as the primary tumor type that Cu-ATSM hypoxia-selectivity is reduced to low or even undetectable levels [27, 28]. This current study was undertaken in human prostate tumor lines to better examine the reasons for the lack of hypoxia-selectivity of Cu-ATSM in prostate tumors.
Biologically, prostate tumors have some unique characteristics when compared to other malignancies. Prostate cancer is known to have low metabolism, and the most common PET tracer for detecting malignancies, 18F-FDG, is not effective in delineating it from surrounding tissue [43] and also goes to sites of inflammation. Fatty acid synthase (FAS) is a multi-functional enzymatic protein involved in many stages of fatty acid synthesis and has been found to be overexpressed in prostate carcinomas as well as other cancers [44-52]. It is an androgen-regulated enzyme that is overexpressed in the vast majority of prostate tumors and its expression defines distinct molecular signatures in prostate cancer [53]. An increased level of FAS has been found to be indicative of aggressive and late-stage prostatic adenocarcinomas [31] as well as a prognostic indicator for overall survival [54].
As a defense mechanism in prostate cancer cells, the fatty acid synthesis pathway harnesses its oxidizing power for improving the redox balance (i.e., lower NADH/NAD+ ratios) despite conditions of extreme hypoxia [32]. This pathway is able to consume reducing equivalents (i.e., NADPH) as part of its normal processes. Under hypoxic conditions, anaerobic glycolysis creates excessive levels of lactate, which limits the respiratory chain and reduces its oxidizing power. The relationship between the fatty acid synthesis pathway and prostate tumors has been superbly reviewed by Hochachka et al., [32]. In regards to the hypoxia-selectivity of Cu-ATSM, it has been shown that Cu-ATSM enters all cells by passive diffusion, and under normoxic conditions freely exits the cell. Under hypoxic or anoxic conditions, the redox potential of the cell is altered such that the Cu(II) in the complex is reduced to Cu(I) and the complex falls apart [21, 27, 41, 42]. This retention mechanism is reliant on the reduction of the Cu(II) to Cu(I), and if cellular reducing equivalents such as NADPH are consumed by the overexpression of the fatty acid synthesis pathway this chemical reduction, even under conditions of hypoxia, is unlikely to take place.
C75 is a small molecule that binds to and inhibits mammalian FAS and inhibits fatty acid synthesis in human cancer cells. Researchers have shown that C75 inhibits FAS by 89-95% [55]. In this study, C75 was used to inhibit FAS activity in prostate tumor cell lines by blocking this enzymatic cycle's ability to offset the redox balance of a hypoxic cell, therefore, increasing retention of Cu-ATSM under hypoxic conditions. Typically a blocking study is designed to inhibit uptake of the radiotracer; in this case we were attempting to block the FAS so that it is unable to ‘stabilize’ the redox environment of the cell. Its inhibition should allow the anticipated retention of Cu-ATSM in hypoxic regions of the tumor. In vitro results in all cell lines demonstrate that FAS has a significant impact on the retention of 64Cu-ATSM into human prostate tumor cells under oxygen-limited conditions. Inhibition of FAS with a single 1 hour treatment of C75 (50 μM) resulted in increased retention of Cu-ATSM in all four tumor lines tested in vitro (Fig.1), but to a limited extent in the low-FAS-expressing LAPC-4. Results also showed that a linear change in uptake correlated directly to the cellular expression of FAS (Fig 3.). To demonstrate that restoration of retention is directly related to FAS inhibition, a dose response experiment was undertaken (Fig. 4).
Conclusion
Cu-ATSM has been a valuable tool as a marker of hypoxia, and it's possible that the nature of its mechanism of retention, though not completely understood, could lead us to answers for the incongruences in its selectivity. The uncertainty lies in the possibility that other physiological changes caused by malignant progression and hypoxia within the cell could also affect this redox balance regardless of oxygen concentration. If the suggested mechanism of Cu-ATSM uptake holds, then reduction, release and ultimate cellular trapping of Cu(I) would be caused by a decrease in the redox potential of the cell. And, although in most cases this is directly due to a change (reduction) in oxygen concentration within the cell, there are many other processes that could also alter the redox potential of this environment. In prostate tumors, a reduction of 64Cu-ATSM hypoxia-selectivity is demonstrated in a manner that is related to FAS expression. However, if a reduction in Cu-ATSM hypoxia-selectivity is demonstrated in non-prostate tumor lines it cannot be assumed that FAS is involved and each line must be studied on a case-by-case basis. In conclusion, 64Cu-ATSM is a very effective PET agent for clinically delineating many hypoxic human malignancies, but, as with all radiopharmaceuticals, it is not a universal agent. Care should be taken in particular regard to the imaging of prostate tumors.
Acknowledgments
The authors thank Susan Adams for cell preparation. We also thank Drs. Nobuyuki Oyama and Steven Kridel for their helpful discussions. Thanks also to the cyclotron facility staff for radionuclide production. We are grateful for financial support from the Department of Defense (PC040435) and the NIH for salary support for ALV (F32CA110422-03).
This work was supported by the United States Department of Defense (PC040435) and fellowship support from a National Institutes of Health NRSA (F32CA110422-03).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown JM. The hypoxic cell: A target for selective cancer therapy - eighteenth Bruce F. Cain Memorial Award Lecture. Cancer Res. 1999;59:5863–5870. [PubMed] [Google Scholar]
- 2.Tatum JL, Kelloff GJ, Gillies RJ, Arbeit JM, Brown JM, Chao KSC, Chapman JD, Eckelman WC, Fyles AW, Giaccia AJ, Hill RP, Koch CJ, Krishna MC, Krohn KA, Lewis JS, Mason RP, Melillo G, Padhani AR, Powis G, Rajendran JG, Reba R, Robinson SP, Semenza GL, Swartz HM, Vaupel P, Yang D, Croft B, Hoffman J, Liu G, Stone H, Sullivan D. Hypoxia: Importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol. 2006;82:699–757. doi: 10.1080/09553000601002324. [DOI] [PubMed] [Google Scholar]
- 3.Höckel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 4.Crabtree HG, Cramer W. The action of radium on cancer cells I. II. Some factors determining the susceptibility of cancer cells to radium. Proc R Soc Ser B. 1933;113:238–250. [Google Scholar]
- 5.Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. Concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953;26:638–648. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- 6.Tannock I, Guttman P. Responses of chinese hamster ovary cells to anticancer drugs under aerobic and hypoxic conditions. Br J Cancer. 1981;42:245–248. doi: 10.1038/bjc.1981.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teicher BA, Holden SA, Al-Achi SA, Herman TS. Classification of antineoplastic treatments by their differential toxicity toward putative oxygenated and hypoxic tumor subpopulations in vivo in the FSaIIC murine fibrosarcoma. Cancer Res. 1990;50:3339–3344. [PubMed] [Google Scholar]
- 8.Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, Prosnitz LR, Dewhirst MW. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996;56:941–943. [PubMed] [Google Scholar]
- 9.Gatenby RA, Kessler HB, Rosenblum JS, Coia LR, Moldofsky PJ, Hartz WH, Broder GJ. Oxygen distribution in squamous cell carcinoma metastases and its relationship to outcome of radiation therapy. Int J Radiat Oncol Biol Phys. 1988;14:831–838. doi: 10.1016/0360-3016(88)90002-8. [DOI] [PubMed] [Google Scholar]
- 10.Nordsmark M, Bentzen SM, Rudat V, Brizel D, Lartigau E, Stadler P, Becker A, Markus A, Molls M, Dunst J, Terris DJ, Overgaard J. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol. 2005;77:18–24. doi: 10.1016/j.radonc.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 11.Rajendran JG, Krohn KA. Imaging hypoxia and angiogenesis in tumors. Radiologic Clin N Am. 2005;43:169–187. doi: 10.1016/j.rcl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Nunn A, Linder K, Strauss HW. Nitroimidazoles and imaging hypoxia. Eur J Nucl Med. 1995;22:265–280. doi: 10.1007/BF01081524. [DOI] [PubMed] [Google Scholar]
- 13.Rajendran JG, Mankoff DA, O'Sullivan F, Peterson LM, Schwartz DL, Conrad EU, Spence AM, Muzi M, Farwell DG, Krohn KA. Hypoxia and glucose metabolism in malignant tumors: evaluation by [18F]Fluoromisonidazole and [18F]Fluorodeoxyglucose positron emission tomography imaging. Clin Cancer Res. 2004;10:2245–2252. doi: 10.1158/1078-0432.ccr-0688-3. [DOI] [PubMed] [Google Scholar]
- 14.Rajendran JG, Wilson DC, Conrad EU, Peterson LM, Bruckner JD, Rasey JS, Chin LK, Hofstrand PD, Grierson JR, Eary JF, Krohn KA. [18F]FMISO and [18F]FDG PET imaging in soft tissue sarcomas: correlation of hypoxia, metabolism and VEGF expression. Eur J Nucl Med Mol Imaging. 2003;30:695–704. doi: 10.1007/s00259-002-1096-7. [DOI] [PubMed] [Google Scholar]
- 15.Cherk MH, Foo SS, Poon AM, Knight SR, Murone C, Papenfuss AT, Sachinidis JI, Saunder TH, O'Keefe GJ, Scott AM. Lack of correlation of hypoxic cell fraction and angiogenesis with glucose metabolic rate in non–small cell lung cancer assessed by 18F-fluoromisonidazole and 18F-FDG PET. J Nucl Med. 2006;47:1921–1926. [PubMed] [Google Scholar]
- 16.Piert M, Machulla HJ, Picchio M, Reischl G, Ziegler S, Kumar P, Wester HJ, Beck R, McEwan AJB, Wiebe LI, Schwaiger M. Hypoxic-specific tumor imaging with 18F-fluoroazomycin arabinoside. J Nucl Med. 2005;46:106–113. [PubMed] [Google Scholar]
- 17.Sorger D, Patt M, Kumar P, Wiebe LI, Barthel H, Seese A, Dannenberg C, Tannapfel A, Kluge R, Sabri O. [18F]Fluoroazomycinarabinofuranoside (18FAZA) and [18F]Fluoromisonidazole (18FMISO): A comparative study of their selective uptake in hypoxic cells and PET imaging in experimental rat tumors. Nucl Med Biol. 2003;30:317–326. doi: 10.1016/s0969-8051(02)00442-0. [DOI] [PubMed] [Google Scholar]
- 18.Souvatzoglou M, Grosu AL, Roper B, Krause BJ, Beck R, Reischl G, Picchio M, Machulla HJ, Wester HJ, Piert M. Tumour hypoxia imaging with [18F]FAZA PET in head and neck cancer patients: a pilot study. Eur J Nucl Med Mol Imag. 2007;34:1566–1575. doi: 10.1007/s00259-007-0424-3. [DOI] [PubMed] [Google Scholar]
- 19.Vavere AL, Lewis JS. Cu-ATSM: A radiopharmaceutical for the PET imaging of hypoxia. Dalton Trans. 2007;43:4893–4902. doi: 10.1039/b705989b. [DOI] [PubMed] [Google Scholar]
- 20.Dearling JLD, Lewis JS, Mullen GED, Rae MT, Zweit J, Blower PJ. Design of hypoxia-targeting radiopharmaceuticals: Selective uptake of copper-64 complexes in hypoxic cells in vitro. Eur J Nucl Med. 1998;25:788–792. doi: 10.1007/s002590050283. [DOI] [PubMed] [Google Scholar]
- 21.Fujibayashi Y, Taniuchi H, Yonekura Y, Ohtani H, Konishi J, Yokoyama A. Copper-62-ATSM: A new hypoxia imaging agent with high membrane permeability and low redox potential. J Nucl Med. 1997;38:1155–1160. [PubMed] [Google Scholar]
- 22.Dehdashti F, Grigsby PW, Mintun MA, Lewis JS, Siegel BA, Welch MJ. Assessing tumor hypoxia in cevical cancer by positron emission tomography with 60Cu-ATSM: relationship to therapeutic response - a preliminary report. Int J Radiat Biol Phys. 2003;55:1233–1238. doi: 10.1016/s0360-3016(02)04477-2. [DOI] [PubMed] [Google Scholar]
- 23.Dehdashti F, Mintun MA, Lewis JS, Bradley J, Govindan R, Laforest R, Welch MJ, Siegel BA. In vivo assessment of tumor hypoxia in lung cancer with 60Cu-ATSM. Eur J Nucl Med Mol Imag. 2003;30:844–850. doi: 10.1007/s00259-003-1130-4. [DOI] [PubMed] [Google Scholar]
- 24.Dietz DW, Dehdashti FD, Grigsby PW, Malyapa RS, Myerson RJ, Picus J, Ritter J, Lewis JS, Welch MJ, Siegel BA. Tumor hypoxia detected by Positron Emission Tomography with 60Cu-ATSM as a predictor of response and survival in patients undergoing neoadjuvant chemoradiotherapy for rectal carcinoma: a pilot study. Dis Colon Rec. 2007 doi: 10.1007/s10350-008-9420-3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dehdashti F, Grigsby PW, Lewis JS, Laforest R, Siegel BA, Welch MJ. Assessing tumor hypoxia in cervical cancer by positron emission tomography with 60Cu-ATSM. J Nucl Med. 2007 doi: 10.1016/s0360-3016(02)04477-2. in press. [DOI] [PubMed] [Google Scholar]
- 26.Lewis JS, McCarthy DW, McCarthy TJ, Fujibayashi Y, Welch MJ. Evaluation of 64Cu-ATSM in vitro and in vivo in a hypoxic tumor model. J Nucl Med. 1999;40:177–183. [PubMed] [Google Scholar]
- 27.Burgman P, O'Donoghue JA, Lewis JS, Welch MJ, Humm JL, Ling CC. Cell line-dependent differences in uptake and retention of the hypoxia-selective nuclear imaging agent Cu-ATSM. Nucl Med Biol. 2005;32:623–630. doi: 10.1016/j.nucmedbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 28.O'Donoghue JA, Zanzonico P, Pugachev A, Wen B, Smith-Jones P, Cai S, Burnazi E, Finn R, Burgman P, Ruan S, Lewis JS, Welch MJ, Ling CC, Humm JL. Assessment of regional tumor hypoxia using 18F-fluoromisonidazole and 64Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone) positron emission tomography: Comparative study featuring microPET imaging, pO2 probe measurement, autoradiography, and fluorescent microscopy in the R3327-AT and FaDu rat tumor models. Int J Radiat Biol Phys. 2005;61:1493–1502. doi: 10.1016/j.ijrobp.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 29.Vavere AL, Kridel SJ, Wheeler FB, Lewis JS. 1-11C-acetate as a PET radiopharmaceutical for imaging fatty acid synthase expression in prostate cancer. J Nucl Med. doi: 10.2967/jnumed.107.046672. in press. [DOI] [PubMed] [Google Scholar]
- 30.Wakil SJ. Fatty acid synthase, a proficient multifunctional enzyme. Biochemistry. 1989;28:4523–4530. doi: 10.1021/bi00437a001. [DOI] [PubMed] [Google Scholar]
- 31.Myers RB, Oelschlager DK, Weiss HL, Frost AR, Grizzle WE. Fatty acid synthase: An early molecular marker of progression of prostatic adenocarcinoma to androgen independence. J Urology. 2001;165:1027–1032. [PubMed] [Google Scholar]
- 32.Hochachka PW, Rupert JL, Goldenberg L, Gleave M, Kozlowski P. Going malignant: the hypoxia-cancer connection in the prostate. BioEssays. 2002;24:749–757. doi: 10.1002/bies.10131. [DOI] [PubMed] [Google Scholar]
- 33.McCarthy DW, Shefer RE, Klinkowstein RE, Bass LA, Margenau WH, Cutler CS, Anderson CJ, Welch MJ. Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl Med Biol. 1997;24 doi: 10.1016/s0969-8051(96)00157-6. [DOI] [PubMed] [Google Scholar]
- 34.Gillemin K, Krasnow MA. The hypoxic response: huffing and HIFing. Cell Death Differentiation. 1997;89:9–12. doi: 10.1016/s0092-8674(00)80176-2. [DOI] [PubMed] [Google Scholar]
- 35.Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary over a physiologically relevant range of O2 tension. Am J Physiol. 1996;271:C1172–1180. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 36.Höckel M, Schlenger K, Aral B, Mitze M, Shäffer U, Vaupel P. Assocaition between tumor hypoxia and malignant progression in advancer cancer of the uterine cervix. Cancer Res. 1996;56:4509–4515. [PubMed] [Google Scholar]
- 37.Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature (Lond) 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 38.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature (Lond) 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 39.Chao C, Bosch WR, Mutic S, Lewis JS, Dehdashti FD, Mintun MA, Demsey JF, Perez CA, Purdy JA, Welch MJ. A novel approach to overcome hypoxic tumor resistance: Cu-ATSM-guided intensity-modulated radiation therapy. Int J Radiat Biol Phys. 2001;49:1171–1182. doi: 10.1016/s0360-3016(00)01433-4. [DOI] [PubMed] [Google Scholar]
- 40.Yuan H, Schroeder T, Bowsher JE, Hedlund LW, Wong T, Dewhirst MW. Intertumoral differences in hypoxia selectivity of the PET imaging agent 64Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone) J Nucl Med. 2006;47:989–998. [PubMed] [Google Scholar]
- 41.Dearling JLJ, Lewis JS, Mullen GED, Welch MJ, Blower PJ. Copper bis(thiosemicarbazone) complexes as hypoxia imaging agents: structure-activity relationships. J Biol Inorg Chem. 2002;7:249–259. doi: 10.1007/s007750100291. [DOI] [PubMed] [Google Scholar]
- 42.Maurer RI, Blower PJ, Dilworth JR, Reynolds CA, Zheng Y, Mullen GED. Studies on the mechanism of hypoxic selectivity in copper bis(thiosemicarbazone) radiopharmaceuticals. J Med Chem. 2002;45:1420–1431. doi: 10.1021/jm0104217. [DOI] [PubMed] [Google Scholar]
- 43.Fricke E, Mchtens S, Hofmann M, van den Hoff J, Bergh S, Brunkhorst T, Meyer GJ, Karstens JH, Knapp WH, Boerner AR. Positron emission tomography with 11C-Acetate and 18F-FDG in prostate cancer patients. Eur J Nucl Med Mol Imag. 2003;30:607–611. doi: 10.1007/s00259-002-1104-y. [DOI] [PubMed] [Google Scholar]
- 44.Dhanasekaran SM, Barrette TR, Ghosh D, Shah R, Sooryanarayana V, Kurachi K, Pienta KJ, Rubin MA, Chinnaiyan AM. Delineation of prognostic biomarkers in prostate cancer. Nature. 2001;412:822–826. doi: 10.1038/35090585. [DOI] [PubMed] [Google Scholar]
- 45.Swinnen JV, Roskams T, Joniau S, Van Poppel H, Oyen R, Baert L, Heyns W, Verhoeven G. Overexpression of fatty acid synthase is an early and common event in the development of prostate cancer. Int J Cancer. 2002;98:19–22. doi: 10.1002/ijc.10127. [DOI] [PubMed] [Google Scholar]
- 46.Welsh JB, Sapinosa LM, Su AI, Kern SG, Wang-Rodriguez J, Moskaluk CA, Frierson HF, Hampton GM. Analysis of gene expression identifies candidate markers and pharmacological targets in prostate cancer. Cancer Res. 2001;61:5974–5978. [PubMed] [Google Scholar]
- 47.Menendez JA, Lupu R. Oncologic properties of the endogenous fatty acid metabolism: molecular pathology of fatty acid synthase in cancer cells. Curr Opin Clin Nutr Metab Care. 2006;9:346–357. doi: 10.1097/01.mco.0000232893.21050.15. [DOI] [PubMed] [Google Scholar]
- 48.Alo PL, Visca PGT, Mangoni A, Lenti L, Monaco S, Botti C, Serpieri DE, Di Tondo U. Fatty acid synthase (FAS) predictive strength in poly differentiated early breast carcinomas. Tumori. 1999;85:35–40. doi: 10.1177/030089169908500108. [DOI] [PubMed] [Google Scholar]
- 49.Alo PL, Visca P, Marci A, Mangoni A, Botti C, Di Tondo U. Expression of fatty acid synthase (FAS) as a predictor of recurrence in stage I breast carcinoma patients. Cancer. 1996;77:474–482. doi: 10.1002/(SICI)1097-0142(19960201)77:3<474::AID-CNCR8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 50.Gansler TS, Hardman WA, H DA, Schaffel S, Hennigar RA. Increased expression of fatty acid synthase (OA-519) in ovarian neoplasms predicts shorter survival. Human Pathol. 1997;28:686–692. doi: 10.1016/s0046-8177(97)90177-5. [DOI] [PubMed] [Google Scholar]
- 51.Rashid A, Pizer ES, Moga M, Milgraum LZ, Zahurak M, Pasternack GR, Kuhajda FP, Hamilton SR. Elevated expression of fatty acid synthase and fatty acid synthetic activity in colorectal neoplasia. Am J Pathol. 1997;150:201–208. [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Kuhajda FP, Li JN, Pizer ES, Han WF, Sokoll LJ, Chan DW. Fatty acid synthase (FAS) expression in human breast cancer cell culture supernatants and in breast cancer patients. Cancer Lett. 2001;167:99–104. doi: 10.1016/s0304-3835(01)00464-5. [DOI] [PubMed] [Google Scholar]
- 53.Rossi S, Graner E, Febbo P, Weinstein L, Bhattacharya N, Onody T, Bubley G, Balk S, Loda M. Fatty acid synthase expression defines distinct molecular signatures in prostate cancer. Mol Cancer Res. 2003;1:707–715. [PubMed] [Google Scholar]
- 54.Takahiro T, Shinichi K, Toshimitsu S. Expression of fatty acid synthase as a prognostic indicator in soft tissue sarcomas. Clin Cancer Res. 2003;9:2204–2212. [PubMed] [Google Scholar]
- 55.Loftus TM, Jaworsky DE, Frehywot GL, Townsend CA, Ronnett GV, Lane MD, Kuhajda FP. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science. 2000;288:2379–2381. doi: 10.1126/science.288.5475.2379. [DOI] [PubMed] [Google Scholar]