Abstract
Background
This study aims to determine what effect correcting melphalan dosing for ideal body weight (IBW) has on toxicity and response in isolated limb infusion (ILI) in patients with advanced extremity melanoma.
Methods
This was an open observational study examining whether correcting the melphalan dose for IBW will influence response and toxicity in patients undergoing ILI for advanced extremity melanoma in 41 patients undergoing 42 procedures (13 without correction for IBW; and 29 with correction for IBW). Melphalan pharmacokinetics, limb toxicity, serologic toxicity, and response at 3 months were compared.
Results
The corrected group had a lower estimated limb volume (Vesti) to melphalan volume at steady state (Vss) (P < .0001) ratio as well as lower incidence of grade ≥3 regional toxicity, serologic toxicity, and compartment syndrome (P = .0249, P = .027, P = .02). There was a positive correlation of Vesti/Vss to toxicity (P = .0127, r = .382). No significant difference in response (P = .3609) between the groups was found, although there was a trend of association between Vesti/Vss and response (P = .051, r = .3383).
Conclusions
Correcting for IBW in ILI lowers toxicity without significantly altering response rates.
Isolated limb infusion (ILI) is a widely accepted regional treatment for recurrent melanoma of the extremity. This technique was originally developed in Sydney, Australia, as a less complex alternative to hyperthermic isolated limb perfusion (HILP).1,2 Melphalan (l-phenylalanine mustard; LPAM), an alkylating agent, is the most commonly used chemotherapy agent for ILI because of its short half-life, low toxicity, and near-linear dose-dependent relationship to cytotoxicity. Isolation of the extremity in ILI allows for treatment of regionally advanced or in-transit extremity melanoma with levels of melphalan several orders of magnitude higher than what can be achieved systemically in an effort to avoid amputation or disfiguring surgery. Treatment not only can be repeated, but also the regional approach allows for the safe use of other treatments such as hyperthermia and hypoxia, which may augment response rates.3 Response rates to ILI have varied among studies with complete response rates ranging from 23% to 41%.3–5 Our institution has recently described its experience with 61 ILI procedures and found an overall tumor response rate of 44%, with 30% of those patients being complete responders.3
While melphalan pharmacokinetics in the context of HILP has been well studied, little has been published on the melphalan pharmacokinetics in the context of ILI. No studies have been undertaken to try to optimize the therapeutic index of melphalan ILI including whether melphalan dosing should be based on actual body weight or ideal body weight. Based on our previous experience in HILP6 and our preliminary findings in ILI, we hypothesized that correction of the melphalan dose for ideal body weight would reduce toxicity in ILI without altering response. The rationale for this dosing adjustment is based on the finding that the strongest predictor of toxicity in patients undergoing HILP (n = 14) was the ratio of estimated limb volume (Vesti) to steady-state limb drug volume of distribution (Vss).6 All major toxicity was seen in patients whose Vesti/Vss ratio was more than 4. A two-compartmental model for plasma melphalan distribution is generally applied to regional drug delivery including treatments such as HILP and ILI, where plasma melphalan concentration is generally accepted to be a direct measure of the (first) central compartment, which includes rapidly perfused tissues, such as muscle. The proportion of the extremity volume that makes up the muscle compartment generally tends to vary less than the soft tissue and skin compartment. For patients with larger limb volumes and ideal body weights less than actual body weights, giving a reduced melphalan dose attempts to minimize the risk of overdosing the main compartment (muscle) in which the drug is primarily distributed. However, what effect this reduced melphalan dosing might have on tumor response is not clear.
The purpose of this study is to examine whether correcting the melphalan dose for ideal body weight has an effect on response and toxicity parameters in patients undergoing ILI for advanced extremity melanoma.
METHODS
Patients
From December 2003 to May 2007, patients at our institution were treated with isolated limb infusion with melphalan for the management of recurrent or in-transit extremity melanoma. These patients represent a subset of our previously published experience in 61 ILI procedures and are patients who had concurrent melphalan drug pharmacokinetic parameters tested.3 The other patients in that group of 61 did not have plasma drawn for pharmacokinetic analysis. In this study, the initial 13 patients had their melphalan dosage calculated based on limb volume alone, without correction for ideal body weight. The final 29 patients had their melphalan dosage calculated according to a limb volume that was corrected for ideal body weight. All patients were recruited from Duke University Medical Center, and all signed an Institutional Review Board consent form for blood sampling.
Limb Volume and Ideal Body Weight Calculation
Prior to ILI, each patient’s leg or arm circumference was measured at 1.5-cm intervals up to the level of the tourniquet, encompassing the entire area to be infused. Measurements were entered into a limb volume calculation program using Microsoft Excel. The Devine ideal body weight was calculated for 29 patients according to the standard method with males calculated by 50 + 2.3 × (patient height in inches − 60), whereas for females the calculation is 45.5 + 2.3 × (patient height in inches − 60).
Melphalan Dosing
The melphalan dose was calculated using the formula of 7.5 mg/l for the lower extremity and 10 mg/l for the upper extremity.5 The dose of melphalan was calculated according to limb volume alone for our initial 13 patients. We then corrected the remaining 29 for ideal body weight, by determining the limb volume and then multiplying this volume by the ratio of ideal body weight to actual body weight as previously described.3 In all cases, melphalan was given in conjunction with dactinomycin at a dose of 75 µg/l limb volume infused for the lower extremity and 100 µg/l limb volume infused for the upper extremity. In patients whose melphalan dose was corrected for ideal body weight, the dactinomycin dose was also similarly corrected.
Technical Aspects of ILI
The technique of ILI was conducted as previously reported.1,2 On the day of ILI, high-flow 6-Fr arterial and venous catheters were inserted into an uninvolved extremity and positioned in the involved extremity using the Seldinger technique and fluoroscopic guidance in the vascular radiology suite. In the operating room, a warming blanket was wrapped around the extremity and kept in place for the duration of the procedure. Once the patient was fully heparinized to achieve a target activated coagulation time (ACT) ≥350, circulation was begun through the circuit using a 20-cc syringe. Once circulation of blood via the catheters was deemed to be adequate, a pneumatic or Esmarch tourniquet was positioned and inflated or tightened around the proximal portion of the extremity. Once the extremity reached a temperature of at least 37.0°C, the melphalan was rapidly infused (2–5 min) into the arterial line. After the rapid infusion, a 30-min circulation of the chemotherapy was done using a 20-cc syringe. After 30 min of circulation, the limb was flushed with 1 l of normal saline and the effluent collected for disposal. Finally, the tourniquet was let down and the heparinization reversed.
Blood Collection
All blood samples were collected in 4-ml prelabeled lithium-heparin tubes. Blood was collected from the venous stopcock of the infusion circuit at the following 954 N. McMahon et al. time points during circulation of melphalan: 0, 5, 10, 15, 20, 25, and 30 min. An additional systemic sample was collected at 30 minutes. The 0-min time point was taken before melphalan was injected into the circuit. Each tube was placed immediately on wet ice and transferred to the lab where each was centrifuged at 3,000 rpm at 4°C for 10 min. The plasma was removed, aliquoted into freezer tubes, and stored at −80°C.
Melphalan Measurements
The melphalan concentration in plasma was measured in each sample by an improved assay based on a published high-performance liquid chromatography (HPLC)-fluorescence method by Ehrsson et al.7 By introducing chlorambucil as an internal standard, utilizing a short high-resolution HPLC column (Eclipse Plus C18, 4.6 × 150 mm, 1.8 µm, Agilent Technologies, Santa Clara, CA, USA), and using a state-of-art fluorescence detector (W2475, Waters Corporation, Milford, MA, USA), we achieved a high sensitivity (LLQ = 16 ng/ml) and a short (9-min) run time by using only 50 µl of plasma sample. In this study, each analysis run (3-patient sets) was accompanied by a set of quality control samples (.05, .5, 5, and 50 µg/ml) run before and after the patient sample set. An analysis run was considered successful if the accuracy of the quality control samples at all levels was greater than 90%.
Toxicity and Tumor Response Evaluation
Toxicity was evaluated according to the National Cancer Institute Common Toxicity Criteria for Adverse Events version 3.0 (CTCAE v3.0). Both regional limb toxicity and serologic toxicity (creatine phosphokinase levels) were evaluated in both groups.
Tumor response was evaluated at 3 months according to Response Evaluation Criteria in Solid Tumors (RECIST) modified for relevance to cutaneous lesions. A complete response (CR) was given when there was disappearance of all lesions, partial response (PR) indicated a 30% or greater decrease in diameter of target lesions, stable disease (SD) was neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify as progressive disease (PD), and PD was a minimum 20% increase in diameter of target lesions or the appearance of one of more new lesions. Response was evaluated both in the field of treatment, defined as all lesions distal to the level of the tourniquet, and out of the field of treatment, anything proximal to the level of the tourniquet. For the purposes of this study, we looked at any differences in in-field response and any correlation between in-field response and pharmacokinetic parameters of melphalan.
Pharmacokinetic Analysis
The two-compartmental model was used for pharmacological analysis. The plasma melphalan concentrations versus time for each patient were fitted to a biexponential equation using the nonlinear least-square method (Prism 5 for Mac OSX, version 5.0a; GraphPad Software, Inc., La Jolla, CA, USA). The fitting procedure includes the initial estimates from the stripping method with two-step linear regression, giving the bounds of primary parameters from the known half-life of melphalan, then the method of Marquardt and Levenberg for least square fit. Detailed pharmacokinetic equations and parameters have been described previously.6
Statistical Analysis
Area under raw data of concentration-time curve (AUCraw), area under second compartment drug amount-time curve (AUX2), estimated limb volume/steady-state volume distribution (Vesti/Vss), and peak creatine phosphokinase (CPK) between these two groups were compared with the Mann–Whitney test. Additionally, the Mann–Whitney test was used for comparing response grading, CTCAE score, and serologic toxicity grade. For comparing the rate of compartment syndrome and tumor response (CR + PR vs. SD + PD and CR vs. PR + SD + PD), the chi-square test was used. Both groups of patients (uncorrected and corrected for melphalan dosing) were also combined for analysis. Linear relationship among Vesti/Vss, AUCraw, and AUX2 with CTCAE score, tumor response grading, and serologic toxicity grade was studied with Spearman rank correlation. Relation of Vesti/Vss with peak CPK values was analyzed with Pearson correlation.
RESULTS
Patient Characteristics
There were 41 patients who underwent 42 isolated limb infusions for malignant melanoma of the extremity. There were 21 males and 20 females with ages ranging from 38 to 90 years (mean, 65 years; median, 66 years). One of the male patients received 2 ILIs. There were 8 upper extremity treatments and 34 lower extremity treatments. The melphalan dose was not corrected for ideal body weight in the first 13 procedures. Starting in mid-2005, we began correcting melphalan dosing for ideal body weight, and the remaining 29 ILIs examined in this study had their dose corrected. Of those uncorrected patients, all 13 were evaluable for limb and serologic toxicity and 11 were evaluable at 3 months for response. Of those patients who were corrected for ideal body weight, all 29 were evaluable Correcting Melphalan Dose for Ideal Body Weight in ILI 955 for toxicity and 28 were evaluable for response at 3 months.
Ideal Body Weight/Melphalan Dosing
In the uncorrected group of patients (n = 13), the median actual body weight was 84.5 kg, while the median IBW would have been 59.63 kg. All 13 patients had ideal body weights less than actual body weights, which would have resulted in a median reduction of the melphalan dose by 17.4%. In the corrected group of patients (n = 29), the median actual body weight was 84.8 kg, while the median IBW was 65.9 kg. Of the 29 patients, 27 had ideal body weights less than actual body weights, resulting in a median reduction of the melphalan dose by 21.7%.
Limb and Serologic Toxicity
Both regional and serologic toxicity, as measured by CPK levels, were evaluated in the group of patients who were not corrected for IBW (n = 13) and those who were corrected (n = 29). Of the uncorrected group, 5 patients (38%) had grade 3 or higher regional toxicity, while the corrected group had significantly fewer patients (n = 3, 11%) with a grade 3 or higher regional toxicity (P = .027). Of the uncorrected group, 7 patients (54%) had grade 3 or higher serologic toxicity (Table 1), while in the corrected group 6 patients (21%) had grade 3 or higher serologic toxicity (P = .02). Additionally, peak CPK values seen in each group were examined, and it was found that the uncorrected group had a significantly higher peak CPK (range, 52–11,674) compared with the corrected group (range 39–7,114) (P = .0004). Additionally, in the uncorrected group of patients there were 3 instances of compartment syndrome as well as 1 patient with muscle necrosis that required debridement. There were no compartment syndromes or comparable complications in the group of patients who were corrected for IBW. This difference seen in rate of compartment syndrome was also statistically significant (P = .0249).
TABLE 1.
Regional and serologic toxicity in evaluable patients according to dosing modification and clinical response in evaluable patients according to dosing modification
| Toxicity | Dose not corrected for IBW (n =13) | Dose corrected for IBW (n = 28) |
| CTCAE grade ≥3 | 5 (38%) | 3 (11%) |
| CPK grade ≥3 | 7 (54%) | 6 (21%) |
| Compartment syndrome | 3 (23%) | 0 (0%) |
| Response | Dose not corrected for IBW (n = 11) | Dose corrected for IBW (n = 28) |
| Complete response | 3 (27%) | 8 (29%) |
| Partial response | 3 (27%) | 2 (7%) |
| Stable disease | 3 (27%) | 4 (14%) |
| Progressive disease | 2 (18%) | 14 (48%) |
IBW ideal body weight, CTCAE National Cancer Institute Common Toxicity Criteria for Adverse Events, CPK creatine phosphokinase
Clinical Response
Of the 13 patients who were not corrected for ideal body weight, 11 were evaluable for response at 3 months (1 patient had a prophylactic ILI, and 1 patient was lost to follow-up). Twenty-eight patients who were corrected for ideal body weight were evaluable for response (1 patient was lost to follow-up). The results are shown in Table 1. The CR rate in the uncorrected group was 36%, while it was 29% in the corrected group. Additionally, the rate of PD was 18% in the uncorrected group versus 48% in the corrected group. While these differences were noted in these response rates, when all response grades were considered together and compared using the Mann–Whitney test, the differences did not reach statistical significance (P = .2302). Additionally, we used chi-square analysis to compare both the rates of overall response (CR + PR) and CR and found no significant difference between the two groups of patients (P = .3069 and .7086, respectively). The mean duration of complete response in the uncorrected group was 19.9 months (median, 22.0 months). The mean duration of complete response in the corrected group was 14.7 months (median, 19.7 months).
Pharmacokinetics
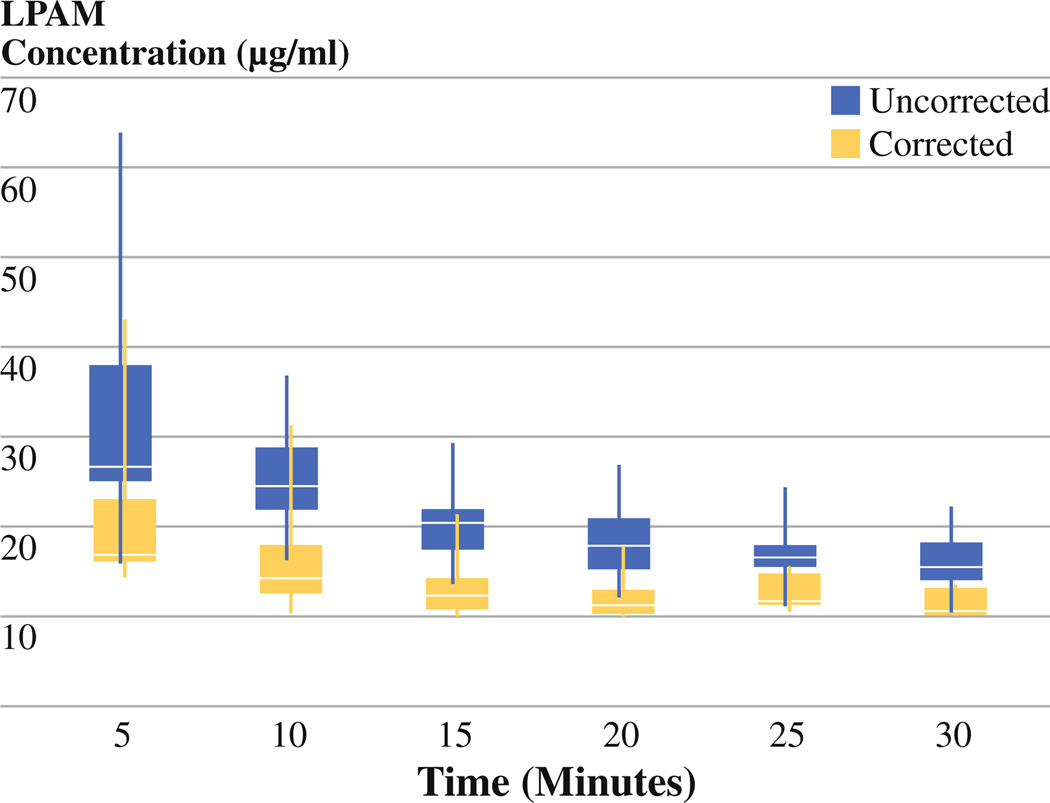
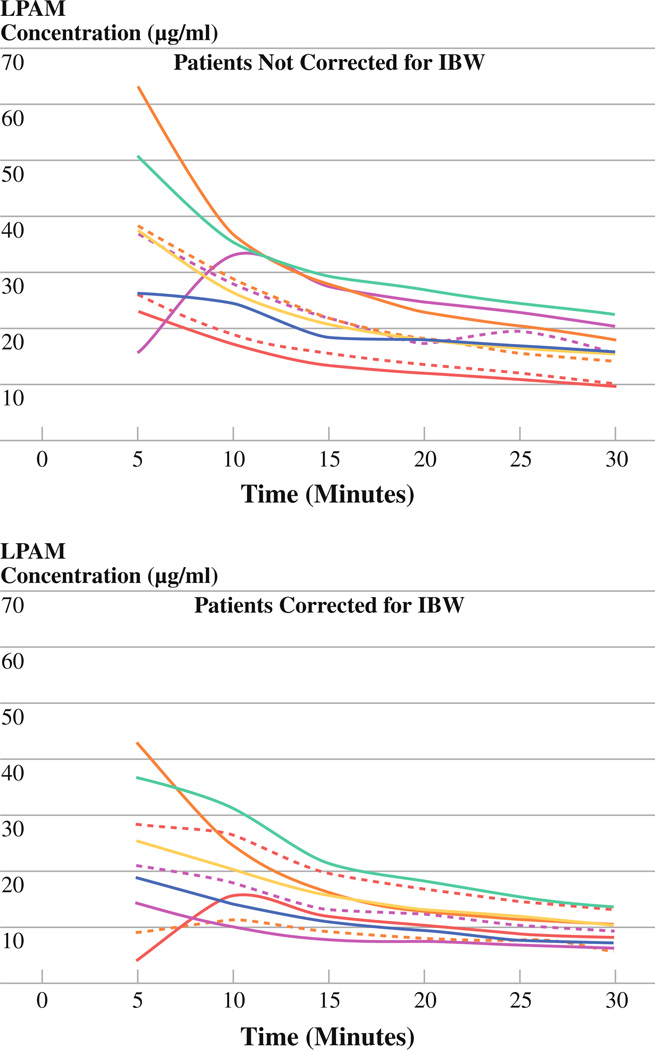
The median melphalan concentrations at 5-min intervals were determined for each group (see Fig. 1). At each time point, the median concentration of melphalan is greater in the group that was not corrected for IBW versus the group that was corrected. When the melphalan levels are plotted for each individual patient, we find the levels were somewhat variable among patients in both groups (see Fig. 2); however, at each time point the standard deviation was lower in the group of patients who were corrected for IBW (P < .0001).
FIG. 1.
Median melphalan plasma concentration at 5-min intervals during ILI with boxes representing the interquartile range and whiskers representing all data points. Data are separated based on patients corrected for IBW versus patients not corrected for IBW
FIG. 2.
Individual melphalan concentrations at 5-min intervals during ILI. Top graph represents patients whose dose was not corrected for IBW, while the bottom represents patients who were corrected. Variability is seen in both groups, but the standard deviations at each time point were lower in the corrected group (P < .0001)
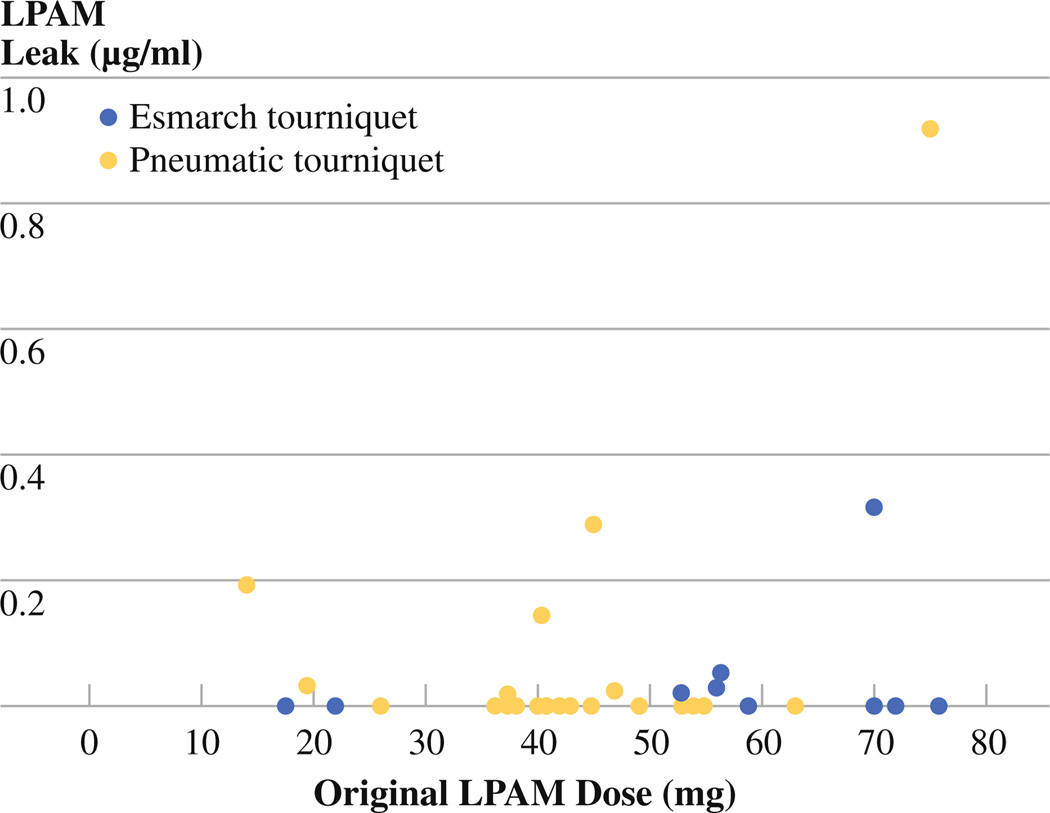
The amount of systemic leak was recorded for all 29 corrected patients, but this information was available only for 4 of the uncorrected patients. Figure 3 shows the incidence of systemic leak compared with original dose of melphalan and is separated based on which type of tourniquet (pneumatic or Esmarch) was used in ILI. The type of tourniquet does not appear to affect the amount of systemic leak. However, it appears that there is more systemic detection of melphalan in patients who were given a higher dose of melphalan, but this correlation was not significant in this set of patients (P = .129).
FIG. 3.
Incidence of systemic leak of LPAM at 30 min after ILI. There is a trend of association between amount of leak and original dose of LPAM, but this does not reach statistical significance (P = .129)
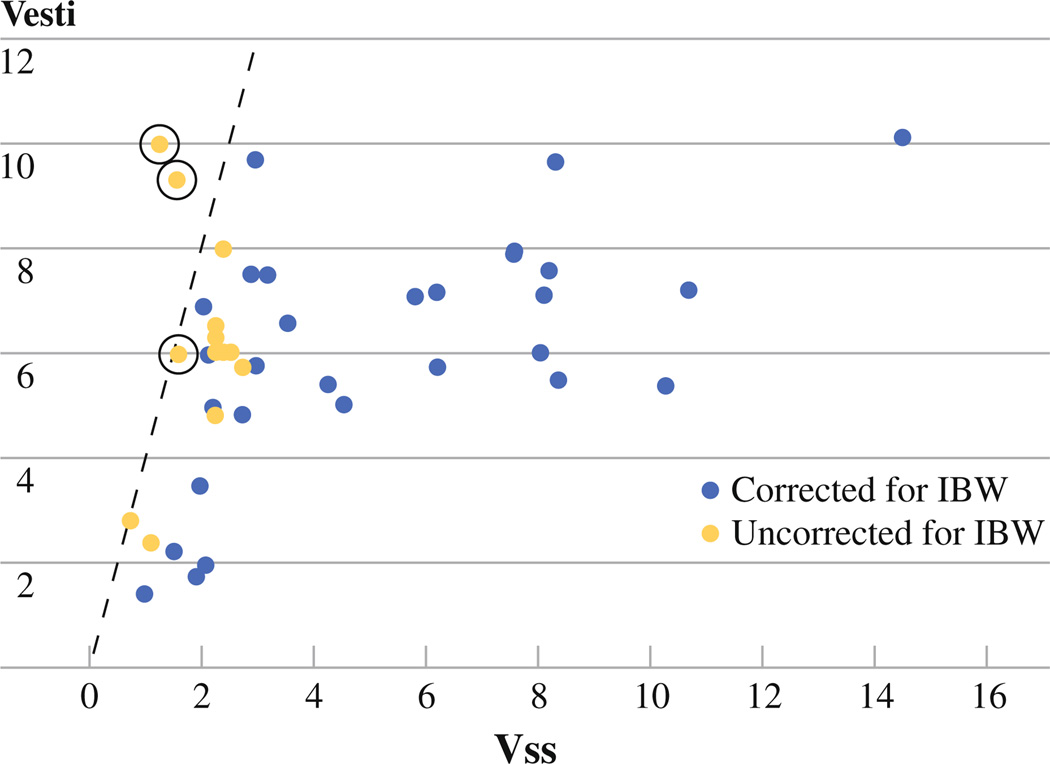
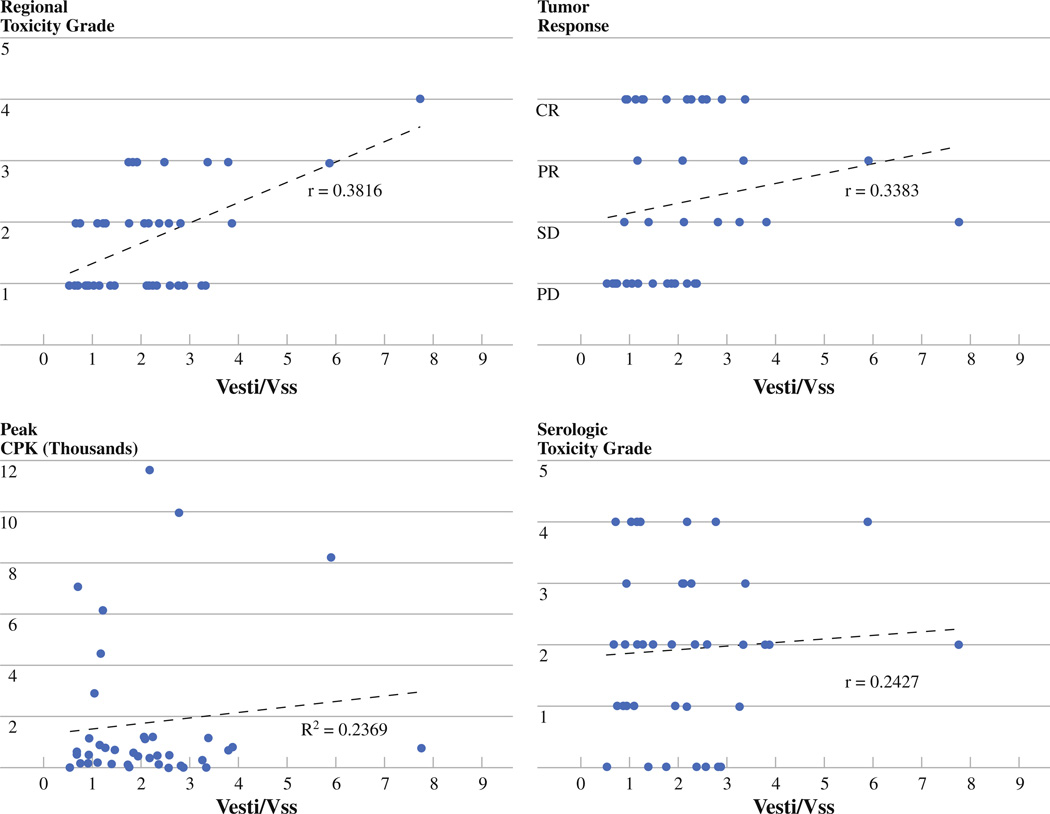
Several pharmacokinetic parameters were evaluated in each group of patients including AUC1, AUX1, AUX2, Vesti, Vss, and Vesti/Vss. This study found that the strongest predictor of toxicity was the ratio of estimated limb volume to steady-state limb drug volume of distribution (Vesti/Vss). None of these pharmacokinetic parameters were found to be significantly correlated with response. The Vesti/Vss ratio was significantly lower in the corrected group of patients with a median (IQR) of 1.216 (.9116–2.046) vs. 2.766 (2.267–3.827) (P < .0001) with all ratios being less than 4 in the uncorrected group (Fig. 4). Additionally, the corrected group of patients had a lower AUCraw with a median (IQR) of 289.3 (252.3–342) vs 515.3 (400.0–591.8) (P < .0001), which indicates a lower overall amount of drug exposure to the body.
FIG. 4.
Relationship between estimated limb volume (Vesti) and steady-state melphalan volume of distribution (Vss) for all patients in the study. The solid line represents Vesti/Vss ratio = 4. The circles represent patients who experienced major toxicity in the form of compartment syndrome
Pharmacokinetic Correlations
In order to examine pharmacokinetic correlations with toxicity and response, both groups of patients were combined. When combined, Vesti/Vss was found to have a statistically significant correlation with regional toxicity (P = .0127; Spearman r = .3816). While this ratio was not significantly correlated with response (P = .051; Spearman r = .3383), it did approach statistical significance. As indicated by the r values in both cases, the correlation was not particularly strong. Additionally, there was a significant correlation of Vesti/Vss and peak CPK peak (P = .0013; r2 = .2369) but no significant correlation between Vesti/Vss and serology grade (P = .1735; Spearman r = .2427). These correlations are shown graphically in Fig. 5.
FIG. 5.
Correlations between Vesti/Vss and regional toxicity, tumor response, serologic toxicity, and peak CPK
DISCUSSION
Melphalan dosing for regional therapy was originally determined based on body weight alone, but this did not take into account the distribution of body mass. To more accurately dose the chemotherapeutic agent, dosing based on limb volume became the standard practice in the 1980s.8 In HILP, the optimal therapeutic index for melphalan was found to occur at a dose of 10 mg/l of limb volume for the lower limb and 13 mg/l of limb volume for the upper limb.8–11 In ILI, 7.5 mg/l for the lower extremity and 10 mg/l for the upper extremity are the generally accepted doses that were suggested by the group that originally described the ILI technique.1 However, no studies have been done to determine if the dosing of melphalan in ILI can be optimized to either improve response or decrease toxicity. Using a correction for IBW to optimize the therapeutic index of regionally administered melphalan was initially explored in the context of HILP and was found to be associated with a decrease in major toxicity.6 After an initial experience with relatively high morbidity using uncorrected melphalan dosing in ILI, we postulated that correcting for ideal body weight in ILI might be a way to decrease regional toxicity without compromising response.
A two-compartmental model for plasma melphalan distribution has been applied to both HILP and ILI with the division of drug distribution into a central (first) compartment and a peripheral (second) compartment. Plasma concentration is generally accepted to be a direct measure of the central compartment, which is also the site of rapidly perfused tissues, such as muscle. Skin, subcutaneous tissue, and tumor are generally considered to be in the second compartment. In HILP, area under the second compartment curve has been shown to be the best predictor of response.6 In this study, no similar parameter was found to correlate strongly with response in ILI. In animal models, a wide range of melphalan plasma concentrations have been examined, and it has been found that levels of approximately 15 µg/ml were always associated with a partial or complete response. Additionally, at levels above 25 µg/ml there was no additional tumor response seen. This suggests that above certain plasma concentrations of melphalan, one will not get any improved tumor response while toxicity will continue to increase.12 As seen in Fig. 1, on average, our patients who had their dose corrected for IBW achieved plasma melphalan concentrations in this ideal range of 15– 25 µg/ml, while those patients who were not corrected achieved plasma concentrations higher than this level. The Sydney Melanoma Unit recently described their 14-year experience with ILI and found that a higher melphalan concentration at the end of ILI was correlated with an increase in response.13 While this trend was not reported in their multivariate analysis, we should acknowledge that there is a melphalan concentration required to achieve response in ILI and that significantly decreasing a patient’s dose could potentially sacrifice some response.
In terms of response, when the two melphalan dosing methods (uncorrected and corrected) were compared separately, there was no significant difference seen in overall response (CR + PR) or in complete response alone. However, when both groups of patients were combined, there was a weak correlation, which approached but did not reach statistical significance, between the Vesti/Vss ratio and response, in that as the ratio increased, there was an increase in response rate. This suggests that correcting for IBW may potentially sacrifice some response in exchange for a lowered toxicity. However, the correlation explained only about 30% of the variation seen in response. In a larger group of ILI patients, many of whom did not have melphalan pharmacokinetics measured, similar response rates were seen regardless of whether melphalan dosing was corrected for IBW or not. In this analysis of 61 patients undergoing ILI, 50 were evaluable for response and the CR, PR, SD, and PD rates were 29%, 18%, 6%, and 47%, respectively, for uncorrected melphalan dosing (n = 17) and 30%, 12%, 12%, and 45%, respectively, for corrected melphalan dosing (n = 33).3 Additionally, a recent analysis of 162 ILIs found no difference in complete response rates in patients corrected for IBW (n = 68) versus those patients without a dose modification (n = 90) (P = .345). This study did find a significant difference in overall response (CR + PR) rate between the two groups, implying there are factors affecting rate of PR between the groups.14 Other factors, both genetic and clinical (including the volume or extent of in situ disease, level of hyperthermia or anoxia achieved during ILI, etc.) may have effects on drug dynamics and tumor response. Furthermore, while the reduced dose of melphalan given to patients with IBWs less than actual body weight would minimize toxicity, the less well-vascularized nature of subcutaneous tissue (especially in patients with a lot of subcutaneous fat) may impair optimal drug delivery to in-transit tumor deposits in the setting of the reduced dose. This may explain the weak correlation between Vesti/Vss and response in patients who had their melphalan dose reduced based on IBW. Therefore, in patients who have a dramatic difference in IBW versus actual body weight, consideration of a limitation on the dose reduction could be considered.
In HILP, pharmacokinetic analysis of plasma LPAM levels has found that the ratio of Vesti to Vss was strongly correlated with acute regional toxicity.6 A high Vesti/Vss ratio (≥4) was found in patients who experienced Wieberdink toxicity of grade 3 or higher. This high Vesti leads to higher than expected drug concentrations in both the tissue and plasma compartments and means that patients who have a lower estimated limb volume compared with their actual effective limb volume (i.e., patients who received an overdose of melphalan) are at risk for increased toxicity.6 In this study, we confirmed this finding for ILI as well in that those patients who were corrected for IBW had a lower incidence of toxicity. In addition, we also confirmed that the Vesti/Vss ratio correlated with toxicity, just as it did for HILP. Adjusting the limb-volume calculation decreased the rate of compartment syndrome, the incidence of grade 3 or higher regional toxicity scores, and peak CPK value.
Because this decrease in toxicity is important clinically in terms of decreased hospital time and the need for fewer interventions, there is motivation to experiment with other factors that could increase response in the setting of lowered toxicity. Hyperthermia has been shown to correlate with increase response rates in ILI, and in ILP.15–20 Using hypoxia and acidosis are other techniques that are possible with ILI that could be used to improve response rates. Additionally, papaverine is routinely used during ILI at the Sydney Melanoma Unit (SMU) to optimize cutaneous blood flow and increase drug delivery to cutaneous lesions.1,2 We have only recently started using papaverine to maximally dilate cutaneous blood vessels in an isolated limb so its effects on melphalan pharmacokinetics, limb toxicity, and tumor response remain to be determined.
Our current approach to improving regional response rates uses strategies that involve targeted agents given systemically to make tumors more susceptible to regionally administered chemotherapy agents. We have recently concluded the phase I portion of a clinical trial involving systemic ADH-1, an N-cadherin targeting agent, to be used in combination with regional melphalan. Preliminary results have found a complete response rate of 50% with no additional toxicity experienced in the first 20 patients treated.21 Another phase I study involving systemic Sorafenib, a multikinase inhibitor, also in combination with regional melphalan is currently underway with impressive initial responses to therapy in patients who have failed a previous melphalan infusion (unpublished data). Finally, we have recently opened a trial using buthionine sulfoximine (BSO) in combination with regional melphalan. This selective inhibitor of gluthathione synthesis is thought to decrease tumor resistance to chemotherapy. Based on these preliminary findings, it appears that using targeted therapies in effort to overcome resistance to melphalan is a promising strategy to improve response.
Finally, in HILP, numerous studies have examined the utility of a planned repeat perfusion, typically 4 weeks after the initial treatment. Results have shown this to be an effective way to increase incidence of complete response rates, with rates ranging from 65% to 77%.22–26 However, many of the patients in these studies were unable to undergo repeat perfusion due to toxicity associated with the first procedure. With ILI, not only does the technique allow for a relatively short interval between treatments, there is significantly lower toxicity compared with HILP.3 Lindner and colleagues studied the outcomes for planned repeat ILI and found a 41% CR rate for patients with a double ILI performed 4 weeks after initial treatment. This response rate was comparable to that seen with single ILI.27 This study did report, however, that repeat ILI is valuable for patients who have recurrent or progressive disease. In both HILP and ILI, the durability of response was not found to be much different than that seen with a single treatment and there was also an increase in toxicity in patients undergoing a planned double ILI. By correcting the melphalan dose for ideal body weight we could lower this toxicity, and when combined with other factors to increase response, this could be an ideal option for repeated treatment of extremity melanoma.
CONCLUSIONS
In an effort to optimally manage patients with regionally advanced melanoma, ILI has emerged as an effective alternative to HILP. Given its relatively recent description, little has been done to optimize the therapeutic index of melphalan delivered via this mechanism. This study has demonstrated the importance of the Vesti/Vss ratio in defining toxicity in ILI and has shown that correcting melphalan dosing for IBW can improve the therapeutic index of melphalan in ILI by decreasing toxicity without sacrificing response. Through a number of other novel studies aimed at improving the effectiveness of regionally administered melphalan, future improvement in the therapeutic index of ILI can be obtained by optimizing tumor responses in the setting of a melphalan dose corrected for IBW to minimize toxicity.
ACKNOWLEDGMENTS
This work was supported in part by the Duke Melanoma Research Fund and a Grant from the Institute of Genomic Sciences and Policy at Duke University Medical Center (DT) and the NIH/NCI Duke Comprehensive Cancer Center Core Grant (5-P30-CA14236-29).
REFERENCES
- 1.Thompson JF, Kam PC, Waugh RC, Harman CR. Isolated limb infusion with cytotoxic agents: a simple alternative to isolated limb perfusion. Semin Surg Oncol. 1998;14:238–247. doi: 10.1002/(sici)1098-2388(199804/05)14:3<238::aid-ssu8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 2.Thompson JF, Waugh RC, Saw RPM, Kam PCA. Isolated limb infusion with melphalan for recurrent limb melanoma: a simple alternative to isolated limb perfusion. Reg Cancer Treat. 1994;7:188–192. [Google Scholar]
- 3.Beasley GM, Petersen RP, Yoo J, McMahon N, Aloia T, Petros W, et al. Isolated limb infusion for in-transit malignant melanoma of the extremity: a well tolerated but less effective alternative to hyperthermic isolated limb perfusion. Ann Surg Oncol. 2008;15:2195–2205. doi: 10.1245/s10434-008-9988-9. [DOI] [PubMed] [Google Scholar]
- 4.Brady MS, Brown K, Patel A, Fisher C, Marx W. A phase II trial of isolated limb infusion with melphalan and dactinomycin for regional melanoma and soft tissue sarcoma of the extremity. Ann Surg Oncol. 2006;13:1123–1129. doi: 10.1245/ASO.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Lindner P, Doubrovsky A, Kam PC, Thompson JF. Prognostic factors after isolated limb infusion with cytotoxic agents for melanoma. Ann Surg Oncol. 2002;9:127–136. doi: 10.1007/BF02557363. [DOI] [PubMed] [Google Scholar]
- 6.Cheng TY, Grubbs E, Abdul-Wahab O, Leu SY, Hung CF, Petros W. Marked variability of melphalan plasma drug levels during regional hyperthermic isolated limb perfusion. Am J Surg. 2003;186:460–467. doi: 10.1016/j.amjsurg.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 7.Ehrsson H, Eksborg S, Lindfors A. Quantitative determination of melphalan in plasma by liquid chromatography after derivatization with N-acetylcysteine. J Chromatogr Biomed Appl. 1986;380:222–228. doi: 10.1016/s0378-4347(00)83648-8. [DOI] [PubMed] [Google Scholar]
- 8.Wieberdink J, Benckhuysen C, Braat RP, van Slooten EA, Olthuis GA. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol. 1982;18:905–910. doi: 10.1016/0277-5379(82)90235-8. [DOI] [PubMed] [Google Scholar]
- 9.Fraker DL. Hyperthermic regional perfusion for melanoma of the limbs. In: Balch CM, Houghton AN, Sober AJ, Soong S, editors. Cutaneous Melanoma. St. Louis, MO: Quality Medical; 1998. pp. 281–300. [Google Scholar]
- 10.Boddie AW, Briele H, Krementz F. A phase I study of melphalan in 40 C isolated limb perfusion using packed red blood cells and lactated ringers perfusate. Proc ASCO. 1992;11:351. [Google Scholar]
- 11.Krementz ET, Carter RD, Sutherlan CM, Muchmore JH, Ryan RF, Creech O., Jr Regional chemotherapy for melanoma: a 35-year experience. Ann Surg. 1994;220:520–535. doi: 10.1097/00000658-199410000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts MS, Wu ZY, Siebert GA, Thompson JF, Smithers BM. Saturable dose-response relationships for melphalan in melanoma treatment by isolated limb infusion in the nude rat. Melanoma Res. 2001;11:611–618. doi: 10.1097/00008390-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Kroon HM, Moncrieff M, Kam PC, Thompson JF. Outcomes following isolated limb infusion for melanoma: a 14-year experience. Ann Surg Oncol. 2008;15:3003–1313. doi: 10.1245/s10434-008-9954-6. [DOI] [PubMed] [Google Scholar]
- 14.Beasley GM, Caudle A, Peterson RP, McMahon N, Mosca P, Zager J, et al. A multi-institutional experience of isolated limb infusion: defining response and toxicity in the United States. Presented in part at the Southern Surgical Association’s Annual Meeting; West Palm Beach, FL. 2008. [Google Scholar]
- 15.Abdel-Wahab OI, Grubbs E, Viglianti B, Cheng TY, Ueno T, Ko S, et al. The role of hyperthermia in regional alkylating agent chemotherapy. Clin Cancer Res. 2004;10:5919–5929. doi: 10.1158/1078-0432.CCR-04-0096. [DOI] [PubMed] [Google Scholar]
- 16.Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43:33–56. doi: 10.1016/s1040-8428(01)00179-2. [DOI] [PubMed] [Google Scholar]
- 17.Stehlin JS. Hyperthermic perfusion with chemotherapy for cancers of the extremity. Surg Gynecol Obstet. 1969;129:305–308. [PubMed] [Google Scholar]
- 18.Skene AL, Bulman AS, Williams TF, Thomas JM, Westbury G. Hyperthermic isolated limb perfusion with melphalan in the treatment of advanced malignant melanoma of the lower limb. Br J Surg. 1990;77:765. doi: 10.1002/bjs.1800770716. [DOI] [PubMed] [Google Scholar]
- 19.Storm FK, Morton DL. Value of therapeutic hyperthermic limb perfusion in advanced recurrent melanoma of the lower extremity. Am J Surg. 1985;150:32–35. doi: 10.1016/0002-9610(85)90006-6. [DOI] [PubMed] [Google Scholar]
- 20.Minor DR, Allen RE, Alberts D, Peng YM, Tardelli G, Hutchinson J. A clinical and pharmacokinetic study of isolated limb perfusion with heat and melphalan for melanoma. Cancer. 1985;55:2638–2644. doi: 10.1002/1097-0142(19850601)55:11<2638::aid-cncr2820551118>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 21.Beasley GM, McMahon N, Sanders G, Zipfel P, Augustine C, Petros W, et al. A Phase I study of systemic ADH-1 in combination with melphalan via isolated limb infusion in patients with locally advanced in-transit malignant melanoma. ASCO abstract. 2008 Jun;9013:486. doi: 10.1002/cncr.24509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noorda EM, Vrouenraets BC, Nieweg OE, Klaase JM, van der Zee J, Kroon BB. Long-term results of a double perfusion schedule using high dose hyperthermia and melphalan sequentially in extensive melanoma of the lower limb. Melanoma Res. 2003;13:395–399. doi: 10.1097/00008390-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Klop WM, Vrouenraets BC, van Geel BN, Eggermont AM, Klaase JM, Nieweg OE, et al. Repeat isolated limb perfusion with melphalan for recurrent melanoma of the limbs. J Am Coll Surg. 1996;182:467–472. [PubMed] [Google Scholar]
- 24.Klaase JM, Kroon BB, Van Geel AN, Eggermont AM, Franklin HR, van Dongen JA. Is there an indication for a double perfusion schedule with melphalan for patients with recurrent melanoma of the limbs? Melanoma Res. 1994;4:13–16. [PubMed] [Google Scholar]
- 25.Klaase JM, Kroon BB, van Geel AN, Eggermont AM, Franklin HR, van Dongen JA. A retrospective comparative study evaluating the results of a single-perfusion versus double-perfusion schedule with melphalan in patients with recurrent melanoma of the lower limb. Cancer. 1993;71:2990–2994. doi: 10.1002/1097-0142(19930515)71:10<2990::aid-cncr2820711017>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 26.Kroon BB, Klaase JM, van Geel BN, Eggermont AM, Franklin HR, van Dongen JA. Results of a double perfusion schedule with melphalan in patients with melanoma of the lower limb. Eur J Cancer. 1993;29A:325–328. doi: 10.1016/0959-8049(93)90377-r. [DOI] [PubMed] [Google Scholar]
- 27.Lindner P, Thompson JF, De Wilt JHW, Colman M, Kam PC. Double isolated limb infusion for recurrent and metastatic limb melanoma. Eur J Surg Oncol. 2004;30:433–439. doi: 10.1016/j.ejso.2004.01.015. [DOI] [PubMed] [Google Scholar]