Summary
Induction of the unfolded protein response (UPR) is recognized as central to fatty liver disease (FLD) pathophysiology. This pathway may be a potential therapeutic target for FLD, as well as other diseases. However, fundamental questions as to how UPR contributes to FLD remain unanswered. Conflicting data suggest that this pathway can both protect against and augment this disease. Here, we review the relationship between protein secretion, endoplasmic reticulum function (ER), and UPR activation. The UPR serves to maintain secretory pathway homeostasis by enhancing the protein folding environment in the ER, and we review data investigating the role for individual UPR players in fatty liver (steatosis). We explore a novel concept in the field that all cases of UPR activation do not equal “ER stress”. Rather, different types of UPRs that can either protect against or cause FLD are discussed. Refining our current understanding of this complex pathway is particularly important, as drugs that affect the protein folding environment in the ER and affect UPR activation are being successful in clinical trials for FLD.
Keywords: Unfolded protein response, Fatty liver disease, Adaptation, ER stress, Ern, Xbp1, BIP, Atf6, Eif2ak3
Introduction
Fatty liver disease (FLD) ranges from lipid accumulation in hepatocytes (steatosis) to steatohepatitis, which can progress to fibrosis, cirrhosis, and ultimately liver failure. Metabolic syndrome and alcohol abuse are the most common causes of FLD, although viral hepatitis, drug toxicity, and some metabolic disorders are also culprits. Whether steatosis qualifies as a bona fide pathology is under debate, but it is widely accepted that steatosis, which may be a surrogate marker of increased free fatty acid influx to the liver, is the prerequisite step to disease progression. Thus, FLD treatment begins with alleviating steatosis.
Despite obvious differences between the multiple FLD etiologies, there are some commonalities. One is that FLD is frequently associated with serum protein deficiencies caused by a defect in hepatocyte secretion. The cellular basis for this longstanding clinical observation is not fully understood, however, there are several clear measures indicating that FLD is associated with suboptimal protein secretion by hepatocytes. This defect in the secretory pathway can induce the unfolded protein response (UPR), and UPR activation is observed in every FLD etiology [1]. Two possible hypotheses explain this finding: (i) lipid accumulation in hepatocytes causes ER dysfunction and UPR activation, and thus it is a consequence of steatosis or (ii) ER dysfunction and unfolded protein accumulation precede steatosis, causing the disease. Some studies support the first theory – that lipotoxicity can cause UPR activation [2] – however works from several groups, including ours, have conclusively demonstrated that accumulation of unfolded proteins in the ER is sufficient to cause steatosis [3–7]. This supports the second model that UPR activation causes steatosis. While this is likely true in some cases, this model is oversimplified. Here, we provide an overview of the UPR, summarize data supporting a role for the UPR in FLD, and present the novel concept that there are different types of UPRs, even a UPR continuum, with dramatically different outcomes in FLD, which can explain the conflicting data in the field. This exciting idea lays the foundation to develop therapies that would divert hepatocytes from a stressed, disease causing UPR to an adaptive, beneficial UPR.
The unfolded protein response: three branches, many outcomes
Secreted proteins begin their journey to the cell surface during translation into the ER. There, proteins are glycosylated, folded, and packaged into vesicles for transport to the Golgi apparatus to become further modified and then targeted to their final destination. Hepatocytes carry out the essential function of secreting serum proteins, including albumin, transferring, and clotting factors. Thus, when hepatocytes become diseased and the secretory pathway is dysfunctional, serum protein deficiency occurs. The clinical consequences of edema and hypocoagulation are frequent complications of advanced liver disease.
The UPR functions in all cell types, but is most active in highly secretory cells like hepatocytes. This pathway serves to both enhance the protein folding capacity of the ER and the protein quality control system that recycles or eliminates improperly folded and thus potentially destructive secretory proteins. Thus, the UPR is essential for hepatocyte homeostasis.
When there is an imbalance between the protein folding capacity of the ER and the unfolded protein load, the UPR is further upregulated to reduce the build up of secretory cargo in the ER. It does this by decreasing the influx of new proteins into the ER, targeting terminally unfolded proteins for destruction and inducing the transcription of hundreds of genes that serve to expand the ER and alleviate the unfolded protein load in the ER and restore homeostasis.
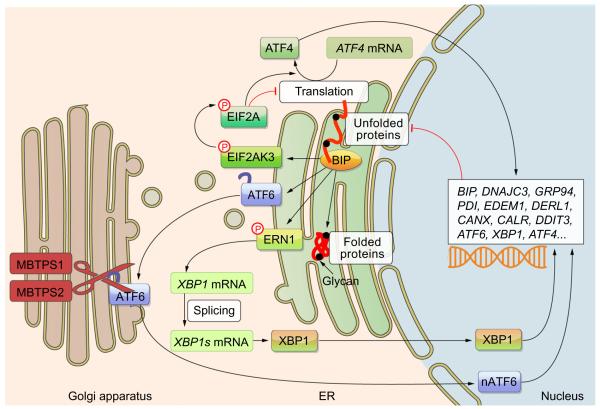
The three major UPR branches (Fig. 1) converge on the X-box binding protein 1 (XBP1), activating transcription factor 4 (ATF4) and ATF6 transcription factors that regulate hundreds of genes that function to augment protein folding. One such category is protein chaperones, which assist in protein folding. The major ER chaperone – BIP – also serves as an unfolded protein sensor. Thus, when unfolded proteins accumulate, they are bound by BIP, releasing the three main UPR mediators that reside in the ER membrane. EIF2AK3 (also called PERK) phosphorylates EIF2A which blocks protein translation en masse while selectively promoting translation of activating transcription factor 4 (ATF4) mRNA. ERN1 splices XBP1 mRNA to produce the active XBP1 transcription factor. Third, ATF6 translocates to the Golgi apparatus where it is cleaved by the MBTPS1 and MBTPS2 proteases to release a fragment that can transit to the nucleus and act as a transcription factor (nATF6). The UPR target genes include chaperones and other factors that serve to reduce the burden of unfolded proteins in the ER as well as some of the major players of the UPR. Once UPR function is ramped up, the unfolded protein burden is reduced and this provides a negative feedback that dials down the UPR.
Fig. 1. The three branches of the UPR.
Each of the most proximal UPR mediators receives an activating signal from the chaperone BIP or from unfolded proteins. Each branch results in the activation of transcription factors that induce a set of genes (a partial list is provided) that then function to reduce the load of unfolded proteins in the ER. This serves as a negative feedback to downregulate the UPR once homeostasis is achieved. p, phosphorylation.
The UPR continuum: from homeostasis to apoptosis
The UPR has largely been studied in yeast or cultured mammalian cells exposed to high doses of drugs that cause an acute and pro-found accumulation of unfolded proteins in the ER. The result is that each UPR branch and most target genes are induced. In this experimental setting, the presence of the stressor is persistent, resulting in an unrelenting accumulation of unfolded proteins and significant UPR activation. This is ER stress, and represents the only one type of UPR. Other types of UPR have different, even beneficial functions.
While these in vitro studies have been useful in identifying UPR target genes, this level of stress falls outside the physiologic range experienced by most cells. Instead, there is a basal level of UPR activity i.e., a homeostatic UPR, which can manage the load of cargo that fall within the physiological demands of hepatocytes in a healthy liver. However, if ER function is compromised, unfolded proteins accumulate, and a stressed UPR ensues. This induces all UPR branches and may not be unlike the stressed UPR that occurs in experiments where cells are exposed to potent stressors. Indeed, intraperitoneal injection of tunicamycin, a potent UPR inducer, causes a stressed UPR in hepatocytes, followed by steatosis [3,6–8]. Both ER stress and steatosis resolve within 72 h in healthy animals because the full induction of the stressed UPR effectively mitigates the transient accumulation of unfolded proteins. Irreparable secretory pathway dysfunction, however, can cause the stressed UPR to transition to a terminal UPR, causing cell death. In contrast, if the UPR can manage the unfolded protein burden, then homeostasis is restored, and, these cells retain an enhanced ER protein folding capacity. This is analogous to the well-documented adaptive response to oxidative stress following recovery, in which an enhanced antioxidant capacity persists. When these adapted cells are re-stressed, UPR induction is lower than in naïve cells.
We propose that the UPR serves as a stress meter for the secretory pathway (Fig. 2). In this model, the UPR is not a single entity, but is a continuum, where the constellation of activated UPR mediators and effectors varies with the nature and duration of the stress. Manipulation between UPR subclasses can serve as the basis for treatments that will adjust the UPR dial. While there are innumerable potential UPRs, grouping them into subclasses (i.e., homeostatic, adaptive, stressed, and terminal) provides a framework for understanding the markedly different outcomes that result from each of them. Importantly, while a stressed UPR may cause steatosis, recent findings have highlighted that all UPRs do not. In contrast, an adaptive UPR protects against FLD [3,9].
Fig. 2. The UPR as a stress-meter.

There are multiple types of UPRs which can be grouped into subclasses. The outcome can be dramatically different – a homeostatic UPR maintains the efficient processing of proteins through the secretory pathway, an adaptive UPR occurs in cells that have recovered from a moderate stress, a stressed UPR signals pathway dysfunction and correlates with steatosis and a terminal UPR occurs when the stress is overwhelming and the cell is eliminated by apoptosis. With the exception of a terminal UPR in which there is a point of no return, the degree of UPR activation in a cell can fluctuate, providing the basis for treatment. A signature for each type of UPR has yet to be defined.
UPR activation and FLD
An exciting advance has come from bridging the common clinical observation of serum protein deficiency in patients with liver disease to an underlying cellular and molecular mechanism that gives rise to this disease, centered on the UPR. Thus, it is not surprisingly to find that ER dysfunction and UPR activation occur with alcohol consumption and obesity [10–14], as well as with steatosis in viral hepatitis [15,16] and even recovery of steatotic allografts following liver transplant [17]. However, as the field matures to incorporate the nuanced and dynamic nature of this pathway, important questions emerge. Namely, when some markers of the UPR are detected in diseased livers, does it reflect a stressed UPR that is contributing to the disease, or, conversely, is it an adaptive UPR at work to restore hepatocyte function? This remains an important issue to be addressed in future studies.
Recent studies have focused mainly on conventional genetic approaches in mice to dissect the role of individual UPR players in FLD. Results from these studies are conflicting (Table 1). For instance, removing BIP from hepatocytes exacerbates FLD caused by alcohol, high fat diet, and drugs [18], while overexpression of BIP in hepatocytes alleviates FLD caused by obesity [19]. This would suggest that BIP loss dials the UPR towards a stressed or terminal UPR whereas BIP overexpression keeps the UPR dial in the adaptive range. Interestingly, BIP heterozygotes are protected from steatosis caused by a high fat diet [9]. This is attributed to the partial loss of BIP resulting in a compensatory activation of an adaptive UPR that renders hepatocytes resistant to the stress induced by obesity.
Table 1.
Genetic manipulation of UPR mediators in mouse models of FLD alters insulin resistance and steatosis.
| Target gene [Ref.] |
Genetic approach (KO: knock-out; OE: overexpression; H: heterozygosity) |
Fatty liver disease model | Liver disease outcome | |||||
|---|---|---|---|---|---|---|---|---|
| Global |
Hepatocyte |
|||||||
| KO | OE | H | KO | OE | H | |||
|
Xbp1 [14] |
X | 17 wk high fat diet | Hyperglycemia Hyperinsulinemia Glucose intolerance Increased insulin resistance |
|||||
|
Xbp1 [9] |
X | none | Decreased plasma lipids No change in steatosis Decreased lipogenesis |
|||||
|
Ern1 [25] |
X | 8, 24, 36, 48, 72 h post IP injection with tunicamycin | Increased lipogenesis UPR activation Increased steatosis Apoptosis |
|||||
|
Atf6 [18,25] |
X | 8, 24, 48, 72 h post IP injection with tunicamycin | Increased steatosis Increased insulin resistance |
|||||
|
Eif2ak3 [20] |
X | 6, 24, 36 h post IP injection with tunicamycin | Apoptosis Increased steatosis Increased lipogenesis |
|||||
|
Dnajc3 [18] |
X | 48 h post IP injection with tunicamycin | Decreased lipogenesis Increased steatosis |
|||||
|
BiP [24] |
X | 20 wk on high fat diet | Hyperglycemia Hyperinsulinemia No steatosis Increased insulin sensitivity |
|||||
|
BiP [6] |
X | 1. 6 wk high fat diet 2. 6-8 wk alcohol |
Steatosis Increased insulin resistance Increased lipogenesis lnflammation Apoptosis |
|||||
|
BiP [7] |
X | Obese (ob/ob) mice 72 h post-injection of adenovirus expressing Bip |
Decreased UPR activation Decreased lipogenesis Insulin sensitivity increased Decreased gluconeogenesis |
|||||
Opposing results are found in other studies investigating the main UPR players in FLD: blocking the ERN1–XBP1 branch by deleting Ern1 in hepatocytes [8] or heterozygosity for Xbp1 [13] worsens FLD, whereas deleting Xbp1 in hepatocytes makes them more resistant to developing steatosis caused by a high carbohydrate diet [20]. We found that knock-down of Atf6 in zebrafish reduces steatosis incidence caused by chronic ER stress but increases it in response to acute stress [3], similar to findings of increased steatosis by tunicamycin injection in Atf6−/− mice [5,21]. Thus, loss of Atf6 has dramatically different effects depending on the nature and duration of the stress. Data on the EIF2AK3 pathway appears more straight forward in suggesting that this pathway serves a protective function, as Eif2ak3−/− mice have increased steatosis [7], whereas keeping this pathway constitutively active in hepatocytes is protective against the effects of a high-fat diet [22].
Initial analysis of these studies presents a confusing picture. For instance, when the stress is chronic, such as during obesity or prolonged alcohol abuse, the effects of UPR depletion are different compared to an acute stress caused by binge drinking or toxin exposure. Thus, it will be important for future studies on UPR and FLD to analyze similar methods of depletion in parallel with multiple FLD models.
The UPR and lipids: what is the link?
Uncovering how this central aspect of the secretory pathway impacts the hepatocyte metabolic machinery is a critical, unanswered question. Whether the UPR has a direct impact on lipid metabolism remains to be determined. Compelling data suggests that UPR activation drives hepatic insulin resistance [13], which can then lead to lipogenesis. This is supported by the finding that XBP1 drives expression of genes involved in triglyceride synthesis [20]. However, in other studies, these genes were unaffected [3] or decreased [5,21] in hepatocytes with ER stress, suggesting that ER stress-induced steatosis does not require this pathway. Loss of ATF6 has effects on expression of genes controlling β-oxidation of fatty acids [21], carbohydrate metabolism [5], and lipoprotein export [21]. Whether any of these genes play a role in steatosis following UPR induction remains to be determined.
Summary and clinical perspective: treating fatty liver disease by targeting the UPR
The ability for cells to transition between UPR subclasses has important implications for treatment. We envision therapies that would dial down a stressed UPR to become an adaptive one. The successful treatment of FLD in rodent models and in humans with chemical chaperones [23–25] is a clinical and scientific break-through, which is rooted in this concept. This presents an exciting new area for developing novel FLD treatments and also highlights how basic research carried out without directives for translational relevance can provide novel, important insights into a highly prevalent liver disease.
Acknowledgments
The underlying research reported in the study was funded by the NIH Institutes of Health.
Financial support National Institutes of Health Grants 1R011AA0188886-02 and p20AA017067 and the American Gastroenterological Association Research Scholar Award support K.C.S. The Training Program in Cellular and Molecular Biology (NIGMS/T32GM08633) supported in part by the Medical Scientist Training Program (T32GM007280) and DI.
Footnotes
Conflict of interest The authors declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
References
- [1].Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2010;54:795–809. doi: 10.1016/j.jhep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Malhi H, Gores GJ. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:360–369. doi: 10.1055/s-0028-1091980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cinaroglu A, Gao C, Imrie D, Sadler KC. Activating transcription factor 6 plays protective and pathological roles in steatosis due to endoplasmic reticulum stress in zebrafish. Hepatology. 2011;54:495–508. doi: 10.1002/hep.24396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yamamoto K, Yoshida H, Kokame K, Kaufman RJ, Mori K. Differential contributions of ATF6 and XBP1 to the activation of endoplasmic reticulum stress-responsive cis-acting elements ERSE, UPRE and ERSE-II. J Biochem (Tokyo) 2004;136:343–350. doi: 10.1093/jb/mvh122. [DOI] [PubMed] [Google Scholar]
- [5].Rutkowski DT, Wu J, Back SH, Callaghan MU, Ferris SP, Iqbal J, et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell. 2008;15:829–840. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lee JS, Zheng Z, Mendez R, Ha SW, Xie Y, Zhang K. Pharmacologic ER stress induces non-alcoholic steatohepatitis in an animal model. Toxicol Lett. 2012;211:29–38. doi: 10.1016/j.toxlet.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Teske BF, Wek SA, Bunpo P, Cundiff JK, McClintick JN, Anthony TG, et al. The eIF2 kinase PERK and the integrated stress response facilitate activation of ATF6 during endoplasmic reticulum stress. Mol Biol Cell. 2011;22:4390–4405. doi: 10.1091/mbc.E11-06-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang K, Wang S, Malhotra J, Hassler JR, Back SH, Wang G, et al. The unfolded protein response transducer IRE1alpha prevents ER stress-induced hepatic steatosis. EMBO J. 2011;30:1357–1375. doi: 10.1038/emboj.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ye R, Jung DY, Jun JY, Li J, Luo S, Ko HJ, et al. Grp78 heterozygosity promotes adaptive unfolded protein response and attenuates diet-induced obesity and insulin resistance. Diabetes. 2010;59:6–16. doi: 10.2337/db09-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Park SW, Zhou Y, Lee J, Ozcan U. Sarco(endo)plasmic reticulum Ca2+-ATPase 2b is a major regulator of endoplasmic reticulum stress and glucose homeostasis in obesity. Proc Natl Acad Sci U S A. 2010;107:19320–19325. doi: 10.1073/pnas.1012044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fu S, Yang L, Li P, Hofmann O, Dicker L, Hide W, et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473:528–531. doi: 10.1038/nature09968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124:1488–1499. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- [13].Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- [14].Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, et al. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134:568–576. doi: 10.1053/j.gastro.2007.10.039. [DOI] [PubMed] [Google Scholar]
- [15].Asselah T, Bieche I, Mansouri A, Laurendeau I, Cazals-Hatem D, Feldmann G, et al. In vivo hepatic endoplasmic reticulum stress in patients with chronic hepatitis C. J Pathol. 2010;221:264–274. doi: 10.1002/path.2703. [DOI] [PubMed] [Google Scholar]
- [16].Tardif KD, Waris G, Siddiqui A. Hepatitis C virus, ER stress, and oxidative stress. Trends Microbiol. 2005;13:159–163. doi: 10.1016/j.tim.2005.02.004. [DOI] [PubMed] [Google Scholar]
- [17].Anderson CD, Upadhya G, Conzen KD, Jia J, Brunt EM, Tiriveedhi V, et al. Endoplasmic reticulum stress is a mediator of posttransplant injury in severely steatotic liver allografts. Liver Transpl. 2011;17:189–200. doi: 10.1002/lt.22220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ji C, Kaplowitz N, Lau MY, Kao E, Petrovic LM, Lee AS. Liver-specific loss of glucose-regulated protein 78 perturbs the unfolded protein response and exacerbates a spectrum of liver diseases in mice. Hepatology. 2011;54:229–239. doi: 10.1002/hep.24368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–1215. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yamamoto K, Takahara K, Oyadomari S, Okada T, Sato T, Harada A, et al. Induction of liver steatosis and lipid droplet formation in ATF6alpha-knockout mice burdened with pharmacological endoplasmic reticulum stress. Mol Biol Cell. 2010;21:2975–2986. doi: 10.1091/mbc.E09-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Oyadomari S, Harding HP, Zhang Y, Oyadomari M, Ron D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7:520–532. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kars M, Yang L, Gregor MF, Mohammed BS, Pietka TA, Finck BN, et al. Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 2010;59:1899–1905. doi: 10.2337/db10-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Xiao C, Giacca A, Lewis GF. Sodium phenylbutyrate, a drug with known capacity to reduce endoplasmic reticulum stress, partially alleviates lipid-induced insulin resistance and {beta}-cell dysfunction in humans. Diabetes. 2011;60:918–924. doi: 10.2337/db10-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]