Abstract
We have previously shown that 3-nitro-1H-1,2,4-triazole-based amines demonstrate significant trypanocidal activity, in particular against T. cruzi, the causative parasite of Chagas disease. In the present work we further expanded our research by evaluating in vitro the trypanocidal activity of nitrotriazole-based piperazines and nitrotriazole-based 2-amino-1,3-benzothiazoles to establish additional SARs. All nitrotriazole-based derivatives were active or moderately active against T. cruzi; however two of them did not fulfill the selectivity criteria. Five derivatives were active or moderately active against T.b. rhodesiense while one derivative was moderately active against L. donovani. Active compounds against T. cruzi demonstrated selectivity indexes (toxicity to host cells/toxicity to T. cruzi amastigotes) from 117-1725 and 12 of 13 compounds were up to 39-fold more potent than the reference compound benznidazole. Detailed SARs are discussed.
Keywords: nitrotriazoles, piperazines, benzothiazoles, Chagas disease, antitrypanosomal agents
1. Introduction
The trypanosomatid protozoan parasites Trypanosoma cruzi (T. cruzi), Trypanosoma brucei (T. brucei), and various Leishmania species are the causative agents of American trypanosomiasis (Chagas disease), human African trypanosomiasis (HAT) and various forms of leishmaniasis, respectively. Over 20 million people are infected by these parasites, resulting in 100,000 deaths per year [1]. Chagas disease, one of the most neglected diseases, is endemic mainly in Latin America and occurs in two phases: acute and chronic. The most important clinical manifestations of the chronic form of Chagas disease are heart insufficiency (Chagasic cardiopathy and arrhythmias, 90% of cases approximately) and gastrointestinal syndromes (megaoesophagus and megacolon). It is estimated that around 100 million people are at risk of infection with T. cruzi in endemic areas in Latin America [2]. Despite the fact that the number of incidences has significantly declined in the past 20 years, primarily due to vector control initiatives, the number of cases in non-endemic regions (United States, Australia, Europe and Japan) is rising [3-5]. Reasons for this include population migration, illegal drug usage and medical practices. With no immediate prospect for vaccines, chemotherapy is the only current method to treat patients affected with Chagas disease.
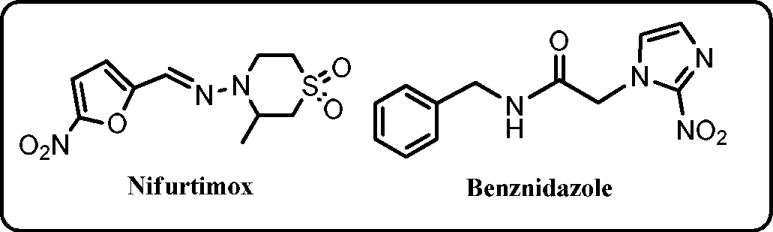
The existing drugs for Chagas, nifurtimox (a nitrofuran; Nfx) and benznidazole (a 2-nitroimidazole; Bnz) (Chart 1) are old, have limited efficacy, significant side effects and some strains are refractory to treatment [6,7]. Recently, inhibitors of the sterol 14 -demethylase enzyme (CYP51), which is part of the ergosterol biosynthesis pathway, represent one form of new treatments under development as effective antichagasic agents [8], though this mechanism of action still remains to be clinically validated. Moreover, the high cost of these inhibitors may limit their use in poor countries where the disease is most prevalent [9]. Therefore, the need for new, affordable and safer drugs to treat Chagas disease is urgent.
Chart 1.
We are interested in the synthesis and development of safer nitroheterocyclics as potential antichagasic agents [10,11]. Nitroheterocyclics function as prodrugs and must undergo activation before mediating their cytotoxic effects. It was demonstrated that an oxygen-insensitive, type I nitroreductase (NTR), present in trypanosomes and absent from most other eukaryotes, is responsible for Nfx and Bnz trypanocidal activity [12-14]. Via a series of 2 electron reduction reactions, this enzyme is involved in the production of toxic metabolites which can selectively kill trypanosomatids [15]. The NTR-mediated activation of nitroheterocyclic prodrugs, which occurs specifically in the trypanosomatids, has led to a renewed interest in the use of such compounds as antiparasitic agents [16-24].
We have recently reported that 3-nitro-1H-1,2,4-triazole-based amines, amides and sulfonamides demonstrate excellent activity against T. cruzi amastigotes in infected L6 cells with no toxicity towards the host cells [10,11]. The IC50 values of these compounds against the intracellular parasite ranged from low nM to less than 4 μM and have selectivity indices ranging from 66 to 2682. In addition, several of these compounds were up to 56 fold more active than the reference drug benznidazole (Bnz), tested in parallel. We have also shown that such nitrotriazoles are activated by the type I NTR and that T.b. brucei parasites overexpressing the enzyme are hypersensitive to these compounds [10,11]. Interestingly, in preliminary in vivo studies, we found that treatment of T. cruzi-infected mice with one nitrotriazole-based aromatic amine, NTLA-1 [25], given at just 2 mg/kg/day × 50 days, resulted in a rapid and persistent drop in peripheral parasite levels and in a fraction of cures [26,27]. More importantly, there was an absolute correlation between treatment efficacy as determined parasitologically and the increase in the fraction of T. cruzi-specific CD8+ T cells with a T central memory phenotype in the peripheral blood of treated mice [26, 27]. More recently, representative nitrotriazoles of different chemical classes have been evaluated for in vivo efficacy by using a fast luminescence assay in mice infected with transgenic parasites that express luciferase [28]. Most of the tested compounds demonstrated in vivo antichagasic activity in this assay, and at least three of them were identified with superior efficacy to benznidazole [29]. In addition, several nitrotriazole-based derivatives were tested in the Ames test, and in contrast to their 2-nitroimidazole analogs, were not mutagenic, at least at non-toxic concentrations [29].
In the present study we have further investigated into two subclasses of 3-nitrotriazole-based amines as antitrypanosomal agents: piperazines and 2-amino-1,3-benzothiazoles. We have synthesized eleven novel piperazine-based derivatives and six 2-amino-1,3-benzothiazoles. For comparison purposes, and to confirm our previous findings, a small number of 2-nitroimidazole-based analogs were included in the above numbers. The synthesis and in vitro evaluation of the compounds as antitrypanosomal agents is described and SARs are discussed.
2. Results and Discussion
2.1. Chemistry
The structures of all compounds are depicted on Table 1. Their synthesis is straightforward and based on well-established chemistry, outlined in Scheme 1. Piperazine derivatives 1-14 were synthesized by nucleophilic attack of an N-monosubstituted piperazine to the appropriate nitro(triazole/imidazole)-alkylbromide [30] in the presence of K2CO3 as described before [10]. N-substituted-2-amino-1,3-benzothiazoles 15-20 were synthesized by coupling of the proper 2-chloro-1,3-benzothiazole with the appropriate nitro(triazole/imidazole)-alkylamine [31] by nucleophilic aromatic substitution (Scheme 1). In general the yields were moderate to good but if we account for the recovered unreacted starting materials, the yields are significantly greater than reported.
Table 1. In vitro screening data against three different trypanosomatids.
| ID No | T.b.rhod.a | SI | T. cruzib | SI | L.don. axen.c | SI | Cytotox. L6 | Bnz/Comp. | Chemical Structure |
|---|---|---|---|---|---|---|---|---|---|
| IC-50 μM | IC-50 μM | IC-50 μM | IC-50 μM | ||||||
| 1 | 1.38 | >14 | 0.34 | >562 | 58.15 | >3 | >191.1 | 4.6 |

|
| 2 | 1.2 | 99 | 0.412 | 287 | 44.45 | 3 | 118.4 | 3.8 |
|
| 3 | 5.33 | 10 | 0.04 | 1320 | 25.92 | 2 | 52.79 | 39.2 |
|
| 4 | 49.6 | 5.2 | 1.678 | 152 | >100 | 1 | >100 | 0.9 |
|
| 5 | 35.65 | 4.3 | 0.848 | 180 | >315 | <1 | 152.68 | 1.9 |
|
| 6 | 1.34 | 114.6 | 8.8 | 17.5 | >315 | <1 | 153.9 | 0.2 |
|
| 7 | 0.231 | 180.2 | 0.048 | 868 | 11.35 | 3.7 | 41.7 | 32.7 |

|
| 8 | 23.85 | 5.1 | 0.419 | 289 | 173.45 | <1 | 121.34 | 3.7 |
|
| 9 | 35.56 | 3.5 | 0.556 | 224.5 | >238.66 | <1 | 124.8 | 2.8 |
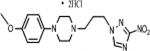
|
| 10 | 10.46 | 10.8 | 0.828 | 137 | 180.2 | <1 | 113.3 | 2.5 |
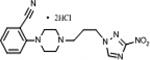
|
| 11 | 0.78 | 42.9 | 0.286 | 117 | 23.9 | 1.4 | 33.5 | 7.1 |

|
| 12 | 15.62 | 13 | 4.7 | 43.1 | >290 | <1 | 202.9 | 0.3 |
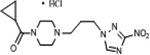
|
| 13 | 62.4 | 2.2 | 34.4 | 4 | 186.4 | <1 | 137.1 | 0.1 |

|
| 14 | 10.74 | 7.4 | 9.4 | 8.4 | 149.5 | <1 | 79.2 | 0.2 |

|
| 15 | 0.204 | 500 | 0.059 | 1725 | 4.49 | 22.7 | 101.9 | 26.6 |
|
| 16 | 0.954 | 170 | 0.566 | 287 | 20 | 8.1 | 162.5 | 3.6 |
|
| 17 | 0.355 | 125.6 | 0.142 | 314 | 4.1 | 10.9 | 44.54 | 14.4 |
|
| 18 | 7.61 | 6.5 | 3.23 | 15.3 | 4.27 | 11.6 | 49.5 | 0.6 |
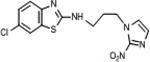
|
| 19 | 52.5 | 3.4 | 4.88 | 36.1 | 11.4 | 15.4 | 176 | 0.4 |
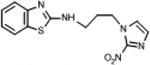
|
| 20 | 3.87 | 11.1 | 1.75 | 24.6 | 7.3 | 5.5 | 43 | 1.2 |
|
STIB 900 trypomastigotes;
Tulahuen C4 amastigotes;
MHOM-ET-67/L82 amastigotes; Cytotoxicity was measured in L6 cells. SI = IC50 in L6 cells / IC50 in each type of parasites. The reference compounds were: Melarsoprol (T. b. rhodesiense; IC50 = 0.011 0.004), Benznidazole (Bnz; T. cruzi; IC50 = 1.85 0.33) and Miltefosine (L. donovani; IC50 = 0.378 0.011). Bnz/comp.: The ratio of IC50 values of Benznidazole and each tested compound against T. cruzi. The IC50 values are the means of two independent assays and varies less than ± 50%. Green color indicates active compounds, light red, moderately active, light blue, toxic and no color, inactive. Compounds 1-3 have been described before [10].
Scheme 1.
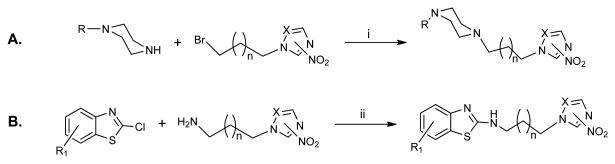
i) K2CO3 (9 eq), CH3CN, RT, 48 h; ii) 1-Propanol, reflux 16-24 h. R: varies (see Table 1); R1: H or 6-Cl; n: 1 or 2; X=C, 2-NO2; X=N, 3-NO2
2.2. Biological Evaluation
The in vitro growth inhibitory properties of compounds 1-14 against T. b. rhodesiense bloodstream form trypomastigotes, T. cruzi amastigotes (in infected L6 myoblasts), axenically cultured L. donovani amastigotes and rat skeletal myoblasts (L6 cells) are shown in Table 1. IC50 values in μM were determined from resultant dose response curves (Table 1). The following criteria were used to interpret activity: for T.b. rhodesiense, compounds with an IC50 < 0.5 μM, were designated as ‘active’, while those yielding an IC50 = 0.5-6.0 μM or an IC50 > 6.0 μM were designated ‘moderately active’ and ‘inactive’, respectively. For T. cruzi, IC50 < 4.0 μM, ‘active’; IC50 = 4.0-60 μM, ‘moderately active’; IC50 > 60 μM, ‘inactive’. For L. donovani, IC50 < 1 μM, ‘active’; IC50 = 1.0-6.0 μM, ‘moderately active’; IC50 > 6.0 μM, ‘inactive’. In addition, to evaluate toxicity, growth inhibition of the mammalian L6 host cells has to be taken into account. Thus, the selectivity index (SI), namely the ratio of IC50 against L6 cells to IC50 against each parasite should be ≥ 100 for T.b. rhodesiense,≥ 50 for T. cruzi and ≥ 20 for L. donovani axenic amastigotes. These criteria were established by the TDR's “compound screeners network”, published in a review [32] and take into consideration the complex life cycles of the parasites and the fact that T. cruzi and L. donovani are (in contrast to T.b. rhodesiense) intracellular parasites.
2.2.1. Analysis of the antitrypanosomal activity of all tested compounds
Based on the above, all but two (6, 12) 3-nitrotriazole-based piperazines and benzothiazoles (87%) were active against T. cruzi and fulfilled the corresponding selectivity criteria (Table 1). In addition, most of them demonstrated antichagasic activity at nanomolar concentrations and a SI > 200 (1-3, 7-9, 15-17). The p-pyrido-piperazine 6 and the cyclopropylcarbonyl-piperazine 12 were moderately active against T. cruzi but also toxic towards the L6 host cells (SI<50). All 2-nitroimidazole based analogs were moderately active against T. cruzi but toxic to the host cells (13, 14, 18-20). Similar behavior has been observed before with other chemical classes of 2-nitroimidazole-based compounds [10].
With regard to T. b. rhodesiense, three 3-nitrotriazole-based analogs were active and selective (7, 15 and 17) and two (6 and 16) were moderately active and selective. The p-trifluoromethylbenzyl-piperazine 2 was moderately active against T. b. rhodesiense and marginally selective (SI =99). In addition, the 3-nitrotriazole-based analogs 1, 3, 11 and the 2-nitroimidazole-based compound 20 were moderately active towards T. b. rhodesiense but toxic to the host cells (Table 1).
Only one compound, the 3-nitrotriazole-based 6-chlorobenzothiazole 15 was moderately active towards L. donovani. In fact, this compound was found to be active towards all tested parasites.
Comparing the antichagasic activity of the tested compounds with that of Bnz, we observed that all but three 3-nitrotriazole-based analogs (4, 6 and 12) (80%) were significantly more potent (2- to 39-fold) than Bnz (Table 1). All 2-nitroimidazole-based analogs were less potent than Bnz with the exception of 20 which was slightly more potent than Bnz (Table 1).
2.2.2. SAR analysis of the antichagasic and anti-HAT activity of nitro(triazole/imidazole)-based piperazines
A closer look at the SARs of nitrotriazole-based piperazines with regard to their antichagasic activity (Table 1) suggests that the most potent compounds were 1-phenyl piperazines, substituted in the para or meta position with electron withdrawing groups. Thus, p-chloro-(7) or p-trifluoromethyl-substituted 1-phenyl piperazines (3) were more active against T. cruzi compared to their p-methyl (8) or p-methoxy- (9) analogues. Indeed, the Hammett substituent constants of –CF3, p-Cl-, m-Cl, CH3- and MeO- groups are 0.54, 0.23, 0.37, -0.17 and -0.27, respectively [33]. Plotting the T. cruzi IC50 values of these compounds versus the constants, we find a good correlation (R2=0.985) between antichagasic activity and electronegativity (plot is not shown). Compound 10 with the electron withdrawing cyano group in the ortho position of the phenyl group demonstrated a significantly greater IC50 against T. cruzi compared to 3 and 7. It is well known that Hammett analysis cannot include ortho-substitution because such substitution introduces steric effects. Thus, it is speculated that since the cyano-group, besides its excellent inductive properties can also function as a hydrogen bond acceptor, it could cause some unfavorable interactions with type I Tc-NTR. Substituted 1-phenyl piperazines were significantly more potent (6-10 fold) against T. cruzi than their 1-benzyl analogs (compare 1 and 2 to 3, and 11 to 7). With regard to anti-HAT activity, both 1-phenyl- and 1-benzylpiperazines substituted with electron withdrawing groups in the para- position were similarly active against T. b. rhodesiense, however only the phenyl analog 7 fulfilled the selectivity criteria (Table 1).
Antichagasic activity was reduced significantly when the 1-phenyl group was replaced with a nitrogen(s) containing heteroaryl ring (4-6). The position of the nitrogen(s) in the ring had an effect on the antichagasic activity of 1-pyridino-/1-pyrimidino-piperazines. Thus, one N in the 2-position (5) resulted in an IC50 against T. cruzi of 848 nM, very similar to that of 1-(2-cyanophenyl)piperazine 10, whereas two N atoms in 2 and 6 positions in 4 decreased the activity by 2-fold compared to 5. When N was in the 4 position (6) the antichagasic activity was reduced by more than 10-fold compared to 5. Compound 6 was the only one among the heteroarylpiperazines tested to show moderate activity against T.b. rhodesiense (Table 1). It is not clear why several 3-nitrotriazole-based piperazines (e.g. heteroaryl-piperazines but not only) showed so great a difference between their antichagasic and anti-HAT activity. Differences in the active site of Tc- and Tb-nitroreductases could account for differences in activation, if we assume that NTRs are the only enzymes that activate this type of compounds in the parasites. These issues are going to be clarified in future studies, in collaboration with the Queen Mary University of London.
Antichagasic activity was significantly reduced when the 1-phenyl group was replaced with an 1-alkylcarbonyl-group (12).
Comparing the 2-nitroimidazole-based p-methoxyphenyl-piperazine 13 with its 3-nitrotriazole counterpart 9, we observed that the antichagasic and anti-HAT activity of 13 was reduced about 62- and 2-fold, respectively, compared to 9. Similarly, comparing the 2-nitroimidazole-based benzylpiperizine 14 with its 3-nitrotriazole-based counterpart 11, the antichagasic and anti-HAT activity of 14 was reduced about 33- and 14-fold, respectively, compared to 11.
2.2.3. SAR analysis of the antichagasic and anti-HAT activity of nitro(triazole/imidazole)-based benzothiazoles
Chloro-substitution in the 6 position of the benzothiazole ring increased the activity against all parasites of either nitrotriazole- or nitroimidazole-based analogs (compare 15 and 17 to 16; 18 and 20 to 19 in Table 1). Increasing the linker between the nitro-bearing ring and 2- aminobenzothiazole resulted in an increase in the antichagasic and anti-HAT activity of the 2- nitroimidazole-based analogs but a decrease in their 3-nitrotriazole-based counterparts (compare 15 to 17 and 18 to 20 in Table 1).
2.2.4. Correlation between the physical properties and antitrypanosomal activity of tested compounds
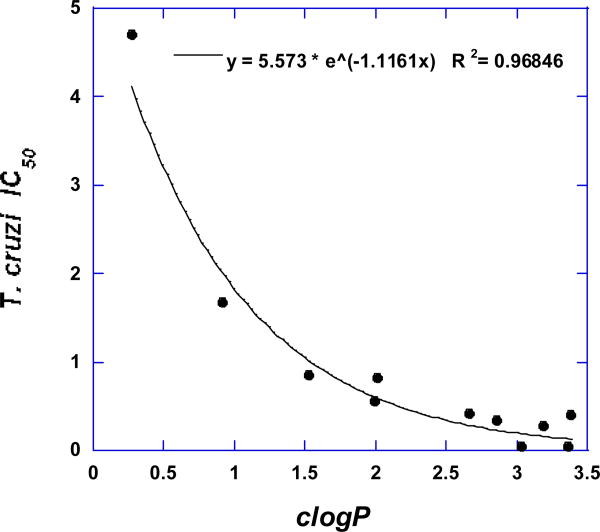
The calculated physical properties of all tested compounds are listed in Table 2. All compounds fulfill the Lipinski rule of 5 [34]. Plotting the T. cruzi IC50 values of all active nitrotriazole-based piperazines versus their clogP values, we observe that a good correlation exists (R2 = 0.97), following an exponential relationship (Fig. 1). Thus, lipophilicity seems to play an important role in the antichagasic activity of 3-nitrotriazole-based piperazines and clogP values from 2.5-3.5 show the best antichagasic activity. No such correlation exists however for the corresponding anti-HAT activity of these compounds.
Table 2.
Calculated physical properties of compounds listed in Table 1.
| ID No | clogP | logD6a | pKa | LR5b | PSAc Å2 | Drug like Prediction | ID No | clogP | logD6a | pKa | LR5b | PSAc A2 | Druglike Prediction |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.86 | 0.56 | 8.33 | Sd | 83.01 | 0.64 | 11 | 3.19 | 1.08 | 8.12 | S | 83.01 | 7.61 |
| 2 | 3.38 | 0.90 | 8.52 | S | 83.01 | -0.83 | 12 | 0.27 | -0.86 | 7.09 | S | 100.08 | 9.86 |
| 3 | 3.03 | 0.89 | 8 | S | 83.01 | -1.74 | 13 | 2.36 | 0.21 | 8.17 | S | 79.35 | 5.39 |
| 4 | 0.91 | -0.77 | 7.38 | S | 108.79 | 9.24 | 14 | 3.56 | 1.46 | 8.11 | S | 70.12 | 7.61 |
| 5 | 1.53 | -0.45 | 7.63 | S | 95.9 | 8.84 | 15 | 3.10 | 3.10 | 3.30 | S | 101.45 | 0.21 |
| 6 | 0.93 | -1.97 | 8.74 | S | 95.9 | 7.18 | 16 | 2.49 | 2.49 | 3.36 | S | 101.45 | -0.5 |
| 7 | 3.36 | 1.33 | 7.74 | S | 83.01 | 6.29 | 17 | 3.61 | 3.61 | 3.3 | S | 101.45 | -1.03 |
| 8 | 2.66 | 0.42 | 8.27 | S | 83.01 | 5.68 | 18 | 3.47 | 3.46 | 3.3 | S | 88.56 | 0.21 |
| 9 | 1.99 | -0.17 | 8.18 | S | 92.24 | 5.39 | 19 | 2.86 | 2.86 | 3.36 | S | 88.56 | -0.5 |
| 10 | 2.01 | 0.14 | 7.57 | S | 106.8 | 3.99 | 20 | 3.98 | 3.98 | 3.3 | S | 88.56 | -1.03 |
logP value at pH 6.
Lipinski rule of 5.
PSA: Polar surface area.
Satisfied. All physical properties were predicted by using the Marvin Calculator (www.chemaxon.com). Compounds 1-14 are piperazines, whereas compounds 15-20 are benzothiazoles. Drug-likeness prediction was calculated by using the Osiris Property Explorer (http://www.organic-chemistry.org/prog/peo/). Most traded drugs demonstrate drug-likeness values in the range of -2 to 5.
Fig. 1.
Correlation between lipophilicity and antichagasic activity in active 3-nitrotriazole-based piperazines.
Plotting the T. cruzi IC50 values of all active nitrotriazole-based piperazines versus their polar surface area (PSA) we observe that, although there is not a good linear correlation (R2 = 0.78), antichagasic activity is decreased with increasing PSA and compounds with the best T. cruzi IC50 values have a PSA value between 80 and 85 Å2 (Fig. 2).
Fig. 2.
Correlation between polar surface area (PSA) in Å2 and antichagasic activity in active 3- nitrotriazole-based piperazines.
Finally, in the same group of compounds, a correlation exists between pKa values and antichagasic activity (Fig. 3) following a second degree polynomial relationship (R2 = 0.93). 3-Nitrotriazole-based piperazines with a pKa around 8 demonstrate the best antichagasic activity. No correlation exists between pKa values and antichagasic activity for the 3-nitrotriazole-based 2-aminobenzothiazoles, perhaps because they demonstrate an acidic rather than a basic character.
Fig. 3.
Correlation between pKa values and antichagasic activity in active 3-nitrotriazole-based piperazines.
In general, with regard to nitro(triazole/imidazole)based benzothiazoles, both antichagasic and anti-HAT activity increased with increasing lipophilicity (Table 2), but this was more apparent with 2-nitroimidazole-based benzothiazoles.
Oral administration and good intestinal permeability are critical parameters for antichagasic drugs. It was recently shown that Partition coefficients (logD and logP) and molecular surface area (PSA) are potential predictors of the intestinal permeability of drugs [34]. According to this study, the logD value at pH 6 (logD6) more accurately predicts intestinal permeability compared to other parameters, and a logD6 > -0.42 (the logD6 value of labetalol) is associated with high permeability [35]. As can be seen in Table 2, most of the active compounds demonstrate logD6 values > -0.42 and thus they may demonstrate good intestinal permeability. However, ADME studies are necessary for reliable predictions with regard to absorption, bioavailability and metabolic stability of these compounds.
Using the Osiris Property Explorer software (http://www.organic-chemistry.org/prog/peo/) we calculated values that indicate the drug-likeness of the compounds 1-20 (Table 2). The drug-likeness value is based on a list of about 5300 distinct substructure fragments with associated druglikeness scores. The distribution of drug-likeness values calculated from 15000 Fluka compounds and from 3300 traded drugs showed that most traded drugs demonstrate drug-likeness values in the range of -2 to 5 whereas the big majority of Fluka chemicals accounts for negative values. Based on the drug-likeness values, all tested compounds contain predominantly fragments which are frequently present in commercial drugs. However, other parameters (e.g. lipophilicity, toxicity, bioavailability, etc.) will determine if a compound qualifies as an acceptable pharmaceutical. Our previous studies with several nitrotriazole-based sulfonamides and amides showed promising results with regard to in vivo efficacy, stability, bioavailability and lack of mutagenicity in the Ames test (in contrast to 2-nitroimidazole analogs) [29] and make us optimistic.
As mentioned earlier, all nitrotriazole-based compounds described in our previous work were very good substrates for the type I NTR [10, 11]. Indeed, the activity of recombinant, purified his-tagged T. b. brucei nitroreductase (TbNTR) using compounds 1, 2 and 3 as substrates, was 420 15, 364 13 and 788 54 nmol NADH oxidized min-1mg-1, respectively, compared to 74 18 and 218 4 nmol NADH oxidized min-1mg-1 for nifurtimox and benznidazole, respectively [10, 11]. Since the enzymatic activity of 1-3 correlates with their antichagasic activity, we anticipate that the rest of the compounds in Table 1 most likely are also substrates of type I NTR. Since the precise nature of the active site of TcNTR and TbNTR still remains unclear, modeling studies are prohibited.
In conclusion, several analogs of 3-nitrotriazole-based piperazines and benzothiazoles (Table 1) have been identified as potential candidates for in vivo studies in T. cruzi infected mice and further development as antichagasic drugs. All of them demonstrate significant activity against T. cruzi amastigotes at low to intermediate nmolar concentrations, have SI values of ≥200, satisfy the Lipinski's rule of 5 and demonstrate drug-like characteristics. In addition, compounds 7, 15 and 17 are good candidates for further evaluation in vivo as anti-HAT agents. However, initial ADMET studies are necessary for better lead optimization and selection for in vivo testing.
3. Experimental
3.1. Chemistry
All starting materials and solvents were purchased from Sigma-Aldrich (Milwaukee, WI), were of research-grade quality and used without further purification. Solvents used were anhydrous and the reactions were carried out under a nitrogen atmosphere and exclusion of moisture. Melting points were determined by using a Mel-Temp II Laboratory Devices apparatus (Holliston, MA) and are uncorrected. Elemental Analyses were obtained by Midwest Microlab, LLC (Indianapolis, IN). Proton NMR spectra were obtained on a Varian Inova-500 or a Bruker Avance-III-500 spectrometer at 500 MHz and are referenced to Me4Si or to the corresponding protonated solvent, if the solvent was not CDCl3. High-resolution electrospray ionization (HRESIMS) mass spectra were obtained on a Agilent 6210 LC-TOF mass spectrometer at 11000 resolution. Thin-layer chromatography was carried out on aluminum oxide N/UV254 or polygram silica gel G/UV254 coated plates (0.2 mm, Analtech, Newark, DE). Chromatography was carried out on preparative TLC alumina GF (1000 microns) or silica gel GF (1500 microns) plates (Analtech). All the amines were purified by preparative TLC chromatography on alumina plates (≥ 95% purity). The results from elemental analysis for C, H, Cl and N were within 0.4 of the theoretical value for piperazines 1-3[10]; from these data and the similar synthetic preparation of the novel piperazine derivatives, it was concluded that all (but piperazine 12) were the dihydrochloride salts.
3.1.1. General Synthetic Procedure of 3-Nitrotriazole/2-Nitroimidazole-based Piperazines
Piperazine derivatives 1-3 are known [10]. Piperazine derivatives 4-14 were synthesized from the commercially available appropriate monoalkylated piperazines (1.44 mmol) and 2-nitro-1H-imidazolyl-propylbromide or 3-nitro-1H-1,2,4-triazolyl-propylbromide (1.485 mmol) [30] in the presence of potassium carbonate (13.24 mmol) in dry acetonitrile (25 mL) as described before [10]. The reaction mixture was stirred under a nitrogen atmosphere at room temperature for 48 h, then filtered to remove the inorganic salts. The organic filtrate was evaporated and the residue extracted from water-chloroform. The organic layer was separated and dried over anhydrous Na2SO4. After filtration, the solvent was evaporated and the residue purified by preparative TLC on alumina plates with ethyl acetate: petroleum ether mixture. The desired product was dissolved in ethyl acetate and converted to its HCl salt by treating with HCl gas in dry ether (1 M solution).
3.1.1.1. 2-{4-[3-(3-nitro-1H-1,2,4-triazol-1-yl)propyl]piperazin-1-yl}pyrimidine dihydrochloride (4)
Light yellow powder (75%): mp > 210 °C (dec); 1H NMR (500 MHz, D2O) δ: 8.65 (s, 1H), 8.44 (dd, J=5.0, 2.5 Hz, 2H), 6.86 (m, 1H), 4.52 (t, J=6.5 Hz, 2H), 3.80-3.50 (br m, 10H), 2.48 (quintet, J=8.0 Hz, 2H). HRESIMS calcd for C13H19N8O2m/z [M+H]+ 319.1625, found 319.1627.
3.1.1.2. 1-[3-(3-nitro-1H-1,2,4-triazol-1-yl)propyl]-4-(pyridin-2-yl)piperazine dihydro-chloride (5)
The free amine formed yellow crystals and the HCl salt was a pale yellow powder (40%): mp 69-71 °C (free amine); 1H NMR of the 2HCl salt (500 MHz, D2O) δ: 8.66 (s, 1H), 8.12 (t, J=9.0 Hz, 1H), 8.01 (d, J=6.5 Hz, 1H), 7.36 (d, J=9.0 Hz, 1H), 7.14 (t, J=7.0 Hz, 1H) 4.54 (t, J=6.5 Hz, 2H), 4.00 (br s, 4H), 3.60 (br s, 4H), 3.39 (m, 2H), 2.49 (quintet, J=8.0 Hz, 2H). HRESIMS calcd for C14H20N7O2m/z [M+H]+ 318.1673, found 318.1675.
3.1.1.3. 1-[3-(3-nitro-1H-1,2,4-triazol-1-yl)propyl]-4-(pyridin-4-yl)piperazine (6)
White crystals (35%). mp 116-118 °C (dec, free amine); 1H NMR (500 MHz, CD3OD) δ: 8.63 (s, 1H), 8.16 (d, J=6.5 Hz, 2H), 6.91 (d, J=7.0 Hz, 2H), 4.42 (t, J=6.5 Hz, 2H), 3.41 (t, J=5.0 Hz, 4H), 2.63 (t, J=5.0 Hz, 4H), 2.47 (t, J=7.5 Hz, 2H), 2.18 (quintet, J=7.5 Hz, 2H). HRESIMS calcd for C14H20N7O2m/z [M+H]+ 318.1673, found 318.1676.
3.1.1.4. 1-(3,4- dichlorophenyl)-4-[3-(3-nitro-1H-1,2,4-triazol-1-yl)propyl]piperazine dihydrochloride (7)
Off white powder (50%): mp 204-206 °C (dec); 1H NMR (500 MHz, D2O) δ: 8.66 (s, 1H), 7.45 (d, J=9.0 Hz, 1H), 7.24 (d, J=2.5 Hz, 1H), 6.98 (dd, J=9.0, 3.0 Hz, 1H), 4.52 (t, J=6.5 Hz, 2H), 3.84 (br d, 2H), 3.71 (br d, 2H), 3.34 (m, 2H), 3.25 (br d, 2H), 3.15 (br d, 2H), 2.47 (quintet, J=8.0 Hz, 2H). HRESIMS calcd for C15H19Cl2N6O2 and C15H18Cl2N6NaO2m/z [M+H]+ and [M+Na]+, respectively 385.0941, 387.0916 and 407.0761, 409.0734 found 385.0945, 387.0917 and 407.0757, 409.0728, respectively.
3.1.1.5. 1-(4-methylphenyl)-4-[3-(3-nitro-1H-1,2,4-triazol-1-yl)propyl]piperazine dihydro chloride (8)
Light yellow powder (40%): mp 185-190 °C (dec); 1H NMR (500 MHz, D2O) δ: 8.65 (s, 1H), 7.24 (d, J=8.5 Hz, 2H), 7.05 (d, J=8.5 Hz, 2H), 4.52 (t, J=6.5 Hz, 2H), 3.70-3.30 (br s, 8H), 3.34 (m, 2H), 2.46 (m, 2H). HRESIMS calcd for C18H25N3O3m/z [M+H]+ 331.1890, found 331.1885.
3.1.1.6. 1-(4-methoxyphenyl)-4-[3-(3-nitro-1H-1,2,4-triazol-1-yl)propyl]piperazine dihydro-chloride (9)
White microcrystallic powder (45%): mp 210 °C (dec); 1H NMR (500 MHz, D2O) δ: 8.66 (s, 1H), 7.14 (d, J=9.0 Hz, 2H), 7.02 (d, J=9.0 Hz, 2H), 4.53 (t, J=6.5 Hz, 2H), 3.82 (s, 3H), 3.60-3.40 (br m, 8H), 3.36 (m, 2H), 2.48 (quintet, J=8.5 Hz, 2H). HRESIMS calcd for C16H23N6O3m/z [M+H]+ 347.1826, lound 347.1828.
3.1.1.7. 2-{4-[3-(3-nitro-1H-1,2,4-triazol-1-yl)propyl]piperazin-1-yl}benzonitrile dihydro-chloride (10)
Off white powder (55%): mp 168-170 °C (dec); 1H NMR (500 MHz, D2O) δ: 8.67 (s, 1H), 7.76 (dd, J=8.0, 1.5 Hz, 1H), 7.67 (t, J=7.5 Hz, 1H), 7.26 (m, 2H), 4.55 (t, J=6.5 Hz, 2H), 3.80-3.30 (br m, 8H), 3.38 (t, J=8.0 Hz, 2H), 2.49 (quintet, J=8.0 Hz, 2H). HRESIMS calcd for C16H20N7O2m/z [M+H]+ 342.1673, found 342.1680.
3.1.1.8. 1-[(3,4-dichlorophenyl)methyl]-4-[3-(3-nitro-1H-1,2,4-triazol-1-yl)propyl]piperazine dihydrochloride (11)
Fine white powder (75%): mp > 220 °C; H NMR (500 MHz, D2O) δ: 8.65 (s, 1H), 7.68 (d, J=2.0 Hz, 1H), 7.66 (d, J=8.0 Hz, 1H), 7.41 (dd, J=8.0, 2.0 Hz, 1H), 4.51 (t, J=7.0 Hz, 2H), 4.35 (s, 2H), 3.60-3.45 (br m, 8H), 3.32 (m, 2H), 2.42 (quintet, J=8.0 Hz, 2H). HRESIMS calcd for C16H21Cl2N6O2m/z [M+H]+ 399.1098, 401.1071, found 399.1112, 401.1086.
3.1.1.9. 1-cyclopropanecarbonyl-4-[3-(3-nitro-1H-1,2,4-triazol-1-yl)propyl]piperazine hydrochloride (12)
White powder (68%): mp 210 °C (dec); 1H NMR (500 MHz, D2O) δ: 8.66 (s, 1H), 4.52 (t, J=6.5 Hz, 2H), 3.80-3.00 (m, 10H), 2.47 (m, 2H), 1.97 (m, 1H), 0.92 (quintet, J=2.5 Hz, 2H), 0.89 (quintet, J=2.5 Hz, 2H). HRESIMS calcd for C13H21N6O3 and C13H20N6NaO3m/z [M+H]+ and [M+Na]+ 309.1670 and 331.1489, found 309.1664 and 331.1490.
3.1.1.10. 1-(4-methoxyphenyl)-4-[3-(2-nitro-1H-imidazol-1-yl)propyl]piperazine dihydro-chloride (13)
Off white powder (55%): mp 165-169 °C (dec); 1H NMR (500 MHz, D2O) δ: 7.54 (s, 1H), 7.27 (s, 1H), 7.18 (br s, 2H), 7.07 (br s, 2H), 4.62 (t, J=7.5 Hz, 2H), 3.86 (s, 3H), 3.62-3.38 (br m, 10H), 2.45 (quintet, J=8.5 Hz, 2H). HRESIMS calcd for C17H24N5O2 and C17H23N5NaO2m/z [M+H|+ and [M+Na]+ 346.1874 and 368.1693, found 346.1871 and 368.1690.
3.1.1.11. 1-[(3,4-dichlorophenyl)methyl]-4-[3-(2-nitro-1H-imidazol-1-yl)propyl]piperazine dihydrochloride (14)
White powder (75%): mp 185-188 °C (dec); 1H NMR (500 MHz, D2O) δ: 7.72 (d, J=2.0 Hz, 1H), 7.69 (d, J=8.0 Hz,1H), 7.50 (s, 1H), 7.44 (dd, J=8.5, 2.0 Hz, 1H), 7.23 (s, 1H), 4.59 (t, J=7.0 Hz, 2H), 4.47 (s, 2H), 3.65 (br s, 8H), 3.41 (m, 2H), 2.40 (quintet, J= 7.5 Hz, 2H). HRESIMS calcd for C17H22Cl2N5O2m/z [M+H]+ 398.1145, 400.1119, found 398.1142, 400.1115.
3.1.2. General Synthetic Procedure of 2-Amino-substituted 1,3-Benzothiazoles (15-20)
The appropriate commercially available 2-chloro-1,3-benzothiazole (1.24 mmol), was coupled with 2-nitro-1H-imidazolyl-alkylamine (1.24 mmol) [31] or 3-nitro-1H-1,2,4-triazolyl-alkylamine (1.24 mmol) [31] by refluxing in absolute 1-propanol (7-10 ml) for 16-24 h in the presence of 5 fold excess triethylamine. In most cases the desired product was precipitated upon cooling of the reaction mixture and then extracted from ethyl acetate-water. The organic layer was dried over Na2SO4 and upon evaporation gave the final product. Additional product was separated from the reaction mixture by preparative TLC on alumina by using ethyl acetate as eluent, occasionally containing 1% MeOH.
3.1.2.1. 6-chloro-N-[3-(3-nitro-1H-1,2,4-triazol-1-yl)propyl]-1,3-benzothiazol-2-amine (15)
Orange powder (63%): mp 193-195 °C 1H NMR (500 MHz, CD3COCD3) δ: 8.71 (s, 1H), 7.70 (d, J=2.5 Hz, 1H), 7.39 (d, J=8.5 Hz, 1H), 7.35 (br s, 1H), 7.25 (dd, J=8.5, 2.5 Hz, 1H), 4.57 (t, J=7.0 Hz, 2H), 3.62 (t, J=6.0 Hz, 2H), 2.39 (quintet, J=6.5 Hz, 2H). HRESIMS calcd for C12H12ClN6O2S m/z [M+H]+ 339.0425, 341.0398, found 339.0427, 341.0395.
3.1.2.2. N-[3-(3-nitro-1H-1,2,4-triazol-1-yl)propyl]-1,3-benzothiazol-2-amine (16)
Light orange powder (45%): mp 155-156 °C; 1H NMR (500 MHz, CD3COCD3) δ: 8.73 (s, 1H), 7.63 (d, J=7.5 Hz, 1H), 7.43 (d, J=8.0 Hz, 1H), 7.26 (t, J=8.5 Hz, 1H), 7.22 (br s, 1H), 7.05 (t, J=8.5 Hz, 1H), 4.58 (t, J=7.0 Hz, 2H), 3.62 (t, J=6.0 Hz, 2H), 2.39 (quintet, J=7.0 Hz, 2H). HRESIMS calcd for C12H13N6O2S m/z [M+H]+ 305.0815, found 305.0814.
3.1.2.3. 6-chloro-N-[4-(3-nitro-1H-1,2,4-triazol-1-yl)butyl]-1,3-benzothiazol-2-amine (17)
Bright yellow crystals (75%): mp 116-118 °C; 1H NMR (500 MHz, CD3COCD3) δ: 8.68 (s, 1H), 7.68 (d, J=2.5 Hz, 1H), 7.38 (d, J=8.5 Hz, 1H), 7.25 (br s, 1H), 7.25 (dd, J=8.5, 2.0 Hz, 1H), 4.50 (t, J=7.0 Hz, 2H), 3.56 (t, J=5.5 Hz, 2H), 2.10 (quintet, J=7.5 Hz, 2H), 1.78 (quintet, J=7.5 Hz, 2H). HRESIMS calcd for C13H14ClN6O2S m/z [M+H]+ 353.0582, 355.0555, found 353.0574, 355.0549.
3.1.2.4. 6-chloro-N-[3-(2-nitro-1H-imidazol-1-yl)propyl]-1,3-benzothiazol-2-amine (18)
Light yellow powder (60%): mp 147-149 °C; 1H NMR (500 MHz, CD3COCD3) δ: 7.69 (d, J=1.5 Hz, 1H), 7.64 (s, 1H), 7.40 (d, J=8.5 Hz, 1H), 7.33 (br s, 1H), 7.25 (dd, J=8.5, 2.0 Hz, 1H), 7.13 (s, 1H), 4.66 (t, J=7.5 Hz, 2H), 3.61 (m, 2H), 2.33 (quintet, J=7.0 Hz, 2H). HRESIMS calcd for C13H13ClN5O2S and C13H12ClN5NaO2S m/z [M+H]+ and [M+Na]+ 338.0473, 340.0446 and 360.0292, 362.0265, found 338.0470, 340.0444 and 360.0290, 362.0265.
3.1.2.5. N-[3-(2-nitro-1H-imidazol-1-yl)propyl]-1,3-benzothiazol-2-amine (19)
Bright yellow powder (35%): mp 124-126 °C; 1H NMR (500 MHz, CD3COCD3) δ: 7.66 (s, 1H), 7.63 (d, J=7.5 Hz, 1H), 7.43 (d, J=8.5 Hz, 1H), 7.25 (t, J=8.5 Hz, 1H), 7.18 (br s, 1H), 7.05 (t, J=7.5 Hz, 1H), 4.66 (t, J=7.0, 2H), 3.61 (m, 2H), 2.33 (quintet, J= 7.0 Hz, 2H). HRESIMS calcd for C13H14N5O2S and C13H13N5NaO2S m/z [M+H]+ and [M+Na]+ 304.0863 and 326.0682, found 304.0861 and 326.0683.
3.1.2.6. 6-chloro-N-[4-(2-nitro-1H-imidazol-1-yl)butyl]-1,3-benzothiazol-2-amine (20)
Light yellow powder (52%): mp 137-139 °C; 1H NMR (500 MHz, CD3COCD3) δ: 7.68 (d, J =2.0 Hz,1H), 7.57 (s, 1H), 7.38 (d, J=8.5 Hz, 1H), 7.25 (br s, 1H), 7.24 (dd, J=8.5, 2.5 Hz, 1H), 7.11 (s, 1H), 4.58 (t, J=7.0 Hz, 2H), 3.56 (m, 2H), 2.03 (quintet, J=7.5 Hz, 2H), 1.78 (quintet, J=7.5 Hz, 2H). HRESIMS calcd for C14H15ClN5O2S and C14H14ClN5NaO2S m/z [M+H]+ and [M+Na]+ 352.0629, 354.0603 and 374.0449, 376.0422, found 352.0621, 354.0594 and 374.0436, 376.0412.
3.2.In vitrobiological evaluation
In vitro activity against T. cruzi, Trypanosoma b. rhodesiense, Leishmania donovani axenic amastigotes and cytotoxicity assessment using L6 cells (rat skeletal myoblasts) was determined using a 96-well plate format as previously described [36]. Data were analyzed with the graphic program Softmax Pro (Molecular Devices, Sunnyvale, CA, USA), which calculated IC50 values by linear regression from the sigmoidal dose inhibition curves.
Acknowledgments
The authors thank Dr. Y. Wu for obtaining the NMR spectra of the compounds, and M. Cal and S. Sax (Swiss TPH) for parasite assay results. This work was supported in part by an NIH Challenge Grant: 1R01AI082542 – 01, Subaward No: RU374-063/4693578. For the work described in this paper, DNDi received financial support from the Bill & Melinda Gates Foundation (BMGF). The donors had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Abbreviations: T. cruzi, Trypanosoma cruzi; T. brucei, Trypanosoma brucei; HAT, human African trypanosomiasis; Nfx, nifurtimox (4-(5-nitrofurfurylindenamino)-3-methylthio-morpholine-1,1-dioxide); Bnz, benznidazole (N-benzyl-2-(2-nitro-1H-imidazol-1-yl)acetamide); NTR, type I nitroreductase; TbNTR, T. brucei NTR; IC50, concentration for 50% growth inhibition; SI, selectivity index; SARs, structure-activity relationships; TDR, Tropical diseases research (http//www.who.int/tdr/en/).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stuart K, Brun R, Croft S, Fairlamb A, Gürtler RE, McKerrow J, Reed S, Tarleton R. J Clin Invest. 2008;118:1301. doi: 10.1172/JCI33945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sánchez-Sancho F, Campillo NE, Páez JA. Curr Med Chem. 2010;17:423. doi: 10.2174/092986710790226101. [DOI] [PubMed] [Google Scholar]
- 3.Schmunis GA, Yadon ZE. Acta Trop. 2010;115:14. doi: 10.1016/j.actatropica.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Rassi A, Jr, Rassi A, Marin-Neto JA. Lancet. 2010;375(9723):1388. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 5.Leslie M. Science. 2011;333:934. doi: 10.1126/science.333.6045.934. [DOI] [PubMed] [Google Scholar]
- 6.Bern CN. Engl J Med. 2011;364:2527. doi: 10.1056/NEJMct1014204. [DOI] [PubMed] [Google Scholar]
- 7.Urbina JA. Acta Trop. 2010;115:55. doi: 10.1016/j.actatropica.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Urbina JA. Mem Inst Oswaldo Cruz. 2009;104(Suppl 1):311. doi: 10.1590/s0074-02762009000900041. [DOI] [PubMed] [Google Scholar]
- 9.Clayton J. Nature. 2010;465:S12. doi: 10.1038/nature09224. [DOI] [PubMed] [Google Scholar]
- 10.Papadopoulou MV, Bourdin Trunz B, Bloomer WD, McKenzie C, Wilkinson SR, Prasittichai C, Brun R, Kaiser M, Torreele E. J Med Chem. 2011;54(23):8214. doi: 10.1021/jm201215n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papadopoulou MV, Bloomer WD, Rosenzweig HS, Chatelain E, Kaiser M, Wilkinson SR, McKenzie C, Ioset JR. J Med Chem. 2012;55(11):5554. doi: 10.1021/jm300508n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilkinson SR, Taylor MC, Horn D, Kelly JM, Cheeseman I. PNAS. 2008;105(13):5022. doi: 10.1073/pnas.0711014105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alsford S, Eckert S, Baker N, Glover L, Sanchez-Flores A, Leung KF, Turner DJ, Field MC, Berriman M, Horn D. Nature. 2010;482:232. doi: 10.1038/nature10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker N, Alsford S, Horn D. Mol Biochem Parasitol. 2011;176:55. doi: 10.1016/j.molbiopara.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilkinson SR, Bot C, Kelly JM, Hall BS. Curr Top Med Chem. 2011;11:2072. doi: 10.2174/156802611796575894. [DOI] [PubMed] [Google Scholar]
- 16.Baliani A, Jimenez Bueno G, Stewart ML, Yardley V, Brun R, Barrett MP, Gilbert IH. J Med Chem. 2005;48:5570. doi: 10.1021/jm050177+. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez J, Aran VJ, Boiani L, Olea-Azar C, Lavaggi M, González M, Cerecetto H, Maya JD, Carrasco-Pozo C, Cosoy HS. Bioorg Med Chem. 2009;17:8186. doi: 10.1016/j.bmc.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 18.Boiani L, Gerpe A, Aran VJ, Torres de Ortiz S, Serna E, Vera de Bilbao N, Sanabria L, Yaluff G, Nakayama H, Rojas de Arias A, Maya JD, Morello JA, Cerecetto H, González M. Eur J Med Chem. 2009;44:1034. doi: 10.1016/j.ejmech.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 19.Hall BS, Wu X, Hu L, Wilkinson SR. Antimicrob Agents Chemother. 2010;54:1193. doi: 10.1128/AAC.01213-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bot C, Hall BS, Bashir N, Taylor MC, Helsby NA, Wilkinson SR. Antimicrob Agents Chemother. 2010;54(10):4246. doi: 10.1128/AAC.00800-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu L, Wu X, Han J, Lin Chen L, Vass SO, Browne P, Hall BS, Bot C, Gobalakrishnapillai V, Searle PF, Knox RJ, Wilkinson SR. Bioorg Med Chem Lett. 2011;21(13):3986. doi: 10.1016/j.bmcl.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Torreele E, Bourdin Trunz B, Tweats D, Kaiser M, Brun R, Mazué G, Bray MA, Pécoul B. PLoS Negl Trop Dis. 2010;4(12):e923. doi: 10.1371/journal.pntd.0000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaiser M, Bray MA, Cal M, Bourdin Trunz B, Torreele E, Brun R. Antimicrob Agents Chemother. 2011;55(12):5602. doi: 10.1128/AAC.00246-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bourdin Trunz B, Jędrysiak R, Tweats D, Brun R, Kaiser M, Suwiński J, Torreele E. Eur J Med Chem. 2011;46:1524. doi: 10.1016/j.ejmech.2011.01.071. [DOI] [PubMed] [Google Scholar]
- 25.Rosenzweig HS, Papadopoulou MV, Bloomer WD. Oncol Res. 2005;15:219. doi: 10.3727/096504005776382288. [DOI] [PubMed] [Google Scholar]
- 26.Bustamante JM, Evans A, Papadopoulou MV, Tarleton R. 12th Woods Hole Immunoparasitology Meeting; Woods Hole, MA. 2008. [Google Scholar]
- 27.Canavaci AMC, Bustamante JM, Padilla AM, Perez Brandan CM, Simpson LJ, Boehlke CL, Tarleton RL. PLos Negl Trop Dis. 2010;4(7):e740. doi: 10.1371/journal.pntd.0000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andriani G, Chessler ADC, Courtemanche G, Burleigh BA, Rodriguez A. PLoS Negl Trop Dis. 2011;5(8):e1298. doi: 10.1371/journal.pntd.0001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papadopoulou MV, Bloomer WD, Rosenzweig HS, Ashworth R, Wilkinson SR, Kaiser M, Andriani G, Rodriguez A. Future Med Chem. 2013 doi: 10.4155/fmc.13.108. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cowan DSM, Panicucci R, McClelland RA, Rauth AM. Radiat Res. 1991;127:81. [PubMed] [Google Scholar]
- 31.Papadopoulou MV, Bloomer WD. Drugs of the Future. 1993;18:231. [Google Scholar]
- 32.Nwaka S, Ramirez B, Brun R, Maes L, Douglas F, Ridley R. PLoS Negl Trop Dis. 2009;3(8):e440. doi: 10.1371/journal.pntd.0000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansch C, Leo A, Taft RW. Chem Rev. 1991;91:165. [Google Scholar]
- 34.Lipinski CA. Drug Discovery Today: Technologies. 2004;1(4):337. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Shawahna R, Rahman NU. DARU. 2011;19(2):83. [PMC free article] [PubMed] [Google Scholar]
- 36.Orhan I, Sener B, Kaiser M, Brun R, Tasdemir D. Mar Drugs. 2010;8:47. doi: 10.3390/md8010047. [DOI] [PMC free article] [PubMed] [Google Scholar]