Abstract
The synthesis and characterization of a new class of bioinspired carbohydrate amphiphiles that modulate Pseudomonas aeruginosa biofilm formation are reported. The carbohydrate head is an enantiopure poly-amido-saccharide (PAS) prepared by a controlled anionic polymerization of β-lactam monomers derived from either glucose or galactose. The supramolecular assemblies formed by PAS amphiphiles are investigated in solution using fluorescence assays and dynamic light scattering. Dried samples are investigated using X-ray, infrared spectroscopy, and transmission electron microscopy. Additionally, the amphiphiles are evaluated for their ability to modulate biofilm formation by the Gram-negative bacterium Pseudomonas aeruginosa. Remarkably, from a library of eight amphiphiles, we identify a structure that promotes biofilm formation and two structures that inhibit biofilm formation. Using biological assays and electron microscopy, we relate the chemical structure of the amphiphiles to the observed activity. Materials that modulate the formation of biofilms by bacteria are important both as research tools for microbiologists to study the process of biofilm formation and for their potential to provide new drug candidates for treating biofilm-associated infections.
Introduction
Amphiphiles containing a hydrophilic carbohydrate headgroup and a hydrophobic tail play a variety of essential biological roles.1 Studies examining the effect of oligo- and polysaccharide headgroup on assembly and bioactivity have highlighted the importance of carbohydrate-carbohydrate interactions in the assembled structures.2–10 As seen in natural and synthetic systems, self-assembled structures may possess unique or increased bioactivity in comparison to their individual, unassembled building blocks.11–13 Therefore, the ability to prepare a wide-range of carbohydrate amphiphiles that display variations in assembly is a promising route to dynamic, bioactive structures. However, glycolipids containing oligo- or polymeric headgroups can be challenging to prepare by traditional synthetic means14 despite recent advances in carbohydrate chemistry.15–17 Recognizing this need, we describe a synthetic strategy to prepare bioinspired poly-amido-saccharide (PAS)18, 19 amphiphiles that offer many potential structures that are readily accessible through monomer and initiator selection. Specifically, we report the synthesis of eight new polymeric amphiphiles (Fig. 1) that form supramolecular assemblies in aqueous solution. Additionally, we report the ability of PAS amphiphiles to modulate biofilm formation of Pseudomonas aeruginosa, a Gram-negative bacterium known to cause biofilm-associated infections.
Figure 1.
A. Schematic representations of PAS amphiphiles. B. Cartoon of biofilm modulation showing biofilm inhibition (left) and biofilm promotion (right).
Biofilms are surface-adhered bacterial communities surrounded by an extracellular matrix.20 In biofilms, pathogenic bacteria become more tolerant to antibiotic treatment and the host immune response.20 The ability to influence biofilm formation selectively, without affecting the bacterial growth rate, is important for studying the mechanisms that govern biofilm formation. Additionally, the ability to inhibit biofilm formation provides opportunities to develop new therapies for biofilm-associated illness. It has been shown that synthetic amphiphilic macromolecules21–27, including those inspired by antimicrobial peptides28–31, can exhibit potent and selective activity against a variety of microbes.32 Given that carbohydrate amphiphiles, such as lipopolysaccharide and rhamnolipids, play important roles in bacterial communities, we reasoned that P. aeruginosa would be an excellent model organism to explore the bioactivity of PAS amphiphiles.33
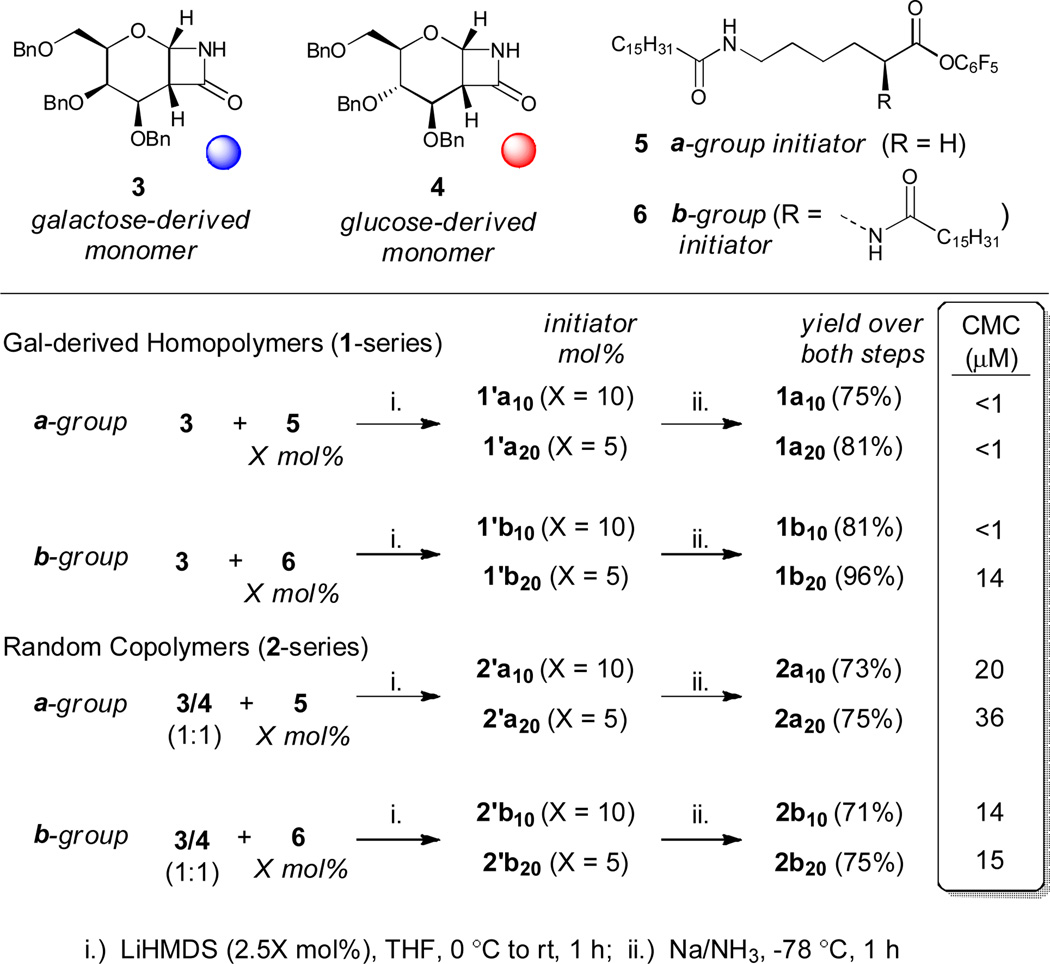
PASs are enantiopure synthetic polymers containing sugar-derived repeat units joined by an amide bond.18 Herein, we use a polymerization initiator that contains one or two palmitamide chains, derived from the saturated long-chain fatty acid palmitic acid, to synthesize PAS amphiphiles (Fig. 2) in a two-step process - polymerization followed by deprotection. Using this approach, a set of amphiphilic macromolecules are prepared that vary in three important ways: (1) the “a-group” of compounds has a single palmitamide chain joined to the PAS polymer by a 6-aminohexanoic acid linker, whereas the “b-group” has two palmitamide chains joined to the PAS polymer by an L-lysine linker; (2) the 1-series has a headgroup synthesized from only galactose(gal)-derived monomers, whereas the 2-series has a headgroup formed by the random copolymerization of a 1:1 mixture of gal- and glucose(glc)-derived polymers; and (3) two lengths of headgroup are prepared, one with a theoretical degree of polymerization (DPth) of 10 and one with a DPth of 20.
Figure 2.
PAS amphiphile synthesis.
Results and Discussion
PAS Amphiphile Synthesis and Characterization
Our synthetic approach to PAS amphiphiles relies on the controlled anionic ring-opening polymerization of a β-lactam monomer34, 35, specifically monomers 3 (gal-derived) and 4 (glc-derived) (Fig. 2), both of which are prepared via a one-step procedure.36 The polymerization is initiated by pentafluorophenol ester 5 or 6, which have one or two palmitamide chains, respectively. Amphiphiles with DPth’s of 10 and 20 were prepared by using either 10 or 5 mol% of initiator, respectively. After polymerization, the benzyl ether protecting groups were removed using sodium metal in liquid ammonia and the deprotected polymers were purified by dialysis. The yields, reported over both steps, ranged from 71–96%.
All the protected amphiphiles were characterized using 1H- and 13C-NMR. Due to the polymeric structure, the NMR spectra were broadened. The molecular weights of the protected amphiphiles were determined using gel permeation chromatography (GPC) (Table S1†). The Mn-values were in good agreement with the theoretical values and the measured dispersities (–) were between 1.1 and 1.2.
Following deprotection, the amphiphiles were characterized using 1H-NMR and IR. DPs were estimated using 1H-NMR integrations and were in good agreement with theoretical values (Table S2†). The molecular weights of amphiphiles, 1b20, 2a10, and 2a20, were confirmed using MALDI-tof and were found to be in good agreement with the theoretical mass of the sodium adduct and showed the expected spacings of 189 amu between species with different DPs (Fig. S1†). Because of their amphiphilic nature and propensity to assemble, the deprotected amphiphiles were not characterized by GPC. However, we have previously shown that deprotection has no adverse effect on PAS molecular weight.18, 19
Characterization of Supramolecular Assemblies
The critical micelle concentrations (CMC) of the amphiphiles were determined by monitoring pyrene fluorescence (Fig. 2, far right).37 However, amphiphiles 1a10, 1a20, and 1b10 formed aggregates that readily precipitated, making it difficult to accurately measure the CMC using this method. Noticeable precipitate could be observed by eye at concentrations as low as 1 µM, and therefore we report this as an upper bound. The other amphiphiles formed visibly clear, stable suspensions in water and phosphate buffered saline (PBS), and the values are reported for PBS. The general understanding of how amphiphile structure affects CMC is that increasing the hydrophilic headgroup size relative to the hydrophobic tail size raises the CMC, and vice versa. Based on the CMCs measured, the 1-series does not behave as a typical amphiphile. Hato et al. commented that certain oligosaccharide headgroups can engage in attractive carbohydrate-carbohydrate interactions that cause them not to behave as typical hydrophilic groups, as appears to be the case with the 1-series but not with the 2-series.3 The 2-series follows the trends generally observed for amphiphiles. The CMC of 2a20 (36 µM) is significantly higher than 2a10 (20 µM), hence for 2a increasing the hydrophilic portion of the amphiphile decreases its tendency to assemble in solution. The CMC values for 2b10 and 2b20 are similar, suggesting that increasing the PAS headgroup length has less effect when the endgroup is more hydrophobic. As would be expected for a typical amphiphile, increasing the number of fatty acid chains in the tail of the 2-series amphiphiles decreases the CMC, as evidenced by comparing the CMCs of the a-group (1 chain) to the b-group (2 chains).
The turbidity of the dispersions (1 mg/mL) formed by 1b20, 2a10, 2a20, 2b10, and 2b20 was monitored at room temperature and at 37 °C by recording the absorbance due to scattering at 330 nm (Fig. S2†). Only 1b20 showed additional assembly into larger structures, which are more effective at scattering light. Heating to 37 °C was required for this process to occur in a timespan of hours, as 1b20 did not show increased scattering within the first 30 hours at room temperature.
To investigate the size of the aggregates formed by the dispersible amphiphiles, the apparent hydrodynamic diameter (Dh) was determined by dynamic light scattering (DLS) in PBS buffer at an amphiphile concentration of 1.0 mg/mL. In general, the amphiphiles showed nanoscale assemblies that are relatively heterogeneous based on the measured polydispersities. Amphiphile 1b20 initially displayed small structures with a diameter of 34 nm. After incubation of the sample at 37°C for 16 hours the size of the structures increased 3-fold to 105 nm.
To examine the crystalline order of the assemblies, we performed wide-angle X-ray scattering (WAXS) on powder samples prepared by lyophilizing flash-frozen aqueous suspensions of the amphiphiles at concentrations above the CMC (Fig. 3). The amphiphiles that were the least dispersable (1a10, 1b10, and 1a20) produced the most structured patterns. Focusing on these amphiphiles, we can observe correlations between the molecular structure of the amphiphile and the crystalline order observed in the aggregates. The fact that 1a10 and 1b10 show similar diffraction patterns with consistent d-spacings suggests that the crystalline order observed resides in the gal-derived PAS headgroup, which is the same in both, rather than the hydrophobic tail, which differs significantly between the two species. The hydrophobic tails may have crystalline order, but as they comprise a relatively small part of the molecule it is difficult to discern from the WAXS patterns. Additionally, the peaks appear more intense in 1a10 compared to 1b10, suggesting that a tail with a single palmitamide promotes order relative to a tail with two fatty acid chains. Comparing 1a10 to 1a20 suggests that a shorter headgroup (DPth=10) promotes order relative to a longer headgroup (DPth=20). In contrast to 1a10, 1b10, and 1a20, the amphiphiles that formed stable suspensions gave WAXS patterns without defined peaks. The 2-series all gave similar, unstructured patterns, hence only a single representative profile (2a10) is shown in Fig. 3.
Figure 3.
A. WAXS of powder samples of amphiphiles. Sample 1b20* was incubated overnight at 37 °C in PBS (4 mg/mL), dialyzed, and then lyophilized. The red arrows point to the peaks suggesting increased crystalline order. B. IR spectra of select amphiphiles. The amide I (green), amide II (orange), and OH/NH (yellow) regions are highlighted.
To further understand the relationship between the 1-series and 2-series, we focus on the amphiphiles with DPth=10. The fact that 2a10 and 2b10 show no defined scattering peaks, whereas 1a10 and 1b10 show clear crystalline order, highlights the importance of carbohydrate stereochemistry on assembly. Amphiphiles 1a10 and 2a10 are structurally the same except that the stereochemistry at the 4-position (see Fig. 1, purple dashed oval) of the pyranose ring has been randomly varied in 2a10; the same relationship exists between 1b10 and 2b10. Therefore, we conclude that the PAS amphiphiles with headgroups composed only of gal-derived repeat units can pack into an ordered, partially crystalline assembly, and that this packing is prevented by the introduction of disorder in the form of a headgroup made of a random copolymer.
Amphiphile 1b20 appears to violate the trend of ordered packing in the PAS headgroup displayed by the 1-series, given that its WAXS pattern was more similar to that of the 2-series amphiphiles. However, the increased turbidity observed in dispersions of 1b20 upon heating could be caused by the amphiphiles rearranging into a more ordered assembly. Therefore, we lyophilized a dispersion of 1b20 after incubation in PBS at 37 °C for 16 hours followed by dialysis, and were able to observe subtle diffraction peaks (Fig. 3, red arrows) that correspond with the major peaks of 1a10, 1b10, and 1a20, indicating that the crystalline order increased with incubation. We suggest that 1b20 forms assemblies with ordered PAS headgroups more slowly than other 1-series members because it contains two fatty acid chains and has a DPth=20, two structural factors that appear to disfavor ordered headgroups in this system.
The powder samples used for WAXS were also investigated by IR, and 1a10 and 1b10 gave spectra with sharper bands in comparison to the other amphiphiles (Fig. 3B, Fig. S3† for all spectra). In contrast to WAXS, IR does not directly examine crystalline order, however the narrowing of bands among spectra of similar samples is understood to result from an increase in structural order. We observe that in the region of the OH and NH stretches (yellow), 1a10 and 1b10 show evidence of ordered H-bonding based on the structure of the band and a shift to lower energy. A comparison of the spectrum of 1b20 before and after incubation at 37 °C (1b20*) confirms an increase in order, as was observed with WAXS. The amide-I band (orange) occurs in the range of 1665–1675 cm−1 and the amide-II band (green) shifts to lower energies and splits into two bands in the more crystalline samples (see 1a10, 1b10, and 1b20*). For peptides, the amide-I and amide-II bands can be used to evaluate the amount and type of hydrogen bonding. However, we are hesistant to make such interpretations in the spectra of PASs because they contain a hemiamidal structure that may not obey the trends seen in peptides. Nonetheless, the IR spectra of 1a10 and 1b10 support the conclusion that the carbohydrate headgroups are ordered, and confirm that carbohydrate-carbohydrate interactions, such as hydrogen bonding, are playing an important role in aggregate formation.
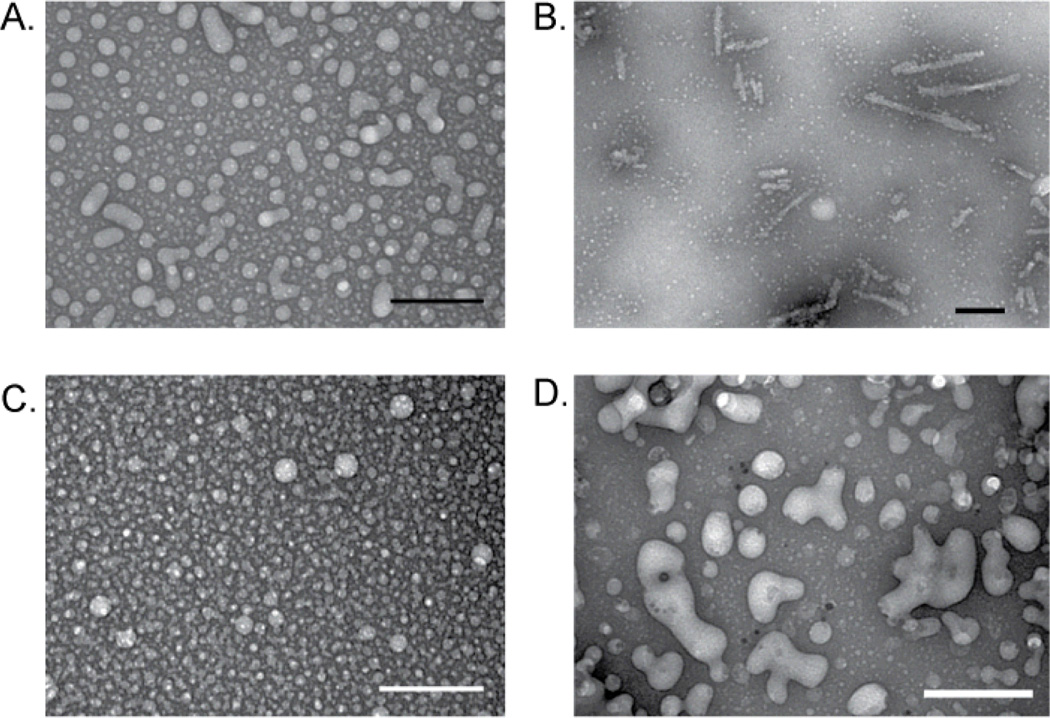
Finally, we used transmission electron microscopy (TEM) to visualize the assemblies formed by the PAS amphiphiles. TEM micrographs of 1b20, 2a10, and 2a20 are shown in Fig. 4 and additional micrographs of the other amphiphiles are available as supplementary information, Fig. S4†. Amphiphiles 1a20, 2a10 (Fig. 4C), 2a20 (Fig. 4D), and 2b20 appeared as globular micellar aggregates with diameters less than 100 nm. In contrast, the assemblies of 1b10 and 2b10 were elongated worm-like micelles. Interestingly, 1b20 transformed from a globular aggregate (Fig. 4A) to a worm-like aggregate (Fig. 4B) following incubation for 16 hrs at 37°C based on the TEM micrographs. Amphiphile 1a10, the most structured based on WAXS, appeared as particles with an unusual internal structure. The observation that 1b10 and 2b10 form worm-like micelles can be rationalized based on the larger b-group tail promoting less curvature. In the case of 1b20, we conclude that the transition from a globular to worm-like micellar state results from better packing of the headgroups following incubation at 37°C. As the headgroups pack more closely they are less hydrated and therefore require less space, thus promoting the transition to a structure with less curvature.
Figure 4.
TEM micrographs of 1b20 before (A) and after (B) incubation at 37°C for 16 hours showing the transition from globular micelles to worm-like micelles. Amphiphiles 2a10 (C) and 2a20 (D) appear as gobular micelles by TEM. All samples were negatively stained with 1% ammonium molybdate pH 7.0 and sample concentrations were 1.0 mg/mL for A, 4.0 mg/mL for B, 2.0 mg/mL for C, and 0.8 mg/mL for D. Scale bars = 100 nm.
Pseudomonas aeruginosa Biofilm Modulation
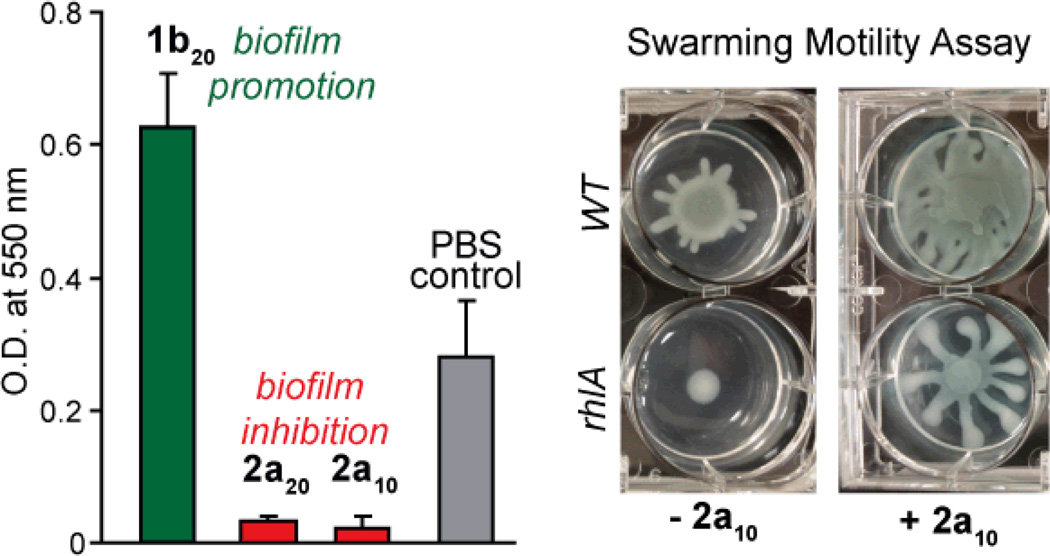
The amphiphiles that formed stable suspensions (1b20, 2a10, 2a20, 2b10, 2b20) were tested for their ability to influence biofilm formation by P. aeruginosa strain PA1438, a clinical isolate with high virulence. Biofilm formation was quantified using a previously described assay that measures crystal violet absorbance at 550 nm to assess surface-attached biomass.39 Amphiphiles 2a10 and 2a20 showed similar abilities to inhibit biofilm formation (Fig. 5). In contrast, 1b20 promoted biofilm formation, and it was found that to reliably observe this activity, the stock solution of 1b20 (4 mg/mL) had to be incubated at 37 °C overnight. Amphiphiles 2b10 and 2b20 showed little activity (not shown). The activity of 1b20, 2a10, and 2a20 was concentration dependent, with 1b20 showing biofilm promotion at and above 0.50 mg/mL and 2a10 and 2a20 showing biofilm inhibition at and above 0.25 mg/mL. Bacterial growth, measured in a separate assay, was not significantly affected by 1b20, 2a10, and 2a20 at their active concentrations (Fig. S5†). In addition, the corresponding PAS gal-derived homopolymer and 1:1 random copolymer of a similar molecular weight, but lacking the hydrophobic endgroup, showed no activity in either the biofilm or growth assays, confirming that the amphiphilic structure is essential to the observed bioactivity (not shown). Because the active amphiphiles show activity at concentrations significantly above their CMC and because the headgroups lacking a hydrophobic tail are inactive, we conclude that the supramolecular assemblies are important for the observed bioactivity.
Figure 5.
(bar graph) The relative amount of P. aeruginosa PA14 biofilm formation measured using crystal violet stain is shown for 0.5 mg/mL solutions of 1b20, 2a10, and 2a20 (incubation time 8 hours, n = 3, error bars show std. dev.). (right) A swarming assay showing the effect of 2a10 (0.5 mg/mL) on wild type (WT) and rhamnolipid-deficient mutant (rhlA) strains. Addition of 2a10 restored the swarming behavior in the rhlA strains, a result consistent with 2a10 acting as a surfactant to inhibit biofilm formation.
In exploring the mechanism by which 2a10 and 2a20 inhibit biofilm formation, we considered two possibilities. First, given our previous observation that rhamnolipid surfactants produced by P. aeruginosa can inhibit biofilm formation, we explored the possibility that 2a10 and 2a20 act as surfactants.33 It is understood that rhamnolipids disrupt biofilm formation by affecting cell-surface and cell-cell interactions33 by forming assemblies that lower the surface tension of water at interfaces.40 To evaluate the surfactant properties of the dispersible PAS amphiphiles reported here, we measured their effect on surface tension using a hanging drop assay (Fig. S6†). At a concentration of 1.0 mg/mL in PBS buffer, both 2a10 and 2a20 significantly lowered the surface tension in comparison to PBS buffer. In contrast, 1b20, 2b10, and 2b20 displayed no or little ability to decrease the surface tension. Many amphiphiles act as surfactants,41 however amphiphiles of the b-group are significantly less effective at lowering the surface tension of water relative to amphiphiles of the a-group based on our measurements. As 2b10 and 2b20 share the same headgroup as 2a10 and 2a20, we conclude that the additional palmitamide chain in the b-group tail is responsible for the lack of surfactant activity, and thus, their lack of activity at inhibiting biofilm formation. To further confirm that 2a10 and 2a20 are mimicking the surfactant activity of rhamnolipids, we tested whether they also promote bacterial swarming motility. Swarming motility is a process by which P. aeruginosa move across a semi-solid surface, and it requires that the bacterium produce its own rhamnolipid surfactant to lower the surface tension of water at the surface.42 Using a plate-based assay, 2a10 (Fig. 5) and 2a20 (not shown) rescued swarming motility in a rhamnolipid deficient mutant (rhlA). Thus, 2a10 and 2a20 mimic the surfactant activity of rhamnolipid under these conditions, further supporting our hypothesis that the mechanism of biofilm inhibition is related to their surfactant properties. A second possibility was that 2a10 and 2a20 reduced levels of c-di-GMP, an intracellular signaling molecule required for biofilm formation in P. aeruginosa.43 However, no difference in c-di-GMP levels in bacteria treated with the active amphiphiles (1b20, 2a10, and 2a20) compared to PBS-treated controls was observed (not shown).
The ability of 1b20 to promote biofilm formation was unexpected. Galactose was chosen for investigation because other galactose containing structures, such as dendrons, have shown the ability to inhibit biofilm formation in P. aeruginosa by interaction with a bacterial surface lectin.44, 45 However, we did not observe this activity in the amphiphiles or in the unassembled polymer headgroups lacking the hydrophobic tail. As we have recently reported, nonamphiphilic gal-derived PASs are not recognized by the gal-specific lectin on the surface of hepatocytes.19 We suspect that the difference in structure between the gal-derived PAS repeat unit and natural galactose derivatives is large enough to prevent interaction with gal-specific lectins. Additionally, if the activity we observe here resulted from an interaction between PAS gal-residues and bacterial receptors, we would expect to see some level of activity from 2b10 and 2b20, which have headgroups with half of their repeat units derived from galactose. Therefore, we conclude that a specific interaction between a gal-derived repeat unit and a bacterial surface lectin is likely not responible for the biofilm promotion activity.
As previously mentioned, the 1b20 suspension requires incubation overnight at 37°C in order to show consistent biofilm promotion. We have demonstrated that larger aggregates with more highly ordered headgroups are formed during incubation at 37°C using turbidity measurements, DLS, WAXS, IR, and TEM. Based on TEM, these aggregates are rod-like extended structures (Fig. 4B). It is known that many bacteria, including P. aeruginosa, utilize fibrillar adherence proteins in the process of biofilm formation.46 Recently, B. subtilis, a Gram positive bacterium, was shown to use endogenously produced amyloid fibers for cell adherence during biofilm formation.47 Based on these observations, we suggest that the ordered aggregates of 1b20 may be promoting biofilm formation by an analogous process. Currently, we are working to better understand the specific mechanism of biofilm promotion by 1b20 using a genetic approach that employs mutant screening to elucidate the molecular pathways that are affected.
Conclusions
In summary, we report that PAS amphiphiles containing a headgroup with gal- or gal/glc-derived repeat units form assembled structures in aqueous solution. The assemblies prepared from the 1-series of polymeric amphiphiles result from hydrophobic interactions between tail groups and attractive interactions between the headgroups, such as hydrogen bonding. In contrast, the random copolymer headgroups of the 2-series are more hydrated and have more repulsive, and less attractive, interactions. From the WAXS patterns and IR, we gain additional insights about the relationship between the structure and the crystalline order present in the PAS headgroup, specifically: (1) for the 1-series, the a-group (one palmitamide chain) promotes more ordered structures than the b-group (two palmitamide chains); (2) for the 1-series, the amphiphiles with DPth=10 form more ordered structures than those with DPth=20; (3) the 2-series amphiphiles do not form assemblies with crystalline order in the headgroup because of the disorder introduced by the PAS random copolymer. Amphiphiles 2a10 and 2a20 inhibited biofilm formation of P. aeruginosa and rescued swarming in a mutant strain of P. aeruginosa unable to produce rhamnolipid biosurfactants, suggesting they are acting via a similar mechanism to rhamnolipids by lowering the surface tension of water.33, 42 In contrast, 1b20 promoted biofilm formation by a mechanism that is related to its ability to form rod-like aggregates and which is under further investigation. The novelty of the structures presented here and their practical synthesis suggest that PAS amphiphiles are a promising new class of bioinspired synthetic glycolipids. Future work is underway to investigate the effect of varying the structure of both the PAS headgroup and the hydrophobic tail to further understand how structure affects assembly and the ability of the assemblies to modulate biofilm formation.
Supplementary Material
Table 1.
Sizing of Assemblies by Dynamic Light Scattering
| PAS Amphiphilea | Dh (nm)b | polydispersity |
|---|---|---|
| 1b20 | 34 | 0.29 |
| 1b20* (16 h at 37°C) | 105 | 0.25 |
| 2a10 | 209 | 0.35 |
| 2a20 | 111 | 0.39 |
| 2b10 | 40 | 0.3 |
| 2b20 | 80 | 0.35 |
Concentration 1.0 mg/mL in PBS buffer, measurements performed at 25°C.
Apparent hydrodynamic diameter.
Acknowledgements
We thank Dr. J. Sparks for collecting WAXS patterns and L. Filkins for assistance with the TEM studies. E.L.D. acknowledges receipt of NIH fellowship 1F32GM097781 and G.A.O NIH grant R01AI083256.
Footnotes
Electronic Supplementary Information (ESI) available: Experimental procedures, supplementary tables, figures, and NMR spectra. See DOI: 10.1039/b000000x/
references
- 1.Holst O. In: Glycoscience. Fraser-Reid B, Tatsuta K, Thiem J, editors. ch. 39. Berlin Heidelberg: Springer; 2008. pp. 1603–1627. [Google Scholar]
- 2.Takeoka S, Sou K, Boettcher C, Fuhrhop J-H, Tsuchida E. J. Chem. Soc., Faraday Trans. 1998;94:2151–2158. [Google Scholar]
- 3.Hato M, Minamikawa H, Tamada K, Baba T, Tanabe Y. Ad. Colloid Interface Sci. 1999;80:233–270. doi: 10.1016/s0001-8686(98)00085-2. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka M, Schneider MF, Brezesinski G. Chem Phys Chem. 2003;4:1316–1322. doi: 10.1002/cphc.200300791. [DOI] [PubMed] [Google Scholar]
- 5.Tirelli N. Macromol. Biosci. 2006;6:575–578. doi: 10.1002/mabi.200600146. [DOI] [PubMed] [Google Scholar]
- 6.Houga C, Giermanska J, Lecommandoux S, Borsali R, Taton D, Gnanou Y, Le Meins J-F. Biomacromolecules. 2008;10:32–40. doi: 10.1021/bm800778n. [DOI] [PubMed] [Google Scholar]
- 7.Birchall LS, Roy S, Jayawarna V, Hughes M, Irvine E, Okorogheye GT, Saudi N, De Santis E, Tuttle T, Edwards AA, Ulijn RV. Chem. Sci. 2011;2:1349–1355. [Google Scholar]
- 8.Upadhyay KK, Le Meins J-F, Misra A, Voisin P, Bouchaud V, Ibarboure E, Schatz C, Lecommandoux S. Biomacromolecules. 2009;10:2802–2808. doi: 10.1021/bm9006419. [DOI] [PubMed] [Google Scholar]
- 9.de Medeiros Modolon S, Otsuka I, Fort S, Minatti E, Borsali R, Halila S. Biomacromolecules. 2012;13:1129–1135. doi: 10.1021/bm3000138. [DOI] [PubMed] [Google Scholar]
- 10.Smith DK. In: Supramolecular Chemistry: From Molecules to Nanomaterials. Gale PA, Steed JW, editors. vol. 7. New York: John Wiley & Sons, Ltd; 2012. pp. 3169–3182. [Google Scholar]
- 11.Barnard A, Smith DK. Angew. Chem. Int. Ed. 2012;51:6572–6581. doi: 10.1002/anie.201200076. [DOI] [PubMed] [Google Scholar]
- 12.Levine PM, Carberry TP, Holub JM, Kirshenbaum K. Med. Chem. Comm. 2013;4:493–509. [Google Scholar]
- 13.Petkau-Milroy K, Brunsveld L. Org. Biomol. Chem. 2013;11:219–232. doi: 10.1039/c2ob26790j. [DOI] [PubMed] [Google Scholar]
- 14.Hato M, Minamikawa H. Langmuir. 1996;12:1658–1665. [Google Scholar]
- 15.Hsu C-H, Hung S-C, Wu C-Y, Wong C-H. Angew. Chem. Int. Ed. 2011;50:11872–11923. doi: 10.1002/anie.201100125. [DOI] [PubMed] [Google Scholar]
- 16.Krock L, Esposito D, Castagner B, Wang C-C, Bindschadler P, Seeberger PH. Chem. Sci. 2012;3:1617–1622. [Google Scholar]
- 17.Wang L-X, Davis BG. Chem. Sci. 2013;4:3381–3394. doi: 10.1039/C3SC50877C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dane EL, Grinstaff MW. J. Am. Chem. Soc. 2012;134:16255–16264. doi: 10.1021/ja305900r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dane EL, Chin SL, Grinstaff MW. ACS Macro Lett. 2013:887–890. doi: 10.1021/mz400394r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costerton JW, Stewart PS, Greenberg EP. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 21.Liu D, Choi S, Chen B, Doerksen RJ, Clements DJ, Winkler JD, Klein ML, DeGrado WF. Angew. Chem., Int. Ed. 2004;43:1158–1162. doi: 10.1002/anie.200352791. [DOI] [PubMed] [Google Scholar]
- 22.Chu-Kung AF, Bozzelli KN, Lockwood NA, Haseman JR, Mayo KH, Tirrell MV. Bioconjugate Chem. 2004;15:530–535. doi: 10.1021/bc0341573. [DOI] [PubMed] [Google Scholar]
- 23.Meyers SR, Juhn FS, Griset AP, Luman NR, Grinstaff MW. J. Am. Chem. Soc. 2008;130:14444–14445. doi: 10.1021/ja806912a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oda Y, Kanaoka S, Sato T, Aoshima S, Kuroda K. Biomacromolecules. 2011;12:3581–3591. doi: 10.1021/bm200780r. [DOI] [PubMed] [Google Scholar]
- 25.Papadopoulos A, Shiao TC, Roy R. Mol. Pharm. 2011;9:394–403. doi: 10.1021/mp200490b. [DOI] [PubMed] [Google Scholar]
- 26.Xue X, Pasparakis G, Halliday N, Winzer K, Howdle SM, Cramphorn CJ, Cameron NR, Gardner PM, Davis BG, Fernández-Trillo F, Alexander C. Angew. Chem. Int. Ed. 2011;50:9852–9856. doi: 10.1002/anie.201103130. [DOI] [PubMed] [Google Scholar]
- 27.Fukushima K, Tan JPK, Korevaar PA, Yang YY, Pitera J, Nelson A, Maune H, Coady DJ, Frommer JE, Engler AC, Huang Y, Xu K, Ji Z, Qiao Y, Fan W, Li L, Wiradharma N, Meijer EW, Hedrick JL. ACS Nano. 2012;6:9191–9199. doi: 10.1021/nn3035217. [DOI] [PubMed] [Google Scholar]
- 28.Mowery BP, Lindner AH, Weisblum B, Stahl SS, Gellman SH. J. Am. Chem. Soc. 2009;131:9735–9745. doi: 10.1021/ja901613g. [DOI] [PubMed] [Google Scholar]
- 29.Lienkamp K, Tew GN. Chem. Eur. J. 2009;15:11784–11800. doi: 10.1002/chem.200900049. [DOI] [PubMed] [Google Scholar]
- 30.Lienkamp K, Madkour AE, Tew GN. Adv. Polym. Sci. 2011:1–32. [Google Scholar]
- 31.Wu H, Niu Y, Padhee S, Wang RE, Li Y, Qiao Q, Bai G, Cao C, Cai J. Chem. Sci. 2012;3:2570–2575. [Google Scholar]
- 32.Mintzer MA, Dane EL, O’Toole GA, Grinstaff MW. Mol. Pharm. 2011;9:342–354. doi: 10.1021/mp2005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davey ME, Caiazza NC, O'Toole GA. J. of Bacteriol. 2003;185:1027–1036. doi: 10.1128/JB.185.3.1027-1036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Kissounko DA, Lee SE, Gellman SH, Stahl SS. J. Am. Chem. Soc. 2009;131:1589–1597. doi: 10.1021/ja8069192. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Gellman SH, Stahl SS. Macromolecules. 2010;43:5618–5626. [Google Scholar]
- 36.Kałuża Z, Abramski W, Bełżecki C, Grodner J, Mostowicz D, Urbański R, Chmielewski M. Synlett. 1994:539–541. [Google Scholar]
- 37.Astafieva I, Zhong XF, Eisenberg A. Macromolecules. 1993;26:7339–7352. [Google Scholar]
- 38.Rahme L, Stevens E, Wolfort S, Shao J, Tompkins R, Ausubel F. Science. 1995;268:1899–1902. doi: 10.1126/science.7604262. [DOI] [PubMed] [Google Scholar]
- 39.O'Toole GA, Kolter R. Mol. Microbio. 1998;28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 40.Sánchez M, Aranda FJ, Espuny MJ, Marqués A, Teruel JA, Manresa Á, Ortiz A. J. Colloid Interface Sci. 2007;307:246–253. doi: 10.1016/j.jcis.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 41.Ramanathan M, Shrestha LK, Mori T, Ji Q, Hill JP, Ariga K. Phys. Chem. Chem. Phys. 2013;15:10580–10611. doi: 10.1039/c3cp50620g. [DOI] [PubMed] [Google Scholar]
- 42.Déziel E, Lépine F, Milot S, Villemur R. Microbiology. 2003;149:2005–2013. doi: 10.1099/mic.0.26154-0. [DOI] [PubMed] [Google Scholar]
- 43.Römling U, Galperin MY, Gomelsky M. Microbiol. Mol. Biol. Rev. 2013;77:1–52. doi: 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kadam RU, Bergmann M, Hurley M, Garg D, Cacciarini M, Swiderska MA, Nativi C, Sattler M, Smyth AR, Williams P, Cámara M, Stocker A, Darbre T, Reymond J-L. Angew. Chem. Int. Ed. 2011;50:10631–10635. doi: 10.1002/anie.201104342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reymond J-L, Bergmann M, Darbre T. Chem. Soc. Rev. 2013;42:4814–4822. doi: 10.1039/c3cs35504g. [DOI] [PubMed] [Google Scholar]
- 46.Tomich M, Planet PJ, Figurski DH. Nat. Rev. Micro. 2007;5:363–375. doi: 10.1038/nrmicro1636. [DOI] [PubMed] [Google Scholar]
- 47.Romero D, Aguilar C, Losick R, Kolter R. Proc. Natl. Acad. Sci. USA. 2010;107:2230–2234. doi: 10.1073/pnas.0910560107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.