Abstract
One aim of stem cell-based therapy is to utilize pluripotent stem cells (PSCs) as a supplementary source of cells to repair or replace tissues or organs that have ceased to function due to severe tissue damage. However, PSC-based therapy requires extensive research to ascertain if PSC derivatives are functional without the risk of tumorigenicity, and also do not engender severe immune rejection that threatens graft survival and function. Recently, the suitability of induced pluripotent stem cells applied for patient-tailored cell therapy has been questioned since the discovery of several genetic and epigenetic aberrations during the reprogramming process. Hence, it is crucial to understand the effect of these abnormalities on the immunogenicity and survival of PSC grafts. As induced PSC-based therapy represents a hallmark for the potential solution to prevent and arrest immune rejection, this review also summarizes several up-to-date key findings in the field.
Keywords: Pluripotent stem cell, differentiation, immunogenicity, genetic, epigenetic, MHC I, MHC II, NK cell, T cell.
INTRODUCTION
PSCs are capable of differentiating into almost all types of cells and hold great promise as an alternative source for cell-based therapy. Before large scale expansion of clinical compatible PSCs, direct differentiation into a given cell type, and structural and functional integration into transplanted organs can be clinically applied, concerns over immune rejection and potential tumorigenesis will need to be addressed.
IMMUNOGENICITY AND VARIABILITY OF PLURIPOTENT STEM CELLS
The assumption that patient-derived or syngeneic induced PSCs (iPSCs) will be immune-tolerated has since been disputed by studies completed by Zhao et al. [1]. This study demonstrated that both retroviral and episomal-derived iPSCs showed immune rejection after transplantation into C57BL/6 mice, compared to embryonic stem cells (ESCs). Expression analysis revealed that regressing teratomas commonly overexpressed two genes that contribute to an increase in immunogenicity, Hormad1 and Zg16. Evidently, more recent studies have been conducted that contradict these initial findings. Concerns over the method of immunogenicity assessment, number of clones tested, and reprogramming efficacy have led to corrective measures and a more critical evaluation of the immunogenicity of iPSC-derived tissues. A subsequent study assessed immunogenicities of ESCs and iPSCs using three assays - teratoma formation, skin transplantation and bone marrow transplantation - on five ESC and seven iPSC lines to obtain more conclusive results [2]. Reports revealed that differentiated cells developed from iPSCs elicit limited immune responses, with no physiologic correlation between basal, endogenous expression of Hormad1 and Zg16 and syngeneic graft survival [2, 3].
Nevertheless, further investigation into the immunogenicity of iPSC-derived tissue will be needed before use in a clinical setting. For example, variation among iPSC clones due to partial reprogramming or differential developmental stages can trigger an immune response during transplantation [2]. One study revealed that the human immune system possesses a natural ability to detect pluripotency antigen Oct4 through memory T cells [4]. It seems that residual undifferentiated cells would need to be eliminated before transplantation to avoid an immune response to Oct4 as well as teratoma formation. In addition, there are still concerns over the influence of genetic background on the reprogramming process, as well as the introduction of genetic instability during this process. Reports have demonstrated that iPSC lines generated from the same individual show expression signatures more similar to one another than to those from different individuals [5], and that certain mouse strains were more efficient at generating iPSCs than others [6]. Furthermore, reprogramming methods that do not involve genomic integration have been shown to be less prone to immune attacks and have a lower teratoma-forming propensity after transplantation [1, 7]. Nonetheless, single nucleotide polymorphism and whole genome copy number variation analyses have revealed a higher frequency of genomic variations that arise after reprogramming, during the prolonged iPSC maintenance, and as a result of differentiation [8, 9].Therefore, establishing standardized methods of reprogramming that elicit a minimal immune response would be beneficial before applications in a clinical setting.
As cell replacement therapy would involve transplantation of differentiated iPSCs into patients, another concern is increased immunogenicity involved with the differentiation process. Work with ESCs has shown variability in MHC expression and increased immunogenicity after differentiation [10, 11]. As a precaution, immunosuppressive drug regimens can be used to manipulate the recipients’ immune system to accommodate transplantation of iPSC-derived tissue. However, there are several pitfalls to this, such as an increased risk for opportunistic infections, drug toxicities, and potential inhibition of graft maturation and function [12-14]. If in vitro modifications to iPSCs can be avoided, chance of host rejection will be reduced. Therefore, quality controls to avoid changes in antigen presentation and in genetic alterations during differentiation of iPSCs in combination with immunosuppressive measures will be instrumental in promoting graft acceptance.
UNDIFFERENTIATED PSCS EXPRESS LOW LEVELS OF MAJOR HISTOCOMPATIBILITY COMPLEX ANTIGENS AND CO-STIMULATORY MOLECULES
Major histocompatibility complex (MHC) molecules in mouse or human leukocyte antigens (HLAs) in human have been identified as one of the major impediments in the development of transplantation. High polymorphism of MHC molecules attributes pertinently to the immunological barrier between organ donors and recipients, and incompatibility of MHCs leads to acute graft rejection [15, 16].
Although the immunogenicity of PSCs and their derivatives remains elusive, it has been shown that undifferentiated but not differentiated PSCs possess immune privilege properties. Early studies have demonstrated that human ESCs (hESCs) have low expression of MHC class I, and complete absence of MHC class II antigens and co-stimulatory molecules (CD80 and CD86) [17-19]. Yet, when MHC molecules are up-regulated during ESC differentiation and/or during in vitro interferon-gamma (IFNγ) stimulation, immune rejection is accelerated [17, 18]. Mouse ESC-derived insulin producing cell clusters were shown to have higher MHC expression, compared to undifferentiated ESCs of origin. In addition to differentiation, increased immunogenicity of undifferentiated ESCs after IFNγ treatment was reported by several studies, all of which concurred to similar results that no teratomas or only quickly regressing teratomas were formed [19-21] (Fig. 1). These findings suggest the possibility of PSC-derived graft failure, if transplanted into an unfavorable environment that promotes the upregulation of MHC molecules [22].
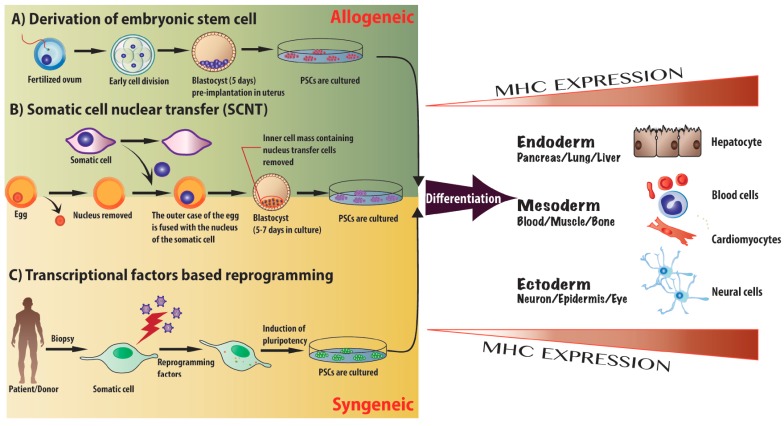
Fig. (1).
Different approaches for the induction of pluripotency. A) Allogeneic PSCs can be derived by isolating the inner cell mass of a blastocyst 4-6 days post-fertilization and pre-implantation (ESCs). B) The SCNT method: transfer of the nucleus from patient somatic cells into enucleated donor oocytes, the inner cell mass containing nucleus transfer cells is removed from the blastocyst after 5 to 7 days, and subsequently cultured to generate patient specific PSCs. C) Four transcriptional factors, Sox2, Oct3/4, c-Myc and Klf4, are transduced into patient’s somatic cells via viral vectors. Induced pluripotent stem cells (iPSCs, syngeneic patient specific PSCs) can be generated by repetitive passaging and subculture. Once the PSC cell line has been established from different sources, the cells have the capacity of self-renewal and unlimited division in a theoretical setting. PSCs can be directed to preferentially differentiate into three different germ layers – endoderm, mesoderm and ectoderm – and potentially applied to clinical therapies. Coinciding with processes of differentiation, the major histocompatibility complex (MHC) expression is robustly up-regulated, and therefore potentiates the immune rejection response post-transplantation.
Successful generation of iPSCs seems to herald the possibility for development of “customized” patient-specific cells and tissues to circumvent both ethic concerns and MHC barriers. Ideally, autologous iPSCs will be a perfect immunological match due to their lack of alloantigens to trigger graft rejection. Similar to ESCs, Suárez-Álvarez and colleagues have confirmed the low expression of MHC class I and β2m, as well as the lack of MHC class II in iPSCs. During the process of differentiation, an upsurge of MHC expression was observed, particularly with a drastic boost in MHC class I and β2m, after formation of embryoid body (EB) structures (a common procedure used in PSC differentiation) [23]. Overall, these studies suggest that MHC expression on PSCs is inducible and dynamically regulated, consequently implicating the immunogenic potential of PSCs and their progeny after tissue specification and cytokine treatment.
EPIGENETIC REGULATION CONTRIBUTES TO INCREASED IMMUNE RECOGNITION OF DIFFERENTIATED PSCS
Extending the notion of increased MHC expression during differentiation of iPSCs and ESCs demands for an in-depth investigation of its regulation mechanisms. Suárez-Álvarez et al. proposed an epigenetic mechanism to regulate MHC expression during differentiation. In undifferentiated hESCs and iPSCs, as a major epigenetic repression mechanism in gene expression, MHC class II and CIITA genes (the essential transcriptional factor for MHC class II expression) were found to be hypermethylated. Bisulfide sequencing and analysis of CpG methylation profiles all concluded that the absence of MHC class II expression in hESCs and iPSCs was controlled via heavy DNA methylation of these genes. Importantly, this hypermethylation status of MHC class II and its associated genes was barely changed after differentiation. This finding is in agreement with the previous observation that MHC class II was neither expressed nor up-regulated during differentiation or after IFNγ stimulation [23]. Considering the restricted expression pattern of MHC class II in nature, if PSC differentiation does not enhance MHC class II expression, then PSC-derived grafts are unlikely to carry professional antigen presenting cells (APCs) that could directly comprise engraftment via acute rejection.
In contrast to MHC class II genes, components of MHC class I antigen processing machinery genes showed demethylation, which corresponds to enhanced expression in differentiated iPSCs; indicative of an increased susceptibility to rejection. Although MHC molecules act critically in antigen presentation, the complexity of this process requires signals other than MHC antigens. Early efforts have examined the expression of costimulatory molecules (CD40, CD80 and CD86) on PSCs. Similar to MHC class II expression, both differentiation and IFNγ treatment did not increase the expression of costimulatory molecules in human ESCs, and therefore regulation is possibly through a DNA methylation mechanism [17, 23, 24]. Presumably, the lack of costimulatory molecule expression on PSCs further impairs their ability to function as APCs.
In addition to DNA methylation, histone modification provides alternative strategies leading to the MHC expression profile unique in ESCs and iPSCs. Posttranslational modification of the amino-terminal tails of core histones acts in the regulation of gene expression and other diverse biological processes. Depending on the type and number of histone modifications, epigenetic modified histone could either activate or repress gene expression. By performing ChiP assays, Suárez-Álvarez and colleagues showed that H3K9me3 (repressive histone marker) maintained a high degree at MHC class II loci during ESC differentiation. In contrast, 60-fold greater H3K4me3 (active promoter marker) at MHC class I and β2m regions was observed in differentiated iPSCs and hESCs, coincident with the increased β2m mRNA level. It is known that lack of β2m in cells will limit the expression of MHC class I trimeric molecule on the cell surface. Histone modification of H3K4me3 in MHC class I genes post differentiation facilitated chromatin relaxation and allowed gene expression [23]. Expression of Tapasin (TPN), an antigen processing machinery family member, is also repressed by H3K9me3 in undifferentiated PSCs while upregulated by H3K4me3 in differentiated PSCs. TPN is a TAP (transporter associated with antigen processing)-associated glycoprotein and an ER chaperone. It is uniquely dedicated to MHC class I biosynthesis [23]. TPN binds MHC class I molecules and integrates into peptide-loading complexes. Formation of an "optimal peptide" bond allows MHC class I to be released and exported to the cell surface for presentation to T cells [24-26] (Fig. 2). The repression on TPN in undifferentiated PSCs could contribute to low MHC class I expression. It is undeniable that epigenetic regulation is fundamentally associated with controlling MHC expression in ESCs and iPSCs, and provides tantalizing strategies for inducing tolerance for stem cells grafts. However, this study has yet to directly assess the time points for the series of events that lead to enhanced MHC expression in comparison with other embryonic antigen expression. The risk posed by presenting embryonic antigens to a host immune system could result in the destruction of even syngeneic iPSCs by NK cells and T cell-mediated rejection due to an inherent ability to recognize embryonic antigens, as described above.
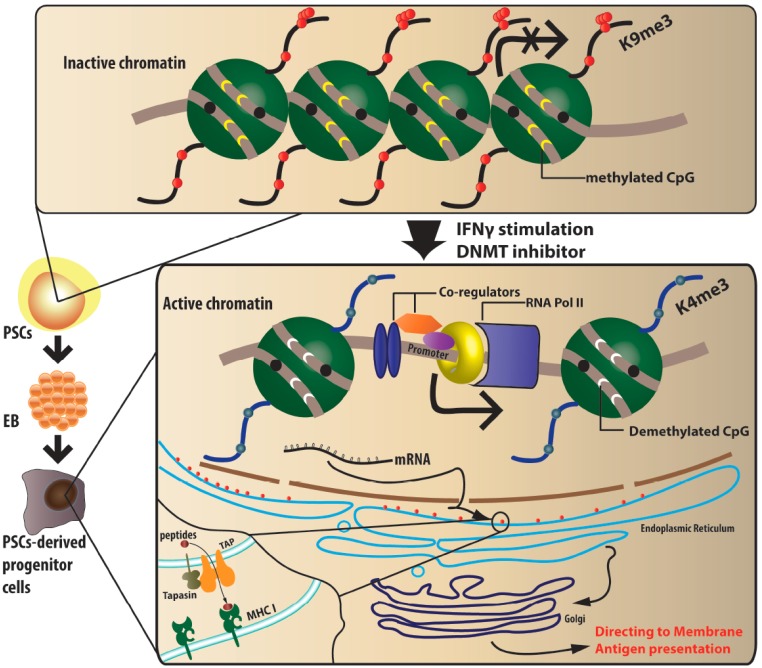
Fig. (2).
Epigenetic mechanisms regulating MHC expression in PSCs. PSCs have low expression of MHC Class I, and complete absence of MHC Class II and co-stimulatory molecules. In undifferentiated PSCs, the genes of MHC and associated transcriptional factors are fully methylated. CpG islands contained in MHC genes are also in a complete state of methylation. Additionally, post-translational modifications of the amino terminal tails of the core histone in MHC genes results in H3K9 trimethylation, which withholds MHC expression in undifferentiated PSCs and early EBs. Differentiation of PSCs into desired therapeutic cells leads to an up-regulation of MHC gene expression seen in chromatin relaxation. MHC class I genes are demethylated in differentiated cells, including CpG sites. Post-transcriptional modification of H3K4 trimethylation results in chromatin relaxation and subsequent MHC gene expression. Furthermore, TAP-1, TAP2 and tapasin molecules, that participate in peptide loading onto MHC class I, also show a significant increase after differentiation, which further contributes to increased immune recognition. In addition to differentiation, in vitro IFNγ stimulation and DNA methyltranferase treatment would up-regulate MHC expression in undifferentiated PSCs.
EPIGENETIC MEMORY
Gene profiling studies have revealed some aberrant epigenetic patterns and even residual epigenetic memories from the cell type of origin in iPSCs [27]. With emerging evidence, it raises the concerns of whether disrupted epigenetic patterns in iPSCs will compromise their suitability for clinical application in regenerative medicine.
Differentiated somatic cells have distinctive epigenetic patterns to maintain their cell identity, whereas cellular reprogramming works to break the epigenetic barrier of differentiated cells back to an undifferentiated state [27]. Somatic cell nucleus transfer (SCNT) techniques facilitate a transfer of somatic cell nuclei into enucleated unfertilized or recently fertilized oocytes [27]. Of the current reprogramming methods, SCNT is believed to generate iPSCs closest to the ESC pluripotent state. Kim and colleagues compared iPSCs generated from SCNT and the typical Yamanaka transcription factor-based reprogramming method for alternations in DNA methylation [28]. Strikingly, there were remarkable epigenetic signatures left over from the somatic cell of origin, even though iPSCs from both methods established the defined ESC phenotype. The epigenetic landscape of SCNT-generated iPSCs revealed relatively much less deviation from those of authentic ESCs. They further investigated the differentiation potential of iPSCs derived from the two methods, which alternatively confirmed residual epigenetic memory in iPSCs. There was high propensity for iPSCs to differentiate into their cell type of origin; osteogenic and hematopoietic cells. Nonetheless, epigenetic abnormalities caused by forced expression of the four Yamanaka factors in early reprogramming process could be ameliorated by repetitive subculture and subsequent passaging [28]. However, certain epigenetic defects would not be completely corrected, and consequently, this reduces the flexibility of iPSCs and limits their future application in regenerative medicine.
Ultimately, it is relevant to ask whether it is really possible to coerce iPSCs to a pluripotency “ground-zero” state, similar to ESCs. The goal for using iPSCs is to utilize their capacity of self-renewal and differentiation to generate patient-specific cells and tissues. Rather than focusing on how close iPSCs are to matching authentic ESCs, one could take advantage of epigenetic memory. If the target treatment demands for a specific cell type, iPSC lines based from close lineage would eliminate difficulties associated with targeted differentiation. For example, Bar-Nur et al. demonstrated that beta cell-derived iPSCs have a greater propensity to differentiate into insulin-producing cells [29]. Moreover, it seems that low immunogenic lineages could be generated from iPSCs derived from cells with inherent low immunogenicity. Recently, Liu et al. have successfully generated a population of neural progenitor cells with relatively low immunogenicity from iPSCs derived from umbilical cord mesenchymal cells (UMCs). As the cell of origin, UMCs have long been proven to be less immunogenic than other somatic fibroblasts used commonly in reprogramming. Authors showed that UMC-derived iPSCs and their differentiated lineages retain low immunogenicity of UMCs [30]. This observation reflects the presence of epigenetic memory in iPSCs and may hold new promises to develop stem cell-based therapy with low immunogenicity by utilizing epigenetic memories.
PLURIPOTENT STEM CELLS MEET THE IMMUNE SYSTEM: INNATE IMMUNE RESPONSES TO PSCS
Innate immunity is the first line of defense and is comprised of fast responding immune cells, including all granulocytes and natural killer (NK) cells. It carries the responsibility of generating an immediate wave of inflammatory reaction towards a graft after transplantation [22, 31, 32]. Even though innate immune cells do not require antigen specificity, they function as a bridge for full activation of the adaptive immune system through the process of antigen presentation.
MHC molecules create a sense of “self” in host immune systems. Both ESCs and iPSCs are characterized to be immune privileged by their low expression of MHC molecules to evade T cell mediated immune responses [15, 19]. However, loss of “self” entity on cell surfaces, similar to tumor cells, is the specialized forte of NK cell killing. Absence or mismatch of MHC class I on ESC-derived tissue would therefore lead to the premise of increased graft susceptibility to NK cell recognition and damage. Hanna et al. first studied the immunologic barrier of NK cells in iPSC-derived hematopoietic progenitor cells (HPCs) and found a rejection of engraftment [33]. In support of this finding, others later reported that undifferentiated ESCs and ESC-derived progenitor cells were more prone to NK cell attack due to expression of NK cell activating ligands. One study compared the susceptibility of undifferentiated ESCs and ESC-derived HPCs in the matter of NK mediated lysis. High levels of H60, an activating ligand to Natural Killer group 2 D (NKG2D), contributed to NK cell mediated destruction of ESC-derived HPCs in a perforin-granzyme dependent manner [34]. Admittedly, balance of MHC expression on PSCs and their derivatives is critical for the involvement of NK cells in graft rejection. Tabayoyong et al. showed that the enhancement of MHC class I expression via IFNγ stimulation would be protective for HPCs against NK cell cytotoxicity [34]. One study further concluded that IFNγ mediated up-regulation of MHC class I is critical in syngeneic transplant survival of ESC-derived vascular progenitor cells by evading NK cell-mediated lysis [26] (Fig. 3A).
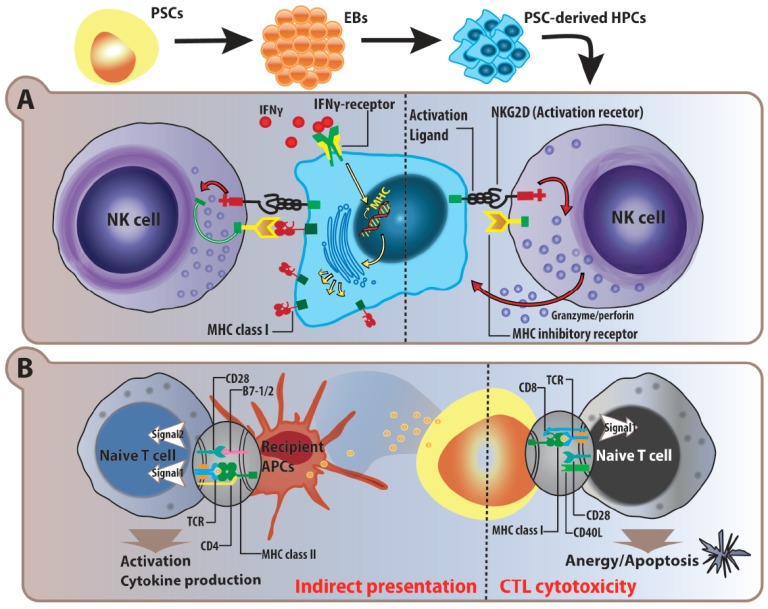
Fig. (3).
Simplified schematic of natural killer and T cell immune responses towards PSCs and their progeny. A) PSCs and PSC-derived hematopoietic progenitor/stem cells (HPC) express low levels of MHC class I molecules, which make them susceptible to NK cell recognition and cytotoxicity. Even though PSC-derived HPCs have a low expression of NK activation ligands, the lack of MHC molecules for inhibitory signal alone will trigger NK cell killing by the release of granzyme and perforin. In vitro IFNγ stimulation augments MHC expression on PSC-derived HPCs, and is in turn, protective against NK cell cytotoxicity via binding of MHC class I on the NK inhibitory receptor. B) Due to the lack of co-stimulatory signals in the activation process, CD8+ cytotoxic T lymphocytes (CTLs) specific to PSC antigens are anergized, and subsequently results in apoptotic cell death. As in the indirect allogeneic recognition, recipient professional antigen presenting cells (APCs) can pick up PSC-derived embryonic antigens, and present to specific CD4+ T helper cells. With complete signal 1 from TCRs and signal 2 from co-stimulatory molecules, indirect recognition against PSCs will trigger immune rejection responses.
Contrary to the aforementioned findings, in the scenario of fetal development, absence of MHC class II and low levels of MHC class I at the maternal-fetal interface promotes maternal immunosuppression [35, 36]. Additional signals, besides MHC class I, are also utilized to establish fetomaternal tolerance. For instance, HLA-G and HLA-E are non-classical MHC class Ib molecules that are essential in the acceptance of an embryo. To investigate whether PSCs also take advantage of these molecules, a study conducted by Yen et al. revealed that hESC-derived mesenchymal progenitor cells (MPCs) were resistant to NK cell lysis, coinciding with high HLA-G expression on the surface [37]. Furthermore, several reports claimed that this resistance to NK cell cytotoxicity exists, by showing contradicting results in NKG2D ligand expression. Frenzel et al. revealed that NKG2D ligands were expressed on murine ESCs but not on ESC-derived cardiomyocytes (ESCM), protecting ESC-derived progeny from NK cell recognition and lysis [38].
To analyze these disparities, it is important to consider the differential responses posed by different NK cell subtypes, and account for the level of activation in the setting of in vitro assays. Given the challenges in grasping the balance of inhibitory and stimulatory signals in NK cells and the dynamic regulation of MHC expression, it is likely that discrepancies on whether PSCs promote/evade NK cell-mediated cytotoxicity will continue.
Granulocytes, macrophages and neutrophils, contribute to early inflammation attacks as an immediate immune response after transplantation. Neutrophils and macrophages are instantly recruited and infiltrated into the site of ESC-derived insulin producing cell cluster implants with robust inflammatory cytokine and chemokine excretion [20, 21]. Although innate responses are likely to eventually subside, it is unclear as to what extent adaptive immunity has been evoked and graft function has been damaged after this inflammatory assault. It is clear that the immune system cannot simply be dichotomized into two isolated compartments. Given that early innate inflammatory responses lay the basis for later adaptive immune activation, effective control of innate inflammatory responses should improve long-term engraftment.
PLURIPOTENT STEM CELLS MEET THE IMMUNE SYSTEM: ADAPTIVE IMMUNE RESPONSES TO UNDIFFERENTIATED PSCS AND THEIR DIFFERENTIATED PROGENIES
Adaptive immunity generates antigen specific immune responses. Without immunosuppression, the outcome of allogeneic transplantation is graft rejection mediated by CD4 and/or CD8 T cells [39]. Activation of the adaptive immune system facilitates a subsequent expansion of specific recipient T helper cells via either donor or recipient APC. As aforementioned, PSCs are unlikely to function as APCs owing to the absence of MHC class II and costimulatory molecules. However, the immunogenic outcomes of engraftment remain uncertain, especially for PSC-derived epithelial cells. Under certain conditions, epithelial cells, the unconventional APCs, could acquire antigen presentation capacity leading to graft failure by directly activating allogeneic T cells [40]. Nevertheless, in stem cell-based transplantation, graft damage and subsequent functional failure are most likely due to indirect antigen presentation-mediated T cell response. CD4+ T helper cells do not directly lyse target cells expressing allogeneic MHC molecules. Rather, they orchestrate the entire immune rejection response by aiding activation of donor specific immune cells; B cell and cytotoxic T lymphocytes (CTL). The majority of graft damage and succeeding functional failure is caused by CTL-induced lysis through binding to MHC class I on donor cells [15-17, 39]. In general, ESCs and iPSCs exhibit low immunogenicity with low expression levels of MHC class I molecules and undetectable levels of MHC class II and costimulatory molecules. Early studies have suggested that undifferentiated hESCs are incapable of inducing allogeneic T cell proliferation in mixed leukocyte reactions in vitro. Similarly, undifferentiated murine ESCs and early EB cells expressing lymphocytic choriomeningitis virus (LCMV) peptides showed resistance to LCMV-specific CTL lysis [41]. In contrast, Dressel et al. argued that murine ESCs loaded with OVA peptide were subjected to OVA-specific TCR transgenic T cell recognition and subsequent antigen-specific lysis [42]. In this experimental setting, they used a pure OVA specific T effector cell population instead of a T cell mixture with diverse specificity, which might undermine inhibitory effects observed by other groups [43]. It is possible that indirect allorecognition of iPSCs and ESCs in prolonged engraftment may take place when recipient APCs pick up ESC- or iPSC-derived antigens to CD4+ T cells (Fig. 3B).
Several potential mechanisms for suppressing host immune response mediated by PSCs have been proposed. Inhibition on T cell proliferation and activation is thought to occur via Arginase I expression on the surface of ESCs. Consumption of L-arginine in the localized environment impairs proper activation of T cells, which would theoretically be reversed by supplementing L-arginine back into culture media [44]. Our group found that addition of high levels of L-arginine did not ameliorate suppression on T cell proliferation, suggesting other coexisting mechanisms. We found that, similar to intact cells, the cellular protein extracts from both undifferentiated mouse and human ESCs retain immunomodulation properties. ESC-derived soluble factors were able to suppress T cell proliferation and to impede DC maturation. T cells exposed to soluble ESC cellular factors also showed a reduced Th1 cytokine production and transcriptional profile. The potential mechanism was likely through inhibition of PKC-θ phosphorylation[45, 46]. In addition, ESCs have been reported to regulate the local microenvironment to favor T regulatory (Treg) cell polarization to acquire immune privilege. Treg is a unique subset of T cells, which can suppress T effector cells with diverse antigen specificities. Induction or recruitment of Treg into the graft is an ideal outcome in transplantation. Once activated, Treg will reinforce an establishment of tolerance and acquirement of immune privilege [47-49]. In one study, a transplanted murine EB graft was accepted by an allogeneic recipient only in combination with non-depleting anti-CD8 antibody [49-51]. The greater proportion of infiltrating T cells in the tolerated graft was found to be FoxP3+. Even so, future studies should be conducted to clarify how ESCs interfere with T cell polarization, and what the underlying mechanisms are.
Taken together, the majority of reports and proposed mechanisms support a reduced immunogenicity of undifferentiated PSCs. One would ask whether their differentiated progeny is still capable of retaining these properties after an increase in MHC class I expression. In the context of stem cell-based therapy, the immunological properties of the cell type of interest after differentiation from either ESCs or iPSCs should be assessed to determine their suitability in clinical application. Currently, few studies have been reported regarding whether iPSCs possess the same immune inhibitory property as do ESCs. Kim and colleagues showed that iPSC-derived HPCs induce anergy in allogeneic CTL [52]. They speculated that induced T cell anergy was due to poor expression of MHC and co-stimulatory molecules. Though MHC class I expression was augmented with IFNγ stimulation, the co-stimulatory molecule was unaffected. It would be interesting to examine whether anergy can be generated in iPSC-derived tissues other than HPC/immune cells. In a number of clinical reports, co-transplantation of donor HPC/bone marrow together with other tissues has been shown to enhance engraftment [53-55]. To this context, co-transplantation of hESC-derived HPC or immune cells together with other hESC-derivatives has been considered as one strategy to reduce immune rejection. It is possible that long-term protection from CTL-mediated killing could be an optimistic outlook in the future development of ESC- or iPSC-based therapeutics.
TO BEHAVE AS A CANCER CELL OR NOT TO BEHAVE
One promising aspect of iPSC technology is the potential use in cell replacement therapy to treat numerous debilitating diseases. However, concerns over the immunogenicity and tumorigenicity of these cells has proven challenging when considering clinical applications. Immune evasion is a desired characteristic for iPSCs, but problematic when utilized by cancer cells, as it promotes tumor growth and development.
Whereas iPSC technology is struggling with complications involving immunogenicity, cancer cells are constantly evolving to escape or silence immune responses in order for a tumor to thrive in its microenvironment. Initially the immune system is able to protect the host against tumor development through immunosurveillance, which inadvertently leads to immune evasion by progression through three stages of immunoediting; elimination, equilibrium, escape [56-58]. There are several strategies that tumor cells employ during immune evasion, some of which include MHC class I structural alterations/downregulation [59, 60], mutations of Fas or TRAIL [61, 62], and inhibition of T cell receptors and/or development of Treg [63, 64]. Many of the same mechanisms are used by undifferentiated pluripotent stem cells, however, clinical transplantations would involve differentiated progenies that do not share these characteristics, and therefore additional steps need to be taken to ensure reduced immunogenicity after transplantation.
Not only is there an immunogenicity concern for iPSC use in clinical therapy, but there is also an inherent tumorigenicity risk associated with iPSCs as well. Evidently, many of the genes responsible for pluripotency induction are aberrantly expressed in cancer [65, 66]. Gene expression microarray studies done by Riggs et al. found that both iPSCs and oncogenic foci upregulated the glycolysis pathway and had substantial overlap in gene expression changes, indicating that induced pluripotency and oncogenic transformation are related processes [67]. Supporting this notion, another study revealed that both iPSCs and cancer cells suppress Lefty expression, a tumor suppressor protein, whereas ESCs show normal expression levels [68]. iPSC-based therapies pose the risk of introducing chromosomal damage and genomic instability during iPSC derivation, selecting for tumorigenic clones, producing incompletely differentiated and aberrantly methylated cells during differentiation and culturing, as well as contaminating therapeutic cells and reactivating pluripotency transgenes during transplantation (reviewed by Lee et al.) [69].
Despite these clinical hurdles to iPSC-based therapies, progress has been made towards decreasing the risk of tumor formation after transplantation. One method involves removing the oncogene c-Myc, which contributes to tumorigenesis through overstimulating cell growth/metabolism and promoting genomic instability during reprogramming [70, 71]. Additionally, a shift towards insertion-free procedures for generating iPSCs has already been developed, given that viral methods are a risk for mutagenesis and subsequent tumorigenesis [72]. Together with other attempts to improve the quality of iPSCS, such as specific protein inhibitors [73], substitution [70, 71], use of microRNAs [74, 75] or even small molecules [76], advances in this area look promising. Lastly, precaution during selection of the somatic origin of iPSCs should be exercised, as Miura et al. found that cell lines derived from mouse tail-tip fibroblasts and hepatocytes had a higher teratoma-forming propensity than other tissue origins [7, 77]. This is likely due to an increased resistance to differentiation in these cell lines, which may be associated with epigenetic regulation machinery.
IPSCS FOR POTENTIAL TUMOR THERAPY, NOT INITIATION
Despite concern for tumorigenesis, iPSC technology has generated enthusiasm in the field of cancer therapy. In particular, iPSCs can be differentiated into immune cells, such as T cells and NK cells. By taking advantage of epigenetic memory, it may be possible to reprogram T cells with certain tumor antigens into iPSCs, then differentiate them back and expand the population before infusion back into a patient [78, 79]. Indeed, Themeli et al. have successfully combined iPSC and chimeric antigen receptor (CAR) technologies to generate T cells targeting CD19 that was expressed by malignant B cells, which potently inhibits tumor growth in a xenograft model [80]. Several studies have also focused on generating NK cells because of their anti-tumor effects without the need for antigen matching or previous exposure [81, 82]. iPSC-mediated immunotherapy can prevent relapse and immune rejection, and decrease tumor burden [83]. In addition, Li et al. investigated the possibility of using PSCs to develop a cancer vaccine [84]. In this paper, the premise that several oncofetal antigens are shared between cancer and stem cells led to the hypothesis that the immune response against these embryonic antigens would be cross reactive in targeting cancer antigens, and therefore generate protective immunity against tumors. Indeed, mice immunized with hESCs and iPSCs evoked stronger cellular and humoral immune responses against colon carcinoma through an expansion of tumor specific IFNγ producing cells and a coinciding reduction of myeloid suppressive cells. Although the vaccination with iPSCs to relieve tumor burden or establish tumor protection was not fully successful, this area is likely a promising modality that warrants further investigation. Using less heterogenic iPSCs and combining other immune approaches may help to achieve this goal. Lastly, another area where iPSCs will be useful is for replacement of damaged tissue caused by radiation, chemotherapy, or surgery [78]. Successful engraftment of liver has already been shown to be effective [85]. In summary, provided that the necessary steps are taken to circumvent tumorigenicity, iPSCs could prove to be an efficient and effective means for cancer treatment.
ACKNOWLEDGEMENTS
We apologize to all those colleagues whose important work could not be cited owing to space limitations. This work is supported by operating grants from the Canadian Institutes of Health Research MOP-111224, Canadian Breast Cancer Foundation-Ontario Region, and Heart Stroke Foundation of Ontario NA7186 to LW.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Zhao T, Zhang Z-N, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–5. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 2.Araki R, Uda M, Hoki Y, et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013;494:100–4. doi: 10.1038/nature11807. [DOI] [PubMed] [Google Scholar]
- 3.Guha P, Morgan JW, Mostoslavsky G, Rodrigues NP, Boyd AS. Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell Stem Cell. 2013;12:407–12. doi: 10.1016/j.stem.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Dhodapkar KM, Feldman D, Matthews P, et al. Natural immunity to pluripotency antigen OCT4 in humans. Proc Natl Acad Sci USA. 2010;107:8718–23. doi: 10.1073/pnas.0915086107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mills JA, Wang K, Paluru P, et al. Clonal genetic and hematopoietic heterogeneity among human-induced pluripo-tent stem cell lines. Blood. 2013;122:2047–51. doi: 10.1182/blood-2013-02-484444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schnabel LV, Abratte CM, Schimenti JC, Southard TL, Fortier LA. Genetic background affects induced pluripotent stem cell generation. Stem Cell Res Ther. 2012;3:30. doi: 10.1186/scrt121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miura K, Okada Y, Aoi T, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743–5. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- 8.Laurent LC, Ulitsky I, Slavin I, et al. Dynamic Changes in the Copy Number of Pluripotency and Cell Proliferation Genes in Human ESCs and iPSCs during Reprogramming and Time in Culture. Cell Stem Cell. 2011;8:106–18. doi: 10.1016/j.stem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang Y, Zhang H, Feng Q-S, et al. The propensity for tumorigenesis in human induced pluripotent stem cells is related with genomic instability. Chin J Cancer. 2013;32:205–12. doi: 10.5732/cjc.012.10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyd AS, Wood KJ. Variation in MHC Expression Between Undifferentiated Mouse ES Cells and ES Cell-derived Insulin-producing Cell Clusters. Transplantation. 2009;87:1300–4. doi: 10.1097/TP.0b013e3181a19421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swijnenburg R-J, Tanaka M, Vogel H, et al. Embryonic stem cell immunogenicity increases upon differentiation after transplantation into ischemic myocardium. Circulation. 2005;112:I166–172. doi: 10.1161/CIRCULATIONAHA.104.525824. [DOI] [PubMed] [Google Scholar]
- 12.Charpentier B, Rostaing L, Berthoux F, et al. A three-arm study comparing immediate tacrolimus therapy with antithymocyte globulin induction therapy followed by tacrolimus or cyclosporine A in adult renal transplant recipients. Transplantation. 2003;75:844–51. doi: 10.1097/01.TP.0000056635.59888.EF. [DOI] [PubMed] [Google Scholar]
- 13.Preynat-Seauve O, de Rham C, Tirefort D, Ferrari-Lacraz S, Krause K-H, Villard J. Neural progenitors derived from human embryonic stem cells are targeted by allogeneic T and natural killer cells. J Cell Mol Med. 2009;13:3556–69. doi: 10.1111/j.1582-4934.2009.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng CY, Madsen JC, Rosengard BR, Allan JS. Immunosuppression for lung transplantation. Front Biosci Landmark Ed. 2009;14:1627–41. doi: 10.2741/3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyd AS, Rodrigues NP, Lui KO, Fu X, Xu Y. Concise Review Immune Recognition of Induced Pluripotent Stem Cells. STEM CELLS. 2012;30:797–803. doi: 10.1002/stem.1066. [DOI] [PubMed] [Google Scholar]
- 16.Game DS, Lechler RI. Pathways of allorecognition: implications for transplantation tolerance. Transpl Immunol. 2002;10:101–8. doi: 10.1016/s0966-3274(02)00055-2. [DOI] [PubMed] [Google Scholar]
- 17.Li L. Human Embryonic Stem Cells Possess Immune-Privileged Properties. Stem Cells. 2004;22:448–56. doi: 10.1634/stemcells.22-4-448. [DOI] [PubMed] [Google Scholar]
- 18.Wu DC, Boyd AS, Wood KJ. Embryonic stem cells and their differentiated derivatives have a fragile immune privilege but Still Represent Novel Targets of Immune Attack. Stem Cells. 2008;26:1939–50. doi: 10.1634/stemcells.2008-0078. [DOI] [PubMed] [Google Scholar]
- 19.Drukker M. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc Natl Acad Sci. 2002;99:9864–9. doi: 10.1073/pnas.142298299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyd AS, Wood KJ. Variation in MHC Expression Between Undifferentiated Mouse ES Cells and ES Cell-derived Insulin-producing. Cell Clusters Transplantation. 2009;87:1300–4. doi: 10.1097/TP.0b013e3181a19421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okamura RM, Lebkowski J, Au M, Priest CA, Denham J, Majumdar AS. Immunological properties of human embryonic stem cell-derived oligodendrocyte progenitor cells. J Neuroimmunol. 2007;192:134–44. doi: 10.1016/j.jneuroim.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 22.De Almeida PE, Ransohoff JD, Nahid A, Wu JC. Immunogenicity of Pluripotent Stem Cells and Their Deriv-atives. Circ Res. 2013;112:549–61. doi: 10.1161/CIRCRESAHA.111.249243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suárez-Álvarez B, Rodriguez RM, Calvanese V, et al. Epigenetic Mechanisms Regulate MHC and Antigen Processing Molecules in Human Embryonic and Induced Pluripotent Stem Cells. PLoS ONE. 2010;5:e10192. doi: 10.1371/journal.pone.0010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drukker M, Katchman H, Katz G, et al. Human Embryonic Stem Cells and Their Differentiated Derivatives Are Less Susceptible to Immune Rejection Than Adult Cells. Stem Cells. 2006;24:221–9. doi: 10.1634/stemcells.2005-0188. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman J. Antigen processing and presentation: Evolution from a bird’s eye view. Mol Immunol. 2013;55:159–61. doi: 10.1016/j.molimm.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma M, Ding S, Lundqvist A, et al. Major Histocompatibility Complex-I Expression on Embryonic Stem Cell-Derived Vascular Progenitor Cells Is Critical for Syngeneic Transplant Survival. STEM CELLS. 2010;28:1465–75. doi: 10.1002/stem.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muchkaeva IA, Dashinimaev EB, Terskikh VV, Sukhanov YV, Vasiliev AV. Molecular Mechanisms of In-duced Pluripotency. Acta Naturae. 2012;4:12–22. [PMC free article] [PubMed] [Google Scholar]
- 28.Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–90. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bar-Nur O, Russ HA, Efrat S, Benvenisty N. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell. 2011;9:17–23. doi: 10.1016/j.stem.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Liu P, Chen S, Li X, et al. Low Immunogenicity of Neural Progenitor Cells Differentiated from Induced Pluripotent Stem Cells Derived from Less Immunogenic Somatic Cells. PLoS ONE. 2013;8:e69617. doi: 10.1371/journal.pone.0069617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dressel R, Nolte J, Elsner L, Novota P, et al. Pluripotent stem cells are highly susceptible targets for syngeneic. allogeeic.and xenogeneic natural killer cells. FASEB J . 2010; 24:2164–77. doi: 10.1096/fj.09-134957. [DOI] [PubMed] [Google Scholar]
- 32.Boyd AS, Wood KJ. Characteristics of the Early Immune Response Following Transplantation of Mouse ES Cell Derived Insulin-Producing Cell Clusters. PLoS ONE. 2010;5:e10965. doi: 10.1371/journal.pone.0010965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanna J, Wernig M, Markoulaki S, et al. Treatment of Sickle Cell Anemia Mouse Model with iPS Cells Generated from Autologous Skin. Science. 2007;318:1920–3. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 34.Tabayoyong WB, Salas JG, Bonde S, Zavazava N. HOXB4-Transduced Embryonic Stem Cell-Derived Lin-c-kit+ and Lin-Sca-1+ Hematopoietic Progenitors Express H60 and Are Targeted by NK Cells. J Immunol. 2009;183:5449–57. doi: 10.4049/jimmunol.0901807. [DOI] [PubMed] [Google Scholar]
- 35.Petroff MG, Chen L, Phillips TA, Hunt JS. B7 Family Molecules: Novel Immunomodulators at the Maternal-Fetal Interface. Placenta. 2002;23:S95–S101. doi: 10.1053/plac.2002.0813. [DOI] [PubMed] [Google Scholar]
- 36.Telugu BP, Adachi K, Schlitt JM, et al. Comparison of extravillous trophoblast cells derived from human embryonic stem cells and from first trimester human placentas. Placenta. 2013;34:536–43. doi: 10.1016/j.placenta.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yen M-L, Hou C-H, Peng K-Y, et al. Efficient Derivation and Concise Gene Expression Profiling of Human Embryonic Stem Cell-Derived Mesenchymal Progenitors (EMPs). Cell Transplant. 2011;20:1529–45. doi: 10.3727/096368910X564067. [DOI] [PubMed] [Google Scholar]
- 38.Frenzel LP, Abdullah Z, Kriegeskorte AK, et al. Role of Natural-Killer Group 2 Member D Ligands and Intercellular Adhesion Molecule 1 in Natural Killer Cell-Mediated Lysis of Murine Embryonic Stem Cells and Embryonic Stem Cell-Derived Cardiomyocytes. Stem Cells. 2009;27:307–16. doi: 10.1634/stemcells.2008-0528. [DOI] [PubMed] [Google Scholar]
- 39.Swijnenburg R-J, Schrepfer S, Govaert JA, et al. Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proc Natl Acad Sci. 2008;105:12991–6. doi: 10.1073/pnas.0805802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kreisel D, Krupnick AS. Non-hematopoietic allograft cells directly activate CD8+ T cells and trigger acute rejection: an alternative mechanism of allorecognition. Nat Med. 2002;8:233–9. doi: 10.1038/nm0302-233. [DOI] [PubMed] [Google Scholar]
- 41.Abdullah Z, Saric T, Kashkar H, et al. Serpin-6 expression protects embryonic stem cells from lysis by antigen-specific CTL. J Immunol. 2007;178:3390–9. doi: 10.4049/jimmunol.178.6.3390. [DOI] [PubMed] [Google Scholar]
- 42.Dressel R, Guan K, Nolte J, et al. Multipotent adult germ-line stem cells. like other pluripotent stem clls.can be killed by cytotoxic T lymphocytes despite low expression of major histocompatibility complex class I molecules. Biol Direct . 2009;4:31. doi: 10.1186/1745-6150-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.English K, Wood KJ. Immunogenicity of embryonic stem cell-derived progenitors after transplantation. Curr Opin Organ Transplant. 2011;16:90–5. doi: 10.1097/MOT.0b013e3283424faa. [DOI] [PubMed] [Google Scholar]
- 44.Yachimovich-Cohen N, Even-Ram S, Shufaro Y, Rachmilewitz J, Reubinoff B. Human Embryonic Stem Cells Suppress T Cell Responses via Arginase I-Dependent Mechanism. J Immunol. 2009;184:1300–8. doi: 10.4049/jimmunol.0804261. [DOI] [PubMed] [Google Scholar]
- 45.Mohib K, AlKhamees B, Zein HS, Allan D, Wang L. Embryonic Stem Cell-Derived Factors Inhibit T Effector Activation and Induce T Regulatory Cells by Suppressing PKC-? Activation. PLoS ONE. 2012;7:e32420. doi: 10.1371/journal.pone.0032420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mohib K, Allan D, Wang L. Human Embryonic Stem Cell-extracts Inhibit the Differentiation and Function of Monocyte-derived Dendritic Cells. Stem Cell Rev Reports. 2010;6:611–21. doi: 10.1007/s12015-010-9185-7. [DOI] [PubMed] [Google Scholar]
- 47.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell a jack of all trades master of regulation. Nat Immunol. 2008;9:239–44. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robertson NJ, Brook FA, Gardner RL, Cobbold SP, Waldmann H, Fairchild PJ. Embryonic stem cell-derived tissues are immunogenic but their inherent immune privilege promotes the induction of tolerance. Proc Natl Acad Sci. 2007;104:20920–5. doi: 10.1073/pnas.0710265105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lui KO, Boyd AS, Cobbold SP, Waldmann H, Fairchild PJ. A Role for Regulatory T Cells in Acceptance of ESC-Derived Tissues Transplanted Across an Major Histocom-patibility Complex Barrier. STEM CELLS. 2010;28:1905–14. doi: 10.1002/stem.506. [DOI] [PubMed] [Google Scholar]
- 50.Qin S, Wise M, Cobbold SP, et al. Induction of tolerance in peripheral T cells with monoclonal antibodies. Eur J Immunol. 1990;20:2737–45. doi: 10.1002/eji.1830201231. [DOI] [PubMed] [Google Scholar]
- 51.Honey K, Cobbold SP, Waldmann H. CD40 ligand blockade induces CD4+ T cell tolerance and linked sup-pression. J Immunol. 1999;163:4805–10. [PubMed] [Google Scholar]
- 52.Kim E-M, Manzar G, Zavazava N. Human iPS cell-derived hematopoietic progenitor cells induce T-cell anergy in in vitro-generated alloreactive CD8+ T cells. Blood. 2013;121:5167–75. doi: 10.1182/blood-2012-11-467753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trivedi HL, Vanikar AV, Modi PR, et al. Allogeneic Hematopoietic Stem-Cell Transplantation Mixed Chimerism and Tolerance in Living Related Donor Renal Allograft Reci-pients. Transplant Proc. 2005;37:737–42. doi: 10.1016/j.transproceed.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 54.Rao AS, Fontes P, Zeevi A, et al. Enhancement of donor cell chimerism in whole organ allograft recipients by adjuvant bone marrow transplantation. Transplant Proc. 1995;27(6):3387–8. [PMC free article] [PubMed] [Google Scholar]
- 55.Shapiro R, Rao AS, Fontes P, et al. Combined kidney/bone marrow transplantation—evidence of augmentation of chimerism. Transplantation. 1995;59:306. doi: 10.1097/00007890-199501000-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 57.Vesely MD, Schreiber RD. Cancer immunoediting antigens mechanisms and implications to cancer immunotherapy. Ann N Y Acad Sci. 2013;1284:1–5. doi: 10.1111/nyas.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lakshmi Narendra B, Eshvendar Reddy K, Shantikumar S, Ramakrishna S. Immune system a double-edged sword in cancer. Inflamm Res. 2013;62(9):823–34. doi: 10.1007/s00011-013-0645-9. [DOI] [PubMed] [Google Scholar]
- 59.Garcia-Lora A, Martinez M, Algarra I, Gaforio JJ, Garrido F. MHC class I-deficient metastatic tumor variants immunoselected by T lymphocytes originate from the coordi-nated downregulation of APM components. Int J Cancer. 2003;106:521–7. doi: 10.1002/ijc.11241. [DOI] [PubMed] [Google Scholar]
- 60.Watson NFS, Ramage JM, Madjd Z. Immunosurveillance is active in colorectal cancer as downregulation but not complete loss of MHC class I expression correlates with a poor prognosis. Int J Cancer. 2006;118:6–10. doi: 10.1002/ijc.21303. [DOI] [PubMed] [Google Scholar]
- 61.Real LM, Jimenez P, Kirkin A. Multiple mechanisms of immune evasion can coexist in melanoma tumor cell lines derived from the same patient. Cancer Immunol Immunother CII. 2001;49:621–8. doi: 10.1007/s002620000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shin MS, Kim HS, Lee SH. Mutations of tumor necrosis factor-related apoptosis-inducing ligand receptor 1 (TRAIL-R1) and receptor 2 (TRAIL-R2) genes in metastatic breast cancers. Cancer Res. 2001;61:4942–6. [PubMed] [Google Scholar]
- 63.Simpson-Abelson MR, Loyall JL, Lehman HK. Human ovarian tumor ascites fluids rapidly and reversibly inhibit T cell receptor-induced NF-?B and NFAT signaling in tumor-associated T cells. Cancer Immun. 2013;13:14. [PMC free article] [PubMed] [Google Scholar]
- 64.Elpek KG, Lacelle C, Singh NP, Yolcu ES, Shirwan H. CD4+CD25+ T regulatory cells dominate multiple immune evasion mechanisms in early but not late phases of tumor development in a B cell lymphoma model. J Immunol. 2007;178:6840–8. doi: 10.4049/jimmunol.178.11.6840. [DOI] [PubMed] [Google Scholar]
- 65.Liu Y-H, Li Y, Liu X-H. A signature for induced pluripotent stem cell-associated genes in colorectal cancer. Med Oncol. 2013;30 doi: 10.1007/s12032-012-0426-2. [DOI] [PubMed] [Google Scholar]
- 66.Luo W, Li S, Peng B, Ye Y, Deng X, Yao K. Embryonic Stem Cells Markers SOX2. OCT4 and Nanog Ex-pression and Their Correlations with Epithelial-Mesenchymal Transition in Nasopharyngeal Carcinoma. . PLoS ONE. 2013;8:e56324. doi: 10.1371/journal.pone.0056324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riggs JW, Barrilleaux BL, Varlakhanova N, Bush KM, Chan V, Knoepfler PS. Induced pluripotency and oncogenic transformation are related processes. Stem Cells Dev. 2013;22:37–50. doi: 10.1089/scd.2012.0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saito A, Ochiai H, Okada S, Miyata N, Azuma T. Suppression of Lefty expression in induced pluripotent cancer cells. FASEB J Off Publ Fed Am Soc Exp Biol. 2013;27:2165–74. doi: 10.1096/fj.12-221432. [DOI] [PubMed] [Google Scholar]
- 69.Lee AS, Tang C, Rao MS, Weissman IL, Wu JC. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med. 2013;19:998–1004. doi: 10.1038/nm.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wernig M, Meissner A, Cassady JP, Jaenisch R. c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell. 2008;2:10–2. doi: 10.1016/j.stem.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 71.Fang I-M, Yang C-M, Yang C-H, Chiou S-H, Chen M-S. Transplantation of induced pluripotent stem cells without C-Myc attenuates retinal ischemia and reperfusion injury in rats. Exp Eye Res. 2013;113:49–59. doi: 10.1016/j.exer.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 72.Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–9. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silva J, Barrandon O, Nichols J, Kawaguchi J, Theunissen TW, Smith A. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 2008;6:e253. doi: 10.1371/journal.pbio.0060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miyoshi N, Ishii H, Nagano H. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell. 2011;8:633–8. doi: 10.1016/j.stem.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 75.Lin S-L, Ying S-Y. Mechanism and method for generating tumor-free iPS cells using intronic microRNA miR-302 induction. Methods Mol Biol Clifton NJ. 2013;936:295–312. doi: 10.1007/978-1-62703-083-0_23. [DOI] [PubMed] [Google Scholar]
- 76.Hou P, Li Y, Zhang X. Pluripotent Stem Cells Induced from Mouse Somatic Cells by Small-Molecule Compounds. Science. 2013;341:651–4. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- 77.Okano H, Nakamura M, Yoshida K. Steps toward safe cell therapy using induced pluripotent stem cells. Circ Res. 2013;112:523–33. doi: 10.1161/CIRCRESAHA.111.256149. [DOI] [PubMed] [Google Scholar]
- 78.Sharkis SJ, Jones RJ, Civin C, Jang Y-Y. Pluripotent stem cell-based cancer therapy promise and challenges. Sci Transl Med. 2012;4:127ps9. doi: 10.1126/scitranslmed.3003920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang L. Long-term in vivo provision of antigen-specific T cell immunity by programming hematopoietic stem cells. Proc Natl Acad Sci. 2005;102:4518–23. doi: 10.1073/pnas.0500600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Themeli M, Kloss CC, Ciriello G. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat Biotechnol. 2013;31:928–33. doi: 10.1038/nbt.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Knorr DA, Ni Z, Hermanson D. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl Med. 2013;2:274–83. doi: 10.5966/sctm.2012-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Watarai H, Yamada D, Fujii S, Taniguchi M, Koseki H. Induced pluripotency as a potential path towards iNKT cell-mediated cancer immunotherapy. Int J Hematol. 2012;95:624–31. doi: 10.1007/s12185-012-1091-0. [DOI] [PubMed] [Google Scholar]
- 83.Knorr DA, Kaufman DS. Pluripotent stem cell-derived natural killer cells for cancer therapy. Transl Res J Lab Clin Med. 2010;156:147–54. doi: 10.1016/j.trsl.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Y, Zeng H, Xu R-H, Liu B, Li Z. Vaccination with human pluripotent stem cells generates a broad spectrum of immunological and clinical responses against colon cancer. Stem Cells Dayt Ohio. 2009;27:3103–11. doi: 10.1002/stem.234. [DOI] [PubMed] [Google Scholar]
- 85.Choi SM, Kim Y, Liu H, Chaudhari P, Ye Z, Jang Y-Y. Liver engraftment potential of hepatic cells derived from patient-specific induced pluripotent stem cells. Cell Cycle Georget Tex. 2011;10:2423–7. doi: 10.4161/cc.10.15.16869. [DOI] [PMC free article] [PubMed] [Google Scholar]