Abstract
Background:
Mortality rates for leukemia are high despite considerable improvements in treatment. Since polyphenols exert pro-apoptotic effects in solid tumors, our study investigated the effects of polyphenols in haematological malignancies. The effect of eight polyphenols (quercetin, chrysin, apigenin, emodin, aloe-emodin, rhein, cis-stilbene and trans-stilbene) were studied on cell proliferation, cell cycle and apoptosis in four lymphoid and four myeloid leukemic cells lines, together with normal haematopoietic control cells.
Methods:
Cellular proliferation was measured by CellTiter-Glo® luminescent assay; and cell cycle arrest was assessed using flow cytometry of propidium iodide stained cells. Apoptosis was investigated by caspase-3 activity assay using flow cytometry and apoptotic morphology was confirmed by Hoescht 33342 staining.
Results:
Emodin, quercetin, and cis-stilbene were the most effective polyphenols at decreasing cell viability (IC50 values of 5-22 µM, 8-33 µM, and 25-85 µM respectively) and inducing apoptosis (AP50 values (the concentration which 50% of cells undergo apoptosis) of 2-27 µM, 19-50 µM, and 8-50 µM respectively). Generally, lymphoid cell lines were more sensitive to polyphenol treatment compared to myeloid cell lines, however the most resistant myeloid (KG-1a and K562) cell lines were still found to respond to emodin and quercetin treatment at low micromolar levels. Non-tumor cells were less sensitive to all polyphenols compared to the leukemia cells.
Conclusions:
These findings suggest that polyphenols have anti-tumor activity against leukemia cells with differential effects. Importantly, the differential sensitivity of emodin, quercetin, and cis-stilbene between leukemia and normal cells suggests that polyphenols are potential therapeutic agents for leukemia.
Keywords: Apoptosis, cell cycle, cell proliferation, leukemia, polyphenols.
1. INTRODUCTION
Leukemia affects millions of people worldwide each year, resulting in almost one-third of all cancer deaths [1]. Leukemia is a complex disease affecting all blood cell lineages. Each classification is based, in part, on specific chromosomal and oncogenic rearrangements. Leukemia affects all age groups; it is the most common cancer in children and adolescents [1]. T- and B-cell lymphoblastic leukemia is the most common childhood disease [1, 2], whilst Bcr-Abl-positive chronic myeloid leukemia (CML) is the most common in adults [2, 2]. The treatment regimens for leukemia will depend upon the leukemia type and the patient’s age and health. Treatments include chemotherapy, radiotherapy, immuno-therapy and bone marrow transplantation [1, 2]. Newer therapies also being used include tyrosine kinase inhibitors such as Imatinib, which specifically target the constitutively active tyrosine kinase domain of Bcr-Abl fusion gene present in the majority of chronic myeloid leukemia [3]. Despite considerable improvements in tolerance and efficacy of these treatments, the mortality rate of leukemia still remains high [1, 2]. Chemotherapies are by far the most commonly used treatments, however, many are expensive, mutagenic, carcinogenic or teratogenic [2]. Patients often experience considerable side effects, which are so severe that patients sometimes withdraw themselves from treatment, which results in poor prognosis [1, 2]. In addition, patients often fail to get complete disease remission, due to increased occurrence of drug resistance. It is for this reason, that it is important to find new treatments that can improve patient survival rates [1, 2].
These problems with current treatments have led to the search for new compounds for the treatment of leukemia. One area that has received great interest is the use of bio-active agents from natural sources [4-10]. Two groups of bioactive components that have shown potential are the polyphenols and polyacetylenes [4-10]. Epidemiological data has shown that diets rich in polyphenols significantly improve the quality of life and survival rates of patients with a range of chronic diseases, including cancer [11, 12]. Furthermore, these polyphenols are found naturally in a variety of foods and are well tolerated, with few side effects [11-13]. The selected polyphenols used in this study are representative of 3 different classes of polyphenols, which have been previously shown to have anti-proliferative, pro-apoptotic and/or prevent the progression of solid tumors [9, 11-16] and a handful of leukemic cell lines, with the most commonly studied being the human promyleocytic: HL-60 cells [17-21]. The polyphenols investigated include the flavonol (quercetin), flavones (apigenin and chrysin), anthraquinones (emodin, aloe-emodin and rhein); and two stilbene isomers (cis-stilbene and trans-stilbene) (Table 1).
Table 1.
The chemical structure and classification of each selected polyphenols.
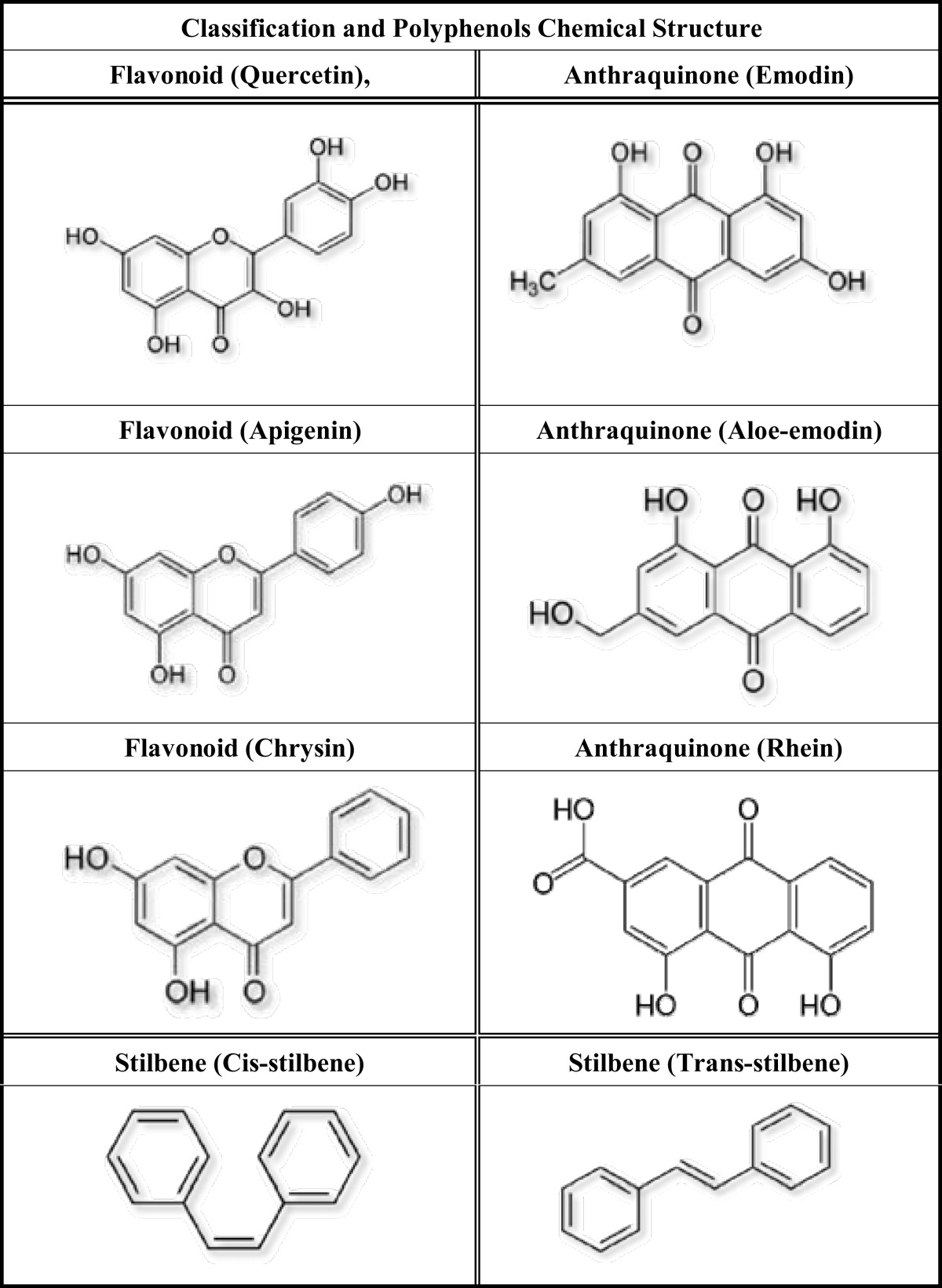 |
This table shows the chemical structure of the flavonoids: flavonol (quercetin) and flavones (apigenin and chrysin); the anthraquinones (emodin, aloe-emodin and rhein); plus the two stilbene isomers (cis-stilbene and trans-stilbene).
Previous work has demonstrated the pro-apoptotic and anti-cancerous activity of polyphenols in a number of solid tumors [11-16] and a selection of leukemic cell lines [17-21]. However, there has not been a comprehensive comparison of the action of polyphenols within a wide range of leukemic cell lines. From previous studies, it is difficult to determine which polyphenols have the greatest potential for the treatment of leukemia. There are no direct comparisons of the IC50 values (the concentration which inhibits 50% of cell proliferation) or AP50 values (the concentration at which 50% of cells undergo apoptosis) for each polyphenol. Furthermore, it is unclear whether a single polyphenol is affective in all leukemia types; or whether specific polyphenols are only useful in single type or subset of leukemia. For this reason we compared the anti-proliferative and pro-apoptotic effects of the 8 polyphenols that have previously shown potential in solid and leukemic cell lines, on a panel of leukemic cell lines that represent the major leukemia types. These included four myeloid (KG-1a, HL-60, THP-1 and K562), three lymphoid (Jurkat, CCRF-CEM and MOLT-3) human leukemic cell lines and one histocytic lymphoma cell line (U937). In addition, for the first time we evaluated the action of these polyphenols on non-tumor hematopoietic stem progenitor cells (CD34+) from cord blood.
The aim of this study was to determine which polyphenols were the most effective at inhibiting cell proliferation and inducing apoptosis in each of the eight leukemic cell lines, whilst having a limited effect on the non-tumor cells. A direct comparison was made of the IC50 and AP50 values of each polyphenol in each cell line. Furthermore, we determined the action of each polyphenol on cell-cycle progression.
2. MATERIALS AND METHODS
2.1. Leukemia Cell Lines
Four myeloid human leukemia cell lines (HL-60 (Human promyelocytic leukemia) (ATCC: CCL-240, Middlesex, UK), THP-1 (acute monocytic leukemia) (ATCC: TIB-202, Middlesex, UK), K562 (chronic myeloid leukemia) (ATCC: CCL-243, Middlesex, UK) and KG-1a (acute mylogenous leukemia)), three human lymphoid cell lines (Jurkat (peripheral blood T cell leukemia) (ATCC: TIB-152, Middlesex, UK), MOLT-3 (acute lymphoblastic leukemia patient released following chemotherapy) (ATCC: CRL-1552, Middlesex, UK), and CCRF-CEM (acute lymphoblastic leukemia) (ATCC: CCL-119, Middlesex, UK)) and one histocytic lymphoma cell line (U937) (ATCC: CRL-1593.2, Middlesex, UK) together with the non-tumor cord blood (CD34+) cells (Stem cell Technologies, Grenoble, France), were used in this study. All leukemia cell lines except MOLT-3 are p53-deficient, being either null, or containing mutant p53 [22-24]. MOLT-3 cells express wild type p53 [25], but are mutant for PTEN [26]. All cells were tested for mycoplasma contamination using the MycoAlert TM mycoplasma detection kit (Lonza Walkersville, Inc) and were all tested negative throughout the study.
2.2. Culture Conditions
Two million cells per milliliter were seeded in T75cm² flasks (Invitrogen, Paisley, UK) in RPMI 1640 medium (Invitrogen, Paisley, UK) supplemented with 10% (v/v) fetal bovine serum, 1.5mM L-Glutamine and 100 µg/ml penicillin/streptomycin (complete RPMI) and incubated at 37ºC with 5% CO2.
2.3. CellTiter-Glo® Luminescent Cell Viability Assay
The CellTiter-Glo® Luminescent Cell Viability Assay Kit (Promega, Southampton, UK) was used as a homogeneous method to determine the number of viable cells in culture was based on a quantification of ATP levels. This assay was used to determine the effect of each polyphenols on cellular proliferation in each of the cell lines. Cells were seeded into white 96-well plates (Fisher Scientific, Loughborough, UK) at 2.5 x 103 cells per well and treated with each polyphenol dissolved in ethanol: quercetin, apigenin, chrysin, emodin, aloe-emodin, rhein, cis-stilbene and trans-stilbene (Sigma, Poole, UK) at concentrations between 2 - 500 µM for 24, 48 and 72 h together with ethanol vehicle controls at 0.1 % (v/v) ethanol. All treatments were performed in triplicate, in three independent experiments. Following treatments, cellular proliferation was measured as per manufacturer’s instructions. The IC50 was determined for each polyphenol in each cell line. This was defined as the treatment concentration at which 50% reduction in cellular proliferation was observed. This was calculated from a linear regression equation of each standard curve for each polyphenol with each cell line. The IC25 was also determined in order to provide treatment ranges for apoptosis detection, and cell cycle treatments, but were not used to determine the effectiveness of treatments.
2.4. Cell Cycle Analysis using Propidium Iodide (PI) and Flow Cytometry
The effect of polyphenols on the progression of the cell cycle was studied using flow cytometric analysis using Propidium Iodide (PI) stain. Propidium Iodide emits red fluorescence when intercalated with double stranded nucleic acids. It can be used to quantify the proportion of cells in each phase of cell cycle (G0/G1, S and G2/M); and determine whether cells are accumulated in a specific phase. For cell cycle analysis, cells were seeded in 12 well plates at 0.5 x 106 cells per well and treated for 24 h with the IC50 concentrations of each polyphenol determined by CellTiter-Glo® assay. Following treatment cells were harvested and centrifuged at 400 g for 5 min. The supernatant was removed, and cells were washed twice in 100 µl cold PBS. Cells were fixed by adding 100 µl of 80% ethanol/H2O (v/v) and stored overnight at -20(C. Then, cells were washed twice with cold PBS prior to addition of 300 µl of 50 µg/mL PI (Sigma, Poole UK) and 50 µl of 0.1 unit/mL RNase (Sigma, Poole UK). Samples were PI stained overnight at 4ºC and analyzed on the flow cytometer with BD FACS Calibur instrument. Ten thousand events were acquired per sample and the DNA histogram of cell cycle phase was analyzed with FlowJo software using the Waston (pragmatic) equation (Tree Star, Ashland, OR, USA).
2.5. Apoptotic Analysis
Cells were seeded in 12 well plates 0.5 x 106 cells per well and treated for 24 h with dose ranges between IC25 and IC50 for each polyphenol as determined from the CellTiter-Glo®. Apoptosis was assessed using the NucView caspase 3 activity assay (Cambridge Bioscience, Cambridge, UK) and morphological assessment of Hoescht 33342 stained cells (Sigma, Poole, UK).
2.5.1. NucView Caspase 3 Activity Assay by Flow Cytometry
The NucView caspase 3 activity assay is a novel cell membrane permeable fluorogenic caspase substrate designed for detecting caspase 3 activity; which is believed to play a key role in the initiation of cellular events during early apoptosis. Using this method, it was possible to determine the AP50 concentrations for each polyphenol in each leukemic cell line. This was defined as the treatment concentration at which 50% of treated cells had undergone apoptosis. Following treatments 200 µl of each cell suspension was transferred to a flow cytometry tube and 5 µl of caspase 3 activity assay (0.2 mM) (Promega, Southampton, UK) was added. This was incubated for 10 min in the dark, and then each sample was analyzed on the flow cytometer using a BD FACS Calibur instrument (BD, Oxford, UK). Ten thousand events were acquired per sample and the data was analyzed using Flow Jo software (Tree Star, Ashland, OR, USA).
2.5.2. Hoechst 33342 Nuclear Morphological Analysis by Fluorescence Microscopy
Apoptotic cells and nuclear morphology was assessed by fluorescence microscopy following Hoechst 33342 nucleic acid staining. Following polyphenol treatments, cells from each culture well was transferred to eppendorf tubes and centrifuged for 5 min at 400 g at 4ºC. The supernatant was removed, and cells washed in 100 µl PBS. The cells were fixed in 4% (w/v) paraformaldhyde/PBS and cytospins formed (Shandon Cytospin 3 Centrifuge, Thermo, US). Samples were air dried and then stained in 50 µl of 10 μg/ml Hoescht 33342 staining (Sigma, Poole, UK) for 10 min in the dark. Slides were mounted in immersion oil and examined using a fluorescence microscope (Olympus, BX60, UK). Two hundred cells (live and apoptotic) were counted and the percentage of apoptotic nuclei determined for each sample. Images were captured using LabWorks 4.0 (UVP BioImaging Systems, Loughborough, UK).
2.6. Statistical Analysis
The means and standard deviations (STD) were calculated. Stats Direct software (Stats Direct Ltd, England) was used to test whether data followed a normal distribution using a Shapiro Wilke test. Data which did not follow a normal distribution, was transformed using the logit transformation and statistically analyzed using one way ANOVA and Tukey post hoc tests to investigate significant differences. Results were considered statistically significant when P ≤ 0.05.
3. RESULTS
3.1. Effects of Polyphenol Treatments on Cell Proliferation in Leukemia Cell Lines
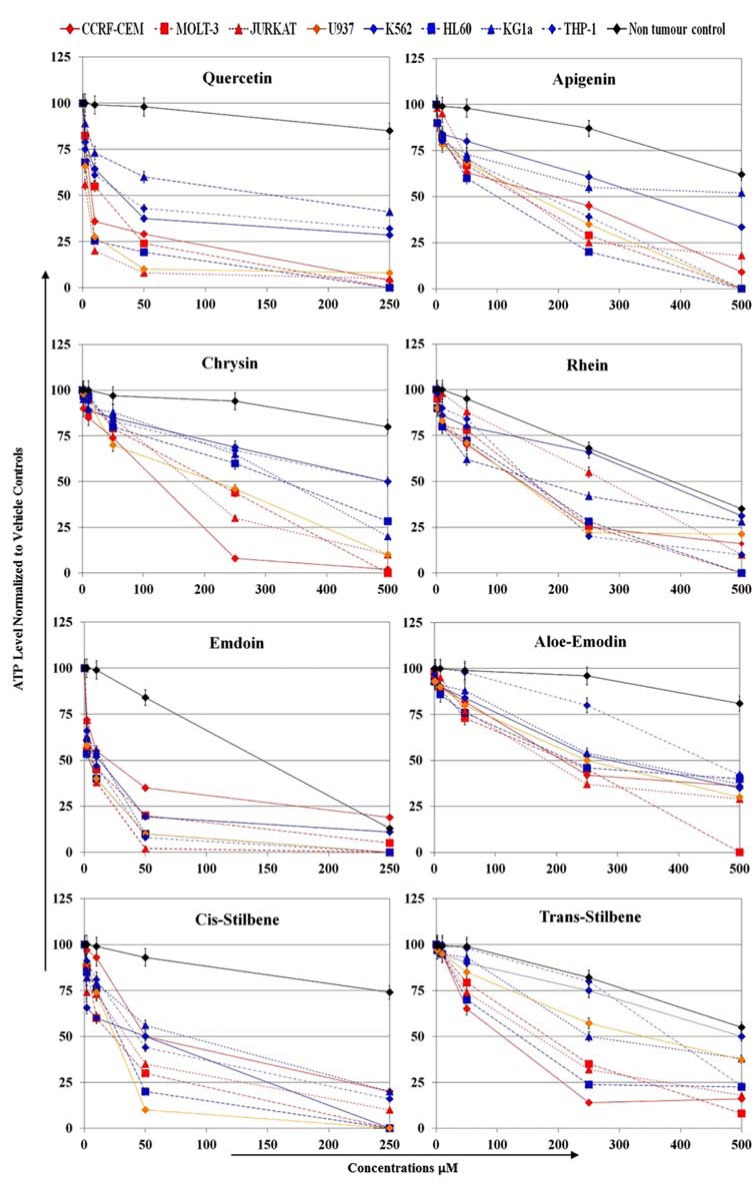
Treatment with polyphenols for 24 h resulted in reduced cell proliferation in all 8 leukemia cell lines to a greater extent than in non-tumor cells (Fig. 1 and Tables 2, 3). Using the lowest dose of polyphenols at which there was a significant inhibition on cellular proliferation (Table 2) and IC50 values (Table 3); it was possible to rank the polyphenols in order of effectiveness. The most effective polyphenols at significantly reducing cell proliferation compared to vehicle controls (p<0.05) were emodin, quercetin, and cis-stilbene (Fig. 1 and Table 2). A more moderate affect was shown by apigenin and rhein; and the least effective polyphenols were aloe-emodin, trans-stilbene and chrysin. Both lymphoid and myeloid leukemia cell lines were sensitive to emodin, quercetin, and cis-stilbene treatment (Fig. 1 and Table 2). However, it is important to note that each leukemia cell line demonstrated differing sensitivity with the remaining polyphenols. Generally, the lymphoid cell lines were usually more sensitive to polyphenol treatment than myeloid cell lines (Fig. 1 and Tables 2, 3).
Fig. (1).
Effect of eight polyphenols (quercetin, apigenin, chrysin, rhein, emodin, aloe-emodin, cis-stilbene and trans-stilbene) on cellular proliferation of three lymphoid leukemia (CCRF-CEM, MOLT-3, JURKAT; red lines), one histocytic lymphoma (U937; orange lines), four human myeloid leukemia cell lines (K562, HL-60, KG-1a , THP-1; blue lines), and one non-tumor normal progenitor cells (CD34+; black line). This was evaluated by CellTiter-Glo® assay. Cells were treated with 0, 2, 10, 50, 250 µM of quercetin, emodin, cis-stilbene; and with 0, 2, 10, 50, 250, 500 µM of apigenin, chrysin, rhein, aloe-emodin, transstilbene for 24 h. Data was normalized to the vehicle control which was assigned 100% cell viability. The data is expressed as mean ± STD (three independent experiments, each in triplicate). The statistical significance was determined by comparison with the vehicle control, statistical significance was set at p<0.05 and determined by one way ANOVA and Tukey post-hoc test. Statistical results are summarised in Table 2 which shows the lowest dose that induced significant inhibition compared to vehicle control. All concentrations above these points were also statistically significant. The IC50 for each polyphenol in each cell line were determined and shown in Table 3.
Table 2.
The lowest dose of polyphenols that induced a significant decrease in cellular proliferation compared to the vehicle controls, p<0.05. Polyphenol treatments were: 0, 2, 10, 50, 250, 500 µM for 24 h.
| Cell Types | The lowest dose of polyphenols (µM) at which there was a significant inhibition of cell proliferation compared to the vehicle control. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quercetin | Apigenin | Chrysin | Rhein | Emodin | Aloe - Emodin | Cis- Stilbene | Trans-Stilbene | |||
| Cell lines | Lymphoid leukaemia | JURKAT | 2 | 50 | 50 | 50 | 2 | 50 | 2 | 50 |
| CCRF-CEM | 2 | 10 | 50 | 50 | 2 | 50 | 10 | 50 | ||
| MOLT-3 | 2 | 50 | 50 | 50 | 2 | 50 | 2 | 50 | ||
| Myeloid leukaemia | HL60 | 2 | 10 | 50 | 50 | 2 | 50 | 2 | 50 | |
| THP-1 | 2 | 10 | 250 | 50 | 2 | 250 | 2 | 250 | ||
| K562 | 2 | 10 | 250 | 50 | 2 | 50 | 2 | 50 | ||
| KG1a | 10 | 10 | 250 | 50 | 2 | 50 | 2 | 50 | ||
| Histocytic lymphoma | U937 | 2 | 10 | 50 | 50 | 2 | 50 | 2 | 50 | |
| Peripheral blood cells | Non-tumour control cells | CD34+ | 250 | 500 | 500 | 250 | 50 | 500 | 250 | 250 |
The polyphenols were ranked in order of activity with respect to significant reduction of cellular proliferation in lymphoid cells (emodin = quercetin > cis-stilbene > apigenin > rhein = trans-stilbene = aloe-emodin = chrysin); and in myeloid cells (emodin = cis-stilbene ≥ quercetin > apigenin > rhein > aloe-emodin = trans-stilbene > chrysin). Note that the treatment doses that caused significant inhibition of cellular proliferation in all leukemic cell lines were much lower than in the non-tumor cells (CD34+). Due to the wide range of concentrations used and the number of cell lines investigated, it was not possible to indicate significance levels on Fig. (1), and thus, Table 2 indicates the lowest dose of polyphenol at which significance was obtained for each cell line, providing the statistical analysis for Fig. (1).
Table 3.
The IC50 values responsible for 50% inhibition of cellular proliferation in each leukemic and non-tumor control cell line following 24 h treatment with each polyphenols.
| Cell Types | Polyphenols IC50 in µM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quercetin | Apigenin | Chrysin | Rhein | Emodin | Aloe - Emodin | Cis- Stilbene | Trans-Stilbene | |||
| Cell lines | Lymphoid leukaemia | JURKAT | 10 | 143 | 180 | 277 | 9 | 185 | 38 | 163 |
| CCRF-CEM | 10 | 195 | 128 | 140 | 22 | 211 | 53 | 109 | ||
| MOLT-3 | 20 | 140 | 217 | 158 | 8 | 220 | 25 | 180 | ||
| Myeloid leukaemia | HL60 | 8 | 100 | 328 | 150 | 5 | 225 | 32 | 135 | |
| THP-1 | 37 | 180 | 500 | 158 | 10 | 450 | 45 | 380 | ||
| K562 | 33 | 350 | 340 | 380 | 13 | 309 | 53 | 500 | ||
| KG1a | 155 | 500 | 335 | 169 | 15 | 310 | 85 | 250 | ||
| Histocytic lymphoma | U937 | 8 | 160 | 217 | 135 | 7 | 250 | 30 | 340 | |
| Peripheral blood cells | Non-tumour control cells | CD34+ | >500 | >500 | >500 | 380 | 150 | >500 | >500 | 500 |
This was determined by CellTiter-Glo® Luminescent assay. The polyphenols were ranked in order of activity with respect to inhibition of 50% proliferation in lymphoid cells (emodin = quercetin > cis-stilbene > apigenin > trans-stilbene ≥ chrysin = rhein > aloe-emodin); and in myeloid cells (emodin = cis-stilbene ≥ quercetin > apigenin = rhein > aloe-emodin = trans-stilbene = chrysin). In Non-tumour cells (CD34+), did not reach 50% inhibition until the polyphenol treatments excessed 500≥M, the only exceptions were emodin and rhein. Note that the highest doses of aloe-emodin, chrysin, rhein and trans-stilbene would be clinically impractical, while quercetin emodin, cis-stilbene had much lower doses and thus are potentially more clinically useful.
Emodin consistently gave the lowest IC50 values (5-22 μM) (Table 3), showing a significant effect on cellular proliferation of all leukemia cell lines, with a slightly greater effect on lymphoid than myeloid cells. Emodin also significantly reduced proliferation in the non-tumor cells (p<0.05). However, the IC50 in the non-tumor cells (~150 μM) was much greater than that seen for all the leukemia cells, demonstrating selectivity towards leukemia cell lines (Fig. 1 and Tables 2, 3). Similarly, quercetin had a more potent effect on lymphoid cell line (IC50 value 8-20 μM) than myeloid cell lines (IC50 33-155 μM). The least sensitive leukemia cell line to quercetin treatment with an IC50 of 155 μM was the acute myelogenous leukemia KG-1a cell line (Fig. 1 and Table 3). However the human promyelocytic leukemia (HL-60) cell line had a much lower IC50 value (8 μM), which was similar to those values seen in lymphoid cells (Fig. 1 and Table 3). Cis-stilbene demonstrated IC50 values of 25-85 μM (Fig. 1 and Tables 2, 3) and affected both lymphoid and myeloid cells equally (Fig. 1).
Apigenin and rhein had a moderate effect on cellular proliferation. Apigenin demonstrated a greater effect on the lymphoid cells (IC50 140-195 μM) compared to the myeloid cells (IC50 100-500 μM) (Fig. 1 and Table 3). Rhein demonstrated a significant decrease in cellular proliferation of all leukemia cell lines and the non-tumor cells (p<0.05), with a similar effect seen in both lymphoid and myeloid cell lines (Fig. 1 and Tables 2, 3).
Aloe-emodin, chrysin and trans-stilbene were the least effective polyphenols on cellular proliferation. Aloe-emodin had IC50 values between 180-450 μM; more than ten times higher than emodin. Aloe-emodin, like emodin, showed a greater effect on lymphoid cell lines than myeloid cell lines (Fig. 1 and Tables 2, 3). Similarly, chrysin demonstrated comparatively high IC50 values, and again was more effective on lymphoid cells (IC50 chrysin 128-217 μM) compared to the myeloid cells (IC50 335-500 μM respectively) (Fig. 1 and Table 3). Trans-stilbene had some of the highest IC50 values ranging between 109-500 μM. These were much higher than those values found with its isomer, cis-stilbene (Fig. 1 and Tables 2, 3). Despite the differing effects on the leukemia cells, trans-stilbene did not affect the cellular proliferation of the non-tumor cells, until the treatment dose reached 500 μM (Fig. 1).
3.2. Cell Cycle Accumulation Following Polyphenol Treatments in Leukemia Cells
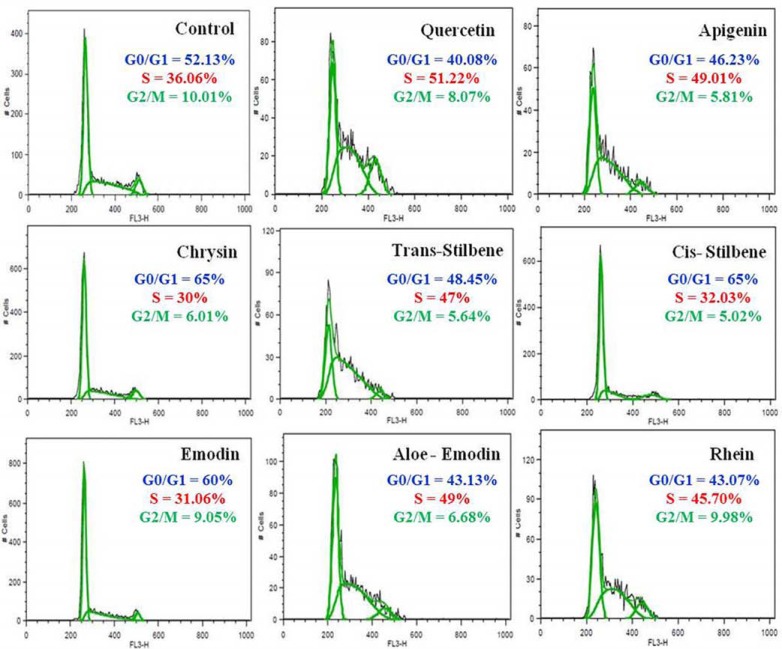
Treatment of leukemic cell lines at the IC50 as determined by CellTiter-Glo® assay of each polyphenol for 24 h significantly induced cell cycle arrest in all leukemia cell lines (p<0.05) (Table 4). There was however no significant arrest in cell cycle in the non-tumor progenitor cells (CD34+) within the IC50 treatment ranges used for leukemic cell lines (Table 4). The phase of cell cycle accumulation varied according to polyphenol treatment and cell line (Table 4). For example, Jurkat cells demonstrated cell cycle accumulation in S-phase following quercetin, apigenin, rhein, aloe-emodin and trans-stilbene treatments (p<0.05) (Fig. 2, Table 4), whilst cells accumulated in G0/G1 phase following chrysin, emodin and cis-stilbene treatment (p<0.05) (Fig. 2 and Table 4). A more consistent effect was seen following emodin treatment, which accumulated the cells at G0/G1 phase in all leukemia cell lines. Similarly cis-stilbene and chrysin also induced cells accumulation at G0/G1 phase in 7 out of the 8 leukemia cell lines (Table 4). Generally, polyphenols appeared to cause G0/G1 phase accumulation in most of leukemic cell lines (Table 4).
Table 4.
The effect of polyphenol treatment on the cell cycle progression in myeloid and lymphoid cell lines.
| Cell lines | Percentage of cells in all phases of cell cycle | The percentage of cells in the phases of cell cycle at which there was a significant accumulation of cells when compared to vehicle controls after treatment with IC50 dose of each polyphenol following 24h. |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vehicle Control | Quercetin | Apigenin | Chrysin | Rhein | Emodin | Aloe - Emodin | Cis- Stilbene | Trans-Stilbene | ||
| Lymphoid leukaemia | JURKAT | G0/G1 = 52.13% S = 36.06% G2/M = 10.01% | S = 51% | S = 49% | G0/G1 = 65% | S = 45.70% | G0/G1 = 60% | S = 49% | G0/G1 =65% | S = 47% |
| CCRF-CEM |
G0/G1 = 41.9% S = 47.2% G2/M = 7.8% |
S = 51.4% | G0/G1 =52.2% | G0/G1 =49.4% | G0/G1 =54% | G0/G1 =50% | G0/G1 =49% | G0/G1 =51.1% | G0/G1 =49.1% | |
| MOLT-3 |
G0/G1 = 64.5% S = 24.8% G2/M = 7.5% |
G2/M =19% | S =35% | G0/G1 =69.3% | G0/G1=72% | G0/G1 =70% | G0/G1 = 68% | G2/M = 15% | G0/G1 = 69% | |
|
Myeloid leukaemia |
HL60 |
G0/G1 = 56.8% S = 33.6% G2/M = 8.45% |
G0/G1 =66.2% | G0/G1=67% | S =45.5% | G0/G1=65% | G0/G1 =67.7% | S =35% | G0/G1 =70.9% | S =41.9% |
| THP-1 |
G0/G1 = 42.3% S = 31.1% G2/M = 26.4% |
G0/G1 =52% | G2/M =32% | G0/G1 =52.1% | G0/G1 =47% | G0/G1= 50.8% | G0/G1 =52% | G0/G1=50% | G0/G1 =53% | |
| K562 |
G0/G1 = 53.7% S = 32.06% G2/M = 13.6% |
G0/G1=60% | S =50.2% | G0/G1 =59% | S =48%% | G0/G1 =66% | G2/M =20% | G0/G1 =64% | G2/M =24.5% | |
| KG1a |
G0/G1 = 44.6% S = 34.6% G2/M = 18.7% |
G0/G1=52% | S = 50% | G0/G1 =50% | G0/G1 =49% | G0/G1 =52.9% | G0/G1 =50% | G0/G1 =51% | G2/M =23.7% | |
| Histocytic lymphoma | U937 |
G0/G1 = 44.03% S = 40.1% G2/M = 19.1% |
G2/M =25% | G0/G1 =59% | G0/G1 =70% | G0/G1 =55% | G0/G1 =60% | G0/G1 =53% | G0/G1 =64% | G0/G1 =62% |
| Non-tumour control cells | CD34+ |
G0/G1 = 53% S = 25.8% G2/M = 20.7% |
No Arrest <50µM | No Arrest ≤250µM | No Arrest <250µM | No Arrest <50µM | No Arrest <50µM | No Arrest <500µM | No Arrest ≤250µM | No Arrest ≤500µM |
The cell cycle phase was assessed by flow cytometric analysis of propidium iodide (PI) stained cells, and the percentage of cells accumulation in each phase of cell cycle (G0/G1, S, G2/M) was determined from the DNA histograms of each sample analysing by FlowJo software using Waston (pragmatic) equation. The data shows the phases of cell cycle in which each cell type was significantly accumulated when compared with the vehicle control, when treated for 24 h with IC50 concentration for each polyphenol, as determined by CellTiter- Glo® assay (p<0.05). The table shows the percentage of cells in each phases of cell cycle at which there was a significant accumulation. No significant arrest in cell cycle was observed in the non-tumor progenitor cells (CD34+) within the IC50 ranges used to treat the leukemic cell lines.
Fig. (2).
An example of the cell cycle phases (G0/G1, S, G2/M) for the acute T cell leukemia (Jurkat) cells after treatment with IC50 concentration of each polyphenol following 24 h as determined by CellTiter-Glo® assay. The percentage of cells in each phase was analyzed with Flow Jo software using Watson pragmatic model. Each polyphenol caused a significant accumulation of cells in cell cycle comparing to the vehicle control. Quercetin, apigenin, trans-stilbene and aloe-emodin and rhein significantly induced accumulation in S-phase (p<0.01), in contrast emodin, cis-stilbene and chrysin significantly induced accumulation in G0/G1 phase (p<0.01).
3.3. Induction of Apoptosis Following Polyphenol Treatments in Leukemia Cells
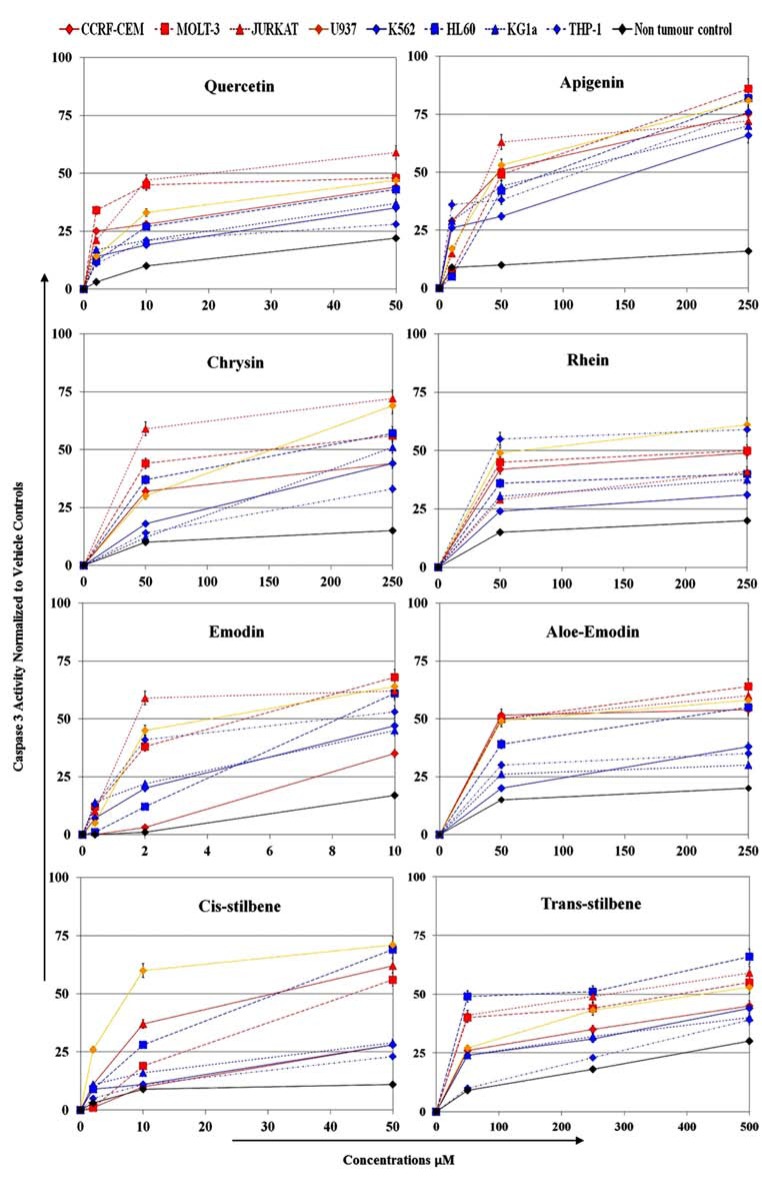
All eight polyphenols induced significantly higher levels of apoptosis determined by caspase 3 activity in all leukemia cell lines compared to the non-tumour cells (p<0.05) (Fig. 3 and Table 5). Emodin, quercetin, cis-stilbene and apigenin were the most effective polyphenols at inducing apoptosis with AP50 values ranging between 2-27 μM, 19-50 μM, 8-50 μM, 35-150 μM, respectively, in all leukemia cell lines (Table 6). The leukemia cell lines demonstrated differing sensitivity to the polyphenols; Jurkat lymphoid cells were most greatly affected, whilst THP-1 myeloid cells were the least affected to all polyphenols treatments (Fig. 3).
Fig. (3).
Effect of eight polyphenols (quercetin, apigenin, chrysin, rhein, emodin, aloe-emodin, two cis-stilbene and trans-stilbene) on apoptosis of three lymphoid leukemia (CCRF-CEM, MOLT-3, and JURKAT; red lines), one histocytic lymphoma (U937; orange lines), four human myeloid leukemia cell lines (K562, HL-60, KG-1a , THP-1; blue lines) and the non-tumor normal progenitor cells (CD34+; black line). Apoptosis was assessed using a caspase 3 activity assay and analyzed by flow cytometry. Cells were treated with range of concentrations for each polyphenol for 24 h and the range of IC25 and IC50 as determined by CellTiter-Glo® assay. The treatment concentrations for emodin were 0, 0.4, 2, 10, 50 µM, for quercetin and cis-stilbene were 0, 2, 10, 50 µM; and for apigenin, chrysin, aloe-emodin, rhein and trans-stilbene were 0, 10, 50, 250 µM. All data was normalized to the vehicle-only control, which was assigned a 0% apoptotic level. The data is expressed as mean ± STD (three independent experiments, each in triplicate). The statistical significance was determined by comparison with the vehicle control, statistical significant was set at p<0.05 and determined by one way ANOVA and Tukey post-hoc test. Statistical results are summarised in Table 5 which shows the lowest dose that induced significant inhibition compared to vehicle control. All concentrations above these points were also significant. The AP50 for each polyphenol in each cell line were determined and shown in Table 6.
Table 5.
The lowest dose of polyphenols which induced significant induction of caspase 3 activity, compared to the control (p<0.05).
| Cell Types | The lowest dose of polyphenols (µM) at which there was a significant induction of apoptosis compared to the vehicle control. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quercetin | Apigenin | Chrysin | Rhein | Emodin | Aloe - Emodin | Cis- Stilbene | Trans-Stilbene | |||
| Cell lines | Lymphoid leukaemia | JURKAT | 2 | 50 | 50 | 50 | 2 | 50 | 10 | 50 |
| CCRF-CEM | 2 | 10 | 50 | 50 | 10 | 50 | 50 | 50 | ||
| MOLT-3 | 10 | 50 | 50 | 50 | 2 | 50 | 10 | 50 | ||
| Myeloid leukaemia | HL60 | 10 | 50 | 50 | 50 | 10 | 50 | 10 | 50 | |
| THP-1 | 10 | 10 | 250 | 50 | 2 | 50 | 50 | 250 | ||
| K562 | 10 | 10 | 250 | 50 | 2 | 50 | 50 | 50 | ||
| KG1a | 10 | 10 | 250 | 50 | 2 | 50 | 10 | 50 | ||
| Histocytic lymphoma | U937 | 2 | 50 | 50 | 50 | 2 | 50 | 2 | 50 | |
| Peripheral blood cells | Non-tumour control cells | CD34+ | 50 | 250 | 250 | 50 | 10 | 250 | 250 | 250 |
Apoptosis was assessed by caspase 3 activity assay. The polyphenols were ranked in order of activity with respect to significant induction of apoptosis in lymphoid cells (emodin = quercetin ≥ cis-stilbene > apigenin > rhein = trans-stilbene = aloe-emodin = chrysin); and in myeloid cells (emodin > quercetin > cis-stilbene = apigenin > rhein = aloe-emodin ≥ trans-stilbene > chrysin). Note that the treatment doses which caused significant induction of apoptosis in all leukemic cell lines were much lower than of the non-tumor cells (CD34+). Due to the wide range of concentrations used and the cell lines investigated, it was not possible to indicate significance levels on Fig. (3) and thus Table 5 provides the lowest doses of polyphenol at which significance was obtained.
Table 6.
The AP50 values responsible for 50% induction of apoptosis, determined by: Caspase 3 activity assay (C3) and Hoechst 33342 staining (Hoe).
| Cell Types | Polyphenols AP50 in µM | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quercetin | Apigenin | Chrysin | Rhein | Emodin | Aloe - Emodin | Cis- Stilbene | trans-Stilbene | |||||||||||
| C3 | Hoe | C3 | Hoe | C3 | Hoe | C3 | Hoe | C3 | Hoe | C3 | Hoe | C3 | Hoe | C3 | Hoe | |||
| Cell lines | Lymphoid leukaemia | JURKAT | 19 | 25 | 35 | 90 | 40 | 30 | >250 | >500 | 2 | 9 | 50 | 130 | 31 | 50 | 250 | 310 |
| CCRF-CEM | 50 | 50 | 50 | 100 | 250 | 40 | 200 | 250 | >10 | >500 | 50 | 150 | >50 | >500 | >500 | 200 | ||
| MOLT-3 | 50 | 50 | 50 | 125 | 140 | 38 | 200 | 265 | 5 | 9 | 50 | 150 | 44 | 50 | 350 | 400 | ||
| Myeloid leukaemia | HL60 | 50 | 50 | 84 | 129 | 175 | 75 | >250 | >500 | 8.5 | 6 | 185 | 190 | 31 | 49 | 40 | 50 | |
| THP-1 | >50 | >500 | 110 | 220 | >250 | >500 | 50 | 60 | 7.8 | 10 | >250 | 283 | >50 | >500 | >500 | >500 | ||
| K562 | >50 | 205 | 150 | 190 | >250 | >500 | >250 | >500 | >10 | >500 | >250 | 500 | >50 | 410 | >500 | 460 | ||
| KG1a | >50 | 125 | 89 | 235 | >250 | >500 | >250 | >500 | >10 | >500 | >250 | 360 | >50 | 350 | >500 | 360 | ||
| Histocytic lymphoma | U937 | 50 | 50 | 45 | 130 | 150 | 32 | 60 | 140 | 4 | 27 | 50 | 195 | 8 | 20 | 200 | 225 | |
| Peripheral blood cells | Non-tumour control cells | CD34+ | >50 | >500 | >250 | >500 | >250 | >500 | >250 | >500 | >10 | >500 | >250 | >500 | >50 | >500 | >500 | >500 |
The polyphenol were ranked in order of induction of 50% apoptosis in lymphoid cells (quercetin ≥ emodin = cis-stilbene > apigenin > aloe-emodin > chrysin ≥ rhein > trans-stilbene). The HL-60 human promyelocytic leukemia cell line was the only myeloid cell to reach an AP50. The non-tumour cells (CD34+) did not reach 50% apoptosis with any of the treatment doses investigated. THP-1, K562 and KG-1a myeloid cell lines were the most resistant cell lines, although did they reached an AP50 with apigenin treatment.
Quercetin, apigenin, emodin, aloe-emodin and chrysin demonstrated a greater toxicity towards lymphoid leukemia cell lines than myeloid leukemia cell lines (Fig. 3 and Tables 5, 6). In contrast, rhein, cis-stilbene and trans-stilbene demonstrated similar sensitivity to both myeloid and lymphoid cell lines. Some cell lines were more resistant to polyphenol treatment. The THP-1 myeloid cell line was only sensitive to emodin, rhein and apigenin treatment (Fig. 3 and Tables 5, 6); whilst the myeloid cell lines (K562 and KG-1a) were only sensitive to apigenin treatment (Fig. 3 and Table 6).
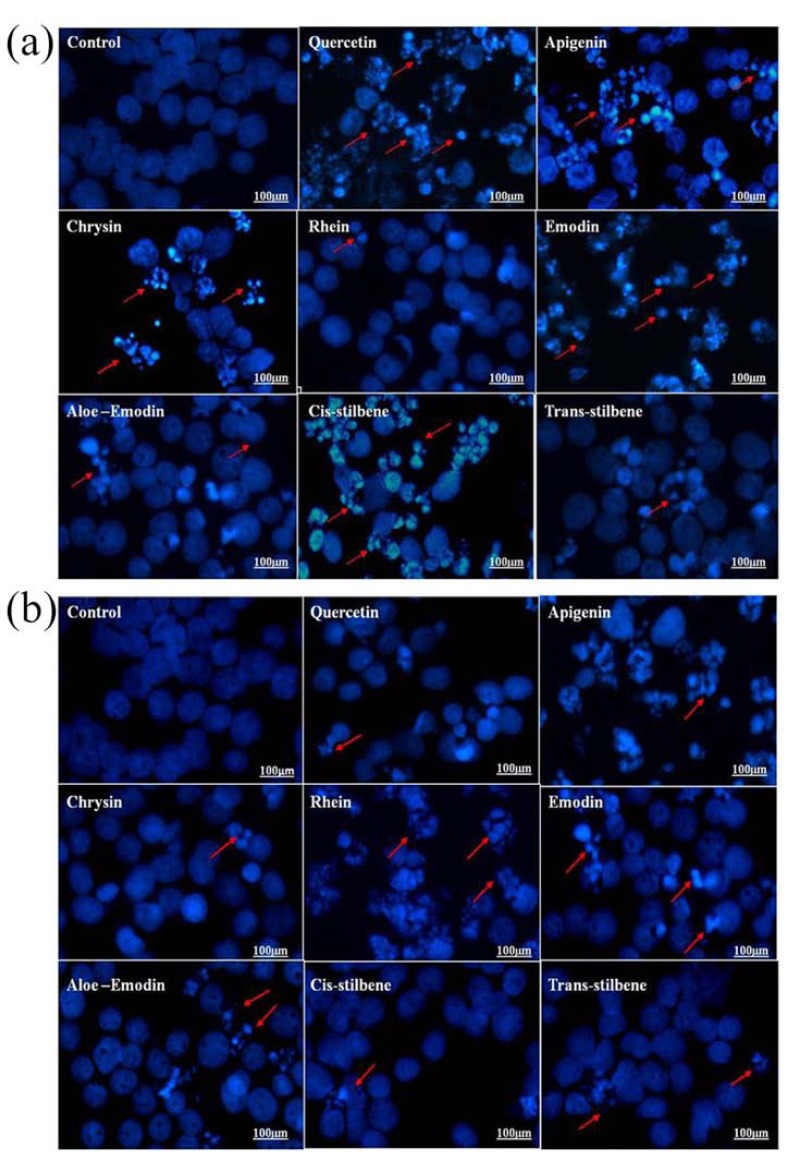
Morphological assessment of apoptosis by Hoechst 33342 staining confirmed the patterns of apoptosis induction seen in caspase 3 activity assays, although the AP50 values determined using this method were higher demonstrating that the progression to the later stages of apoptosis required a greater treatment dose (Fig. 4 and Table 6).
Fig. (4).
A typical example of morphological assessment of apoptosis using Hoechst 33342 nuclear staining, after treatment with the IC50 concentration for each polyphenol for 24 h: (A) Shows the Jurkat lymphoid leukemia cells, which were one of the most sensitive cell lines to polyphenol treatment and (B) Shows THP-1 myeloid leukemia cells, which were one of the most resistant cell lines to polyphenol treatment. Apoptotic cells were identified by their irregular shape, intensely stained nuclei, cell shrinkage, blebbing and chromatin condensation and the formation of apoptotic bodies. Scale bar = 100 µM. Arrow indicates examples of apoptotic cells.
4. DISCUSSION
Over the past 10 years, researchers have confirmed that dietary polyphenols are capable of inhibiting cell proliferation, inducing cell cycle arrest and apoptosis in a number of solid tumor cell lines [11-16], however there has not been a direct comparison of the effect of polyphenols on leukemia cell lines and non-tumor cells. Here, we directly compared the effect of eight polyphenols (quercetin, apigenin, chrysin, rhein, emodin, aloe-emodin, cis-stilbene and trans-stilbene) on four lymphoid and three myeloid leukemia cell lines; one histocytic leukemia cell line; and the non-tumor blood progenitor cells (CD34+). The effects of these polyphenols were shown to be greater in leukemia cells compared to non-tumor blood progenitor cells (CD34+). When non-tumor cells were treated with quercetin and cis–stilbene, chrysin, apigenin and aloe-emodin, there were no significant decrease on cellular proliferation until the treatment concentration increased to 250-500 µM. There was a significant decrease on proliferation of non-tumor cells when treated with ≥250 µM of emodin, rhein and trans-stilbene; however this is 5-10 times higher than the IC50 values reported for all leukemia cells (Fig. 1 and Tables 2, 3). Consequently, we have shown that each of the polyphenols caused a decrease in proliferation in all leukemia cell lines and can be ranked according to their effectiveness: emodin > quercetin > cis-stilbene > apigenin ≥ rhein > aloe-emodin ≥ trans-stilbene ≥ chrysin. However, it is important to note that this ranking did vary between individual cell lines (Table 3).
Emodin was the most effective polyphenol at reducing cellular proliferation. It was by far the most effective of the anthraquinones investigated. The structural differences between the anthraquinones are slight and, indeed, emodin and aloe-emodin have the same structural formula (C15H10O5), although the orientations of the functional groups vary. The IC50 values for emodin (5-22 µM) were the lowest of all the studied polyphenols; and were comparable with those previously reported in squamous cell carcinoma (SCC-4) cells [27]. Emodin was shown to consistently induce accumulation of cells at G0/G1 phase in all leukemia cell lines, and induced 50% apoptosis in 5 of the 8 leukemia cell lines (Jurkat, MOLT3, HL-60, THP-1 and U937). This is consistent with previous studies in which emodin induced apoptosis in HL-60 [28] and SCC-4 [27] cells.
Quercetin was also a potent polyphenol, with IC50 value ranging between 8-33 µM and induction of apoptosis with AP50 value ranging between 19-50 µM. Quercetin was the most effective of the flavonoids tested and was routinely 5-10 times more potent than apigenin and chrysin. The IC50 values noted are at the lower end of values previously reported (20-278 µM), in breast (MDA-MB-231 and MDA-MB-453 [29, 30], MCF-7 [31]), cervical (HeLa) [32-34], liver (HepG2) [35], lung (A-549) [36] and leukemia cell lines (HL-60 and K562) [17, 37]. Lymphoid cell lines were more susceptible to quercetin treatment than myeloid leukemia cells. The only exception being the promyelocytic leukemia cells (HL-60), which
showed the same level of sensitivity as lymphoid cells. Quercetin demonstrated a differential induction of apoptosis in each leukemia cell line although the AP50 values were consistently low. Previously, quercetin has been reported to induce apoptosis in a range of solid tumors, via a caspase 3-dependent mechanism [29, 31 38], and in HL-60 cells via decreased PI3K/AKT pathway activity [39]. However, there are no reported AP50 values for these studies. Quercetin was found to have a differential effect on the cell cycle in myeloid and lymphoid cell lines. Inducing accumulation of cells at G0/G1 phase in all myeloid cell lines, and either S-phase (Jurkat and CCRF-CEM) or G2/M phase (U937 and MOLT3) accumulation in the lymphoid cell lines (Table 4). This varied effect of quercetin has been previously observed, where it induced accumulation in S-phase in breast cancer (MCF-7) [31] and in G2/M phase in cervical cancer (HeLa) cell lines [32, 33]. Together with our study, this suggests that quercetin causes differential effects on cell cycle dependant on cell type, even in comparatively similar leukemia cell lines. This may reflect expression of different molecular target in myeloid and lymphoid cell lines; or a differential effect on the same pathway in different cell lineages.
Cis-stilbene was much more effective than its isomer trans-stilbene in all leukemia cell lines. This is reflected in IC50 values for cis-stilbene (25-85 µM) and trans-stilbene (109-500 µM); however, these values were considerably higher than those previously reported in solid tumors [40, 41]. Very few studies have investigated the effects of stilbenoids on cell cycle. Cis-stilbene has been reported to induce cells accumulation in G2/M phase in the lung cancer cell line (A549) [42], and in S-phase in one leukemia cell line (HL-60) [19]. Our results have shown that cis-stilbene consistently caused cell accumulation at G0/G1 phase in 7 of the 8 cell lines including the HL-60 cell line. This contrasts with the finding that HL-60 cells when treated with the stilbene derivate 3, 3', 4, 4', 5, 5'-hexahydroxystilbene induced S-phase accumulation [19]. A less consistent effect was found with trans-stilbene treatment, which caused cell accumulation at different phases of cell cycle in all leukemia cell lines. Both cis- and trans-stilbene induced caspase-3 activity of early apoptosis and morphological changes characteristic of late apoptosis in the majority of leukemia cell lines. Cis-stilbene was able to induce apoptosis in three lymphoid cell lines (Jurkat, MOLT3, and U937) with AP50 values ranging between 20-50 µM, the remaining cell lines were more resistant, and did not reach 50% apoptosis even when treated with a maximal treatment dose (500µM). Similarly, trans-stilbene induced apoptosis in the same three lymphoid cell lines, however the AP50 values were much higher (40-460 µM), there was also a similar resistance to treatment in the remaining lymphoid cell lines. Cis-stilbene did not show any significant effect on proliferation of the non-tumor cells, however, trans-stilbene did, but only at high treatment concentration in excess of 250 μM. Previous work has shown that stilbenoids can inhibit cell proliferation and induce apoptosis in lung (A549) [42, 43], prostate (DU145 and PC3), breast (BT-549), colon (HT-29) [40, 41] and one leukemia (HL-60) [19, 20] cell line. Trans-stilbene had a reported IC50 values of 25-98 μM at 24 h in two lung cancer cell lines (A549 and CH27) [43]. A direct comparison of cis-stilbene and trans-stilbene in lung (A549) [42] and leukemia (HL-60) [19] cell lines, demonstrated that cis-stilbene was more effective than trans-stilbene with IC50 values of 0.03 μM and 6.25 µM, respectively, at 24 h [19, 42]. This supports the finding of this study that cis-stilbene is more potent than trans-stilbene in the treatment of leukemia cells. However, the reason for this difference is not clear, but may be related to the stability of the trans- and cis-isomers in culture.
A moderate effect was seen in leukemia cells treated with apigenin, with IC50 values between 100-500 µM. However, in other cell types lower IC50 values have been reported, including 36 µM in human cervical cancer cells (HeLa) [44] and 70 µM in colorectal cancer cells (SW480, HT-29 and Caco-2) following 24 h treatments [45] suggesting differential activity within tumour types. In addition, apigenin induced variable effects on cell cycle, which was dependant on the cell lines investigated. This phenomena has also been seen in solid tumor cell lines, where apigenin induced G0/G1 arrest in human cervical cancer (HeLa) cells [44] and G2/M arrest in human colon carcinoma (SW480, HT-29 and Caco-2) [45]. Apigenin was shown to induce apoptosis in all leukemia cell lines, with AP50 values ranging between 35-130 µM in lymphoid cell lines and 84-235 µM in the myeloid cell lines. In contrast to quercetin, apigenin was capable of inducing both an increase in caspase 3 indicating early apoptosis, plus morphological evidence of late apoptosis, in all leukemia cells lines; including the KG-1a and K562 cells which were resistant to emodin, quercetin and cis-stilbene treatment. This pro-apopotic action of apigenin has been previous demonstrated in MDA-MB-453 breast cells [30]. The other polyphenols investigated; rhein, chrysin, aloe-emodin demonstrated a low potency and thus are unlikely to be of clinical use in leukemia treatment. Similar low potency has also been shown in solid tumors, for example the reported IC50 for chrysin in solid tumor cell lines are between 40 and 100μM [46-48].
Within all the polyphenols agents tested, the leukaemia cells were more sensitive than the CD34+ non-tumour cells. Interestingly, the proliferation rates and percentages of G0/G1 population were comparable in all untreated leukaemia and non-tumour control cells, suggesting sensitivity rates were not related to rates of proliferation. The order of sensitivity within the leukaemia cells was shown to be dependent on the polyphenol investigated. For example, U937 cells were one of the most affected cell lines when treated with quercetin, emodin and cis- stilbene, however they were the were least affected cell line when treated with apigenin. This demonstrates that no single polyphenol is active on all cell lines and that specific polyphenols should be selected for each type of leukaemia.
The cell cycle arrest data showed predominately G0/G1 arrest, however some treatments arrested cells in S-phase and G2M. It is well known that cell cycle is regulated by the coordinated activity of family of protein kinases: cyclin-dependent kinase (CDKs), cyclins and CDK inhibitors (CDKIs) [49]. Cell-cycle can be arrested via protein kinase inhibitors (CDKIs), such as p21waf1 and p27kip1, upon binding to cyclins and CDK complexes and indeed modulation of their activities could be possible targets for the polyphenols. The stage of cell cycle arrest induced by phenolic agents can indicate the molecular mechanisms of action. For example it is well known that cells arrested in G1 phase can be via inhibition of CDK4 and/or CDK6 [49]. S-phase arrest can be caused by inhibition of Cyclin A and Cyclin E through the activation of p21 (via p53 in the presence of DNA damage) and p27 (activated by Transforming Growth Factor of β (TGF- β)) [49]. Arrest of cells in G2/M phase can be caused by inactivation of cyclin B1 with Cdc2 kinase activity through p53 activation [49].
Here, we demonstrated that the majority of polyphenols investigated induce G0/G1 arrest, suggesting that they may inhibit CDK4 and/or -6, however this requires confirmation. Hur, et.al (2004) showed that Jurkat cells and T lymphocytes stimulated with rosmarinic acid induce p56lck (Lck) protein kinase-dependant apoptosis, through the mitochondrial pathway [50]. P56lck is a lymphoid-specific protein tyrosine kinase and is usually expressed on T lymphocytes [50]. This may explain why the lymphoid cell lines were more sensitive than myeloid cell lines. In addition, recent investigations showed that polyphenols such as the flavanoids (apigenin and quercetin) can act as a p56lck (Lck) protein kinase inhibitors [50, 51]. As p56lck is an essential regulator of the cell cycle; modulation of this kinase could lead to the G0/G1 arrest. However, further investigation is essential to determine the molecular mechanisms of each polyphenol.
It is well established that tumor suppressor gene p53 has a role in the regulation of the cell cycle, as well as in the initiation of apoptosis. However the majority of our cell lines were either null or mutated for p53, with the exception of MOLT3 which express wild type p53 [22-25]. MOLT 3 cells however, display PTEN mutations, which results in constitutive activity of AKT [26]. p53 induces Bax, which leads to activation of the intrinsic apoptotic pathway. AKT promotes pro-apoptotic BAD to be sequestered. Therefore a lack of p53 or PTEN both lead to an insensitivity to apoptosis with respect to the intrinsic pathway [52]. This suggests that the p53 status does not influence the effect of polyphenol treatment in this study.
To determine whether the effects of these polyphenols in vitro are relevant to their clinical use, it is essential also to consider their bioavailability and whether these treatment concentrations are achievable in plasma. It has been suggested that physiological concentrations of plasma metabolites will not exceed 10 µM [53-55]. Our study has shown that quercetin, emodin and cis- stilbene induced significant affects at low doses (between 2 to 10 µM) following 24 h of treatment in most of leukemic cell lines. The data available on bioavailability of polyphenols however is still limited, but there is evidence that quercetin obtained from plant products can result in micromolar concentrations in blood plasma [54, 56], supporting the idea that in vivo effects may be possible, through diet. However, quercetin has a reported plasma half-life of 11–28 h; with a 50-100 mg dose causing a plasma concentration of 0.75–1.5 µM in plasma [53-56]. This is further complicated as abundant dietary polyphenols do not necessarily have the best bioavailability profile [53, 55] and they are extensively metabolized by intestinal and hepatic enzymes and microflora [53, 57]. The absorption of polyphenols depends primarily on their chemical structure, and molecular size as well as the degree of glycosylation, esterification, and polymerization with other polyphenols [53, 55, 57, 58].
In conclusion, we have shown that the effectiveness of polyphenols varied depending on the leukemia cell lineage (lymphoid vs. myeloid) and in some cases within the cell lines from the same lineage. We have shown that myeloid cell lines (K562 and KG-1a) were particularly resistant even to the most active polyphenols. This suggests that the molecular mechanism of action of the polyphenols may vary in each cell line and this requires further investigation. Furthermore, we have demonstrated that polyphenols with similar molecular structures such as emodin and aloe-emodin, and even cis- and trans-stilbene do not have the same effect on leukemia cells. These findings suggest that polyphenols have anti-tumor activity against leukemia cells with differential effects. The observed differential sensitivity between leukemia and normal cells suggests that polyphenols have potential in treatment of leukemia. The most potent polyphenols are emodin, quercetin, and cis-stilbene; these polyphenols may have potential in treating leukemia.
ACKNOWLEDGEMENTS
The study was funded by Ministry of Higher Education - Saudi Arabia.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
LIST OF ABBREVIATIONS
AP50 = the concentration which 50% of cells undergo apoptosis.
REFERENCES
- 1.The Leukemia & Lymphoma society. Available at http://www.lls.org/#/ diseaseinformation/leukemia/. Accessed July 26. 2012 [Google Scholar]
- 2.Gerber DEl. Targeted therapies: A new generation of cancer treatments. Am Fam Physician. 2008;77(3):311–319. [PubMed] [Google Scholar]
- 3.Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal G M, Fanning S, et al. Effects of a selective inhibitor of the abl tyrosine kinase on the growth of bcr-abl positive cells. Nature Med. 1996; 2(5): 561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 4.Dahlawi H, Jordan-Mahy N, Clench MR, Le Maitre CL. Bioactive actions of pomegranate fruit extracts on leukemia cell lines in vitro hold promise for new therapeutic agents for leukemia. Nutr. Cancer. 2012;64(1):100–110. doi: 10.1080/01635581.2012.630155. [DOI] [PubMed] [Google Scholar]
- 5.Spagnuolo C, Russo M, Bilotto S, Tedesco I, Laratta B, Russo GL. Dietary polyphenols in cancer prevention: The example of the flavonoid quercetin in leukemia. Ann. NY Acad. Sci. 2012;1259(1):95–103. doi: 10.1111/j.1749-6632.2012.06599.x. [DOI] [PubMed] [Google Scholar]
- 6.Zaini R, Clench MR, Le Maitre CL. Bioactive chemicals from carrot (daucus carota) juice extracts for the treatment of leukemia. J. Med. Food. 2011;14(11):1303–12. doi: 10.1089/jmf.2010.0284. [DOI] [PubMed] [Google Scholar]
- 7.Zaini RG, Brandt K, Clench MR, Le Maitre CL. Effects of bioactive compounds from carrots (daucus carota L.: polyacetyenes beta-carotene and lutein on human lymphoid leukemia cells. Anti-Cancer Agents Med. Chem . 2012; 12(6):640–652. doi: 10.2174/187152012800617704. [DOI] [PubMed] [Google Scholar]
- 8.McDougall GJ, Dobson P, Jordan-Mahy N. Effect of different cooking regimes on rhubarb polyphenols. Food Chem. 2010;119(2):758–764. [Google Scholar]
- 9.Huang Q, Lu G, Shen HM, Chung M, Ong CN. Anti-cancer properties of anthraquinones from rhubarb. Med. Res. Rev. 2007;27(5):609–630. doi: 10.1002/med.20094. [DOI] [PubMed] [Google Scholar]
- 10.Dahlawi H, Jordan-Mahy N, Clench MR, Le Maitre CL. Polyphenols are responsible for the proapoptotic properties of pomegranate juice on leukemia cell lines. Food Sci. Nutr. 2013;1(2):196–208. doi: 10.1002/fsn3.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai J, Mumper RJ. Plant phenolics Extraction analysis and their antioxidant and anticancer properties. Molecules. 2010;15(10): 7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han X, Shen T, Lou H. Dietary polyphenols and their biological significance. Int. J. Mole. Sci. 2007;8(9): 950–988. [Google Scholar]
- 13.Jaganathan SK, Mandal M. Antiproliferative effects of honey and of its polyphenols A review. J Biomed Biotechnol 2009. 2009:830616. doi: 10.1155/2009/830616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: Progress, potential and promise (review). Int. J. Oncol. 2007;30(1):233. [PubMed] [Google Scholar]
- 15.Sharif T, Auger C, Alhosin M, Ebel C, Achour M, Étienne-Selloum N, et al. Red wine polyphenols cause growth inhibition and apoptosis in acute lymphoblastic leukemia cells by inducing a redox-sensitive up-regulation of p73 and down-regulation of UHRF1. Eu. J. Cancer. 2010;46(5): 983–994. doi: 10.1016/j.ejca.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 16.Shukla S, Gupta S. Apigenin A promising molecule for cancer prevention. Pharm. Res. 2010;27(6): 962–978. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang T, Liang N. Studies on the inhibitory effects of quercetin on the growth of HL-60 leukemia cells. Biochem. Pharmcol. 1997;54(9): 1013–1018. doi: 10.1016/s0006-2952(97)00260-8. [DOI] [PubMed] [Google Scholar]
- 18.Ramos AM, Aller P. Quercetin decreases intracellular GSH content and potentiates the apoptotic action of the antileukeamia drug arsenic trioxide in human leukemia cell lines. Biochem. Pharmacol. 2008;75(10):1912–1923. doi: 10.1016/j.bcp.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Saiko P, Horvath Z, Murias M, Handler N, Jaeger W, Erker T, et al. Antitumor effects of 3 3' 4 4' 5 5' -hexahydroxystilbene in HL-60 human promyelocytic leukeamia cells. Nucleoides Nucleotides and Nucleic Acids. 2006; 25(9-11):1013–1017. doi: 10.1080/15257770600890624. [DOI] [PubMed] [Google Scholar]
- 20.Simoni D, Roberti M, Invidiata FP, Aiello E, Aiello S, Marchetti P, et al. Stilbene-based anticancer agents: Resveratrol analogues active toward HL-60 leukeamia cells with a non-specific phase mechanism. Bioorg. Med. Chem. Lett. 2006;16(12): 3245–3248. doi: 10.1016/j.bmcl.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 21.Vargo MA, Voss OH, Poustka F, Cardounel AJ, Grotewold E, Doseff AI. Apigenin-induced-apoptosis is mediated by the activation of PKCd and caspases in leukemia cells. Biochem. Pharmacol. 2006;72(6): 681–692. doi: 10.1016/j.bcp.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 22.An WG, Hwang SG, Trepel JB, Blagosklonny MV. Protease inhibitor-induced apoptosis accumulation of wt p53.p21WAF1/ CIP1 and induction of apoptosis are independent markers of proteasome inhibition. Leukemia. 2000;14(7):1276–1283. doi: 10.1038/sj.leu.2401812. [DOI] [PubMed] [Google Scholar]
- 23.Durland-Busbice S, Reisman D. Lack of p53 expression in human myeloid leukemias is not due to mutations in transcriptional regulatory regions of the gene. Leukemia. 2002;16(10):2165–2167. doi: 10.1038/sj.leu.2402647. [DOI] [PubMed] [Google Scholar]
- 24.Geley S, Hartmann BL, Hattmannstorfer R, Loffler M, Ausserlechner MJ, Bernhard D, Sgonc R, Strasser-Wozak EMC, Ebner M, Auer B, Kofler R. p53-induced apoptosis in the human T-ALL cell line CCRF-CEM. Oncogene. 1997;15(20):2429–2437. doi: 10.1038/sj.onc.1201399. [DOI] [PubMed] [Google Scholar]
- 25.Cai Z, Lin M, Wuchter C, Ruppert V, Dorken B, Ludwig WD, Karawajew L. Apoptotic response to homoharringtonine in human wt p53 leukemic cells is independent of reactive oxygen species generation and implicates Bax translocation, mitochondrial cytochrome C release and caspase activation. Leukemia. 2001;15(4): 567–574. doi: 10.1038/sj.leu.2402067. [DOI] [PubMed] [Google Scholar]
- 26.Medyouf H, Gao XG, Armstrong F, Gusscott S, Liu Q, Geldman A L, Matherly L H, Schultz K R, Pflumio F, You M J, Weng AP. Acute T-Cell Leukemias remain dependent on Notch signalling despite PTEN and INK4A/ARF loss. Blood. 2010;115(6): 1175–1184. doi: 10.1182/blood-2009-04-214718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen YY, Chiang SY, Lin JG, Ma YS, Liao CL, Weng SW, et al. Emodin aloe-emodin and rhein inhibit migration and invasion in human tongue cancer SCC-4 cells through the inhibition of gene expression of matrix metalloproteinase-9. Int J Oncol. 2010;36(5):1113–1120. doi: 10.3892/ijo_00000593. [DOI] [PubMed] [Google Scholar]
- 28.Chen YC, Shen SC, Lee WR, Hsu FL, Lin HY, Ko CH. Emodin induces apoptosis in human promyelocytic leukeamia HL-60 cells accompanied by activation of caspase 3 cascade but independent of reactive oxygen species production. Biochem. Pharmacol. 2002;64(12): 1713–1724. doi: 10.1016/s0006-2952(02)01386-2. [DOI] [PubMed] [Google Scholar]
- 29.Chien SY, Wu YC, Chung JG, Yang JS, Lu HF, Tsou MF. Quercetin-induced apoptosis acts through mitochondrial-and caspase-3-dependent pathways in human breast cancer MDA-MB-231 cells. Human Exp. Toxicol. 2009;28(8): 493–503. doi: 10.1177/0960327109107002. [DOI] [PubMed] [Google Scholar]
- 30.Choi EJ, Kim GH. Apigenin induces apoptosis through a mitochondria/caspase-pathway in human breast cancer MDA-MB-453 cells. J Clin Biochem. Nutr. 2009;44(3):260. doi: 10.3164/jcbn.08-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chou CC, Yang JS, Lu HF, Ip SW, Lo C, Wu CC. Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Arch Pharm Res. 2010;33(8):1181–1191. doi: 10.1007/s12272-010-0808-y. [DOI] [PubMed] [Google Scholar]
- 32.Huang LQ, Zhang W, Yang Y, Tao L. Effects and its mechanism of quercetin on cervical cancer HeLa cells. Zhonghua Fu Chan Ke Za Zhi. 2009;44(6):436–439. [PubMed] [Google Scholar]
- 33.Vidya Priyadarsini R, Senthil Murugan R, Maitreyi S, Ramalingam K, Karunagaran D, Nagini S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-?B inhibition. Eu. J. Pharmacol. 2010;649(1):84–91. doi: 10.1016/j.ejphar.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W, Zhang F. Effects of quercetin on proliferation apoptosis adhesion and migration, and invasion of HeLa cells. Eu J Gynaecol Oncol. 2009;30(1): 60–64. [PubMed] [Google Scholar]
- 35.Granado-Serrano AB, Martín MA, Bravo L, Goya L, Ramos S. Quercetin induces apoptosis via caspase activation, regulation of bcl-2, and inhibition of PI-3-kinase/Akt and ERK pathways in a human hepatoma cell line (HepG2). J Nutr. 2006;136(11):2715–2721. doi: 10.1093/jn/136.11.2715. [DOI] [PubMed] [Google Scholar]
- 36.Robaszkiewicz A, Balcerczyk A, Bartosz G. Antioxidative and prooxidative effects of quercetin on A549 cells. Cell Biol Int. 2007;31(10): 1245–1250. doi: 10.1016/j.cellbi.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Csokay B, Prajda N, Weber G, Olah E. Molecular mechanisms in the antiproliferative action of quercetin. Life Scinces. 1997; 60(24):2157–2163. doi: 10.1016/s0024-3205(97)00230-0. [DOI] [PubMed] [Google Scholar]
- 38.Choi EJ, Bae SM, Ahn WS. Antiproliferative effects of quercetin through cell cycle arrest and apoptosis in human breast cancer MDA-MB-453 cells. Arch Pharmacal Res. 2008;31(10): 1281–1285. doi: 10.1007/s12272-001-2107-0. [DOI] [PubMed] [Google Scholar]
- 39.Yuan Z, Long C, Junming T, Qihuan L, Youshun Z, Chan Z. Quercetin-induced apoptosis of HL-60 cells by reducing PI3K/Akt. Mol Biol Reports. 2012:1–9. doi: 10.1007/s11033-012-1621-0. [DOI] [PubMed] [Google Scholar]
- 40.Shankar S, Siddiqui I, Srivastava RK. Molecular mechanisms of resveratrol (3, 4, 5-trihydroxy-trans-stilbene) and its interaction with TNF-related apoptosis inducing ligand (TRAIL) in androgen-insensitive prostate cancer cells. Mol Cellular Biochem. 2007;304(1):273–285. doi: 10.1007/s11010-007-9510-x. [DOI] [PubMed] [Google Scholar]
- 41.Yang LM, Lin SJ, Hsu FL, Yang TH. Antitumor agents.part 3 Synthesis and cytotoxicity of new trans-stilbene benzenesulfonamide derivatives. Bioorg. Med. Chem. Lett. 2002;12(7):1013–1015. doi: 10.1016/s0960-894x(02)00092-6. [DOI] [PubMed] [Google Scholar]
- 42.Lee EJ, Min HY, Joo Park H, Chung HJ, Kim S, Nam Han Y. G2/M cell cycle arrest and induction of apoptosis by a stilbenoid, 3, 4, 5-trimethoxy-4'-bromo-cis-stilbene, in human lung cancer cells. Life Sciences. 2004;75(23):2829–2839. doi: 10.1016/j.lfs.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Weng CJ, Yang YT, Ho CT, Yen GC. Mechanisms of apoptotic effects induced by resveratrol dibenzoylmethane and their analogues on human lung carcinoma cells. J. Agr. Food Chem. 2009;57(12): 5235–5243. doi: 10.1021/jf900531m. [DOI] [PubMed] [Google Scholar]
- 44.Zheng PW, Chiang LC, Lin CC. Apigenin induced apoptosis through p53-dependent pathway in human cervical carcinoma cells. Life Sciences. 2005;76(12): 1367–1379. doi: 10.1016/j.lfs.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 45.Wang W, Heideman L, Chung CS, Pelling JC, Koehler KJ, Birt DF. Cell-cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Molecular Carcinogenesis. 2000;28(2): 102–110. [PubMed] [Google Scholar]
- 46.Li X, Wang JN, Huang JM, Xiong XK, Chen MF, Ong CN. Chrysin promotes tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) induced apoptosis in human cancer cell lines. Toxicology in vitro. 2011;25(3):630–635. doi: 10.1016/j.tiv.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 47.Khoo BY, Chua SL, Balaram P. Apoptotic effects of chrysin in human cancer cell lines. Int. J. Mol. Sci. 2010;11(5):2188–2199. doi: 10.3390/ijms11052188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parajuli P, Joshee N, Rimando AM, Mittal S, Yadav AK. In vitro antitumor mechanisms of various scutellaria extracts and constituent flavonoids. Planta Medica. 2009;75(1):41. doi: 10.1055/s-0028-1088364. [DOI] [PubMed] [Google Scholar]
- 49.Malumbres M, Barbacid M. Cell cycle CDKs and cancer a changing paradigm. Nat Rev Cancer. 2009;9(3):153–66. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 50.Hur YG, Yun Y, Won J. Rosmarinic acid induces p56lck-dependent apoptosis in Jurkat and peripheral T cells via mitochondrial pathway independent from Fas/Fas ligand interaction. J. Immunol. 2004;172(1):79–87. doi: 10.4049/jimmunol.172.1.79. [DOI] [PubMed] [Google Scholar]
- 51.Fassihi A, Sabet R. QSAR study of p56(lck) protein tyrosine kinase inhibitory activity of flavonoid derivatives using MLR and GA-PLS. Int J Mol Sc. 2008;9(9):1876–1892. doi: 10.3390/ijms9091876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deininger MWN, Goldman JM, Melo JV. The molecular Biology of Chronic Myeloid Leukemia. Blood. 2000;96(10): 3343–3356. [PubMed] [Google Scholar]
- 53.D’Archivio M, Filesi C, Varì R, Scazzocchio B, Masella R. Bioavailability of the polyphenols: Status and controversies. International Journal of Molecular Sciences. 2010;11(4):1321–1342. doi: 10.3390/ijms11041321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hollman PCH, van Trijp JMP, Buysman MNCP, Mengelers MJB, de Vries JHM, Katan MB. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Letters. 1997;418(1):152–156. doi: 10.1016/s0014-5793(97)01367-7. [DOI] [PubMed] [Google Scholar]
- 55.Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols Food sources and bioavailability. Am. J. Clin. Nutr. 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 56.Mendoza EE, Burd R. Quercetin as a systemic chemopreventative agent Structural and functional mechanisms. Mini Rev Med Chem. 2011;11(14):1216–1221. doi: 10.2174/13895575111091216. [DOI] [PubMed] [Google Scholar]
- 57.Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans.II. review of 93 intervention studies. Am. J Clin Nutr. 2005;81(1):243S–255S. doi: 10.1093/ajcn/81.1.243S. [DOI] [PubMed] [Google Scholar]
- 58.Pérez-Jiménez J, Fezeu L, Touvier M, Arnault N, Manach C, Hercberg S, Scalbert A. Dietary intake of 337 polyphenols in french adults. Am J Clin Nutr. 2011;93(6):1220–s1228. doi: 10.3945/ajcn.110.007096. [DOI] [PubMed] [Google Scholar]