Abstract
In this study, we examined the differential thermal tolerance of Strongylocentrotus purpuratus early life history stages by comparing high temperature–induced mortality and the relative levels of the stress-induced gene, hsp70, between S. purpuratus embryos and larvae from adults collected throughout the species range. There was no significant difference between gastrulae and 4-arm plutei mortality from all sites examined. Furthermore, there was little variability in temperature tolerance across the biogeographic range as southern gastrulae and 4-arm plutei exhibited similar tolerances to northern individuals. Relative levels of hsp70 mRNA expression did not differ overall between the two developmental stages at each site. Across sites, all gastrulae and 4-arm plutei exhibited maximum hsp70 expression at approximately 25°C; however, the range of hsp70 expression was narrower in southern individuals, suggesting they are living closer to their upper thermal limit than northern individuals.
Introduction
In marine ecosystems, temperature is known to set species’ distribution limits, creating distribution boundaries on scales that range from the biogeographic (Brown et al. 1996; Pörtner 2002; Sagarin and Gaines 2002) to small scale (Helmuth and Hofmann 2001; Miller et al. 2009). For benthic invertebrates, the effects of temperature have been studied extensively in adult forms (e.g., Stillman 2003; Somero 2005; Stillman and Tagmount 2009). Studies on earlier life history stages have often characterized them as being more vulnerable to environmental stress such as temperature and UV exposure (Adams and Shick 2001; Edmunds et al. 2001; but see Hamdoun and Epel 2007). Given that climate change models predict changes in the abiotic characteristics of oceans (IPCC 2007; Doney et al. 2009), understanding physiological tolerances of larval forms is particularly important. In addition, while oceanographic processes such as current patterns and upwelling often dictate species range boundaries for organisms with planktonic larvae (Gaylord and Gaines 2000; deRivera et al. 2007), physiological temperature tolerances of the larvae, in conjunction with oceanographic processes, strongly influence the adult biogeography (Zacherl et al. 2003; Sanford et al. 2006).
With its broad biogeographic range, extending from southern Alaska to central Baja California, Mexico, the purple sea urchin Strongylocentrotus purpuratus as a species experiences a wide range of environmental temperatures, ranging from 4 to 20°C (Morris et al. 1980) on average, with the potential to experience higher temperatures in shallow intertidal tide pools. S. purpuratus urchins have pelagic, free-swimming larvae that remain in the water column for an average of 29–86 days, depending on the latitude and water temperature (Strathmann 1978; Strathmann 1987). After this planktonic larval duration, the larvae metamorphose and settle to become benthic juveniles. It is during the pelagic duration that embryos and larvae are exposed to variable temperature conditions, moving with and potentially through water masses of different temperatures, while also experiencing daily temperature fluctuations in the surface waters. Within larval ecology, the relationship between temperature and larval performance has been studied extensively at the whole-organism level. Many studies have investigated the relationship between seawater temperature and larval development, correlating warmer temperatures with faster development times (Fujisawa 1989; Fujisawa and Shigei 1990; Sewell and Young 1999; Staver and Strathmann 2002). There are limits, however, when temperatures exceed physiological capabilities, and embryos and larvae are no longer able to develop and function at these extreme temperatures (Sewell and Young 1999). For example, in S. purpuratus specifically, Farmanfarmaian and Giese (1963) noted that embryonic offspring of central Californian adults developed normally between 13 and 20°C; however, eggs fertilized between 5 and 13°C and above 20°C developed abnormally and exposure to 25°C led to mortality shortly after exposure. As embryos and larvae approach these limits of their temperature tolerances, they exhibit a number of responses at both the molecular and whole-organism levels as a result of these temperature extremes.
At the whole-organism level in marine ectotherms, oxygen consumption, heart rate, and mortality are some of the measures that have been used as indices for thermal stress (Stillman and Somero 2000; Pörtner 2002; Stillman 2003). These indices of thermal stress, alongside molecular responses, have been widely studied in adults. The focus more recently has been on relating adult physiological performance to biogeography (Sorte and Hofmann 2004; Osovitz and Hofmann 2005; Somero 2005; see Gaston et al. 2009; Stillman and Tagmount 2009) as macrophysiological studies will provide further insight into how organisms will respond to long-term environmental changes such as climate change (Chown and Gaston 2008). Some larval studies have also explored whole-organism level thermal tolerances and development in relationship to adult habitat and biogeography (Johnson and Babcock 1994; Bingham et al. 1997). Investigating whole-organism level thermal indices, such as mortality, in early life history stages will provide more thorough profiles of marine ectotherm stress responses during their life cycles.
Larvae employ a number of molecular mechanisms to handle thermal stress. One mechanism involved in thermal tolerance is the heat-shock response (HSR). The HSR is a molecular-level gene activation event in response to physiological stresses such as high temperatures (Lindquist 1986) and is induced under thermally stressful conditions in marine organisms (Tomanek and Somero 1999; Hofmann 2005) and terrestrial invertebrates (Lindquist 1986; Krebs and Feder 1998; Goto and Kimura 1998). Of the genes that are activated during the HSR, the inducible form of hsp70 is one that has been used extensively to quantify the HSR. hsp70 is a 70kD family of genes that codes for heat-shock proteins (hsps), molecular chaperones that aide in the refolding or translocation of denatured proteins allowing the cell to maintain proper functions (Lindquist 1986; Morimoto 1998). Many studies have focused their attention on inducible hsp70 in adult organisms; however, fewer studies have been carried out with early developmental stages.
It is important to note that early embryos and larvae go through a vast number of cellular and morphological changes, often over a relatively short time (S. purpuratus spend just a few days transitioning from fertilized egg to pluteus larvae), potentially leaving them differentially susceptible to stress as much of their energy may be allocated to cellular divisions and rearrangements (Krebs and Feder 1998; Morimoto 1998; Hamdoun and Epel 2007). Furthermore, they may respond or manifest defense mechanisms at different capacities. Studies have found that before the gastrula stage, embryos of the Mediterranean sea urchin Paracentrotus lividus exposed to high temperatures produced hsp70 transcript but at such low concentrations it was almost undetectable, and there was no discernable HSR compared to the larvae (Sconzo et al. 1986; Sconzo et al. 1995; Giudice et al. 1999). Similar results were found by Brown et al. (2004) in Ostreola conchaphila oyster embryos and larvae: pre-gastrula embryos lacked a measurable HSR. The absence of a measurable HSR is not surprising as, generally, embryos do not begin to transcribe their genome until they have reached the mid-blastula or gastrula stage (Wolpert 1992; Leptin 2005), transcription that includes inducible hsps. Therefore, we focused on gastrulae as the first stage of interest for this study. As it is one of the earliest points at which hsp70 is expressed in measurable amounts (Sconzo et al. 1986; Sconzo et al. 1995; Giudice et al. 1999), gastrulae may regulate the response differently than older larvae, necessitating an embryo to larva comparison.
In the present study, we examined thermal tolerance of S. purpuratus larvae using two biological measures. At the whole-organism level, embryos and larvae were exposed to a gradient of increasing temperatures and mortality was assessed. At the molecular level, hsp70 mRNA transcript was measured as it is a biological indicator of thermal stress. These two metrics were used to compare S. purpuratus at the gastrula and 4-arm pluteus stages to assess developmental differences in thermal tolerance and hsp70 expression of embryos and larvae spawned from adults collected from locations across the range of S. purpuratus. Because the range of S. purpuratus is relatively large, with a potential 10–15°C difference in the temperature at which oogenesis occurs in nature between the range boundaries (National Data Buoy Center at NOAA; http://www.ndbc.noaa.gov/), embryos and larvae of adults from different thermal regimes may respond differently to thermal stress. Therefore, investigations of thermal tolerance of progeny from different latitudes may elucidate the relationship between larval physiological tolerances and biogeography.
Methods
Urchin collection and larval culturing
Approximately 20 S. purpuratus urchins were collected from 4 sites along the west coast of North America between Oregon and Baja California, Mexico: Fogarty Creek, OR (FC; 44º51′N, 124º00′W), Santa Cruz, CA (SC; 36º53′N, 122º04′W), Santa Barbara, CA (SB; 34º14′N, 119º53′W), and Eréndira, Baja California, Mexico (ER; 31º16′N 116º23′W; Fig. 1). The monthly average temperatures range from 10 to 14°C in central Oregon, 12–15°C near Santa Cruz, 13–17°C in Santa Barbara, and 15–21°C near Eréndira (FC, SC and SB data: National Data Buoy Center at NOAA; http://www.ndbc.noaa.gov; ER: Lluch-Cota et al. 2000). The urchins were collected from the subtidal or very low intertidal zone during the height of the S. purpuratus spawning season relative to each site (ER: January; SB: February; SC: March; FC: late March–early April). Urchins were brought back to the University of California, Santa Barbara and placed in flow-through tanks set to the temperatures at which they were collected (FC: ~10°C; SC: 12–13°C; SB: ~15°C; ER: ~15°C). Urchins were held for approximately 1–4 days prior to spawning. From each sample site, 3 to 4 pairs of one male and one female were spawned, and each pair was handled separately. Urchins were shaken to induce spawning. If shaking was not sufficient, urchins were injected with ~0.5–1.0 mL of 0.5 M KCl. Females were inverted over beakers of 0.22–0.35 μm filtered sea water (FSW) into which they shed their eggs. Males were dry spawned. The sperm were removed, placed into microcentrifuge tubes, and kept cold on ice for no more than 15 min until fertilization. Eggs were fertilized with a sperm concentration of ~20 μL per 100 μL of eggs in a 1-L beaker of FSW. Fertilization success was checked within 5 min of sperm addition and only eggs with a success rate of 90% or greater were used. After fertilization, zygotes from each separate pair were washed with FSW to remove excess sperm, and each group zygotes were divided equally into 4-, 3-L culture containers at an average density of 500,000 eggs per culture. Embryos and larvae were raised at the temperatures at which the adults were collected relative to each site. Each culture was gently stirred by paddles as described in Strathmann 1987. Culture water was changed once every 24 h, any dead embryos or larvae were removed, and once late prism stage was reached (~45–75 h post-fertilization, depending on the seawater temperature), they were fed a mixed diet of algae (Reed-Mariculture; Instant Algae Shellfish Diet 1800: Isochyrsis, Pavlova, Tetraselmis, and Thalassiosira weissflogii at ~2 billion cells mL−1) once before being sampled at the 4-arm pluteus stage (~72–114 h p.f. depending on the seawater temperature).
Fig. 1.
Strongylocentrotus purpuratus collection sites: Fogarty Creek, OR (FC), Santa Cruz, CA (SC), Santa Barbara, CA (SB), Eréndira, Baja California, MX (ER)
Larval sampling
Once cultures reached the gastrula (36–70 h p.f. depending on rearing temperature) or 4-arm pluteus stage (72–114 p.f.), gastrulae and 4-arm plutei from each male–female pair were sampled from their respective cultures and divided into 33-, 20-mL scintillation vials. To do this, gastrulae or larvae from 2 of the 3-L culture containers were combined and mixed (the remaining 2 culture containers were combined, mixed, and then individuals were split into 4-, 3-L cultures to reduce the density for the developing gastrulae that would later be sampled for 4-arm plutei). From this larger stock, 33 samples of either 26,000–32,000 gastrulae or 4,000–5,000 4-arm plutei were measured out volumetrically and concentrated down to 1 mL for each of the 33 vials. The concentrated 1 mL of either gastrulae or larvae was added to a 20-mL scintillation vial that contained 9 mL of FSW. The high densities in each scintillation vial were necessary to ensure enough material was available for the genomic analysis of hsp70 mRNA levels, a method that is not uncommon for attaining adequate levels of genomic material for heat-shock analysis (Bédard and Brandhorst 1986; Sconzo et al. 1995; Sconzo et al. 1997; Brown et al. 2004).
Thermal tolerance trials
A temperature gradient heat block was used to conduct the thermal tolerance experiment. The gradient consisted of 11 rows of wells of different temperatures ranging from 10 to 32°C (Table 1). The following procedure was run separately for each of the 3–4 sets of fertilized eggs: three of the 20-mL vials containing gastrulae or 4-arm plutei were placed in each temperature row, representing replicates of temperature. The embryos or larvae remained at these temperatures for 1 h, at which point they were removed and scored under a light microscope. To score, the embryos or larvae were mixed by gentle stirring and 1 mL was taken from each vial and, using a densitometer and light microscopy, one hundred larvae were counted to obtain percent mortality. Swimming larvae or larvae that exhibited cilia movement were scored as alive, and those with no cilia movement were scored as dead. In order to verify that 1 h was sufficient time for a thermal challenge and to ensure that embryos or larvae scored as dead after 1 h were actually dead, a subset of gastrulae and 4-arm plutei from SB adults were exposed to a 1-h heat shock at temperatures between 15 and 32°C as described in Table 1, followed by recovery periods of 1, 2, 4, and 26 h. As mortality after each recovery period was not significantly different from the mortality observed immediately after the 1-h heat shock (data not shown), a 1-h heat shock was deemed sufficient for assessing mortality.
Table 1.
Temperatures of 1-h heat exposure (°C) for each site
| Site | Temperatures of 1-h heat exposure (ºC) |
|---|---|
| FC | 10.5, 13.0, 15.0, 17.2, 19.3, 21.1, 23.1, 25, 26.9, 28.8, 30.8 |
| SC | 13, 15, 16.8, 18.3, 20.1, 21.1, 23.7, 25.4, 27.2, 29.1, 31.0 |
| SB | 15, 16.9, 18.7, 20.3, 21.8, 23.4, 25, 26.7, 28.4, 30.1, 31.9 |
| ER | 15, 16.9, 18.7, 20.3, 21.8, 23.4, 25, 26.7, 28.4, 30.1, 31.9 |
Total RNA extraction
After gastrulae and 4-arm plutei were counted for the mortality assessments, the FSW was filtered off for total RNA extraction. Each sample was homogenized in 500 μL of TRIzol® Reagent (Invitrogen, Carlsbad CA, USA) and stored at −80°C until the RNA was extracted. Total RNA was extracted following the TRIzol manufacturer’s protocol with the following adjustments: After the initial TRIzol extraction, a second extraction using 100% chloroform was used. In addition to the precipitation of total RNA in the recommended volume of 100% isopropanol, a salt solution for precipitation (initial concentration: 1.2 M NaCl, 0.8 M disodium citrate) of equal volume was used. The RNA was re-suspended in nuclease-free water, and their concentrations were measured using a Nanodrop® ND-1000 full-spectrum UV/vis spectrophotometer.
hsp70 analysis using qPCR
Once the concentrations of total RNA were determined, 100 ng was reverse transcribed for cDNA synthesis using oligo dT and the ImProm-IITM Reverse Transcription System (Promega) following the manufacturer’s protocol. Of the 11 heat-shock temperatures used in the thermal tolerance trials (Table 1), temperatures between 10 and 20°C were excluded for each site for hsp70 analysis because no significant upregulation was anticipated between 10 and 20°C based on previous hsp70 expression data in adult S. purpuratus (Osovitz and Hofmann 2005). Quantitative real-time PCR (qPCR) was used to determine the relative levels of hsp70 mRNA following the method described in Osovitz and Hofmann (2005) with the following adjustments. Elongation factor 1-α (ef1-α) was used as a control gene as its mRNA levels did not change significantly across temperature treatments. Primers for ef1-α and hsp70 were designed using Bio-Rad Beacon designer software with the published S. purpuratus sequences for hsp70 (GenBank accession no. XR_026557.1) and ef1-α (GenBank accession no. NM_001123497.1). Primers for hsp70 were as follows: hsp70 sense—5′-AAGATATGAGGTCCAACCCAAGAT-3′; anti-sense—5′-TGCTGAAGCACTGCTTGACA-3′. Primers for ef1-α were as follows: ef1-α sense—5′-CAACGAAATCGTCAGGGAGGTC-3′; anti-sense—5′-AGATTGGGATGAAGGGCACAG-3′. The concentration of the ef1-α primer used was 0.5 μM. Briefly, quantitative real-time PCR was run in 20-μL SYBR green supermix (Bio-Rad) reactions containing 2 μL of template cDNA. The qPCRs were run with the following protocol: 1 cycle at 95°C for 3 min, 40 cycles at 58°C for 10 s and 95°C for 1 min, 1 cycle at 55°C for 1 min. Relative mRNA levels were calculated as follows. hsp70 and ef1-α Ct values (the value that represents the threshold cycle at which a threshold concentration of transcript is reached) were normalized to a single S. purpuratus larval standard run on every qPCR plate. The hsp70 Ct values were then normalized to the corresponding ef1-α values, resulting in the reported relative hsp70 concentrations.
Statistical analysis
Statistical analyses were performed using JMP 7.0 (SAS). The LT50, the lethal exposure temperatures at which 50% of the embryos and larvae died, was used as a measure of mortality in the thermal tolerance trials. To determine the LT50, logistic regressions were calculated from the binary (alive or dead) mortality data from both gastrulae and 4-arm plutei at each site. For each developmental stage, the upper and lower 95% confidence intervals for the LT50s were determined from the logistic regressions. Linear regressions were calculated within developmental stage to determine latitudinal trends in LT50 temperatures by fitting the LT50 data by latitude, and significant differences were indicated by non-overlapping 95% confidence intervals. A Student’s t-test was used to determine statistical significance between gastrulae and 4-arm plutei LT50s.
The hsp70 expression data were transformed using a Box–Cox transformation to meet the assumptions for a two-way ANOVA with temperature and developmental stage as factors with male–female pair nested within temperature. Tukey–Kramer Honestly Significant Difference tests were run for post hoc analysis to characterize the hsp70 induction profile and identify the thermal set-points for each developmental stage at each site.
Results
Thermal tolerance trials
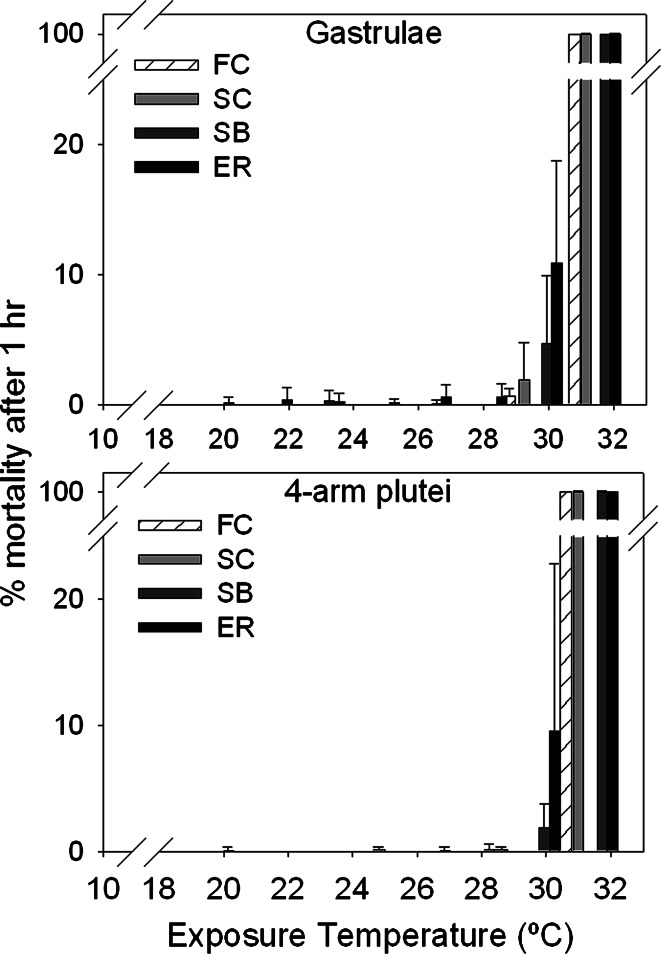
The thermal tolerance range was determined by exposing embryos and larvae to a range of temperatures for 1 h, and thermal tolerance was measured as percent mortality. Overall, embryos and larvae exhibited an abrupt threshold in mortality, regardless of the biogeography of the adults (Fig. 2). For all sites, mortality was consistently very low until the exposure temperature reached 29–30°C, at which point mortality increased significantly and reached 100% mortality within 1–2°C of the initial increase (Fig. 2). Gastrulae and 4-arm plutei from FC and SC adults experienced 100% mortality at approximately 31°C, while gastrulae and 4-arm plutei from SB and ER adults experienced 100% mortality at approximately 32°C.
Fig. 2.
Thermal tolerance plots showing percent mortality in S. purpuratus gastrulae and 4-arm plutei after 1-h heat shock from adults from FC (gastrulae, n = 2; 4-arm plutei, n = 3 where hereafter n represents the number of male–female pairs spawned for each batch of embryos and larvae); SC (gastrulae, n = 3; 4-arm plutei, n = 3); SB (gastrulae, n = 3; 4-arm plutei, n = 4); and ER (gastrulae, n = 3; 4-arm plutei, n = 4). Error bars indicate standard error of male–female pair replicates
Given the sharp increase in mortality and the coarse resolution of mortality temperatures tested, the LT50s were estimated from logistic regression curves. Accordingly, the LT50 temperatures for FC, SC, SB, and ER gastrulae were 30.3, 30.1, 30.7, and 30.9°C, respectively (Table 2). The LT50 temperatures for FC, SC, SB, and ER 4-arm plutei were 29.7, 30.0, 31.0, and 31.0°C, respectively (Table 2). There were no significant differences in mortality between gastrulae and 4-arm plutei for all sites (Student’s t-test assuming unequal variances: P = 0.9121). A linear regression with 95% confidence intervals showed there was a relationship between LT50 and site for 4-arm plutei, although not significant (r 2 = 0.7918, P = 0.1101), and no relationship between LT50 temperature and site for gastrulae (r 2 = 0.4692, P = 0.315).
Table 2.
LT50 temperatures (ºC) for S. purpuratus gastrulae and 4-arm plutei after a 1-h heat exposure
| Stage | FC | SC | SB | ER |
|---|---|---|---|---|
| Gastrulae | 30.3 | 30.1 | 30.7 | 30.9 |
| 4-arm plutei | 29.7 | 30.0 | 31.0 | 31.0 |
Expression of hsp70 mRNA
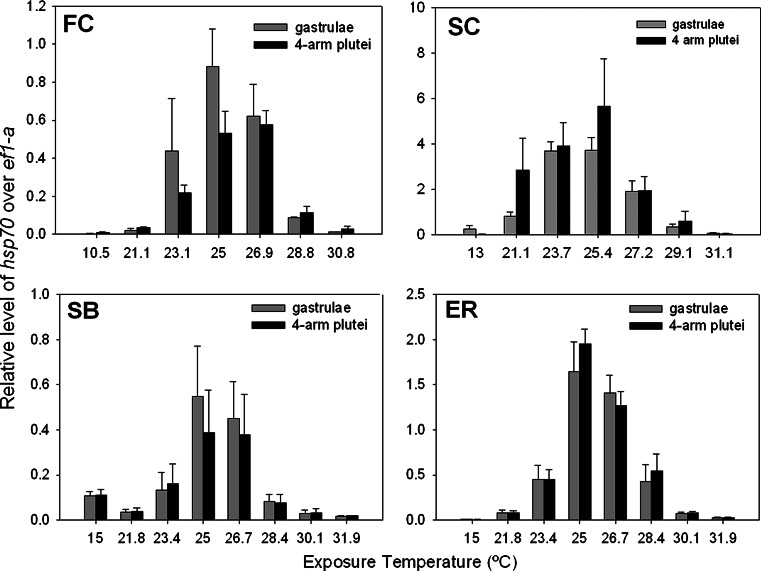
The induction profile for hsp70 mRNA expression was similar between developmental stages and at all four sites (Fig. 3). The overall two-way ANOVA results were significant for each site (P < 0.05) except SB (P = 0.1700). However, temperature had a highly significant effect in SB samples (P = 0.0007; Table 3). The discrepancy may be a result of the anomalously high expression at the control temperature from possible temperature stress during sample handling. The results from the two-way ANOVA for each site showed that temperature was the only significant effect (all P < 0.05) except at SC where both temperature (P < 0.0001) and stage (P = 0.0218) were significant (Table 3). However, the significance in stage at SC was solely driven by differences in expression at 13 and 21.1°C. Variability due to mate-pair was not significant at any site (all P > 0.05), and there were no interactions between factors at any site (all P > 0.05; Table 3). Significant increases in expression above the site-specific controls occurred between 21 and 30°C, with no significant variation in peak expression for each site. Thermal set-points for the initial temperature at which hsp70 expression was significantly different from the control, T on, and peak hsp70 expression, T max, are summarized in Table 4. For the FC samples, the T on was 21.1°C for both developmental stages (Fig. 3). Peak hsp70 expression (T max) occurred at 25°C for gastrulae and 26.9°C for 4-arm plutei; however, the differences in T max were not significantly different (Tukey–Kramer HSD, P = 0.9958; Fig. 3). In the SC samples, the T on occurred at 21.1°C for both stages and the T max of hsp70 expression occurred between 23.7 and 25.4°C for gastrulae and 25.4°C for 4-arm plutei; however, differences between the T max were not significantly different (Tukey–Kramer HSD, P = 1.0; Fig. 3). For the SB samples, the T on occurred between 21.8 and 23.4°C, while the T max occurred between 25 and 26.7°C for both gastrulae and 4-arm plutei (Fig. 3). Lastly, in the ER samples, T on occurred at 21.8°C and peak hsp70 expression occurred at 25°C for both gastrulae and 4-arm plutei (Fig. 3). Due to a shift in the Ct expression values of the internal standard between sites, comparisons in the magnitude of hsp70 expression between sites could not be confidently made.
Fig. 3.
Relative hsp70 mRNA expression levels after 1-h heat shock in S. purpuratus gastrulae and 4-arm plutei of adults from FC (gastrulae, n = 2; 4-arm plutei, n = 3); SC (gastrulae, n = 3; 4-arm plutei, n = 3); SB (gastrulae, n = 3; 4-arm plutei, n = 4); and ER (gastrulae, n = 3; 4-arm plutei, n = 4). Values are relative to a common S. purpuratus larval standard and normalized to ef1-α. Error bars indicate standard error of male–female pair replicates
Table 3.
Two-way ANOVA results testing for the effects of temperature and developmental stage on hsp70 expression in S. purpuratus progeny from FC, SC, SB, and ER
| df | F | p | |
|---|---|---|---|
| FC | |||
| Temperature | 6 | 41.5040 | 0.0001* |
| Stage | 1 | 4.4644 | 0.0791 |
| Mate-pair[temperature] | 14 | 0.9890 | 0.5434 |
| Temperature × stage | 6 | 1.2608 | 0.3928 |
| SC | |||
| Temperature | 6 | 23.4673 | <0.0001* |
| Stage | 1 | 6.7849 | 0.0218* |
| Mate-pair[temperature] | 14 | 0.8158 | 0.6459 |
| Temperature × stage | 6 | 1.3802 | 0.2932 |
| SB | |||
| Temperature | 7 | 6.8587 | 0.0007* |
| Stage | 1 | 0.0236 | 0.8798 |
| Mate-pair[temperature] | 24 | 0.2243 | 0.9995 |
| Temperature × stage | 7 | 0.3497 | 0.9182 |
| ER | |||
| Temperature | 7 | 12.8504 | <0.0001* |
| Stage | 1 | 0.2190 | 0.6476 |
| Mate-pair[temperature] | 24 | 1.6673 | 0.1694 |
| Temperature × Stage | 7 | 0.4658 | 0.8425 |
* Significant at the 0.05 level
Table 4.
hsp70 expression thermal set-points
| Site | Stage | T on (°C) | T max (°C) |
|---|---|---|---|
| FC | Gastrulae | 21.1 | 25 |
| 4-arm plutei | 21.1 | 26.9 | |
| SC | Gastrulae | 21.1 | 23.7–25.4 |
| 4-arm plutei | 21.1 | 25.4 | |
| SB | Gastrulae | 21.8–23.4 | 25–26.7 |
| 4-arm plutei | 21.8–23.4 | 25–26.7 | |
| ER | Gastrulae | 21.8 | 25 |
| 4-arm plutei | 21.8 | 25 |
The initial temperature at which hsp70 expression was significantly different from the control, T on, and peak hsp70 expression, T max
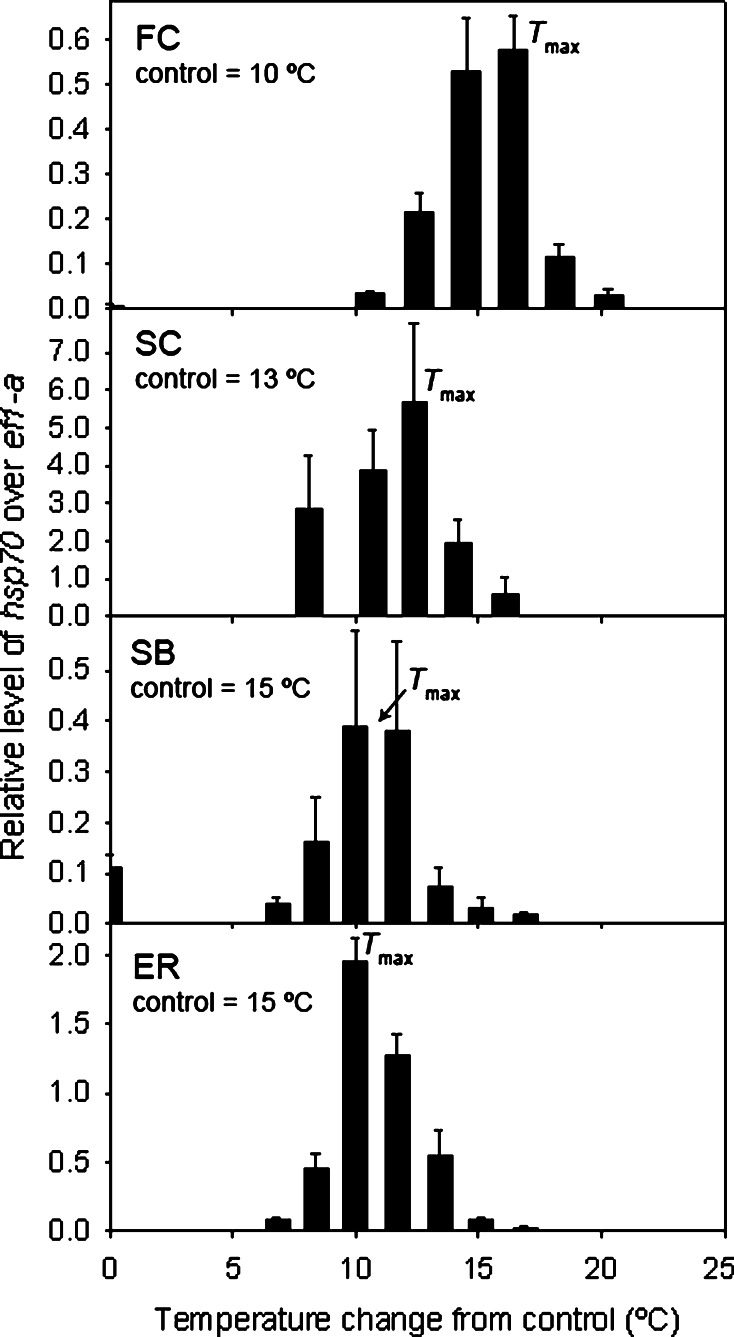
Comparing the range between the absolute site-specific control temperature and T max, gastrulae and 4-arm plutei from FC had a range of ~15°C, SC had a range of ~13°C, and SB and ER both had a range of 10°C (Fig. 4). For simplicity, data were plotted from 4-arm plutei only as there were no significant differences between the two stages in hsp70 expression profiles.
Fig. 4.
Levels of hsp70 mRNA expression in 4-arm plutei from each site relative to a common S. purpuratus larval standard and normalized to ef1-α. Data were plotted from 4-arm plutei only as there were no significant differences between the gastrulae and 4-arm plutei in hsp70 expression profiles. Values along the x-axis represent the change in heat exposure temperature from the control temperature used at each site
Discussion
The aim of this study was to compare the thermal tolerance of two early life history stages of S. purpuratus. Additionally, we sought to examine how the latitude at which the adult females went through oogenesis affected the hsp70 expression of the progeny. As the environmental characteristics encountered by the adults during oogenesis has the potential to affect offspring performance in most organisms (Mousseau and Fox 1998; Bacigalupe et al. 2007), including marine organisms (Li and Brawley 2004; Marshall 2008), for the present study embryos were obtained from adults that originated from various biogeographic locations throughout the recorded range of adult S. purpuratus (Morris et al. 1980; Tegner 2001) during the spawning season. Oogenesis in S. purpuratus typically occurs in autumn/winter (Gonor 1973; Pearse et al. 1986) when the temperatures range from an average of ~12°C in central Oregon (National Data Buoy Center at NOAA; http://www.ndbc.noaa.gov/) to an average of ~19°C near Ensenada, MX (Lluch-Cota et al. 2000). The salient findings of this study were as follows: (1) for thermal tolerance, measured as LT50s, there were no significant latitudinal trends, (2) for the two stages tested, there was no detectable difference in response for gastrulae and larvae, (3) using the expression of hsp70 as a bioindicator of response to acute thermal stress, embryos and larvae displayed similar patterns of gene expression with no patterns detected across the latitudes at which adults were collected, and (4) there were subtle latitudinal trends in hsp70 thermal set-points.
Overall, the results of this study suggest that different early developmental stages have similar whole-organism and molecular-level thermal tolerances across the range of purple urchins along the coast of the northeast Pacific.
Thermal tolerance of early life history stages: LT50s
In this study, we observed acute shifts in survivorship where mortality increased from near zero to 100% within a few degrees. Although, not always reflecting ecological average temperatures, these LT50 assays were employed in order to identify thresholds in tolerance, as has been done in numerous physiological studies (Stillman and Somero 2000; Somero 2002; Miller et al. 2009). Examinations of larval temperature tolerance have found similar abrupt increases in mortality (Brown et al. 2004; Kalosaka et al. 2009; Zippay and Hofmann 2010). For example, Boon-Niermeijer and van de Scheur (1984) exposed Lymnaea stagnalis snail embryos and larvae to 1-h thermal challenges, and all stages studied experienced an abrupt increase in mortality between 39 and 40°C. Sewell and Young (1999) observed abrupt decreases in survival in Echinometra lucunter urchin embryos and larvae exposed to 2-h thermal challenges; all stages experienced 100% survival until 38°C at which point mortality rapidly increased until ~41°C when all stages exhibited 100% mortality. The acute shift in survivorship observed in this study suggests that the embryos and larvae may have reached the critical thermal threshold for the molecular mechanisms regulating thermal tolerance; this is discussed in more detail in the following section.
The mortality results between stages did not differ significantly at any of the sites. Additionally, the LT50s and overall mortality profiles between stages were not significantly different, results that were similar to findings in embryos and larvae of the tropical sea urchin, Echinometra lucunter (Sewell and Young 1999). However, contrasting outcomes have been found in other species. Kalosaka et al. (2009) exposed 6 life history stages of the Mediterranean medfly, Ceratitis capitata, ranging from early embryos to adults to 1-h heat shocks at a range of temperatures and found different patterns of mortality based on life history stage. Sulkin and McKeen (1994) compared temperature-induced mortality in five zoeal stages between four congeneric Cancer crabs raised at 10, 15, and 20°C and found no clear pattern of mortality with temperature between the zoeal stages; different zoeal stages experienced different levels of mortality in all species investigated. The authors suggest that the differences in mortality may lead to dissimilar susceptibilities to thermal stress between developmental stages; however, it was not clear whether these unequal vulnerabilities were ecologically significant. In general, there appear to be no clear patterns in temperature-induced mortality between early life history stages across species; mortality during early development appears to be species specific, and the present data further contributes to our understanding of intraspecific temperature tolerances between early developmental stages. For the S. purpuratus gastrulae and 4-arm plutei investigated here, the similarity in mortality profiles suggests that the likelihood of stage-specific mortality during early development, which may affect settlement and recruitment success, is minimized.
hsp70 expression and developmental stage comparisons
Gastrulae and 4-arm plutei both upregulated hsp70 similarly with no significant differences in expression, irrespective of the characteristic differences between these two life history stages. Gastrulae are swimming embryos that are in the process of forming the most basic structures (mouth, gut, and anus) while 4-arm plutei are feeding, swimming larvae with well-developed systems and defined structures (gastrointestinal system, early larval skeleton, etc.). Previous studies have shown that early urchin embryos do not display a heat-shock response before the gastrula stage (Sconzo et al. 1995; Giudice et al. 1999). This may, in part, be due to the fact that, for the embryo, much of the energy is expended on the proper formation of the germ layers and embryonic and larval structures. Because protein folding and molecular chaperone activities carry large energy requirements (Hochachka and Somero 2002) and because the over-expression of hsps can interfere with or inhibit development (Krebs and Feder 1998; Hamdoun and Epel 2007), the embryo would presumably put less energy toward mounting a defense response like the HSR and put more energy into further development than later, more developed stages. However, given the current data, this does not appear to be the case as once the embryo reached gastrula stage, it expressed hsp70 at equal magnitudes as older, more developed plutei.
While the number of studies investigating stress-induced gene expression between early life history stages is few, the similarity in hsp70 expression profiles between the two developmental stages studied here is similar to findings by previous studies investigating Hsp70 protein expression between early life history stages. When Botton et al. (2006) exposed Limulus polyphemus horseshoe crab embryos and larvae to a 3-h heat shock, both stages had Hsp70 protein present in relatively equal amounts. Additionally, Boon-Niermeyer et al. (1988) found that when Lymnaea stagnalis snail gastrulae and trochophore larvae were exposed to temperature stress, both stages had similar relative values of Hsp70 protein. This suggests that particular stages in early development are similar in their capacity to initiate a HSR through the upregulation of hsp70 and translation of Hsp70 protein. The similarity in expression potentially helps ensure that the embryonic and larval stages are equally equipped to withstand temperature stress until settlement and metamorphosis.
Biogeographic comparisons: macrophysiology, larval performance, and hsp70
Measuring performance across large spatial scales—macrophysiology (Gaston et al. 2009)—has been used to examine response to environmental temperature in a variety of marine invertebrates (e.g. Stillman and Somero 2000; Sorte and Hofmann 2004; Somero 2005). In this study, we measured mortality and LT50 temperatures as indices for thermal tolerance and found no clear latitudinal trend. The lack of a trend suggests S. purpuratus embryos and larvae have similar temperature tolerances at the whole-organism level throughout the species range. The number of studies comparing embryonic and larval thermal tolerances across latitude is few, and a similar study by Zippay and Hofmann (2010) on Nucella ostrina larvae, which develop in egg capsules attached to intertidal substrates, found contrasting results. In that study, Nucella ostrina egg capsules from across a large latitudinal range were collected and exposed to a range of thermal challenges in the laboratory and a strong latitudinal trend in LT50 temperatures was found. It is important to note, however, that the contrast in latitudinal trends between these studies may be a result of planktonic versus non-planktonic early life history stages as discussed below.
In this study, we also measured the expression of hsp70, a common gene in the urchin defensome (Goldstone et al. 2006) that is rapidly upregulated in response to elevated temperature, and compared the expression in gastrulae and larvae across a large spatial scale. The hsp70 expression profiles of both gastrulae and 4-arm plutei in this study did not differ between sites. The overall similarity in thermal set-points (i.e., T on and T max) across sites found in this study contrasts with a similar study by Osovitz and Hofmann (2005) that found a latitudinal trend in thermal set-points in adult S. purpuratus, where southern individuals exhibited a T max of hsp70 expression at a higher temperature relative to northern individuals. Interestingly, studies that have found latitudinal trends in the HSR have done so predominately in adults where the shifts in thermal set-points highlighted local acclimatization along latitudinal or vertical habitat gradients and, thus, highlighted the plasticity in their thermotolerance (Tomanek and Somero 1999; Helmuth and Hofmann 2001; Sorte and Hofmann 2004; Osovitz and Hofmann 2005; Henkel and Hofmann 2008). Additionally, the organisms examined in these studies were predominately benthic or sessile with restricted ranges of movement, conditions under which local acclimatization is more likely to occur.
Planktonic early life history stages, on the other hand, are the dispersal stage and are less likely to have experienced set temperature regimes to which they could become acclimatized to, as they are not localized to any specific area until they settle. Under these circumstances, the lack of a latitudinal trend in this study may suggest that the upregulation of hsp70 and, consequently, the HSR, is a genetically fixed response in S. purpuratus early life history stages. While the thermal set-points of the HSR have been shown to typically display variable yet significant plasticity (Barua and Heckathorn 2004), some exceptions have been found. Similar to the present study, adult Mytilus galloprovincialis mussels from across a large latitudinal gradient exhibited no significant variation in T max after exposure to a 1-h thermal challenge, suggesting a fixed genetic response (Dutton and Hofmann 2009). Alternatively, the relatively identical T max values across latitude found in this study may suggest that the early life history stages have a universal temperature tolerance as a mechanism to increase their likelihood of survival during the planktonic larval duration before settlement.
The similarity between gastrulae and 4-arm plutei in their mortality and hsp70 expression profiles further reflects their similar timing of hsp gene activation and cessation, and their capacity for the HSR. Mortality did not begin to increase significantly until after hsp70 expression reached the T max value and began to cease. After the T max was reached (approximately 25°C), both gastrulae and 4-arm plutei likely exhibited failure of the HSR activation machinery. The HSR is controlled by transcriptional activation via heat-shock transcription factors (HSFs) and, although this regulatory step is known to be subject to some thermal acclimation (Buckley and Hofmann 2002; Buckley and Hofmann 2004), the process likely failed at approximately 25°C in both developmental stages, suggesting that the HSR transcriptional activation machinery is not only a fixed response but is not differentially regulated during different developmental stages. Subsequently, the embryos and larvae in this study reached their critical thermal thresholds at approximately 25°C and mortality increased rapidly, a pattern typical of the HSR (Tomanek and Somero 1999; Brown et al. 2004; Kalosaka et al. 2009).
Embryos and larvae of both SB and ER exhibited a smaller range of hsp70 expression relative to the northern sites. The range of temperatures from the control temperature (which was equal to the temperature at which the adults were collected and therefore similar to conditions the embryos and larvae would have encountered in nature) to the temperature at which hsp70 expression ceased was smaller in the southern sites (15–32°C) than the range for the northern sites (FC: 10–31°C; SC: 13–31°C). More specifically, the range of expression between the site-specific control temperature and the T max of 25°C at each site was smaller for southern individuals. These data suggest that the embryos and larvae from southern sites are closer to their upper thermal limits relative to northern individuals as there was no shift in the thermal set-points of the HSR. The narrower range in tolerance exhibited by the southern progeny would presumably make them more susceptible to increases in environmental temperatures, a similar conclusion drawn from other studies on marine ectotherms living close to their upper thermal limits (Stillman 2003; Tomanek 2008; Somero 2010).
Conclusion
In measuring thermal tolerance and hsp70 gene expression in two early life history stages of S. purpuratus, the results of this study show that gastrulae and 4-arm plutei respond similarly to thermal stress. Additionally, while the temperatures at which both gastrulae and 4-arm plutei began to express maximum hsp70 expression and experienced significant mortality were higher than their average habitat temperature, these responses help characterize the upper thermal limits of the early life history stages and provide insight into how they may respond to temperature changes in surface waters where temperatures are typically significantly warmer and where the embryos and larvae are concentrated. Investigating how marine invertebrate larvae respond to thermal stress at the whole-organism and molecular levels will assist in predicting whether and how early life history stages are likely to adjust to environmental shifts in temperature.
Acknowledgments
The authors would like to thank PISCO members from Oregon State University and Paul Tompkins (UCSC) for the collection of urchins, and Tim Crombie, Elizabeth Hoaglund and Dr. Sarah Lester for field assistance. We would also like to thank Drs. Steven Gaines, Bill Rice and Chris Osovitz for statistical advice. This research was funded by NSF grant (OCE-0425107) to GEH and by an NSF Graduate Research Fellowship to LMH.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Adams NL, Shick JM. Mycosporine-like amino acids prevent UVB-induced abnormalities during early development of the green sea urchin Strongylocentrotus droebachiensis. Mar Biol. 2001;138:267–280. doi: 10.1007/s002270000463. [DOI] [Google Scholar]
- Bacigalupe LD, Araya NM, Carter MJ, Catalana TP, Lardies MA, Bozinovic F. Maternal effects, maternal body size and offspring energetics: a study in the common woodlouse Porcellio laevis. Comp Biochem Physiol A Mol Integr Physiol. 2007;147:349–354. doi: 10.1016/j.cbpa.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Barua D, Heckathorn SA. Acclimation of the temperature set-points of the heat-shock response. J Therm Biol. 2004;29:185–193. doi: 10.1016/j.jtherbio.2004.01.004. [DOI] [Google Scholar]
- Bédard PA, Brandhorst BP. Translational activation of maternal messenger RNA encoding the heat-shock protein HSP90 during sea urchin embryogenesis. Dev Biol. 1986;117:286–293. doi: 10.1016/0012-1606(86)90371-4. [DOI] [PubMed] [Google Scholar]
- Bingham BL, Bacigalupi M, Johnson LG. Temperature adaptations of embryos from intertidal and subtidal sand dollars (Dendraster excentricus, Eschscholtz) Northwest Sci. 1997;71:108–114. [Google Scholar]
- Boon-Niermeijer EK, van de Scheur H. Thermosensitivity during embryonic development of Lymnaea stagnalis (Mollusca) J Therm Biol. 1984;9:265–269. doi: 10.1016/0306-4565(84)90007-X. [DOI] [Google Scholar]
- Boon-Niermeyer EK, Dewaal AM, Souren JEM, Vanwijk R. Heat-induced changes in thermosensitivity and gene expression during development. Dev Growth Differ. 1988;30:705–715. doi: 10.1111/j.1440-169X.1988.00705.x. [DOI] [PubMed] [Google Scholar]
- Botton ML, Pogorzelska M, Smoral L, Shehata A, Hamilton MG. Thermal biology of horseshoe crab embryos and larvae: a role for heat shock proteins. J Exp Mar Biol Ecol. 2006;336:65–73. doi: 10.1016/j.jembe.2006.04.014. [DOI] [Google Scholar]
- Brown JH, Stevens GC, Kaufman DM. The geographic range: size, shape, boundaries, and internal structure. Annu Rev Ecol Syst. 1996;27:597–623. doi: 10.1146/annurev.ecolsys.27.1.597. [DOI] [Google Scholar]
- Brown HM, Briden A, Stokell T, Griffin FJ, Cherr GN. Thermotolerance and Hsp70 profiles in adult and embryonic California native oysters, Ostreola conchaphila (Carpenter, 1857) J Shellfish Res. 2004;23:135–141. [Google Scholar]
- Buckley BA, Hofmann GE. Thermal acclimation changes DNA-binding activity of heat shock factor 1 (HSF1) in the goby Gillichthys mirabilis: implications for plasticity in the heat-shock response in natural populations. J Exp Biol. 2002;205:3231–3240. doi: 10.1242/jeb.205.20.3231. [DOI] [PubMed] [Google Scholar]
- Buckley BA, Hofmann GE. Magnitude and duration of thermal stress determine kinetics of hsp gene regulation in the goby Gillichthys mirabilis. Physiol Biochem Zool. 2004;77:570–581. doi: 10.1086/420944. [DOI] [PubMed] [Google Scholar]
- Chown SL, Gaston KJ. Macrophysiology for a changing world. Proc R Soc B Biol Sci. 2008;275:1469–1478. doi: 10.1098/rspb.2008.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derivera CE, Hitchcock NG, Teck SJ, Steves BP, Hines AH, Ruiz GM. Larval development rate predicts range expansion of an introduced crab. Mar Biol. 2007;150:1275–1288. doi: 10.1007/s00227-006-0451-9. [DOI] [Google Scholar]
- Doney SC, Fabry VJ, Feely RA, Kleypas JA. Ocean acidification: the other CO2 problem. Annu Rev Mar Sci. 2009;1:169–192. doi: 10.1146/annurev.marine.010908.163834. [DOI] [PubMed] [Google Scholar]
- Dutton JM, Hofmann GE. Biogeographic variation in Mytilus galloprovincialis heat shock gene expression across the eastern Pacific range. J Exp Mar Biol Ecol. 2009;376:37–42. doi: 10.1016/j.jembe.2009.06.001. [DOI] [Google Scholar]
- Edmunds PJ, Gates RD, Gleason DF. The biology of larvae from the reef coral Porites astreoides, and their response to temperature disturbances. Mar Biol. 2001;139:981–989. doi: 10.1007/s002270100634. [DOI] [Google Scholar]
- Farmanfarmaian A, Giese AC. Thermal tolerance and acclimation in the western purple sea urchin, Strongylocentrotus purpuratus. Physiol Zool. 1963;36:237–243. [Google Scholar]
- Fujisawa H. Differences in temperature-dependence of early development of sea urchins with different growing seasons. Biol Bull. 1989;176:96–102. doi: 10.2307/1541576. [DOI] [Google Scholar]
- Fujisawa H, Shigei M. Correlation of embryonic temperature sensitivity of sea urchins with spawning season. J Exp Mar Biol Ecol. 1990;136:123–140. doi: 10.1016/0022-0981(90)90191-E. [DOI] [Google Scholar]
- Gaston KJ, Chown SL, Calosi P, Bernardo J, Bilton DT, Clarke A, Clusella-Trullas S, Ghalambor CK, Konarzewski M, Peck LS, Porter WP, Portner HO, Rezende EL, Schulte PM, Spicer JI, Stillman JH, Terblanche JS, van Kleunen M. Macrophysiology: a conceptual reunification. Am Nat. 2009;174:595–612. doi: 10.1086/605982. [DOI] [PubMed] [Google Scholar]
- Gaylord B, Gaines SD. Temperature or transport? Range limits in marine species mediated solely by flow. Am Nat. 2000;155:769–789. doi: 10.1086/303357. [DOI] [PubMed] [Google Scholar]
- Giudice G, Sconzo G, Roccheri MC. Studies on heat shock proteins in sea urchin development. Dev Growth Differ. 1999;41:375–380. doi: 10.1046/j.1440-169x.1999.00450.x. [DOI] [PubMed] [Google Scholar]
- Goldstone JV, Hamdoun A, Cole BJ, Howard-Ashby M, Nebert DW, Scally M, Dean M, Epel D, Hahn ME, Stegeman JJ (2006) The chemical defensome: environmental sensing and response genes in the Strongylocentrotus purpuratus genome. Dev Biol 300:366–384 [DOI] [PMC free article] [PubMed]
- Gonor JJ. Reproductive cycles in oregon populations of the echlnoid, Strongylocentrotus purpuratus (Stimpson). I. Annual gonad growth and ovarian gametogenic cycles. J Exp Mar Biol Ecol. 1973;12:45–64. doi: 10.1016/0022-0981(73)90037-3. [DOI] [Google Scholar]
- Goto SG, Kimura MT. Heat- and cold-shock responses and temperature adaptations in subtropical and temperate species of Drosophila. J Insect Physiol. 1998;44:1233–1239. doi: 10.1016/S0022-1910(98)00101-2. [DOI] [PubMed] [Google Scholar]
- Hamdoun A, Epel D. Embryo stability and vulnerability in an always changing world. Proc Natl Acad Sci USA. 2007;104:1745–1750. doi: 10.1073/pnas.0610108104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmuth BST, Hofmann GE. Microhabitats, thermal heterogeneity, and patterns of physiological stress in the rocky intertidal zone. Biol Bull. 2001;201:374–384. doi: 10.2307/1543615. [DOI] [PubMed] [Google Scholar]
- Henkel S, Hofmann G. Differing patterns of hsp70 gene expression in invasive and native kelp species: evidence for acclimation-induced variation. J Appl Phycol. 2008;20:915–924. doi: 10.1007/s10811-007-9275-3. [DOI] [Google Scholar]
- Hochachka PW, Somero GN. Biochemical adaptation: mechanism and process in physiological evolution. New York: Columbia University Press; 2002. [Google Scholar]
- Hofmann GE. Patterns of Hsp gene expression in ectothermic marine organisms on small to large biogeographic scales. Integr Comp Biol. 2005;45:247–255. doi: 10.1093/icb/45.2.247. [DOI] [PubMed] [Google Scholar]
- IPCC . Climate change 2007: synthesis report contributions of working groups I, II and III to the fourth assessment report of the intergovernmental panel on climate change. Switzerland: IPCC Geneva; 2007. [Google Scholar]
- Johnson LG, Babcock RC. Temperature and the larval ecology of the crown-of-thorns starfish, Acanthaster planci. Biol Bull. 1994;187:304–308. doi: 10.2307/1542287. [DOI] [PubMed] [Google Scholar]
- Kalosaka K, Soumaka E, Politis N, Mintzas AC. Thermotolerance and HSP70 expression in the Mediterranean fruit fly Ceratitis capitata. J Insect Physiol. 2009;55:568–573. doi: 10.1016/j.jinsphys.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Krebs RA, Feder ME. Hsp70 and larval thermotolerance in Drosophila melanogaster: how much is enough and when is more too much? J Insect Physiol. 1998;44:1091–1101. doi: 10.1016/S0022-1910(98)00059-6. [DOI] [PubMed] [Google Scholar]
- Leptin M. Gastrulation movements: the logic and the nuts and bolts. Dev Cell. 2005;8:305–320. doi: 10.1016/j.devcel.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Li R, Brawley SH. Improved survival under heat stress in intertidal embryos (Fucus spp.) simultaneously exposed to hypersalinity and the effect of parental thermal history. Mar Biol. 2004;144:205–213. doi: 10.1007/s00227-003-1190-9. [DOI] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Lluch-Cota SE, Pacheco-Ayub CA, Bautista-Romero JJ, Hernández-Vázquez S, Lluch-Cota DB (2000) Colección de información ambiental para el Pacífico mexicano. CD-ROM CIBNOR, SC/CONACYT
- Marshall DJ. Transgenerational plasticity in the sea: context-dependent maternal effects across the life history. Ecology. 2008;89:418–427. doi: 10.1890/07-0449.1. [DOI] [PubMed] [Google Scholar]
- Miller LP, Harley CDG, Denny MW. The role of temperature and desiccation stress in limiting the local-scale distribution of the owl limpet, Lottia gigantea. Funct Ecol. 2009;23:756–767. doi: 10.1111/j.1365-2435.2009.01567.x. [DOI] [Google Scholar]
- Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- Morris RH, Abbott DP, Haderlie EC. Intertidal invertebrates of California. Stanford: Stanford University Press; 1980. [Google Scholar]
- Mousseau TA, Fox CW. The adaptive significance of maternal effects. Trends Ecol Evol. 1998;13:403–407. doi: 10.1016/S0169-5347(98)01472-4. [DOI] [PubMed] [Google Scholar]
- Osovitz CJ, Hofmann GE. Thermal history-dependent expression of the hsp70 gene in purple sea urchins: biogeographic patterns and the effect of temperature acclimation. J Exp Mar Biol Ecol. 2005;327:134–143. doi: 10.1016/j.jembe.2005.06.011. [DOI] [Google Scholar]
- Pearse JS, Pearse VB, Davis KK. Photoperiodic regulation of gametogenesis and growth in the sea urchin Strongylocentrotus purpuratus. J Exp Zool. 1986;237:107–118. doi: 10.1002/jez.1402370115. [DOI] [Google Scholar]
- Pörtner HO. Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Physiol A Mol Integr Physiol. 2002;132:739–761. doi: 10.1016/S1095-6433(02)00045-4. [DOI] [PubMed] [Google Scholar]
- Sagarin RD, Gaines SD. Geographical abundance distributions of coastal invertebrates: using one-dimensional ranges to test biogeographic hypotheses. J Biogeogr. 2002;29:985–997. doi: 10.1046/j.1365-2699.2002.00705.x. [DOI] [Google Scholar]
- Sanford E, Holzman SB, Haney RA, Rand DM, Bertness MD. Larval tolerance, gene flow, and the northern geographic range limit of fiddler crabs. Ecology. 2006;87:2882–2894. doi: 10.1890/0012-9658(2006)87[2882:LTGFAT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Sconzo G, Roccheri MC, La Rosa M, Oliva D, Abrignani A, Giudice G. Acquisition of thermotolerance in sea-urchin Paracentrotus lividus embryos correlates with the synthesis and age of the heat shock proteins. Cell Differ. 1986;19:173–178. doi: 10.1016/0045-6039(86)90093-X. [DOI] [PubMed] [Google Scholar]
- Sconzo G, Ferraro MG, Amore G, Giudice G, Cascino D, Scardina G. Activation by heat shock of HSP70 gene transcription in sea urchin embryos. Biochem Biophys Res Commun. 1995;217:1032–1038. doi: 10.1006/bbrc.1995.2873. [DOI] [PubMed] [Google Scholar]
- Sconzo G, Amore G, Capra G, Giudice G, Cascino D, Ghersi G. Identification and characterization of a constitutive HSP75 in sea urchin embryos. Biochem Biophys Res Commun. 1997;234:24–29. doi: 10.1006/bbrc.1997.9996. [DOI] [PubMed] [Google Scholar]
- Sewell MA, Young CM. Temperature limits to fertilization and early development in the tropical sea urchin Echinometra lucunter. J Exp Mar Biol Ecol. 1999;236:291–305. doi: 10.1016/S0022-0981(98)00210-X. [DOI] [Google Scholar]
- Somero GN. Thermal physiology and vertical zonation of intertidal animals: optima, limits, and costs of living. Integr Comp Biol. 2002;42:780–789. doi: 10.1093/icb/42.4.780. [DOI] [PubMed] [Google Scholar]
- Somero G. Linking biogeography to physiology: evolutionary and acclimatory adjustments of thermal limits. Front Zool. 2005;2:1. doi: 10.1186/1742-9994-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somero GN. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J Exp Biol. 2010;213:912–920. doi: 10.1242/jeb.037473. [DOI] [PubMed] [Google Scholar]
- Sorte CJB, Hofmann GE. Changes in latitudes, changes in aptitudes: Nucella canaliculata (Mollusca: Gastropoda) is more stressed at its range edge. Mar Ecol Prog Ser. 2004;274:263–268. doi: 10.3354/meps274263. [DOI] [Google Scholar]
- Staver JM, Strathmann RR. Evolution of fast development of planktonic embryos to early swimming. Biol Bull. 2002;203:58–69. doi: 10.2307/1543458. [DOI] [PubMed] [Google Scholar]
- Stillman JH. Acclimation capacity underlies susceptibility to climate change. Science. 2003;301:65. doi: 10.1126/science.1083073. [DOI] [PubMed] [Google Scholar]
- Stillman JH, Somero GN. A comparative analysis of the upper thermal tolerance limits of eastern Pacific porcelain crabs, genus Petrolisthes: influences of latitude, vertical zonation, acclimation, and phylogeny. Physiol Biochem Zool. 2000;73:200–208. doi: 10.1086/316738. [DOI] [PubMed] [Google Scholar]
- Stillman JH, Tagmount A. Seasonal and latitudinal acclimatization of cardiac transcriptome responses to thermal stress in porcelain crabs, Petrolisthes cinctipes. Mol Ecol. 2009;18:4206–4226. doi: 10.1111/j.1365-294X.2009.04354.x. [DOI] [PubMed] [Google Scholar]
- Strathmann R. Length of the pelagic period in echinoderms with feeding larvae from Northeast Pacific. J Exp Mar Biol Ecol. 1978;34:23–27. doi: 10.1016/0022-0981(78)90054-0. [DOI] [Google Scholar]
- Strathmann MF (1987) Reproduction and development of marine invertebrates of the Northern Pacific coast: data and methods for the study of eggs embryos and larvae. University of Washington Press, Seattle, pp XII + 670P
- Sulkin SD, McKeen G. Influence of temperature on larval development of 4 co-occurring species of the brachyuran genus Cancer. Mar Biol. 1994;118:593–600. doi: 10.1007/BF00347506. [DOI] [Google Scholar]
- Tegner MJ. The ecology of Strongylocentrotus franciscanus and Strongylocentrotus purpuratus. In: Lawrence JM, editor. Edible sea urchins: biology and ecology. New York: Elsevier; 2001. pp. 307–331. [Google Scholar]
- Tomanek L. The importance of physiological limits in determining biogeographical range shifts due to global climate change: the heat-shock response. Physiol Biochem Zool. 2008;81:709–717. doi: 10.1086/590163. [DOI] [PubMed] [Google Scholar]
- Tomanek L, Somero GN. Evolutionary and acclimation-induced variation in the heat-shock responses of congeneric marine snails (genus Tegula) from different thermal habitats: implications for limits of thermotolerance and biogeography. J Exp Biol. 1999;202:2925–2936. doi: 10.1242/jeb.202.21.2925. [DOI] [PubMed] [Google Scholar]
- Wolpert L (1992) Gastrulation and the evolution of development. Development 116:7–13 [PubMed]
- Zacherl D, Gaines SD, Lonhart SI. The limits to biogeographical distributions: insights from the northward range extension of the marine snail, Kelletia kelletii (Forbes, 1852) J Biogeogr. 2003;30:913–924. doi: 10.1046/j.1365-2699.2003.00899.x. [DOI] [Google Scholar]
- Zippay ML, Hofmann GE. Physiological tolerances across latitudes: thermal sensitivity of larval marine snails (Nucella spp.) Mar Biol. 2010;157:707–714. doi: 10.1007/s00227-009-1354-3. [DOI] [PMC free article] [PubMed] [Google Scholar]