Abstract
A bottom-mounted upward-facing 38-kHz echo sounder was deployed at ~400 m and cabled to shore in Masfjorden (~60°52′N, ~5°24′E), Norway. The scattering layers seen during autumn (September–October) 2008 were identified by trawling. Glacier lanternfish (Benthosema glaciale) were mainly distributed below ~200 m and displayed three different diel behavioral strategies: normal diel vertical migration (NDVM), inverse DVM (IDVM) and no DVM (NoDVM). The IDVM group was the focus of this study. It consisted of 2-year and older individuals migrating to ~200–270 m during the daytime, while descending back to deeper than ~270 m during the night. Stomach content analysis revealed increased feeding during the daytime on overwintering Calanus sp. We conclude that visually searching glacier lanternfish performing IDVM benefit from the faint daytime light in mid-waters when preying on overwintering Calanus sp.
Introduction
Myctophidae is the most abundant family of mesopelagic fish (Moser and Ahlstrom 1974; Valinassab et al. 2007). They are important in marine food webs worldwide (Tyler and Pearcy 1975; Shreeve et al. 2009; Cherel et al. 2010) as predators on zooplankton (Gjøsæter 1973b; Moku et al. 2000; Shreeve et al. 2009), and as prey for fish (Hansen and Pethon 1985; Giske et al. 1990; Walker and Nichols 1993), sea birds (Hedd et al. 2009) and marine mammals (Doksæter et al. 2008).
Mesopelagic fish form acoustic backscattering layers, and their behavior can thus be studied using echo sounders (Holton 1969; Godø et al. 2009; O’Driscoll et al. 2009). Echo sounders have particularly been used in studies of diel vertical migration (DVM) of mesopelagic scattering layers, of which myctophids are a prevailing part (Valinassab et al. 2007; Godø et al. 2009; Kaartvedt et al. 2009; O’Driscoll et al. 2009). Under the normal DVM pattern (NDVM), the organisms forage on abundant plankton in upper waters at night and hide from visual predators at depth during the day (Pearre 2003; Kahilainen et al. 2009). Another, less common type of DVM is inverse DVM (IDVM). This behavior is characterized by organisms moving to shallower waters during the daytime and descending towards deeper waters during the night (Pearre 2003). IDVM has commonly been ascribed to zooplankton species avoiding NDVM predators (Ohman et al. 1983, Ohman 1990; Lagergren et al. 2008). It has been described for fish (Neilson and Perry 1990), although rarely (Jensen et al. 2011), and has only recently been documented in mesopelagic fishes (Kaartvedt et al. 2009).
Glacier lanternfish (Benthosema glaciale) is the most abundant species of myctophids (myctophidae) in the Atlantic Ocean north of 35°N and is together with pearlside (the Sternoptychidae Maurolicus muelleri) the dominating mesopelagic fish in fjords along the coast of Norway (Aksnes et al. 2004; Kristoffersen and Salvanes 2009). In Masfjorden, Norway, glacier lanternfish are mostly distributed below 200 m during the daytime, while the population is spread throughout the water column during the night (Kaartvedt et al. 1988; Giske et al. 1990; Bagøien et al. 2001). By means of a bottom-mounted echo sounder at ~400 m in Masfjorden, Kaartvedt et al. (2009) observed three different modes of diel behavior in the population of glacier lanternfish. One part of the population exhibited NDVM, one part IDVM and one part did not migrate (NoDVM) (Kaartvedt et al. 2009).
Glacier lanternfish feed primarily on calanoid copepods, especially Calanus (Sameoto 1988, 1989; Balino and Aksnes 1993; Bagøien et al. 2001), although other plankton are frequently observed in the stomach contents (Gjøsæter 1973b; Roe and Badcock 1984; Sameoto 1988, 1989). In Norwegian fjords glacier lanternfish exert a strong predation pressure on overwintering Calanus finmarchicus (Bagøien et al. 2001). During the autumn, the majority of the Calanus sp. population is distributed in the depth intervals 0–50 and 150–250 m (Bagøien et al. 2001). The deepest group of Calanus sp. thus overlaps with the observed IDVM group of glacier lanternfish. Mesopelagic fish with dark-adapted eyes may spot their plankton prey even at several hundred meters depth in daylight (Warrant and Locket 2004; Turner et al. 2009), although feeding efficiency generally increases with available light. Kaartvedt et al. (2009) hypothesized that glacier lanternfish with IDVM ascend to forage on overwintering Calanus finmarchicus in the better light conditions in the middle layers of the water column during the daytime. We here address this hypothesis by examining the vertical distribution of plankton and fish in combination with gut content analyses during the day and night the year subsequent to the study of Kaartvedt et al. (2009). A group performing IDVM was also recorded this year, and these fish are the main focus of this study.
Materials and methods
This study was conducted in September–October 2008 in the deepest basin of Masfjorden (~60°52′N, ~5°24′E), Norway, using R/V “Trygve Braarud” (for a map and further description of the locality, see Kaartvedt et al. 1988; Balino and Aksnes 1993).
Temperature and salinity were measured with a CTD (Conductivity, Temperature, Depth; Falmouth Scientific Inc.).
Zooplankton was sampled with a WP2 net with 200-μm mesh size. Sampling was done in five depth intervals (0–50, 50–100, 100–200, 200–300 and 300–400 m). Two replicates from each depth interval were sampled during the day. Due to time constraints, only two replicates from the upper 50 m were sampled during the night. Samples were fixed in 4% formalin for later identification and numeration. Sub-sampling was done with a modified Folsom splitter. Common zooplanktons were identified to genus level.
Trawling for fish was conducted from 3 October to 7 October 2008. A modified fry trawl with a multisampler opening and closing cod-end permitting depth-stratified sampling was used (Engås et al. 1997). The multisampler had three bags attached that could be opened and closed separately by acoustical signals between R/V “Trygve Braarud” and the multisampler (Scanmar HCL hydro acoustic two-way communication link). The trawl opening was 100 m2. Trawling was conducted for approximately 10 min per bag at approximately 2 knots. Eight successful trawl tows (i.e., 24 trawl samples) between 80 and 400 m during the daytime (from 12:00 to 17:00) gave a minimum of three samples (replicates) from each 50-m depth interval (50–100 to 350–400 m). Four trawl tows were conducted at night (from 19:00 to 23:00), resulting in six samples between 0 and 50 m, three between 200 and 250 m, and two between 300 and 350 m (11 nocturnal samples in total).
The trawl catch was immediately sorted by species, counted, weighed, marked and frozen for later analyses. Total catch in separate depth intervals was evaluated as g min−1 trawling acting as a relative, not quantitative, estimate of species abundance. Depth intervals of 50 m were chosen to represent the vertical distribution of the catch. In most cases the trawl did not completely cover the 50 m interval. For example, trawling in the depth interval 250–300 m was conducted between 250 and 270 m, where the acoustical data suggest a dense concentration of glacier lanternfish.
The size of the individual lanternfish usually influences its choice of vertical position (Halliday 1970; Roe and Badcock 1984). Thirty (when possible) intact individuals from each trawl tow bag were measured for total length (Sameoto 1988) and dissected for analyses of stomach contents. When fewer than 30 individuals were caught, all the intact individuals were measured. Length distribution by depth was statistically examined using Kruskal–Wallis tests.
A total of 664 individuals were analyzed for stomach contents. The stomachs were removed as described by Sameoto (1988, 1989). Degree of stomach fullness and digestion was categorized from 1 to 5 (1: empty; 5: full/distended; 1: fresh; 5: fully digested/unrecognizable; Fotland et al. 2000). A stereo microscope with 10× and 40× magnification was used for the stomach analyses. Stomach contents were identified to the nearest possible taxon, with increasing uncertainty as the degree of digestion increased. Selective preference for a certain prey takes place when a predator consumes more of certain species than other co-existing species (Jacobs 1974). A measurement of prey selectivity (D i) was calculated using a modified Ivlev selectivity index, D i (Jacobs 1974). This index indicates if a predator has negative selection (D i from −1 to 0) or positive selection (D i from 0 to +1) for a specific prey type. The concentration (p) of prey in an area and the concentration (r) of the same prey in the stomach contents are used when calculating D i. The stomach contents of glacier lanternfish caught at different depth intervals at different times of day were compared statistically with Kruskal–Wallis tests followed by Tukey posthoc analyses. The number of prey items per stomach was not normally distributed; thus, the fourth root of the number of prey identified per stomach was used in the posthoc analyses.
A bottom-mounted, upward-facing, calibrated Simrad EK60 38-kHz split-beam echo sounder was deployed at ~400 m depth in Masfjorden, and cabled to the shore for power and continuous transmission of data. Further description of the acoustical setup is given by Kaartvedt et al. (2009). Acoustical data from 23 September, 2 October and 6 October 2008 (a time period overlapping with the period of sampling) were analyzed using the Sonar 5 pro version 5.9.7 software (Balk and Lindem 2007). Echograms illustrating the total volume backscattering (S v) were made in MATLAB.
During the daytime glacier lanternfish are distributed deeper than pearlside in Masfjorden (Giske et al. 1990; Bagøien et al. 2001; Kaartvedt et al. 2009, this study). Since pearlside were found mainly above ~250 m, it appears relatively safe to say that the biomass of mesopelagic fish below 250 m is glacier lanternfish. The scattering layers seen at 38 kHz are present even at 18 kHz (Kaartvedt et al. 2008), which suggests that mesopelagic fishes dominate the acoustical scattering layers addressed here (Love et al. 2004).
Estimation of the total volume backscattering coefficient (S v) in a given interval was done by echo integration in Sonar 5. When both S v and the average echo strength of individual fish (TS) are known, the concentration of individuals can be estimated (for calculations, see MacLennan and Simmonds 1992). Difference in concentration of glacier lanternfish in the 270–300-m interval where the IDVM group is distributed at night was assessed by echo integration to test for diel differences. The S v values in this vertical interval were integrated over 1-h periods. Echo integration was done by subtracting integration results at a S v threshold of −64 dB from results at a S v threshold of −90 dB to exclude organisms larger than glacier lanternfish (Bagøien et al. 2001). The average TS of glacier lanternfish (−58 dB in this study), as measured directly by the split-beam echosounder at a range of 10–50 m (i.e., ~340–380 m depth) from the bottom-mounted echo sounder, was used to calculate the concentration (individuals m−3) from the measured volume backscatter (S v).
R 2.9.0, Microsoft Excel 2003 and SPSS 17.0 were used for statistical calculations.
Results
Hydrography
The salinity increased from 30 in the surface to stable values around 35 below ~100 m (Fig. 1). The highest temperatures were measured in the surface (14.2°C) (Fig. 1). Below ~60 m temperatures were stable at 8.5°C (Fig. 1).
Fig. 1.
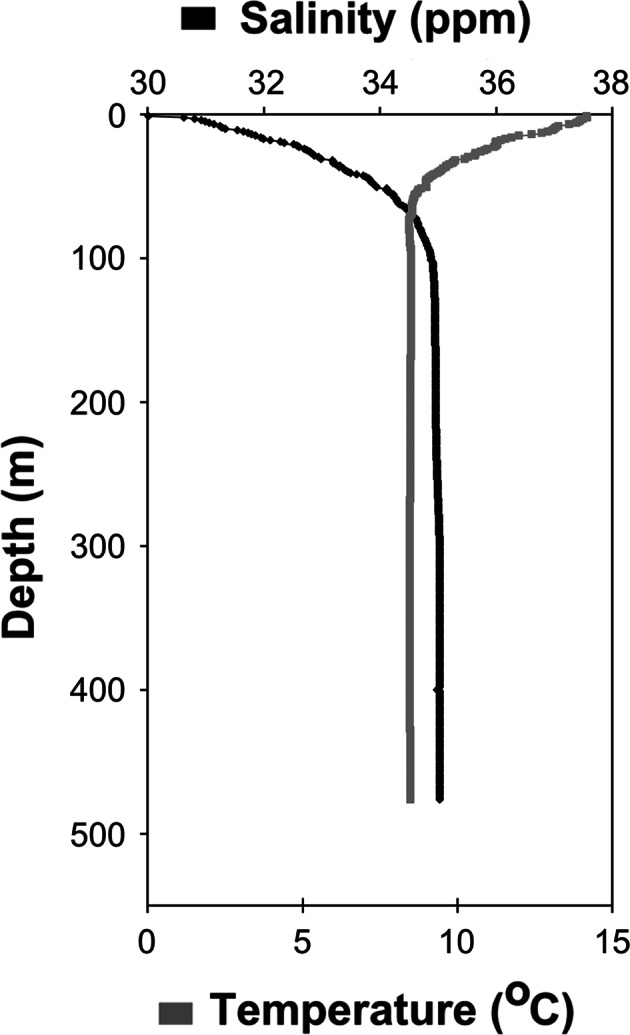
Vertical temperature (gray) and salinity (black) profiles
Distribution of zooplankton
Zooplankton had a bimodal distribution with the highest concentrations between 0–50 and 200–300 m (Table 1). Ninety-five percent of the zooplankton were copepods, with Oithona being the most common taxon (Table 1). Oithona and Acartia dominated in the upper 50 m, while Oithona and Calanus dominated between 200 and 300 m depth (Table 1).
Table 1.
Concentration of crustacean zooplankton (individuals m−3) caught in the WP-II net during the day and night. The estimates in each interval are the average of two replicates sampled
| Depth (m) | Calanus sp. | Oithona sp. | Chiridius sp. | Metridia sp. | Para/Pseudo/Microcalanus sp. | Acartia sp. | Ostracoda | Other | Total |
|---|---|---|---|---|---|---|---|---|---|
| Day | |||||||||
| 0–50 | 5 | 30 | 2 | 1 | 23 | 94 | 0 | 5 | 160 |
| 50–100 | 6 | 61 | 1 | 0 | 2 | 2 | 1 | 2 | 75 |
| 100–200 | 4 | 24 | 1 | 3 | 2 | 0 | 0 | 1 | 35 |
| 200–300 | 34 | 91 | 12 | 8 | 9 | 1 | 3 | 11 | 169 |
| 300–400 | 9 | 11 | 2 | 4 | 4 | 0 | 3 | 4 | 37 |
| Night | |||||||||
| 0–50 | 7 | 147 | 3 | 3 | 38 | 43 | 0 | 12 | 253 |
Trawl catches
Glacier lanternfish dominated the trawl catches deeper than 250 m during both day and night (Fig. 2a–d). During the daytime they were distributed deeper than ~150 m, while some individuals were caught in the surface at night (Fig. 2a). Pearlside were mainly caught in the upper 250 m, with large near-surface catches at night (Fig. 2b). Shrimps (Pasiphea multidentata and Seregestes arcticus) were caught below 200 m during the day and throughout the water column at night (Fig. 2c). Krill (mainly Meganyctiphanes norvegica) was caught between 150 and 250 m at daytime and in the surface at night (Fig. 2d). Mysids (Boreomysis arctica) were less common, but some were caught below 200 m. Some jellyfish (Scyphozoa; Cyanea capillata and Periphylla periphylla) were caught below 300 m.
Fig. 2.
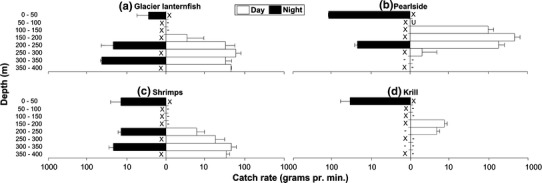
Vertical distribution of a glacier lanternfish, b pearlside, c shrimps and d krill caught during night and day, presented as catch in g min−1 trawling. The Standard deviations are illustrated by lines from the columns, except for the night catch in the depth interval 300–350 m, where the lines illustrate the maximum catch in the trawl tows (since only two successful samples were obtained). Dash indicates that catch is <1 g min−1, cross marks indicate no trawling during the day and night, while U indicates an unknown amount of pearlside larvae
Glacier lanternfish occurred in three size cohorts (1.5–3.3, 3.5–5.4 and 5.5–7.8 cm) (Fig. 3a). The length of individuals increased with depth during both day and night (Kruskal–Wallis, p < 0.001). The smallest individuals were caught between 150 and 250 m during the daytime and never below 250 m (Fig. 3b–d). At night the smallest individuals were caught in the surface and between 200 and 250 m (Fig. 3b–c). The medium-sized length group was more evenly distributed, with 83% found between ~200–300 m during the daytime and 17% below 300 m (Fig. 3b–d). The medium-sized fish made up 45% of the catch between 200 and 250 m during the night (Fig. 3c). The largest size group was distributed below ~200 m (Fig. 3b–d). Between 200 and 300 m, 51% of the captured glacier lanternfish were individuals of the largest size group, while between 300 and 400 m the largest size group constituted 80–90% of the catch (Fig. 3c–d).
Fig. 3.
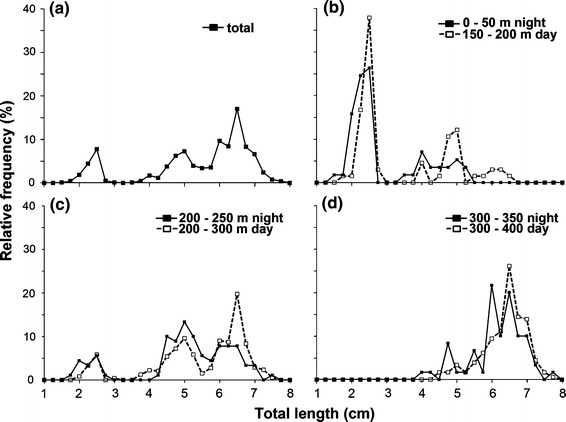
Length distribution of glacier lanternfish. a All captured individuals, b individuals caught from 0 to 50 m at night and from 150 to 200 m at daytime, c individuals caught from 200 to 250 m at night and 200 to 300 at daytime, and d individuals caught from 300 to 350 m at night and 300 to 400 m at daytime. The values of the y-axis are given as relative frequency (%) of total catch in the given interval
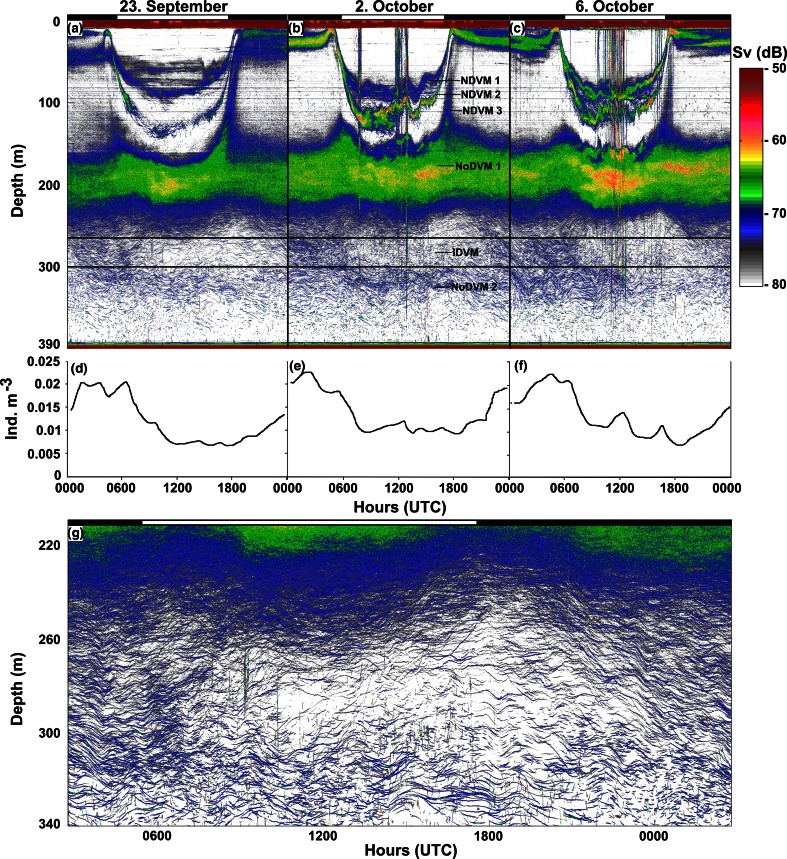
Acoustic scattering layers
At 38 kHz, applying a threshold of −80 dB, most backscattering is due to fish, as shrimps and krill are comparatively weak acoustic targets. The trawl catches suggest that pearlside made up the main part of backscattering in the upper 200 m, while echoes from glacier lanternfish dominated the backscatter deeper than 250 m.
Three different modes of diel behaviors were apparent: NDVM, IDVM and NoDVM (Fig. 4a–c). Three separate scattering layers displayed the NDVM pattern (Fig. 4b). The trawl catches show that the two shallowest SLs mainly consisted of pearlside (NDVM 1 and NDVM 2), while the deepest NDVM SL also contained some glacier lanternfish (NDVM 3) (Fig. 4b). A layer without any apparent migration between 150 and 220 m (NoDVM1) was dominated by pearlside, while also some glacier lanternfish were captured (Fig. 4b).
Fig. 4.
a–c Twenty-four-hour echograms from the bottom-mounted 38-kHz echo sounder on 23 September, 2 October and 6 October 2008, with different DVM modes annotated in b; d–f acoustic abundance estimates of glacier lanternfish (individuals m−3) at 270–300 m (interval marked with horizontal lines in a–c); g zoom on ~200–340 m from ~03:00 to 03:00 on 23–24 September 2008. The coloration in echograms refers to volume backscattering (S v), where red illustrates the strongest and white the weakest backscatter. Black and white bars above echograms (a–c, g) depict night and day, separated by times for sunrise and sunset. Time is given in UTC (local standard time-1 h)
Deeper than ~200 m, two behavioral modes were observed (Fig. 4a–c). One SL ascribed to lanternfish displaying the IDVM pattern was distributed between 250 and 300 m at night and migrated towards the NoDVM1 layer in the morning, leading to a void in the echograms as fish swam up from this interval during the day (Fig. 4a–c). At night individuals of this layer migrated back to deeper waters. Individuals of a deeper SL distributed below ~300 m appeared not to migrate (NoDVM2) (Fig. 4a–c). Based on the trawl results, the individuals that made up these two layers were of the two largest size groups of glacier lanternfish (Fig. 3a–d).
The IDVM pattern was evident in the acoustical data on all days (Fig. 4a–c). Acoustic abundance estimates suggested a daytime concentration reduction of approximately 60–70% in the interval between 270 and 300 m as fish ascended above this level (Fig. 4d–f).
The swimming speeds of glacier lanternfish while performing IDVM were derived by measuring slopes of individual organism traces in the echograms. The vertical relocations were slow, with average speed of ~0.004 m s−1, both when moving upwards and downwards. This is equivalent to ~1/16 body length s−1 for the largest size group. The slow relocation of individuals displaying the IDVM pattern was reflected in the timing of fluctuating densities in the 270–300-m depth interval. The number of fish continued to increase after midnight as fish still returned to deeper waters after their daytime ascent (left of Fig. 4d–f), and, correspondingly, fish had not reached the maximum number after the daytime low the following midnight (right of Fig. 4d–f). The persisting nocturnal descent of fish to deep waters well after midnight is illustrated in Fig. 4g, where the time axis of a diel echogram has been shifted (from ~03:00 to ~03:00) to center the void in the acoustic backscatter created by the IDVM.
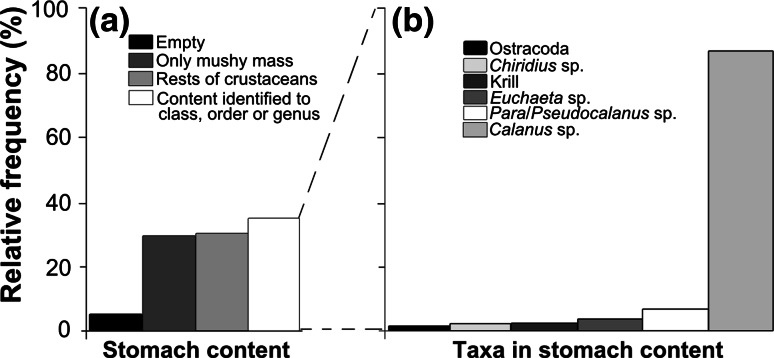
Feeding
Most fish had no or unidentifiable stomach contents (Fig. 5a), but 35% (230 individuals) of the examined fish had stomach contents that could be identified to a taxonomic group (Fig. 5a). Of the individuals with identifiable stomach contents, 31% contained only one prey item. There was a tendency of an increasing number of prey with increasing length of the fish.
Fig. 5.
Stomach contents of glacier lanternfish. a Percentage of empty stomachs and different types of stomach contents in dissected glacier lanternfish. b Identified prey allocated to taxa. The shrimp Sergestes sp. (0.2%), and the copepods Oithona sp. (0.1%) and Metridia sp. (0.5%) are not included in the figure (b)
A total of 191 fish contained Calanus sp., and Calanus sp. accounted for 86% of the identified prey (Fig. 5b). The much larger krill and shrimps were identified in 1.4 and 0.2% of the stomachs, respectively (Fig. 5b). Other prey included various copepods and ostracods (Fig. 5b). Only 39 fish had identifiable stomach contents without containing Calanus sp. The glacier lanternfish had positive selection only for Calanus sp. (D i = 0.76), while other copepods were selected against. A maximum of 24 Calanus sp. were registered per stomach. Individuals of the two largest size groups seemed to feed on more Calanus sp. than individuals belonging to the smallest size group (posthoc, Tukey test, p < 0.001). Due to Calanus sp. domination in the diet, subsequent analyses focus on this species.
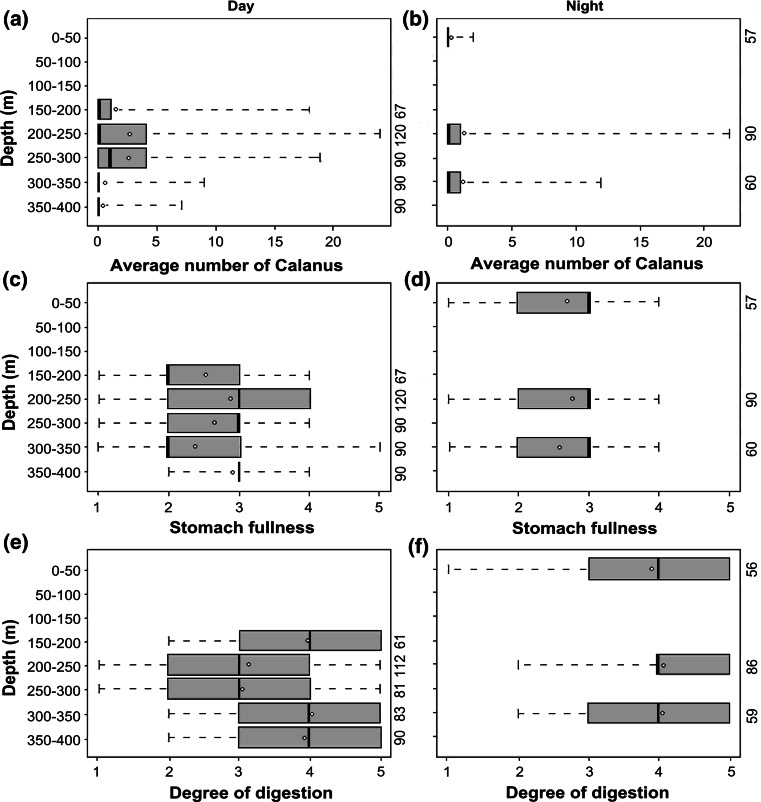
Fish caught between 200–250 and 250–300 m during the daytime had significantly more Calanus sp. in their stomachs than those from 300 to 400 m (posthoc, Tukey test, p < 0.001) (Fig. 6a). There was also significantly more Calanus sp. in the stomach contents of individuals caught between 200–250 m (posthoc, Tukey test, p < 0.01) and 250–300 m (posthoc, Tukey test, p < 0.001) than for fish caught between 150 and 200 m (Fig. 6a). Stomach fullness was highest in fish from 200 to 250 m, but also high in fish from 250 to 300 m during the daytime (Fig. 6c–d).
Fig. 6.
Stomach contents of glacier lanternfish at different depth intervals day (left) and night (right). a, b Number of Calanus sp. in stomach contents at different depth intervals during the day (a) and at night (b); c, d degree of stomach fullness during the day (c) and night (d); e, f degree of digestion of stomach contents during the day (e) and night (f). Vertical axis on the right side on the figures depicts number of glacier lanternfish analyzed for the different depth intervals. The boxes illustrate the 25% quartile, the 50% median and the 75% quartile. The dotted lines illustrate the maximum/minimum values of number of Calanus sp. (a, b), stomach fullness (c, d) or degree of digestion (e, f). The rings mark the average values
Calanus sp. was generally more abundant in stomach contents during the day than during the night (Kruskal–Wallis, p = 0.03) (Fig. 6a–b). During the day more than one quarter of the fish contained two or more Calanus sp. During the night stomachs contained few Calanus sp., with a notable exception for the 22:00 period. Between 23:00 and 11:00 there was no trawling, and thus stomach analysis from this period does not exist. Fish caught during the daytime between 200 and 300 m also had the lowest recorded digestion state (Fig. 6e–f).
In total there were only small differences in the degree of stomach fullness for the whole water column between day and night (Fig. 6c–d), but fish caught at night had less identifiable stomach contents than fish caught during the day (Fig. 6e–f).
Discussion
Acoustical data from a bottom-mounted 38-kHz echo sounder combined with trawling showed that glacier lanternfish are the main cause of backscattering below 250 m in Masfjorden. The distribution of glacier lanternfish overlaps with that of pearlside in the ~200–250-m depth interval. This is supported by previous studies in Masfjorden (Kaartvedt et al. 1988; Giske et al. 1990; Bagøien et al. 2001; Kaartvedt et al. 2009). Three distinct behavioral strategies in the population of glacier lanternfish were identified during autumn.
The smallest individuals carry out NDVM or may not migrate at all (NoDVM1; Fig. 4b) during autumn, while the larger individuals always stay deeper than 200 m. The deep-living components of the population showed two different behavioral strategies, IDVM and NoDVM. The IDVM individuals swim upwards towards 200–270 m during the daytime to feed mainly on Calanus. Kaartvedt et al. (2009) observed similar behaviors in this population during fall the previous year.
Feeding and digestion by glacier lanternfish
Many of the lanternfish had empty stomachs, which seems typical for this species (Albikovskaya 1988; Giske et al. 1990; Balino and Aksnes 1993; Pusch et al. 2004). No individuals with everted stomachs were observed, so we excluded stress and regurgitated stomach contents as potential errors in this study. The glacier lanternfish feeds more regularly during spring and summer than during autumn and winter (Gjøsæter 1973b; Sameoto 1988). This could potentially help explain the high percentage of individuals with empty stomachs in this study.
Calanus sp. was the only prey that was positively selected for. The genus Calanus is known to form a significant part of the diet of myctophids (Gjøsæter 1973b; Sameoto 1989; Pusch et al. 2004; Shreeve et al. 2009). Other calanoid copepods, krill, shrimps and ostracods were also found in the guts, but these taxa only comprised ~14% of the total numerical content. These taxa are commonly part of myctophids’ diet in oceans around the world, although each taxa’s dietary importance varies (Roe and Badcock 1984; Hopkins et al. 1996; Moku et al. 2000; Shreeve et al. 2009). In our study krill was not a numerically important prey. This supports the findings of Giske et al. (1990), but differs from the findings of Gjøsæter (1973b). It is reasonable to assume that Calanus sp. is easier to catch than krill because of the concentration of inactive (Hirche 1983), overwintering Calanus sp. and their smaller size, but at the same time, the dividend will be much greater when catching the two orders of magnitude larger krill (Falk-Petersen 1981; Tande 1982). Krill was more common in the diet of the 2-year group and older individuals. Gjøsæter (1973b) made similar observations, and the same trend has been observed in studies of other myctophids (Pearcy et al. 1979; Pusch et al. 2004).
Digestion time in mesopelagic fish is not well known (Dalpadado and Gjøsæter 1988), and how long copepods can stay undigested in fish stomachs is not known (Bagøien et al. 2001). Nevertheless, a large number of identifiable and undigested prey indicate recent feeding (Dalpadado and Gjøsæter 1988). The largest number of prey in the stomachs and the lowest degree of digestion were observed during the daytime. Stomach fullness might also be high during the night, but then the food was more digested, indicating that it had been a while since feeding. For logistic reasons, samples from night time were restricted to the time period between 19:00 and 23:00; thus, we have no data to tell how glacier lanternfish feed throughout the night. Previous studies from eastern and northwestern parts of the Atlantic Ocean have shown that glacier lanternfish normally feeds in surface waters at night, but also at their daytime depth (Roe and Badcock 1984; Sameoto 1988, 1989). Previous reports from Masfjorden conclude that glacier lanternfish mainly feeds during the daytime (Giske et al. 1990; Balino and Aksnes 1993) or have found no diel pattern in feeding (Bagøien et al. 2001). From our study it seems that individuals with IDVM feed during the daytime (Figs. 4a–c, g, 6a–f), mainly on overwintering Calanus (Fig. 5b), while others (NDVM) probably feed mainly during the night. Thus, depending on which behavioral group is in focus, different conclusions about feeding periodicity can be reached.
Possible explanations for the diel behavior
Most studies have concluded that glacier lanternfish perform NDVM (Roe and Badcock 1984; Sameoto 1988, 1989). However, our study and previous studies have shown reduced levels of NDVM during autumn (Gjøsæter 1973b; Sameoto 1988; Kaartvedt et al. 2009), and Kaartvedt et al. (2009) and this study add IDVM to the behavioral repertoir. The current study identified three size cohorts of the glacier lanternfish population, and possible explanations for the varying migration patterns might be the difference in abilities and motivation between the cohorts and the vertical distribution of prey.
The zooplankton concentration found in this study reflected that sampling was done during the end of the productive season, yet before the winter low. The zooplankton concentration was about fourfold that found during previous winter studies in Masfjorden (Giske et al. 1990; Balino and Aksnes 1993) and almost half of reported summer concentrations (Rasmussen and Giske 1994). Small copepods dominated in the upper 50 m, while Calanus sp., which was found to be the main prey of glacier lanternfish, occurred at the highest concentrations between 200 and 300 m, at similar depths as in previous studies, albeit at lower densities (Giske et al. 1990; Balino and Aksnes 1993; Bagøien et al. 2001). Due to the low concentration of larger zooplankton at the surface, glacier lanternfish will gain little from swimming to the surface in the autumn. However, some individuals of the smallest size group (1.5–3.3 cm) and the smallest individuals of the medium size group (3.5–5.4 cm), which correspond to the 0-year class and the 1-year class, respectively (Halliday 1970; Gjøsæter 1973a), performed NDVM to the upper 50 m at night. The IDVM was mainly carried out by individuals greater than 5 cm, corresponding to the 2-year class or older (Halliday 1970; Gjøsæter 1973a), since these were the ones dominating at depths below 250 m.
In glacier lanternfish and other mesopelagic fish, body size typically increases with depth (Willis and Pearcy 1980; Roe and Badcock 1984; Gartner et al. 1987; Auster et al. 1992). Such size distributions have often been ascribed to smaller mesopelagic fish being less visible to predators (Giske et al. 1990) and more risk taking (Giske and Aksnes 1992) than bigger individuals. For juveniles increased mortality risk in shallower waters might be compensated by higher potential feeding rates, and warmer water implies both decreased digestion time and higher potential growth rates (Wurtsbaugh and Neverman 1988), and consequently earlier maturation than in deeper waters (Rosland and Giske 1997). Also, small individuals will lose a higher percentage of body mass compared to individuals of greater size when starving and therefore have to take greater risks (normally associated with staying shallower) to find food (Krause et al. 1998). On the other hand, individuals that have reached a certain size and maturation state can afford avoiding this risk by staying deeper (Hays et al. 2001). Additionally, they have larger eyes than smaller individuals, and can therefore better detect prey in deeper and darker waters (Warrant and Locket 2004). In sum, young individuals might seek a higher growth rate, but increased mortality risk in shallower waters, while adults prioritize lower mortality risk, but less potential (or less visible) prey in deeper waters (Rosland and Giske 1997).
By remaining in deeper and colder water masses during the night, individuals can minimize their energy loss (Sogard and Olla 1996) and reduce their exposure to predators. This behavior (NoDVM) has previously been observed for glacier lanternfish (Albikovskaya 1988; Kaartvedt et al. 2009) and other myctophids (Pearcy et al. 1979; Gartner et al. 1987; Moku et al. 2000). While low surface concentrations of prey during autumn may explain why a large proportion of the population stays in deeper waters through the whole day, it cannot explain IDVM.
The IDVM group co-occurs with the overwintering component of Calanus sp. Glacier lanternfish fed most actively in this overlapping layer during the daytime. Similarly, Bagøien et al. (2001) observed overlapping distribution between mesopelagic fishes and overwintering Calanus sp. and documented a strong predation pressure on overwintering Calanus sp. in Masfjorden.
Most overwintering Calanus sp. are inactive (Hirche 1983). As a result of predation from mesopelagic fish, a pronounced numerical reduction of Calanus sp. populations is expected during autumn and winter (Kaartvedt 1996; Bagøien et al. 2001). The glacier lanternfish is able to forage visually (Giske et al. 1990; Bagøien et al. 2001) in deeper waters (Roe and Badcock 1984; Sameoto 1988, 1989), but light typically limits food consumption more, through its effect on detection distance, than the concentration of prey (Aksnes and Giske 1993). We hypothesize that the IDVM group swims upward during the daytime to improve the light conditions and thereby their chances of locating prey. Glacier lanternfish ascend and descend in a stop and go manner (Kaartvedt et al. 2008), possibly explaining the slow swimming speed found in the current study.
We reject the alternative explanation that the inverse vertical migration may relate to metabolic advantages (cf. Wurtsbaugh and Neverman 1988) since the water column in the deep water of Masfjorden is homogenous with no vertical temperature gradients. We also reject that the migration relates to exploitation of water currents as a mechanism for retention or horizontal transport (Smith et al. 2001; Bennett et al. 2002). The currents in deeper waters in Masfjorden are weak as the water body is enclosed behind the 75-m-deep sill (Aksnes et al. 1989), and glacier lanternfish in Masfjorden drift slowly back and forth with weak tidal currents at a period that is shorter than the day/night cycle (Kaartvedt et al. 2009).
In conclusion, this study documents that glacier lanternfish is capable of localizing and prey on overwintering Calanus sp. during the daytime. In accordance with Kaartvedt et al. (2009), the most likely explanation for IDVM is that visually foraging individuals during the daytime actively seek an interval with better light conditions, thereby increasing the availability of prey, before returning to deeper waters at night.
Acknowledgments
We would like to thank Anders Røstad for acoustic data processing and ideas for the manuscript, and Josefin Titelman for feedback and comments. We are grateful to the crew on R/V “Trygve Braarud” and Rita Amundsen for making the cruise and sampling possible. Finally, thanks to Arved Staby for sharing data and helping out during the cruise.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Eivind Dypvik, Phone: +966-0544-701841, Email: eivind.dypvik@kaust.edu.sa.
Thor A. Klevjer, Email: thor.klevjer@kaust.edu.sa
Stein Kaartvedt, Email: stein.kaartvedt@kaust.edu.sa.
References
- Aksnes DL, Giske J. A theoretical model of aquatic visual feeding. Ecol Model. 1993;67:233–250. doi: 10.1016/0304-3800(93)90007-F. [DOI] [Google Scholar]
- Aksnes DL, Aure J, Kaartvedt S, Magnesen T, Richard J. Significance of advection for the carrying capacities of fjord populations. Mar Ecol Prog Ser. 1989;50:263–274. doi: 10.3354/meps050263. [DOI] [Google Scholar]
- Aksnes DL, Nejstgaard J, Sædberg E, Sørnes T. Optical control of fish and zooplankton populations. Limnol Oceanogr. 2004;49:233–238. doi: 10.4319/lo.2004.49.1.0233. [DOI] [Google Scholar]
- Albikovskaya LK. Some aspects of the biology and distribution of glacier lanternfish (Benthosema glaciale) over the slopes of Flemish Cap and Eastern Grand Bank. NAFO Sci Count Stud. 1988;12:37–42. [Google Scholar]
- Auster PJ, Griswold CA, Youngbluth MJ, Bailey TG. Aggregations of myctophid fishes with other pelagic fauna. Environ Biol Fishes. 1992;35:133–139. doi: 10.1007/BF00002187. [DOI] [Google Scholar]
- Bagøien E, Kaartvedt S, Aksnes DL, Eiane K. Vertical distribution and mortality of overwintering Calanus. Limnol Oceanogr. 2001;46:1494–1510. doi: 10.4319/lo.2001.46.6.1494. [DOI] [Google Scholar]
- Balino BM, Aksnes DL. Winter distribution and migration of the sound-scattering layers, zooplankton and micronekton in Masfjorden, western Norway. Mar Ecol Prog Ser. 1993;102:35–50. doi: 10.3354/meps102035. [DOI] [Google Scholar]
- Balk H, Lindem T. Sonar 4 & Sonar 5_Pro Post processing systems. Operator manual 5.9.7. Norway: Lindem Data Acquisition, University of Oslo; 2007. [Google Scholar]
- Bennett WA, Kimmerer WJ, Burau JR. Plasticity in vertical migration by native and exotic estuarine fishes in a dynamic low-salinity zone. Limnol Oceanogr. 2002;47:1496–1507. doi: 10.4319/lo.2002.47.5.1496. [DOI] [Google Scholar]
- Cherel Y, Fontaine C, Richard P, Labat JP. Isotopic niches and trophic levels of myctophid fishes and their predators in the Southern Ocean. Limnol Oceanogr. 2010;55:324–332. doi: 10.4319/lo.2010.55.1.0324. [DOI] [Google Scholar]
- Collins MA, Partridge JC, Douglas RH. Vision in lanternfish (Myctophidae): adaptations for viewing bioluminescence in the deep-sea. Deep Sea Res Part 1 Oceanogr Res Pap. 2009;56:1003–1017. doi: 10.1016/j.dsr.2009.01.007. [DOI] [Google Scholar]
- Dalpadado P, Gjøsæter J. Feeding ecology of the laternfish Benthosema pterotum from the Indian Ocean. Mar Biol. 1988;99:555–567. doi: 10.1007/BF00392563. [DOI] [Google Scholar]
- Doksæter L, Olsen E, Nøttestad L, Fernö A. Distribution and feeding ecology of dolphins along the Mid-Atlantic Ridge between Iceland and the Azores. Deep-Sea Res (2 Top Stud Oceanogr) 2008;55:243–253. doi: 10.1016/j.dsr2.2007.09.009. [DOI] [Google Scholar]
- Engås A, Skeide R, West CW. The ‘MultiSampler’: a system for remotely opening and closing multiple codends on a sampling trawl. Fish Res. 1997;29:295–298. doi: 10.1016/S0165-7836(96)00545-0. [DOI] [Google Scholar]
- Falk-Petersen S. Ecological investigations on the zooplankton community of Balsfjorden, Northern Norway: seasonal changes in body weight and the main biochemical composition of Thysanoessa inermis (Krøyer), T. raschii (M. Sars), and Meganyctiphanes norvegica (M. Sars) in relation to environmental factors. J Exp Mar Biol Ecol. 1981;49:103–120. doi: 10.1016/0022-0981(81)90065-4. [DOI] [Google Scholar]
- Fotland Å, Borge A, Gjøsæter H, Mjanger H. Håndbok for prøvetaking av fisk og krepsdyr. Bergen: Institute of Marine Research; 2000. pp. 79–103. [Google Scholar]
- Gartner JV, Jr, Hopkins TL, Baird RC, Milliken DK. The lanternfishes (Pisces: Myctophidae) of the eastern Gulf of Mexico. Fish Bull. 1987;85:81–98. [Google Scholar]
- Giske J, Aksnes DL. Ontogeny, season and trade-offs: vertical distribution of the mesopelagic Maurolicus muelleri. Sarsia. 1992;77:253–261. [Google Scholar]
- Giske J, Aksnes DL, Balino BM, Kaartvedt S, Lie U, Nordeide JT, Salvanes AGV, Wakili SM, Aadnesen A. Vertical-distribution and trophic interactions of zooplankton and fish in Masfjorden, Norway. Sarsia. 1990;75:65–81. [Google Scholar]
- Gjøsæter J (1973a) Age, growth, and mortality of the myctophid fish, Benthosema glaciale (Reinhardt), from western Norway. Sarsia 52:1–14
- Gjøsæter J (1973b) Food of the myctophid fish, Benthosema glaciale (Reinhardt), from western Norway. Sarsia 52:53–58
- Godø OR, Patel R, Pedersen G. Diel migration and swimbladder resonance of small fish: some implications for analyses of multifrequency echo data. ICES J Mar Sci. 2009;66:1143–1148. doi: 10.1093/icesjms/fsp098. [DOI] [Google Scholar]
- Halliday RG. Growth and vertical distribution of the glacier lanternfish, Benthosema glaciale, in the northwestern Atlantic. J Fish Res Bd Can. 1970;27:105–116. doi: 10.1139/f70-011. [DOI] [Google Scholar]
- Hansen LP, Pethon P. The food of atlantic salmon, Salmo salar L., caught by long-line in northern Norwegian waters. J Fish Biol. 1985;26:553–562. doi: 10.1111/j.1095-8649.1985.tb04296.x. [DOI] [Google Scholar]
- Hays GC, Kennedy H, Frost BW. Individual variability in diel vertical migration of a marine copepod: why some individuals remain at depth when other migrate. Limnol Oceanogr. 2001;46:2050–2054. doi: 10.4319/lo.2001.46.8.2050. [DOI] [Google Scholar]
- Hedd A, Montevecchi WA, Davoren GK, Fifield DA. Diets and distributions of Leach’s storm-petrel (Oceanodroma leucorhoa) before and after an ecosystem shift in the Northwest Atlantic. Can J Zool Rev Can Zool. 2009;87:787–801. doi: 10.1139/Z09-060. [DOI] [Google Scholar]
- Hirche HJ. Overwintering of Calanus finmarchicus and Calanus helgolandicus. Mar Ecol Prog Ser. 1983;11:281–290. doi: 10.3354/meps011281. [DOI] [Google Scholar]
- Holton AA. Feeding behavior of a vertically migrating lanternfish. Pac Sci. 1969;23:325–331. [Google Scholar]
- Hopkins TL, Sutton TT, Lancraft TM. The trophic structure and predation impact of a low latitude midwater fish assemblage. Prog Oceanogr. 1996;38:205–239. doi: 10.1016/S0079-6611(97)00003-7. [DOI] [Google Scholar]
- Jacobs J. Quantitative measurement of food selection. Oecologia. 1974;14:413–417. doi: 10.1007/BF00384581. [DOI] [PubMed] [Google Scholar]
- Jensen OP, Hansson S, Didrikas T, Stockwell JD, Hrabik TR, Axenrot T, Kitchell JF. Foraging, bioenergetic and predation constraints on diel vertical migration: field observations and modelling of reverse migration by young-of-the-year herring Clupea harengus. J Fish Biol. 2011;78:449–465. doi: 10.1111/j.1095-8649.2010.02855.x. [DOI] [PubMed] [Google Scholar]
- Kaartvedt S. Habitat preference during overwintering and timing of seasonal vertical migration of Calanus finmarchicus. Ophelia. 1996;44:145–156. [Google Scholar]
- Kaartvedt S, Aksnes DL, Aadnesen A. Winter distribution of macroplankton and micronekton in Masfjorden, western Norway. Mar Ecol Prog Ser. 1988;45:45–55. doi: 10.3354/meps045045. [DOI] [Google Scholar]
- Kaartvedt S, Torgersen T, Klevjer TA, Røstad A, Devine JA. Behavior of individual mesopelagic fish in acoustic scattering layers of Norwegian fjords. Mar Ecol Prog Ser. 2008;360:201–209. doi: 10.3354/meps07364. [DOI] [Google Scholar]
- Kaartvedt S, Røstad A, Klevjer TA, Staby A. Use of bottom-mounted echo sounders in exploring behavior of mesopelagic fishes. Mar Ecol Prog Ser. 2009;395:109–118. doi: 10.3354/meps08174. [DOI] [Google Scholar]
- Kahilainen KK, Malinen T, Lehtonen H. Polar light regime and piscivory govern diel vertical migrations of planktivorous fish and zooplankton in a subarctic lake. Ecol Freshw Fish. 2009;18:481–490. doi: 10.1111/j.1600-0633.2009.00363.x. [DOI] [Google Scholar]
- Krause J, Loader SP, McDermott J, Ruxton GD. Refuge use by fish as a function of body length-related metabolic expenditure and predation-risks. Proc R Soc Lond B Biol Sci. 1998;265:2373–2379. doi: 10.1098/rspb.1998.0586. [DOI] [Google Scholar]
- Kristoffersen JB, Salvanes AGV. Distribution, growth, and population genetics of the glacier lanternfish (Benthosema glaciale) in Norwegian waters: contrasting patterns in fjords and the ocean. Mar Biol Res. 2009;5:596–604. doi: 10.1080/17451000903042479. [DOI] [Google Scholar]
- Lagergren R, Leberfinger K, Stenson JAE. Seasonal and ontogenetic variation in diel vertical migration of Chaoborus flavicans and its effect on depth-selection behavior of other zooplankton. Limnol Oceanogr. 2008;53:1083–1092. doi: 10.4319/lo.2008.53.3.1083. [DOI] [Google Scholar]
- Love RH, Fisher RA, Wilson MA, Nero RW. Unusual swimbladder behavior of fish in the Cariaco Trench. Deep Sea Res Part 1 Oceanogr Res Pap. 2004;51:1–16. doi: 10.1016/j.dsr.2003.09.004. [DOI] [Google Scholar]
- MacLennan DN, Simmonds EJ. Fisheries acoustics. London: Chapman & Hall; 1992. [Google Scholar]
- Moku M, Kawaguchi K, Watanabe H, Ohno A. Feeding habits of three dominant myctophid fishes, Diaphus theta, Stenobrachius leucopsarus and S. nannochir, in the subarctic and transitional waters of the western North Pacific. Mar Ecol Prog Ser. 2000;207:129–140. doi: 10.3354/meps207129. [DOI] [Google Scholar]
- Moser GH, Ahlstrom E. Role of larval stages in systematic investigations of marine teleosts: the Myctophidae, a case study. Fish Bull. 1974;72:391–413. [Google Scholar]
- Neilson JD, Perry RI. Diel vertical migrations of marine fishes—an obligate or facultative process. Adv Mar Biol. 1990;26:115–168. doi: 10.1016/S0065-2881(08)60200-X. [DOI] [Google Scholar]
- O’Driscoll RL, Gauthier S, Devine JA. Acoustic estimates of mesopelagic fish: as clear as day and night? ICES J Mar Sci. 2009;66:1310–1317. doi: 10.1093/icesjms/fsp015. [DOI] [Google Scholar]
- Ohman MD. The demographic benefits of diel vertical migration by zooplankton. Ecol Monogr. 1990;60:257–281. doi: 10.2307/1943058. [DOI] [Google Scholar]
- Ohman MD, Frost BW, Cohen EB. Reverse diel vertical migration: an escape from invertebrate predators. Science. 1983;220:1404–1407. doi: 10.1126/science.220.4604.1404. [DOI] [PubMed] [Google Scholar]
- Pearcy WG, Lorz HV, Peterson W. Comparison of the feeding-habits of migratory and non-migratory Stenobrachius-Leucopsarus (Myctophidae) Mar Biol. 1979;51:1–8. doi: 10.1007/BF00389025. [DOI] [Google Scholar]
- Pearre S. Eat and run? The hunger/satiation hypothesis in vertical migration: history, evidence and consequences. Biol Rev. 2003;78:1–79. doi: 10.1017/S146479310200595X. [DOI] [PubMed] [Google Scholar]
- Pusch C, Hulley PA, Kock K-H. Community structure and feeding ecology of mesopelagic fishes in the slope waters of King George Island (South Shetland Islands, Antarctica) Deep Sea Res Part 1 Oceanogr Res Pap. 2004;15:1685–1708. [Google Scholar]
- Rasmussen OI, Giske J. Life-history parameters and vertical distribution of Maurolicus muelleri in Masfjorden in summer. Mar Biol. 1994;120:649–664. doi: 10.1007/BF00350086. [DOI] [Google Scholar]
- Roe HSJ, Badcock J. The diel migrations and distributions within a mesopelagic community in the North East Atlantic. 5. Vertical migrations and feeding of fish. Prog Oceanogr. 1984;13:389–424. doi: 10.1016/0079-6611(84)90014-4. [DOI] [Google Scholar]
- Rosland R, Giske J. A dynamic model for the life history of Maurolicus muelleri, a pelagic planktivorous fish. Fish Oceanogr. 1997;6:19–34. doi: 10.1046/j.1365-2419.1997.00023.x. [DOI] [Google Scholar]
- Sameoto DD. Feeding of lantern fish Benthosema glaciale off the Nova Scotia Shelf. Mar Ecol Prog Ser. 1988;44:113–129. doi: 10.3354/meps044113. [DOI] [Google Scholar]
- Sameoto DD. Feeding ecology of the lantern fish Benthosema glaciale in a subarctic region. Polar Biol. 1989;9:169–178. doi: 10.1007/BF00297172. [DOI] [Google Scholar]
- Shreeve RS, Collins MA, Tarling GA, Main CE, Ward P, Johnston NM. Feeding ecology of myctophid fishes in the northern Scotia Sea. Mar Ecol Prog Ser. 2009;386:221–236. doi: 10.3354/meps08064. [DOI] [Google Scholar]
- Smith CL, Hill AE, Foreman MGG, Peña MA. Horizontal transport of marine organisms resulting from interactions between diel vertical migration and tidal currents off the west coast of Vancouver Island. Can J Fish Aquat Sci. 2001;58:736–748. doi: 10.1139/f01-012. [DOI] [Google Scholar]
- Sogard SM, Olla BL. Food deprivation affects vertical distribution and activity of a marine fish in a thermal gradient: potential energy-conserving mechanisms. Mar Ecol Prog Ser. 1996;133:43–55. doi: 10.3354/meps133043. [DOI] [Google Scholar]
- Tande KS. Ecological investigations on the zooplankton community of Balsfjorden, Northern Norway: seasonal changes in body weight and the main biochemical composition of Thysanoessa inermis (Krøyer), T. raschii (M. Sars), and Meganyctiphanes norvegica (M. Sars) in relation to environmental factors. J Exp Mar Biol Ecol. 1982;62:129–142. doi: 10.1016/0022-0981(82)90087-9. [DOI] [Google Scholar]
- Tyler HR, Pearcy WG. The feeding habits of three species of lanternfishes (family myctophidae) off oregon, USA. Mar Biol. 1975;32:7–11. doi: 10.1007/BF00395156. [DOI] [Google Scholar]
- Valinassab T, Pierce GJ, Johannesson K. Lantern fish (Benthosema pterotum) resources as a target for commercial exploitation in the Oman Sea. J Appl Ichthyol. 2007;23:573–577. doi: 10.1111/j.1439-0426.2007.01034.x. [DOI] [Google Scholar]
- Walker MG, Nichols JH. Predation on Benthosema glaciale (Myctophidae) by spawning mackerel (Scomber scombrus) J Fish Biol. 1993;42:618–620. doi: 10.1006/jfbi.1993.1068. [DOI] [Google Scholar]
- Warrant EJ, Locket NA. Vision in the deep sea. Biol Rev. 2004;79:671–712. doi: 10.1017/S1464793103006420. [DOI] [PubMed] [Google Scholar]
- Willis JM, Pearcy WG. Spatial and temporal variations in the population size structure of three lanternfishes (Myctophidae) off Oregon, USA. Mar Biol. 1980;57:181–191. doi: 10.1007/BF00390736. [DOI] [Google Scholar]
- Wurtsbaugh WA, Neverman D. Post-feeding thermotaxis and daily vertical migration in a larval fish. Nature. 1988;333:846–848. doi: 10.1038/333846a0. [DOI] [Google Scholar]