Abstract
Factors contributing to pain following surgery are poorly understood with previous research largely focused on adults. With approximately 6 million children undergoing surgery each year8, there is a need to study pediatric persistent postsurgical pain. The present study includes patients with adolescent idiopathic scoliosis undergoing spinal fusion surgery enrolled in a prospective, multi-centered registry examining post-surgical outcomes. The Scoliosis Research Society Questionnaire- Version 30, which includes pain, activity, mental health, and self-image subscales, was administered to 190 patients prior to surgery and at 1 and 2 years post-surgery. A subset (n=77) completed 5-year post-surgery data. Pain prevalence at each time point and longitudinal trajectories of pain outcomes derived from SAS PROC TRAJ were examined using ANOVAs and post-hoc pairwise analyses across groups. Thirty-five percent of patients reported pain in the moderate-severe range prior to surgery. One year postoperative, 11% reported pain in this range while 15% reported pain at two years post-surgery. At five years post-surgery, 15% of patients reported pain in the moderate to severe range. Among the five empirically-derived pain trajectories, there were significant differences on self-image, mental health, and age. Identifying predictors of poor long-term outcomes in children with postsurgical pain may prevent the development of chronic pain into adulthood.
Keywords: persistent postsurgical pain, pediatric chronic pain, spinal fusion surgery, trajectories, pain prevalence
Introduction
Chronic pain is a significant public health problem22 with one potential trigger being surgery. Research has shown that between 5 and 50% of adults who have undergone surgery are affected by persistent postsurgical pain (PPP)18, 21, pain defined as lasting longer than two months post-surgery28, although this definition has been debated as being overly simplistic22. Studying pain following surgery allows for the identification of risk and protective factors that may predict the course of recovery and alleviate the significant psychosocial and economic burden of chronic pain20.
With research examining PPP in adults rapidly progressing23, identifying its prevalence and associated risk factors among children is gaining momentum and with approximately 15-25% of children affected by recurrent chronic pain,16, 34 this research is important. A cross-sectional study by Fortier and colleagues13 found that of 113 children between 2 and 17 years of age who had undergone general, urological, and orthopedic surgeries within the previous 10 months, 13% reported chronic postoperative pain. Similarly, Page and colleagues30 also found one year following major surgery that 22% of children reported moderate to severe levels of pain. While results of these investigations indicate that chronic pain after surgery may be a problem, conclusions are limited due to the small sample sizes, retrospective data collection, and combining divergent surgeries, precluding the examination of specific risk factors for pediatric PPP2. This last point is important because the type of surgery has different consequences on specific organ functions so PPP should be examined on a procedure-specific basis22. Among spinal fusion patients, high rates of pain following surgery have been reported 44. Recently Landman and colleagues26 drew from a national database of prospective outcomes in children with Adolescent Idiopathic Scoliosis (AIS) (n=295) and found that at one and two years post-surgery, 68.8% and 64.4% of children reported mild to severe postoperative pain in the past six months. Although these findings highlight the need to study postoperative pain in patients with AIS, grouping patients with mild and severe pain does not allow a fair estimation of clinically significant postoperative pain prevalence. In view of over 40% of adults reporting the presence of pain at the surgical site, but only 18% reporting moderate to severe pain, identification of patients with moderate or higher pain intensity may yield more useful information to study its impact on functioning18.
AIS is a fitting population to study pediatric PPP as it is associated with extensive surgical tissue injury that can lead to central sensitization, abnormal neural plasticity and persistent pain24, 25. The current study involves patients with AIS enrolled in the Prospective Pediatric Scoliosis Study (PPSS) at Boston Children’s Hospital who have undergone spinal fusion surgery and who have complete pre-surgical, one and two year post-surgery data; a subset of these patients also have 5-years post-surgery data. First, we examined the prevalence of pain pre-operatively and across the three post-operative time points and hypothesized that a subset of patients would experience moderate to severe pain at 1 and 2 years with an increased prevalence at 5 years post-surgery42. Second, we empirically grouped longitudinal pain trajectories and examined baseline pre-operative characteristics of age, gender, pre-operative pain, self-image, and mental health functioning as well as surgery-related variables including: pre-surgical curve angle (Cobb angle), location of curve (main thoracic, double thoracic, double major, triple major, thoracolumbar/lumbar, thoracolumbar/lumbar/main thoracic), surgical approach (anterior or posterior),and fusion length, associated with trajectory groups. We hypothesized that patients in a persistent pain trajectory would report poor mental health functioning and self-image at baseline as compared to patients with a no-pain trajectory or a decrease in pain trajectory.
Method
Participants
Participants were patients from a large northeast children’s hospital who were enrolled between the years 2003-2007 in the Spinal Deformity Study Group’s Prospective Pediatric Scoliosis Study (PPSS), a multi-centered, longitudinal study aimed at evaluating the surgical treatment of adolescents with idiopathic scoliosis. Patient assent and parent consent was provided. Only patients from our hospital were included in this study to have a consistent surgical treatment regimen. Inclusion criteria for this study include a diagnosis of thoracic, thoracolumbar, and/or lumbar idiopathic scoliosis and a spinal fusion with instrumentation. The study consists of assessments at pre-surgery, 1, 2, and 5-year post-surgery. Of the 260 patients who have baseline data, 190 patients completed follow-up data at 1 and 2 years post-operative and 77 of the 190 completed 5-years post-surgery questionnaire data.
Measure
Scoliosis Research Society-30 (SRS-30; The Scoliosis Research Society). The SRS-30 is a measure developed by the Scoliosis Research Society to assess health-related quality of life among patients with scoliosis. The SRS-30 is a combination of versions 22 and 24, which have been demonstrated to be valid and reliable3, 33. It consists of 23 preoperative questions and 7 postoperative questions and contains four subscales: pain, self-image, activity, and mental health. A higher score on each item and subscale indicates a better outcome (e.g., 1= severe pain; 5=no pain)
Pain
Given that pain is the primary outcome variable in the present study, we examined the total subscale score and each question separately. Specific questions included 1) pain experienced in the past 6 months, 2) pain experienced in the past month, 3) pain experienced at rest, 4) medication usage for pain, 5) sick days from work/school over the past 3 months, and 6) post-operative impact of back treatment on pain. Internal consistencies for the pain subscale in this sample was 0.53 pre-operative, 0.77 1-year post-operative, 0.85 2-year post-operative, and 0.89 5-year post-operative. In examining internal consistency of the pre-operative subscale, pain at rest did not correlate with the other pain subscale items pre-operatively, but all items of the subscale were retained to ensure that pain-at-rest was represented in patient reports of their pain experience.
Self-image
Specific questions included in the self-image subscale included: 1) feelings about current back shape if it remained unchanged for the rest of the patient’s life, 2) perception of how good patient looks in clothes, 3) trunk appearance, 4) back condition affecting personal relationships, 5) feelings of attractiveness with current back condition, 5) 1-9 rating of self-image as well as post-operative questions about how self-image has changed as a result of treatment. Internal consistencies for self-image included 0.73 for preoperative, 0.70 for 1-year postoperative, 0.73 for 2-year post-operative, and 0.77 for 5-year post-operative.
Mental health
Specific questions included: 1) being nervous, 2) feeling down without being able to be cheered up, 3) feeling calm and peaceful, 4) feeling blue, 5) happiness, all in the past 6 months. Internal consistencies for the mental health subscale were also strong with 0.75 at the preoperative time point, 0.80 at 1 year post-surgery, 0.87 at 2 years post-surgery and 0.87 at 5 years post-surgery.
The activity subscale containing a variety of pre- and post-operative questions concerning current activity levels and financial difficulties did not demonstrate internal consistency; thus it was not further examined.
Procedures
Data obtained for secondary data analysis for the present study was approved by the Institutional Review Board. Patients completed the questionnaire at the time of the pre- operative visit 1 to 3 weeks before the procedure. The questionnaires were completed with parental assistance as necessary for the younger patients or independently by the patient depending on the patients’ level of understanding of the questionnaire. Standard post-operative visits occurred at 1, 2, and 5 years after surgery with completion of the questionnaires at the time of the office visit.
Statistical Analyses
All analyses were conducted in SPSS version 19 and SAS. Descriptive statistics were calculated for all demographic and study variables and One-way ANOVAs were used to compare participants with only pre-surgical data to those who had both pre-surgical and follow-up data. Frequencies were obtained on each item of the pain subscale of the SRS-30 to describe pain across time points dichotomized to reflect moderate- severe pain and mild-none pain and often-very often pain at rest and rarely to never having pain at rest. In order to examine those with complete data for those pain items examined (pain in the past month, pain in the past 6 months, pain at rest, medication usage, and school/work attendance) only the 169 of the 190 participants was examined. Additionally, of the 77 with 5-year post-surgery data, only 69 had complete data on these specific items.
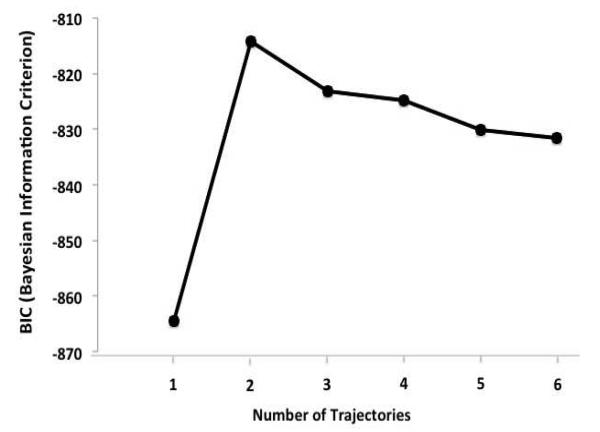
Next, when we looked within the moderate to severe pain and mild to none pain groups we found some who had a resolution of symptoms and others who had a worsening of symptoms and we conducted trajectory analyses in order to examine patterns of pain prevalence. The SAS PROC TRAJ procedure19 was used to determine models of pain across preoperative and postoperative time points. The TRAJ procedure is a mixture model that estimates a regression model for each discrete group within the population. Polynomials were limited to quadratic time due to only having four time points. Individuals with missing observations can be included because PROC TRAJ uses all values available from each case to estimate an individual’s timeline, which allows missing observations to be included in the analyses. Model complexity and overall fit in PROC TRAJ is determined partly on the Bayesian information criterion (BIC), which are negative values in which values closer to 0 indicate a better fit. Trajectory group membership for each individual was then used as the independent variable to compare groups across baseline characteristics using one-way analyses of variance. Post hoc Least Significant Difference tests compared means across multiple trajectories and allowed for the correction of Type I errors.
Results
Preliminary Analyses and Description of the Sample
Of the 260 patients included in this study, 190 patients completed follow-up data at 1 and 2 years post-operative and 77 completed 5-years post-surgery questionnaire data. We examined baseline differences between patients who completed follow-up data (n=190) and those who did not (n=69). There were no significant differences found on age, sex, race/ethnicity. However, there was a significant difference found on the pre-operative pain subscale scores for those who completed follow-up versus those who only had baseline data. Those with post-surgical data reported significantly greater preoperative pain compared to the group who completed only baseline measures (F (1, 246) =10.77, p<.0.001). Additionally, we examined whether pre-operative pain scores were related to surgical variables including: severity of pre-operative curve angle (Cobb angle), location of curve (main thoracic, double thoracic, double major, triple major, thoracolumbar/lumbar, thoracolumbar/lumbar/main thoracic), surgical approach (posterior or anterior) or length of fusion (number of vertebrae fused) and none were found to significantly correlate with preoperative pain scores.
For all subsequent analyses, we examined patients who had one or more follow-up data points (n=190). Age at the time of surgery for this sample was between 8 and 21 years (M=14; SD=2.29). Patients were predominantly female (72%) and Caucasian (82%). The mean preoperative curve angle of the spine was 57.5 degrees (SD= 13.09°), which is considered a curve in the severe range. The majority of patients (90%) underwent a posterior surgical approach. See Table 1.
Table 1.
Participant Characteristics (n=190)
| Variable | Frequency |
|---|---|
| Age | Range= 8-21 |
| Mean=14.35 (2.23) | |
| Gender | |
| Females | 72.3% |
| Males | 22.0% |
| Race | |
| Caucasian | 81.7% |
| Black or African American | 8.4% |
| Asian or Asian American | 3.1% |
| Hispanic | 1.6% |
| Other | 1.6% |
| Surgical Approach | |
| Posterior | 89.9% |
| Anterior | 10.1% |
| Absolute value of largest Cobb Angle measurement | Range= 22-111° |
| Mean= 57.5° (13.09°) | |
| Location of maximum curve (Lenke Classification) | |
| Major thoracic | 48.7% |
| Double thoracic | 23.8% |
| Thoraculumbar/Lumbar | 12.7% |
| Thoraculumbar/Lumbar/Main Thoracic | 5.8% |
| Triple major | 4.8% |
| Double major | 4.2% |
| Fusion length | Range= 0-14 |
| Posterior | Mean= 8.65 (4.15) vertebrae fused |
| Anterior | Mean= 0.89 (2.12) |
Prevalence of pain at each time point (n=169 for pre-operative, 1, & 2 years post-surgery; n=69 for 5 years post-surgery)
In the past six months?
At preoperative evaluation 39% of patients reported pain in the moderate to severe range. One year postoperative 16% reported pain in the moderate to severe range in the past 6 months. At two years postoperative, 16% reported pain in the moderate to severe range). At five years post-surgery, pain in the moderate to severe range was present in 17% of the 69 patients assessed.
In the past month?
Prior to surgery, 35% of patients reported pain in the moderate to severe range in the month prior to surgery. At one year post-operative, 11% of those patients reported pain moderate to severe in the past month and the prevalence increased to 15% at 2 years post-surgery. Fifteen percent of the patients also reported pain in the moderate to severe range over the past month at five years post-surgery.
Pain at rest?
Pain at rest was also reported to be problematic, particularly pre-operatively. Prior to surgery, 43% of participants reported pain often to very often at rest, compared to only 5% at both one year and two-years post-surgery. Eight percent of patients reported pain often to very often when at rest at 5 years post-surgery.
Medication Usage
Overall, this sample did not use medication for back pain prior to or after surgery with frequency of use remaining stable or increasing slightly after surgery.
Opioids
Across all time points, less than 1% of patients reported daily opioid use (e.g., Tylenol #3, Lorocet, Percocet, Darvocet) and 1% or less reported opioid use weekly or less.
Non-opioids
Pre-operatively 3% reported daily use of non-opioid medications (e.g., acetaminophen, non-steroidal anti-inflammatory drug). Post-operatively, rates of daily use increased, but were low at 2%, 4%, and 6% across 1-year 2-year and 5-years. Weekly or less non-opioid use remained stable from 23% at pre-operative to 1-year post-operatively and increased to 25% at 2-years post-surgery and 27% at 5-years post-surgery.
Missed day of work/school due to back pain
Prior to surgery 7% of patients reported missing one or more days of school/work due to back pain in the previous three months. After surgery, patients reported missing one or more days of school/work at slightly higher rates at 1-year 2-year and 5-years post-operative (11%, 11%, and 9% respectively).
Pain Trajectories
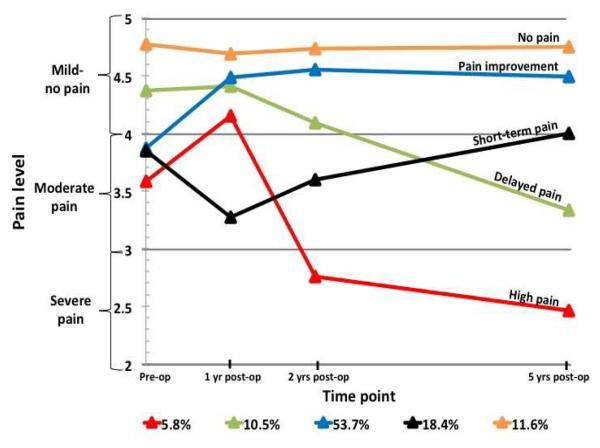
SAS PROC TRAJ was run with 1 to 6 trajectory solutions in order to determine the most appropriate and parsimonious solutions for pain across pre- and post-surgical time points. A logistic model of dropout probability was included for each time point to account for non-random attrition. Similar to cluster and exploratory factor analysis, solutions are selected largely based on judgment so, along with the BIC, an inspection of graphic model curves was employed to determine the number of trajectories to include in analyses. . Despite that there is no statistical test to determine significant differences among BIC scores40 BIC values were inspected for each solution, except for the one trajectory solution, two to seven trajectories were found to have similar BIC values (See Figure 1), providing no clear indication of the superiority from this standpoint. In selecting the number of trajectory solutions, we tried to balance parsimony with clinically relevant hypotheses-generating trajectory paths. Ultimately we chose a five-trajectory solution as each group was clinically interpretable, whereas we felt that with fewer grouping there was unique information lost (e.g., the four trajectory model collapses the short-term pain and delayed pain groups with an overall decline in functioning whereas the five trajectory model teases apart differential outcomes of pain improvement and worsening) and a larger number of trajectory solutions yielded difficult to interpret groups (see Figure 2). The five trajectories consisted of:
“No pain” group: little to no pain before surgery and continued on that trajectory at all follow-up points.
“Pain improvement” group: reported moderate pre-surgical pain and reported decreases over time.
“Short-term pain” group: reported more pain at 1 year post-surgery, but then reported improvements at 2 years post-surgery and reported less pain at 5 years post-surgery.
“Delayed pain” group: did not report pain prior to surgery, reported mild pain at 1 and 2 years post-surgery; however, reported high levels of pain at 5 years post-surgery.
“High pain” group: reported high levels of pain in the moderate range pre-surgically and while some improvement is noted at 1 year post-surgery, pain becomes much worse 2 years post-surgery and then continues to decline 5 years post-surgery into the severe range.
Figure 1.
Bayesian information criterion (BIC) scores were used to interpret trajectories. Bayesian information criterion (BIC), which are negative values in which values closer to 0 indicate a better fit. Although the one trajectory model was clearly a poor fit, the two to six trajectory BIC scores were comparable suggesting the use of additional criteria to choose a solution.
Figure 2.
Examining pain from pre-surgery to 5-years post-surgery yielded five unique trajectories. The largest cohort of patients (54%) reported pain before surgery and experienced a decrease in pain after while the smallest cohort had high levels of pain prior to surgery and maintained this level over the course of five years (6%). Additional unique trajectories of pain suggest that the course of pain improvement or decline is complex and dynamic over time.
Pain trajectory solutions
PROC TRAJ provides individual fit estimates, probabilities that each child belongs to each of the five trajectory groups. Cote et al., (2002) recommend that the average probability for members of a trajectory group should be ≥0.70. The five averages ranged from 0.74 (SD=0.18) for the “Delayed pain” group to 0.93 (SD=.09) for the “High pain” group. These estimates suggest that the average model fit ranged from adequate to excellent for the five trajectories.
Baseline Trajectory Group Differences
We conducted a post-hoc power analysis for the five group one-way ANOVA comparisons. With a significance level of 0.05 and power of 80%, the sample size of 190 is adequate for detecting medium to large effects of 0.25 and higher12. Table 1 shows mean levels of variables by pain trajectory group with test statistics. The pain trajectory groups differed significantly from each other on pre-surgical age, pain, self-image, mental health, and missed days of school/work. The pain trajectory groups did not differ significantly from each other on the surgical variables examined including severity of pre-operative curve angle, curve location (main thoracic, double thoracic, double major, triple major, thoracolumbar/lumbar, thoracolumbar/lumbar/main thoracic), type of surgical approach (posterior vs. anterior), or spinal fusion length.
Post hoc Least Significant Difference tests indicated that those in the “delayed pain group” were significantly older at the time of surgery than the “no pain group” (difference=1.41, p=0.045). The “short-term pain group” was also significantly older compared to the “no pain group” (difference=1.82, p=0.003). The “pain improvement group” was significantly older at the time of surgery compared to the “no pain group” (difference=1.25, p=0.019). Pre-operative pain for the “high pain group” was significantly worse compared to the “delayed pain group” (difference= −0.79, p=0.000), the “pain improvement group” (difference= −0.28, p=0.039), and the “no pain group” (difference= −1.18, p=0.000). The “delayed pain group” had significantly less pre-operative pain compared to the “pain improvement group” (difference=0.50, p=0.000). However, the “delayed pain group” had significantly worse pre-operative pain compared to the “no pain group” (difference= −0.39, p=0.003). The “pain improvement group” had significantly more pre-operative pain compared to the “no pain group” (difference= −0.90, p=0.000). Similarly, the “short-term pain group” had significantly higher pre-operative pain compared to the “no pain group” (difference= −0.92, p=0.000). The “delayed pain group” had significantly better pre-operative self-image compared to the “pain improvement group” (difference=0.42, p=0.004) as well as the “short-term group” (difference= 0.40, p=0.022). The “pain improvement group” had significantly worse pre-surgical self-image compared to the “no pain group” (difference= −.29 p=0.041). The “delayed pain group” also had better pre-surgical mental health functioning compared to “short-term pain group” (difference=0.53, p=0.002) and the “pain improvement group” (difference=0.37, p=0.011). Regarding missed days of school/work, the “high pain group” had significantly more absences compared to the “delayed pain group” (difference=0.36, p=0.017), the “pain improvement group”(difference=0.25, p=0.048) and the “no pain group” (difference=0.36, p=0.016).
Discussion
Approximately 25% of adults referred to chronic pain clinics identify surgery as the antecedent7; however, the examination of persistent postsurgical pain has not been examined systematically in children undergoing surgery20. Given that approximately 6 million children undergo surgery and anesthesia each year in the United States8 ; this is potentially a significant health problem. It is further exaggerated by the fact that chronic pain produces changes in attention, cognition and affect. In childhood these sequelae may result in behavioral disorders, take place at a critical developmental stage, and may continue for decades into adulthood4, 43.
The aim of the present study was to both examine the prevalence of pain in adolescents undergoing spinal fusion surgery across four time points, before surgery and at 1, 2, and 5-years post-surgery, as well as to examine longitudinal pain trajectories with the consideration of pre-surgical variables that impact long-term pain outcomes. Adolescents with idiopathic scoliosis is an appropriate population to study persistent pain following surgery because they are typically otherwise healthy and spinal fusion surgery, which is indicated for correction of curvature of the spine and not pain, is extensive. Additionally, studying one type of surgical procedure and medical condition is ideal22. These factors allow for a well-controlled investigation. This is the first study, to our knowledge, which examines long-term pain outcomes up to five years after surgery and also examines pre-surgical predictors of pain trajectories in a pediatric sample. Although spinal fusion surgery is primarily warranted for severity of the spinal curve and not pain, 35% of the sample reported pain in the moderate to severe range before surgery. While these patients do not necessarily reflect those who fall into the definition of persistent postsurgical pain as outlined by the International Association for the Study of Pain (IASP)28, in that that their pain could be attributed to causes other than the surgery, this finding indicates that pain among this surgical sample is complex. Given that pre-surgical pain is widely known to be a significant predictor of chronic postsurgical pain15 more research is warranted on this relationship. Additionally, it has been noted that although two months or longer postoperatively is the criterion for the diagnosis of persistent postsurgical pain, the definition was not based on procedure-specific data and some surgeries, such as spinal fusion, require longer healing periods thus extending the “acute phase” of pain22. Examining the pain trajectories that emerged within this sample highlights the divergent paths of pain following surgery.
While there was an overall decrease in pain prevalence from pre- to post-surgery, consistent with our hypothesis, a significant proportion of patients reported pain in the moderate to severe range at one and two years with an increase in prevalence at five years post-surgery. Additionally, despite an overall decrease in postoperative pain, medication use remained stable and in some cases, increased post-surgically. While the medication usage in this sample does not appear to be alarming, its increase for back pain several years after surgery is a concern and indicates that other non-pharmacological interventions such as Cognitive Behavioral Therapy (CBT), relaxation, and biofeedback that have been found helpful in treating pediatric chronic pain 10, 31 may be applicable for young people after surgery as well. Such psychological interventions may be useful in pediatric postsurgical populations to also target functioning.
While the sample overall did not miss a lot of work or school due to pain, similar to medication usage, school and work absences increased post-surgically. Reasons for this are unclear from the available measures in this sample. It is possible that after a patient has undergone such an invasive surgery it may impact additional domains of function beyond reported pain (e.g., ability to sit for prolong periods of time in school). Additionally, parents may perceive their child as more vulnerable6. Further assessment that explores their functioning across domains of life as well as tapping parent perceptions and behaviors toward their child is needed. However, the overall low rate of opioid use and good functional status after spinal fusion surgery in adolescents is commonly recognized compared to most of the spine surgeries performed for adults. This lends support to the importance of preventing the continuation of pain into adulthood when overmedication and decreased functioning may be present.
In examining longitudinal pain trajectories, five groups emerged: a no pain group and high pain group across all time points, a pain improvement group, a short-term pain group and a delayed pain group. It is noteworthy that surgical and scoliosis variables, such as severity of and location of pre-operative curve and surgery type, did not significantly impact pain outcomes suggesting the impact of other variables (e.g., mental health, biological) that may influence postsurgical pain outcomes. Although AIS is not considered a painful condition, less than one third of patients reported virtually no pain preoperative. Among these patients, approximately half of them continued to have no pain (12% of sample in no pain group), whereas the other half reported a significant spike in pain at 5-year post-operative (11% of sample in delayed pain group). In examining baseline differences, these two groups significantly differed on age at the time of surgery, with the delayed pain group significantly older than the no pain group. Perhaps, younger age is a protective factor. This is consistent with other findings in the pediatric chronic pain literature, citing that adolescents are at a relative risk compared to younger children in regard to functioning with chronic pain 11, 17. Developmental considerations such as the role of hormones and puberty and developmental milestones that may result in psychosocial stress, such as moving out of the home or going to college, should be further explored as they relate to persistent postsurgical pain.
Among the patients who reported pain at baseline, most reported improvements in pain after surgery with these improvements maintained over time (54% of sample in pain improvement group). These patients, similar to those who experienced a worsening of pain at 1-year, but a rebound at subsequent time points (18% in short-term pain group) reported significantly lower body-image perceptions as compared to the delayed pain group at baseline. Given that pain is a sensory and affective experience 27, 41 improvements in perceived body image after spinal fusion surgery 35 may be tied to a patient’s pain experience.
Lastly, there was a small subgroup of patients (6%) who reported significant pain across all time points. Contrary to our hypothesis, this group did not report significantly poorer mental health functioning or self-image. The distinguishing characteristic was more frequent missed school/work prior to surgery. Functional limitations prior to surgery appear to be a risk factor for persistent pain outcomes over time. Despite the null findings, further assessment of mental health functioning as potential risk factors is needed. The mental health subscale of the SRS-30 is quite broad. The questions are general and do not target variables such as fear of and acceptance of pain and pain catastrophizing, which have been identified in the literature as being important in the maintenance and exacerbation of chronic pain in children 38, 39. Future research should include such mental health constructs when trying to better understand the role of mental health in post-surgical pain outcomes. Another important consideration that was not included in the mental health domain but is an area of important future research is the study of the social context of pain. For children living with chronic pain, parent factors such as distress as well as behavioral responses have been shown to significantly influence children’s pain and functional outcomes 5, 37
Beyond mental health, other variables such as somatosensory phenotype and/or genetic predisposition may be influencing the pain presentation in this high risk group. Beyond psychological and social contextual predictors, there is a need to explore the biological correlates of persistent postsurgical pain. Recent research using Quantitative Sensory Testing (QST) has identified the somatosensory phenotype associated with the development of chronic pain 32 and is known as a reliable marker at detecting chronic pain 36. The influence of a patient’s genetic makeup as a biological variable that contributes to the risk of developing central nervous system sensitization and persistent postsurgical pain has also begun to be examined. Observational and anecdotal evidence suggests that differences in pain perception may be inherited 29 but only recently has proof of the heritability of pain sensitivity in adult humans been obtained identifying individual gene mutations.9 The interplay between psychosocial variables and the neural underpinnings of sensory pain modulation to predict chronic pain outcomes in children has not been examined and is warranted.
The current study must be viewed in light of its limitations. Despite a large sample with longitudinal data, only using one measure to assess pain and functioning is a limit and does not adequately capture all the factors contributing to persistent post-surgical pain. It would have also been beneficial to have data during the acute phase of postoperative pain. As aforementioned, two months postoperatively, as outlined by IASP, is not appropriate for spinal fusion patients as most patients are typically not cleared to return to full functional activities for six months following surgery1; however, having data at 6 months postoperatively would have allowed for a more clear picture of individuals whose pain began after surgery and made the acute to chronic pain transition. Additionally, the database does not separate race and ethnicity, making it difficult to understand the way in which surgical pain outcomes may differentially relate to racial and ethnic minorities. Additionally, this sample was predominantly White and female, which limits conclusions based on males and other under-represented groups. Future research should also further break down location of pain after surgery (e.g. at the fusion site vs. in unfused locations of the spine) in order to elucidate surgical pain from typical “wear and tear”. Despite these limitations, the present study underscores the importance of examining post-surgical pain in children. With recent economic costs of adult chronic pain estimated to be between $560-$635 billion per year 14 research on the role of persistent pain in children is of upmost importance in order to positively impact pre-surgical preparation, postsurgical care, and in potentially preventing disabling pain into adulthood for a population at considerable risk.
Perspective.
This investigation explores the prevalence of pediatric pain following surgery, up to five years post spinal fusion surgery. Five pain trajectories were identified and were distinguishable on pre-surgical characteristics of age, mental health, and self-image. This is the largest study to examine longitudinal pediatric pain trajectories after surgery.
Table 2.
Baseline differences across pain trajectory groups
| No Pain to Low Pain Group at Baseline |
Moderate to Severe Pain Group At Baseline |
||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Variable | Total (n=190) Mean (SD) |
No pain (n=22) Mean (SD) |
Delayed pain (n=20) Mean (SD) |
Pain improvement (n=102) Mean (SD) |
Short term pain (n=35) Mean (SD) |
High pain (n=11) Mean (SD) |
F Ratio |
| Age at enrollment | 14.3 (2.29) | 13.1 (1.85) | 14.5(2.48)e | 14.3 (2.23)e | 14.9 (2.44)e | 13.6(2.21) | 2.56** |
| Pre-operative pain | 4.01 (.54) | 4.76 (.29)a,b,c,d |
4.37 (.39)a,c | 3.87 (.39)a | 3.85 (.52) | 3.58 (.73) | 27.2** |
| Pre-operative self-image | 3.51(.60) | 3.69 (.52)e | 3.82 (.44)c,d | 3.40 (.58) | 3.42 (.70) | 3.77(.65) | 3.34** |
| Pre-operative mental health | 3.98 (.60) | 4.10 (.54) | 4.33 (.39)c,d | 3.95 (.63) | 3.79 (.56) | 3.96 (.71) | 2.82* |
| Pre-operative frequency of missed work/ school days |
.12 (.41) | 0 (0)a | 00 (0a | .11 (.37)a | .20 (.53) | .36(.81) | 2.29 |
Note. Baseline variables that differ significantly at p<.05;
p<.01 across pain trajectory groups are indicated with the superscript of the differing group. (e.g., Patients who had no pain at pre-op had significantly less pain compared to those in groups 1-4.)
= High pain,
= Delayed pain,
= Short-term pain,
= Pain improvement,
= No pain
Acknowledgements
The authors wish to thank Meryl Gold, BA, Eric Riklin, BA, and Aubrey Wasser, MPH for their work on this study. Please note that Dr. Sethna and Dr. Hresko shared senior author/mentoring responsibilities on this manuscript.
This investigation was supported by the Boston Children’s Hospital Career Development Fellowship Award (CS), NIH grant HD067202 (LS), the Sara Page Mayo Endowment for Pediatric Pain Research and Treatment, and the Department of Anesthesiology, Perioperative and Pain Medicine at Boston Children’s Hospital. Data storage for the spinal deformity study group was funded by a grant from Medtronics. This data was retrieved from the SDSG date the warehouse.
Footnotes
Disclosures: There are no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anonymous: Scoliosis Research Society Available at: http://www.srs.org/ Accessed.
- 2.Ahn JC, Fortier MA, Kain ZN. Acute to chronic postoperative pain in children: Does it exist? Pain Management. 2012;2(5):421–3. doi: 10.2217/pmt.12.48. [DOI] [PubMed] [Google Scholar]
- 3.Asher M, Min Lai S, Burton D, Manna B. Scoliosis Research Society-22 patient questionnaire: responsiveness to change associated with surgical treatment. Spine. 2003;28:70–3. doi: 10.1097/00007632-200301010-00016. [DOI] [PubMed] [Google Scholar]
- 4.Brattberg G. Do pain problems in young school children persist into early adulthood? A 13-year follow-<br />up. Eur J Pain. 2004;8:187–99. doi: 10.1016/j.ejpain.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Claar RL, Simons LE, Logan DE. Parental response to children’s pain: the moderating impact of children’s emotional distress on symptoms and disability. Pain. 2008;138(1):172–179. doi: 10.1016/j.pain.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Connelly M, Anthony KK, Schanberg LE. Parent perceptions of child vulnerability are associated with functioning and health care use in children with chronic pain. J Pain Symptom Manage. 2012;43:953–60. doi: 10.1016/j.jpainsymman.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crombie IK, Davies HT, Macrae WA. Cut and thrust: antecedent surgery and trauma among patients attending a chronic pain clinic. Pain. 1998;76(1-2):167–171. [PubMed] [Google Scholar]
- 8.DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat. 2007;13:1–209. [PubMed] [Google Scholar]
- 9.Drenth JP, Waxman SG. Mutations in sodium-channel gene SCN9A cause a spectrum of human genetic pain disorders. J Clin Invest. 2007;117(12):3603–3609. doi: 10.1172/JCI33297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eccleston C, Palermo TM, Williams AC, Lewandowski A, Morley S. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2009;2 doi: 10.1002/14651858.CD003968.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Evans S, Taub R, Tsao J, Meldrum M, Zeltzer L. Sociodemographic factors in a pediatric chronic pain clinic: The roles of age, sex and minority status in pain and health characteristics. J Pain Manag. 2010;3:273–81. [PMC free article] [PubMed] [Google Scholar]
- 12.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 13.Fortier MA, Chou J, Maurer EL, Kain ZN. Acute to chronic postoperative pain in children: Preliminary findings. J Pediatr Surg. 2011;46(1700) doi: 10.1016/j.jpedsurg.2011.03.074. [DOI] [PubMed] [Google Scholar]
- 14.Gaskin D, Richard P. The economic costs of pain in the US. J Pain. 2012;13(8):715. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Gerbershagen H, Özgür E, Dagtekin O, Straub K, Hahn M, Heidenreich A, Sabatowski R, Petzke F. Preoperative pain as a risk factor for chronic post-surgical pain – Six month follow-up after radical prostatectomy. Eur J Pain. 2009;13:1054–61. doi: 10.1016/j.ejpain.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 16.Goodman JE, McGrath PJ. The epidemiology of pain in children and adolescents: a review. Pain. 1991;46(3):247–64. doi: 10.1016/0304-3959(91)90108-A. [DOI] [PubMed] [Google Scholar]
- 17.Huguet A, Miro J. The severity of chronic pediatric pain: an epidemiological study. J Pain. 2008;9:226–36. doi: 10.1016/j.jpain.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Johansen A, Romundstad L, Nielsen C, Schirmer H, Stubhaug A. Persistent postsurgical pain in a general population: Prevalance and predictors in the Tromsø Study. Pain. 2012;153(7):1390–6. doi: 10.1016/j.pain.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Jones B, Nagin D, Roeder K. SAS procedure based on mixture models for estimating developmental trajectories. Sociol Method Res. 2001;29:374–93. [Google Scholar]
- 20.Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother. 2009;9(5):723–744. doi: 10.1586/ern.09.20. [DOI] [PubMed] [Google Scholar]
- 21.Kehlet H, Jensen TS, Woolf CJ. Persisten postsurgical pain: Risk factors and prevention. Lancet. 2006;367(9522):1618–25. doi: 10.1016/S0140-6736(06)68700-X. 13. [DOI] [PubMed] [Google Scholar]
- 22.Kehlet H, Rathmell JP. Persistent postsurgical pain: the path forward through better design of clinical studies. Anesthesiology. 2010;112(3):514–515. doi: 10.1097/ALN.0b013e3181cf423d. [DOI] [PubMed] [Google Scholar]
- 23.Kissin I, Gelman S. Chronic postsurigcal pain: Still a neglected topic? J Pain Res. 2012;5:473–89. doi: 10.2147/JPR.S35145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotzer AM. Factors predicting postoperative pain in children and adolescents following spine fusion. Issues Compr Pediatr Nurs. 2000;23(2):83–102. doi: 10.1080/01460860050121411. [DOI] [PubMed] [Google Scholar]
- 25.LaMontagne LL, Hepworth JT, Cohen F, Salisbury MH. Cognitive-behavioral intervention effects on adolescents’ anxiety and pain following spinal fusion surgery. Nurs Res. 2003;52(3):183–190. doi: 10.1097/00006199-200305000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Landman Z, Oswald T, Sanders J, Diab M, Members of the Spinal Deformity Study Group Prevalence and predictors of pain in surgical treatment of Adolescent Idiopathic Scoliosis. Spine. 2011;36(10):825–29. doi: 10.1097/BRS.0b013e3181de8c2b. [DOI] [PubMed] [Google Scholar]
- 27.Lee M, Tracey I. Neuro-genetics of persistent pain. Current Opinion in Neurobiology. 2012;23:1–6. doi: 10.1016/j.conb.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Macrae WA. Chronic pain after surgery. Br J Anaesth. 2001;87:88–98. doi: 10.1093/bja/87.1.88. [DOI] [PubMed] [Google Scholar]
- 29.Norbury TA, MacGregor AJ, Urwin J, Spector TD, McMahon SB. Heritability of responses to painful stimuli in women: a classical twin study. Brain. 2007;130(Pt 11):3041–3049. doi: 10.1093/brain/awm233. [DOI] [PubMed] [Google Scholar]
- 30.Pagé GM, Stinson J, Campbell F, Isaac L, Katz J. Identification of pain-related psychological risk factors for the development and maintenance of pediatric chronic postsurgical pain. J Pain Research. 2013;6:167–80. doi: 10.2147/JPR.S40846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palermo TM, Eccleston C, Lewandowski AS, Williams AC, Morley S. Randomized controlled trials of psychological therapies for management of chronic pain in children and adolescents: An updated meta-analytic review. Pain. 2010;148(3):387–97. doi: 10.1016/j.pain.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Botefur IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihofner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123(3):231–243. doi: 10.1016/j.pain.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 33.Rothenfluh DA, Neubauer G, Klasen J, Min K. Analysis of internal contruct validity of the SRS-24 questionnaire. Eur Spine J. 2012;21(8):1590–5. doi: 10.1007/s00586-012-2169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roth-Isigkeit A, Thyen U, Stoven H, Schwarzenberger J, Schmucker P. Pain among children and adolescents: restrictions in daily living and triggering factors. Pediatrics. 2005;115(2):152–62. doi: 10.1542/peds.2004-0682. [DOI] [PubMed] [Google Scholar]
- 35.Sanders J, Harrast J, Kuklo T, Polly D, Bridwell K, Diab M, Dormans J, Drummond D, Emans J, Johnston C, Lenke L, McGrath R, Newton P, Richards S. The Spinal Appearance Questionnaire: Results of reliability, validity, and responsiveness testing in patients with idiopathic scoliosis. Spine. 2007;32(24):2719–22. doi: 10.1097/BRS.0b013e31815a5959. [DOI] [PubMed] [Google Scholar]
- 36.Shy ME, Frohman EM, So YT, Arezzo JC, Cornblath DR, Giuliani MJ, Kincaid JC, Ochoa JL, Parry GJ, Weimer LH. Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology: Quantitative sensory testing: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2003;60(6):898–904. doi: 10.1212/01.wnl.0000058546.16985.11. [DOI] [PubMed] [Google Scholar]
- 37.Sieberg CB, Williams S, Simons LE. Do parent protective responses mediate the relation between parent distress and child functional disability among children with chronic pain? J Pediatr Psychol. 2011;36(9):1043–51. doi: 10.1093/jpepsy/jsr043. [DOI] [PubMed] [Google Scholar]
- 38.Simons LE, Sieberg CB, Kaczynski KJ. Measuring parent beliefs about child acceptance of pain: A preliminary validation of the Chronic Pain Acceptance Questionnaire, parent report. Pain. 2011;152(10):2294–300. doi: 10.1016/j.pain.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simons LE, Sieberg CB, Carpino E, Logan D, Berde C. The Fear of Pain Questionnaire (FOPQ): Assessment of pain-related fear among children and adolescents with chronic pain. J Pain. 2011;12(6):677–86. doi: 10.1016/j.jpain.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Singer J, Willett J. Applied Longitudinal Data Analysis. Oxford University Press; London: 2003. [Google Scholar]
- 41.Tracey I, Mantyh P. The cerebral signature for pain percetion and its modulation. Neuron. 2007;55:377–91. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 42.Upasani VV, Caltoum C, Petcharapom M, Bastrom TP, Pawelek JB, Betz RR, Clements DH, Lenke LG, Lowe TG, Newton PO. Adolescent idiopathic scoliosis patients report increased pain at fi ve years compared with two years after surgical treatment. Spine. 2008;33:1107–12. doi: 10.1097/BRS.0b013e31816f2849. [DOI] [PubMed] [Google Scholar]
- 43.Walker LS, Dengler-Crish CM, Rippel S, Bruehl S. Functional abdominal pain in childhood and adolescence increases risk for chronic pain in adulthood. Pain. 2010;150:586–72. doi: 10.1016/j.pain.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong GT, Yuen VM, Chow BF, Irwin MG. Persistent pain in patients following scoliosis surgery. Eur Spine J. 2007;16(10):1551–1556. doi: 10.1007/s00586-007-0361-7. [DOI] [PMC free article] [PubMed] [Google Scholar]