Abstract
The objective of this study was to determine the association between maternal 25-hydroxyvitamin D (25(OH)D) and the risk of spontaneous preterm birth (sPTB) before 35 weeks’ gestation. A random subcohort from the US Collaborative Perinatal Project (1959–1965) was sampled (n = 2,629) and augmented with all remaining cases of sPTB before 35 weeks’ gestation for a total of 767 cases. Banked serum samples collected at 26 weeks’ gestation or earlier were assayed for 25(OH)D. Constructs for vascular histology and inflammatory histology were developed from placental pathology examinations. There was no relationship between 25(OH)D and sPTB among white women. Among nonwhite mothers, serum 25(OH)D levels of 30–<50, 50–<75, and ≥75 nmol/L were associated with reductions of 1.0–1.6 cases of sPTB per 100 live births and 20%–30% reductions in risk of sPTB compared with 25(OH)D levels less than 30 nmol/L after adjustment for prepregnancy body mass index (weight (kg)/height (m)2), season, and other confounders. This association was driven by inflammation-mediated cases of sPTB and sPTB cases without placental lesions. A sensitivity analysis for unmeasured confounding by exercise, fish intake, and skin color suggested some bias away from the null in the conventional results, but conclusions were generally supported. The vitamin D–sPTB relationship should be examined in modern cohorts with detailed data on skin pigmentation and other covariates.
Keywords: gestational age, 25-hydroxyvitamin D, inflammation, placenta, pregnancy, preterm birth
Preterm birth is the most important problem in modern obstetrics. In 2010, more than 1 million infants born preterm (at less than 37 weeks of gestation) died worldwide, making it the second leading cause of death in children under the age of 5 years (1). Preterm infants who survive are at risk of chronic lung disease, deafness, blindness or other visual impairment, and learning and cognitive disabilities (1). The 12% rate of preterm birth in the United States ranks 131st of 184 countries, behind many developing nations (1). The past 3 decades in the United States have seen little decline in preterm births, including the earliest deliveries, which cause the most morbidity and mortality (2, 3). Identifying potential targets for preterm birth prevention is a public health priority (1, 3).
Recently, maternal vitamin D deficiency has been linked to adverse pregnancy outcomes, including preeclampsia and fetal growth restriction (4), and also may be important in preterm birth. Vitamin D is a prohormone that is either ingested orally or produced photochemically in the skin. It affects established physiological pathways in the pathogenesis of preterm birth, including inflammation, immunomodulation, and transcription of genes involved in placental function (5–7). Vitamin D deficiency is common in pregnant women and, like preterm birth (2), is substantially more frequent among non-Hispanic black mothers (8, 9). Nevertheless, little research has been done to explore the relationship between maternal vitamin D status and the risk of preterm birth.
Our objective was to determine the association between maternal vitamin D deficiency at 26 weeks’ gestation or earlier and the risk of spontaneous preterm birth (sPTB) before 35 weeks overall and by placental histology.
MATERIALS AND METHODS
The Collaborative Perinatal Project (CPP) (1959–1965) was the largest prospective cohort study of pregnant women in the United States (10). It involved 55,000 pregnancies of approximately 42,000 women receiving their initial antenatal care visits at 12 US medical centers. As was common at the time the CPP was conducted, women provided a general verbal informed consent for participation at enrollment (11). At the initial prenatal visit, data on maternal characteristics, behaviors, and medical and obstetric history were recorded via in-person interviews. Nonfasting blood samples were collected from mothers every 8 weeks. A labor and delivery summary was recorded by the obstetrician responsible for each patient's care. This study used deidentified data and was exempt from institutional review board approval.
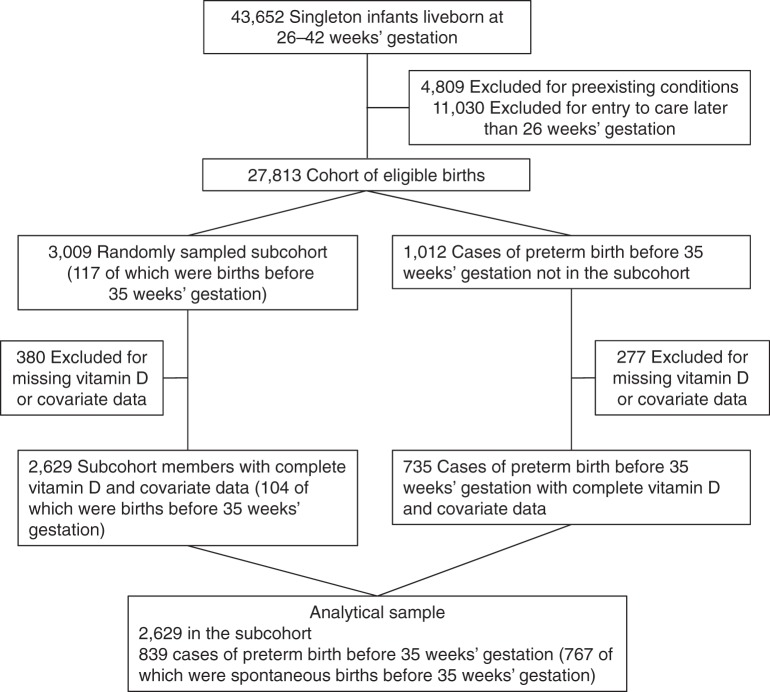
We used a case-cohort design because it allowed us to use the subcohort to estimate the prevalence of vitamin D deficiency in the original cohort and served as a pool of controls for the study of multiple outcomes, including sPTB (12). Figure 1 shows how the sample was selected. There were 27,813 deliveries of singleton, liveborn infants at 26–42 weeks’ gestation to mothers without preexisting medical conditions and with study entry at 26 weeks’ gestation or earlier. From this cohort of eligible women, we randomly selected 11% (n = 3,009) and augmented this subcohort with all remaining cases of preterm birth before 35 weeks’ gestation (n = 1,012, definition below). Some mothers did not provide adequate serum to perform the vitamin D assay, and others were missing data on covariates in the final model (n = 657). In sensitivity analyses, we evaluated the impact of missing data by using multiple imputation (described below). The final analytical sample included 2,629 pregnancies in the subcohort and 839 cases of preterm birth before 35 weeks’ gestation, of which 767 (91%) were sPTBs before 35 weeks’ gestation. A total of 90% of women in the analytical sample had complete placental pathology examinations.
Figure 1.
Study selection flow, case-cohort study in the Collaborative Perinatal Project, 1959–1966.
Definition of sPTB
Information on gestational age was based on the mother's report of the first day of her last menstrual period. Last menstrual period–derived gestational age is error prone, so we used strict gestational age and birth weight limits to reduce the potential for misclassification (13, 14). sPTB was defined as delivery of a liveborn infant at 26–<35 completed weeks’ gestation after spontaneous preterm labor with intact membranes or spontaneous prelabor rupture of the fetal membranes and infant birth weight in the 90th percentile or less based on a standard in which gestational age was documented by ultrasonography (15).
Placental pathology
Pathologists conducted gross examinations of freshly delivered placentas. The microscopic examinations included a full-thickness placental sample taken from a representative block of the central portion of tissue, an umbilical cord sample, a membrane roll sample, and any significant gross abnormalities. In 97% of examinations, pathologists were blinded to clinical course or outcome. Three authors with clinical expertise in placental pathology, maternal-fetal medicine, and pediatrics (M.A.K., W.T.P., and H.N.S.) developed constructs for vascular and inflammatory histologies with the goal of identifying underlying mechanisms of preterm birth and fetal growth restriction. A vascular placental lesion was defined as having any 1 of 12 pathologies, including evidence of placental abruption, infarction, hypoxia, decidual vasculopathy, or thrombosis of fetal vessels. An inflammatory placental lesion was defined as having at least 1 of 8 pathologies, including evidence of bacterial colonies in the epithelium of the amnion or neutrophilic infiltration in the umbilical vein or artery, cord substance, fetal surface, or decidua (Web Table 1, available at http://aje.oxfordjournals.org/). This approach of classifying pathologies in a single construct has been used previously in relationship to pregnancy outcomes (16–19).
Quantification of 25-hydroxyvitamin D concentrations
Maternal serum was stored in glass at −20°C with no recorded thaws. From each woman, we randomly selected 1 banked serum sample drawn at 26 weeks’ gestation or earlier. Sera were shipped to the laboratory of Dr. Michael Holick at Boston University (Boston, Massachusetts), which is Vitamin D External Quality Assessment Scheme–proficient (DEQAS, London, United Kingdom) and Clinical Laboratory Improvement Amendments–certified (Centers for Disease Control and Prevention, Atlanta, Georgia). Samples were assayed for total 25-hydroxyvitamin D (25(OH)D) (calculated as 25(OH)D2 + 25(OH)D3) by using liquid chromatography–tandem mass spectrometry according to the requirements of the National Institute of Standards and Technology (Gaithersburg, Maryland) (20). The assay had a coefficient of variation of 6.0%. Serum 25(OH)D is extremely stable at −20°C for years and is not sensitive to exposure to ultraviolet light or repeated freezing and thawing (21). In a pilot study comparing 25(OH)D in CPP serum samples with serum frozen for 2 years or less, we found that 25(OH)D in the CPP samples was unlikely to show significant degradation (22). There is no universally accepted definition of vitamin D deficiency, so we studied 25(OH)D continuously and by using multiple cutpoints (23, 24).
Covariates
The CPP defined race as white, black, or Puerto Rican. There were few Puerto Rican mothers, and their risks of sPTB and vitamin D deficiency were similar to those of black mothers. Therefore, we classified race as white and nonwhite in the regression models. Data on parity (primiparous, multiparous), maternal age (<20, 20–29, ≥30 years), marital status (unmarried, married), and smoking status at entry (smoker, nonsmoker) were available. Education, occupation, and family income data were previously combined into a composite socioeconomic position score (25). Prepregnancy body mass index (BMI) (weight (kg)/height (m)2) was based on maternal self-reported prepregnancy weight and measured height at enrollment. Season of blood sample collection was defined as winter (December, January, February), spring (March, April, May), summer (June, July, August), or fall (September, October, November).
Statistical analysis
Adjusted risk differences, adjusted risk ratios, and 95% confidence intervals were calculated from multivariable log-binomial regression. All subjects were weighted by using the inverse of the sampling fraction, and a clustered robust variance was used to account for the cases in the subcohort (12). Nonlinearity in 25(OH)D was tested by using restricted cubic splines. Biological interaction between 25(OH)D and race, gestational age at blood collection, and pregravid BMI was tested by using the synergy index (26). When biological interaction was present, statistical interaction terms were included in the model. Potential confounders (race, prepregnancy BMI, trimester of entry to prenatal care, smoking, parity, age, socioeconomic position, marital status, season of blood collection, gestational age at blood collection, and study site) were identified by using theory-based causal models (27). We allowed the confounding effect of socioeconomic position to vary by maternal race by entering an interaction term in the model, but because it did not change the results, it was dropped.
To jointly address the missing data on 25(OH)D concentrations and covariates in the final model, we used multiple imputation. We created 5 imputed data sets that assumed a multivariable normal assumption with a Markov chain Monte Carlo approach (28). We imputed the missing data (all of which were log-transformed) by including all covariates and preterm birth in the imputation model. A comparison of the model results based on multiple imputation (n = 3,463) with those generated by using the complete data set (n = 2,872) revealed no differences in interpretation (data not shown). Therefore, we present results for the complete data set.
Finally, we performed a probabilistic bias analysis for unmeasured confounding by physical activity, fish intake, and skin color/melanin content (among nonwhite mothers only). Leisure-time physical activity and fish intake are unmeasured variables in our data set that have been positively associated with 25(OH)D (29–31) and negatively associated with preterm birth (32, 33). Additionally, skin pigment was not measured but may confound vitamin D–sPTB associations in nonwhite mothers through biological and social pathways. Higher melanin content (pigment) in the skin predicts lower 25(OH)D levels (34). Skin color may also influence sPTB risk through social class or standing (35–37) and may capture additional elements of socioeconomic position that our composite measure did not (38). The Web Appendix provides a detailed description of our bias analysis methods, which were adapted from previous work (39). We compared the risk ratios from the conventional log-binomial regression model of maternal 25(OH)D and sPTB risk with estimates obtained from the sensitivity analysis iterations, which reflected systematic error and random error associated with missing data on each unmeasured confounder (39).
RESULTS
A majority of women in the randomly selected subcohort were 20–29 years of age, married, multiparous, of mid–socioeconomic position and normal weight, and had entered prenatal care in the second trimester (Table 1). Approximately half of the cohort was black and half smoked during pregnancy. The geometric mean maternal serum 25(OH)D level was 43.3 nmol/L (95% confidence interval: 42.4, 44.3), and concentrations of less than 30, less than 50, and less than 75 nmol/L were prevalent in 23.9%, 57.4%, and 84.0% of women in the cohort, respectively.
Table 1.
Characteristics of Women in the Random Sample of the Eligible Cohort and Cases of Spontaneous Preterm Births Before 35 Weeks’ Gestation, Collaborative Perinatal Project, 1959–1966
| Characteristic | Random Sample of the Eligible Cohort, % (n = 2,629) | Spontaneous Preterm Birth Cases, % (n = 767) |
|---|---|---|
| Maternal race/ethnicity | ||
| White | 47 | 28 |
| Black | 46 | 66 |
| Puerto Rican | 7 | 6 |
| Maternal age, years | ||
| <20 | 24 | 34 |
| 20–29 | 60 | 53 |
| ≥30 | 16 | 13 |
| Marital status | ||
| Unmarried | 20 | 28 |
| Married | 80 | 72 |
| Socioeconomic status | ||
| 1 (lowest) | 7 | 9 |
| 2 | 28 | 36 |
| 3 | 33 | 33 |
| 4 | 20 | 17 |
| 5 (highest) | 12 | 6 |
| Parity | ||
| Primiparous | 34 | 30 |
| Multiparous | 66 | 70 |
| Prepregnancy body mass indexa | ||
| <18.5 | 11 | 17 |
| 18.5–24.9 | 72 | 70 |
| 25.0–29.9 | 13 | 10 |
| ≥30.0 | 4 | 3 |
| Smoking status | ||
| Nonsmoker | 52 | 47 |
| Smoker | 48 | 53 |
| Trimester at entry | ||
| First trimester | 27 | 21 |
| Second trimester | 73 | 79 |
| Season of blood collection | ||
| Winter | 23 | 19 |
| Spring | 26 | 29 |
| Summer | 26 | 26 |
| Fall | 25 | 26 |
| Gestational age at blood collection, weeks | ||
| <14 | 16 | 12 |
| 14–<26 | 84 | 88 |
a Weight (kg)/height (m)2.
The incidence of sPTB before 35 weeks’ gestation was 3.2% based on the weighted sample. In unadjusted analyses, mothers who had a sPTB were more likely than mothers in the subcohort to be black, younger than 20 years, unmarried, of lower socioeconomic position, underweight, to have entered prenatal care at 14–26 weeks’ gestation, and to have had their blood samples collected in the spring months (Table 1). sPTB cases were also more likely than mothers in the subcohort to have placental inflammatory lesions (35% vs. 16%) and placental vascular lesions (25% vs. 11%). Among the sPTB cases, the frequency of placental pathologies increased as gestational age decreased (data not shown).
The association between maternal 25(OH)D and sPTB varied by maternal race (P < 0.05). Among nonwhite mothers, the incidence of sPTB before 35 weeks’ gestation decreased significantly as 25(OH)D increased (Table 2, Figure 2; spline terms P < 0.05). After adjustment for maternal race, age, socioeconomic position, parity, marital status, prepregnancy BMI, season, smoking during pregnancy, trimester of entry to prenatal care, and study site, serum 25(OH)D levels of 30–<50, 50–<75, and ≥75 nmol/L were associated with reductions of approximately 1.0–1.6 cases of sPTB per 100 livebirths and approximately 20%–30% reductions in risk of sPTB compared with 25(OH)D levels less than 30 nmol/L. Among white mothers, there was no association between 25(OH)D and sPTB before or after confounder adjustment (Figure 2, Table 2).
Table 2.
Association Between Maternal 25(OH)D Concentrations and the Risk of Spontaneous Preterm Birth Before 35 Weeks’ Gestation by Maternal Race, Collaborative Perinatal Project, 1959–1966
| Serum 25(OH)D Level, nmol/L, by Race | No. of Cases of Spontaneous Preterm Birth Before 35 Weeks’ Gestation | Incidencea of Spontaneous Preterm Birth Before 35 Weeks’ Gestation | Adjustedb Risk Difference | 95% CI | Adjustedb RR | 95% CI |
|---|---|---|---|---|---|---|
| Nonwhite mothers | 556 | 0.045 | ||||
| <30 | 223 | 0.053* | 0.0 | Referent | 1.00 | Referent |
| 30–<50 | 199 | 0.043 | −0.010 | −0.019, −0.0004 | 0.78 | 0.62, 0.99 |
| 50–75 | 94 | 0.037 | −0.016 | −0.026, −0.006 | 0.64 | 0.48, 0.86 |
| >75 | 40 | 0.036 | −0.015 | −0.029, −0.002 | 0.66 | 0.44, 0.98 |
| White mothers | 211 | 0.019 | ||||
| <30 | 29 | 0.019 | 0.0 | Referent | 1.00 | Referent |
| 30–<50 | 64 | 0.019 | 0.0004 | −0.008, 0.009 | 1.0 | 0.64, 1.7 |
| 50–75 | 70 | 0.018 | 0.001 | −0.007, 0.010 | 1.1 | 0.66, 1.7 |
| >75 | 48 | 0.018 | 0.0003 | −0.009, 0.010 | 1.0 | 0.61, 1.7 |
Abbreviations: CI, confidence interval; 25(OH)D, 25-hydroxyvitamin D; RR, risk ratio.
* P < 0.0001.
a Incidence is based on the weighted sample.
b Adjusted for maternal age, socioeconomic position, parity, marital status, prepregnancy body mass index (weight (kg)/height (m)2), smoking during pregnancy, trimester of entry to prenatal care, gestational age at blood sampling, season at blood sampling, and study site.
Figure 2.
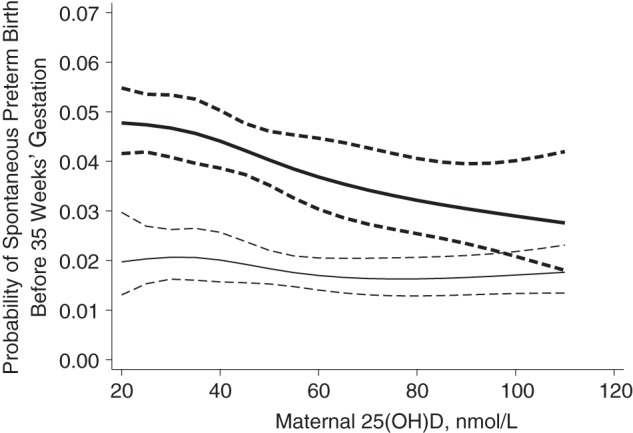
Adjusted probability (solid line) with 95% confidence intervals (dashed lines) of spontaneous preterm birth before 35 weeks’ gestation in relationship to maternal serum 25-hydroxyvitamin D (25(OH)D) (in nmol/L) among nonwhite mothers (bold line) and white mothers, Collaborative Perinatal Project, 1959–1966. 25(OH)D was modeled with a restricted cubic spline, P < 0.001.
After stratification of sPTB cases by placental histology, nonwhite women with serum 25(OH)D levels of 30 nmol/L or more had reduced risks of inflammation-associated sPTB, as well as sPTB without either inflammatory or vascular placental pathologies compared with nonwhite gravidae who had 25(OH)D levels less than 30 nmol/L (Table 3). 25(OH)D was unrelated to sPTB with placental vascular lesions among nonwhite mothers or to any subtypes of sPTB based on placental histology among white mothers. These results did not change when 25(OH)D was more finely categorized.
Table 3.
Association Between Maternal 25(OH)D at 14–26 Weeks’ Gestation and Spontaneous Preterm Birth Before 35 Weeks’ Gestation in the Presence of Inflammatory Pathology Lesions, Placental Vascular Pathology Lesions, or Neither, Collaborative Perinatal Project, 1959–1966
| Serum 25(OH)D Level, nmol/L, by Race | Spontaneous Preterm Birth With Placental Vascular Lesions |
Spontaneous Preterm Birth With Placental Inflammatory Lesions |
Spontaneous Preterm Birth With Neither Placental Pathology |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Cases | Adjusteda RR | 95% CI | No. of Cases | Adjusteda RR | 95% CI | No. of Cases | Adjusteda RR | 95% CI | |
| Nonwhite mothers | 129 | 177 | 257 | ||||||
| <30 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| ≥30 | 0.88 | 0.59, 1.3 | 0.68 | 0.49, 0.95 | 0.74 | 0.56, 0.98 | |||
| White mothers | 66 | 67 | 80 | ||||||
| <30 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| ≥30 | 0.87 | 0.44, 1.7 | 0.75 | 0.39, 1.45 | 1.1 | 0.55, 2.1 | |||
Abbreviations: CI, confidence interval; 25(OH)D, 25-hydroxyvitamin D; RR, risk ratio.
a Adjusted for maternal age, socioeconomic position, parity, marital status, prepregnancy body mass index (weight (kg)/height (m)2), smoking during pregnancy, trimester of entry to prenatal care, gestational age at blood sampling, season at blood sampling, and study site.
The formal sensitivity analysis indicated that systematic error due to unmeasured confounding by physical activity, fish intake, or skin color (among nonwhite women only) biased the conventional modeling results away from the null (Table 4; results for white women are not shown). For example, the adjusted risk ratio of 0.73 (95% confidence interval: 0.59, 0.89) for sPTB before 35 weeks obtained from the conventional analysis was attenuated to 0.84 (95% simulation interval: 0.65, 1.1) after accounting for systematic error due to unmeasured confounding by skin color. This suggests that nonwhite women with 25(OH)D levels of 30 nmol/L or more were 0.84 times as likely as nonwhite women with 25(OH)D levels of less than 30 nmol/L to spontaneously deliver before 35 weeks’ gestation, and that the true risk ratio falls between 0.65 and 1.1. In general, the bias analysis findings supported the conclusions arrived at from the conventional analysis.
Table 4.
Summary of Estimates Yielded by the Conventional Analysis and the Sensitivity Analysis of the Effect of 25-hydroxyvitamin D ≥30 nmol/L Versus <30 nmol/L on the Risk of Spontaneous Preterm Birth Among Nonwhite Mothers, Collaborative Perinatal Project, 1959–1966
| Preterm Birth by Placental Histology | Conventional Analysis |
Bias Analysis |
||||||
|---|---|---|---|---|---|---|---|---|
| Adjusted RR | 95% CI | UC by Physical Activity |
UC by Fish Intake |
UC by Skin Color |
||||
| Point Estimate | 95% BSAI | Point Estimate | 95% BSAI | Point Estimate | 95% BSAI | |||
| sPTB before 35 weeks | 0.73 | 0.59, 0.89 | 0.78 | 0.63, 0.97 | 0.83 | 0.66, 1.1 | 0.84 | 0.65, 1.1 |
| With placental vascular lesions | 0.88 | 0.59, 1.3 | 0.94 | 0.63, 1.4 | 1.0 | 0.66, 1.5 | 1.0 | 0.66, 1.5 |
| With placental inflammatory lesions | 0.68 | 0.49, 0.95 | 0.73 | 0.52, 1.0 | 0.78 | 0.55, 1.1 | 0.79 | 0.54, 1.1 |
| With neither placental lesion | 0.74 | 0.56, 0.98 | 0.79 | 0.59, 1.1 | 0.85 | 0.63, 1.1 | 0.84 | 0.60, 1.2 |
Abbreviations: BSAI, bootstrapped sensitivity analysis interval; CI, confidence interval; RR, risk ratio; sPTB, spontaneous preterm birth; UC, unmeasured confounding.
None of our results varied by prepregnancy BMI or gestational age at blood sampling. Findings were consistent when we used multiple imputation, when we excluded Puerto Rican mothers, and when we substituted maternal education for socioeconomic position in the models (data not shown). To evaluate the potential impact of vitamin D degradation during sample collection, we excluded 2 sites that had mean 25(OH)D concentrations lower than what was expected based on their latitude and racial makeup. The results were not meaningfully different (data not shown).
DISCUSSION
Evidence from this large, diverse, multicenter US pregnancy cohort suggested that low maternal serum 25(OH)D at 26 weeks’ gestation or earlier was associated with a reduced risk of sPTB before 35 weeks only among nonwhite mothers. This association was strongest for inflammation-associated sPTB and sPTB without evidence of placental pathology. These findings were robust to adjustments for measured confounders, such as prepregnancy BMI, season, and socioeconomic position, as well as unmeasured confounders, including skin color, exercise, and fish intake.
Research on maternal vitamin D status and risk of preterm birth is limited. In the only randomized controlled trial of vitamin D supplementation that evaluated preterm birth, there was no effect of daily doses of 2000 IU or 4000 IU of vitamin D3 from 12–16 weeks’ gestation to delivery on the incidence of preterm birth before 37 weeks without preeclampsia in 504 South Carolina mothers (40). Preterm birth without preeclampsia was more common among women with concentrations of 25(OH)D less than 80 nmol/L compared with concentrations of 80 nmol/L or more at the end of pregnancy (17.2% vs. 10.6%, P = 0.07). However, reverse causality likely explains these findings, because a shorter gestation limits the duration of supplementation and time to achieve the same 25(OH)D concentrations (41). Results were not stratified by race/ethnicity.
Observational results have been mixed, with some studies reporting that maternal 25(OH)D is negatively associated with risk of preterm birth (42) or shortened gestational age (43) and others reporting no association (44–46). Previous research has focused on twin gestations (42), US mothers with a history of preterm birth (44), women at high risk for preeclampsia (45), and human immunodeficiency virus–infected pregnant Tanzanian mothers (46), and it is not known whether these findings apply to racially diverse samples of US women in the obstetric population. The preterm birth phenotypes that investigators have evaluated previously have been limited, likely because of too few cases to stratify by timing of delivery and/or pathological features. Our study included more than 700 cases, the largest number to date, which may have provided us with the statistical power needed to detect a modest vitamin D effect within subtypes of sPTB.
Our placental histology–based classification of sPTB may help to identify critical pathways for vitamin D action in this complex, heterogeneous syndrome and to target intervention efforts. Our finding that vitamin D–deficient nonwhite mothers may be susceptible to inflammation-mediated sPTB before 35 weeks’ gestation is consistent with previous work linking maternal vitamin D deficiency to bacterial vaginosis (47, 48), a well-established risk factor for early sPTB resulting from intrauterine bacterial infection (49). Vitamin D biology is critical for a healthy-host innate immune response. Toll-like receptors are critical initiators of host innate immune defense against microbial pathogens, and vitamin D deficiency impairs toll-like receptor induction of the antimicrobial peptide cathelicidin from systemic macrophages, increasing the susceptibility to infection (50). The human placenta and decidua express the vitamin D–activating enzyme 1-α hydroxylase, as well as the vitamin D receptor (51). Trophoblastic vitamin D plays a pivotal role in controlling placental inflammation in response to lipopolysaccharide (7), a stimulus with direct relevance to microbial origins of preterm birth.
Our finding that higher concentrations of 25(OH)D in nonwhite mothers were associated with a reduced risk of sPTB before 35 weeks’ gestation in the absence of placental pathological lesions raises the possibility of alternative biological contributions to preterm birth. In a human myometrial model system, vitamin D attenuates myometrial contractile-associated protein expression in response to labor stimulus (52). Targeted basic studies designed to determine whether vitamin D influences the sensitivity of the myometrium to endogenous and exogenous labor stimuli appear justified.
Racial differences in associations between 25(OH)D and adverse health outcomes like the one we noted here have been reported previously (23) and may be due to heterogeneity in action of parathyroid hormone or expression of the vitamin D receptor, the vitamin D binding protein, or the enzymes involved in its metabolism (53). It may also be that there were not enough cases of sPTB among white women with 25(OH)D levels less than 30 nmol/L (n = 29) to detect an effect. The presence of a vitamin D–sPTB association only among black and Puerto Rican mothers in our study raises concern regarding residual confounding by social status and wealth (38). Therefore, we evaluated systematic error caused by unmeasured data on skin color, a strong biological determinant of 25(OH)D levels (54) and a marker of social status (35, 36). Our probabilistic bias analysis for unmeasured confounding by skin color, as well as fish intake and exercise, suggested that the conventional risk ratio somewhat overestimated the protective effect of 25(OH)D levels of 30 nmol/L or more but did not alter conclusions substantially. The bias analysis results are conditional on the accuracy of the distributions assigned to the parameters, and these may not apply to our population. Our planned work genotyping key vitamin D metabolic loci, including ancestry informative markers to estimate skin color in this cohort, should lead to a more rigorous assessment of confounding and yield insight into potential biological underpinnings of this association.
Preterm birth defined on the basis of menstrual dating has imperfect sensitivity and specificity that are likely to be nondifferential by vitamin D status after controlling for race and socioeconomic position. Our use of strict gestational age and birth weight criteria was intended to reduce the likelihood of outcome misclassification. Indeed, when we defined preterm birth as before 37 weeks’ gestation, our results were substantially attenuated (data not shown). In the 1960s, when the CPP was conducted, medically indicated preterm births were rare. Births in the CPP that likely would be medically indicated in the modern era were either spontaneous deliveries of liveborn infants at later gestational ages (and perhaps classified as term births in our study) or stillbirths (which would have been excluded from our study). Furthermore, although the high vitamin D deficiency rates in the CPP are similar to those of racially diverse cohorts today (8, 55, 56), other factors that we measured (e.g., obesity, smoking) and did not measure (e.g., physical labor, diet) differ. For these reasons, it is critical that we test the vitamin D–preterm birth hypothesis in a contemporary cohort of medically indicated and spontaneous preterm deliveries to evaluate the generalizability of our results.
Major strengths of our study include a large number of preterm birth cases, rich data from placental pathology examinations performed by pathologists blinded to clinical outcomes, and a geographically diverse population of black and white mothers. Serum 25(OH)D was measured by using the “gold standard” method after a careful evaluation of the stability of the analyte in stored samples (22). Finally, the similarity between the multiple imputation and complete-case analyses suggests that the missing data on 17% of women selected into our study were not a major source of bias.
Vitamin D may have relevance to the prevention of particular subtypes of sPTB, but more work is needed to rigorously evaluate this association with large contemporary cohorts of mothers from a wide range of racial and ethnic backgrounds with detailed data on skin pigment and other covariates. Exploring the role of vitamin D in sPTB, particularly among African American mothers, is critical because it may be an important mediator of the racial/ethnic inequality in preterm birth.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, University of Pittsburgh Graduate School of Public Health, Pittsburgh, Pennsylvania (Lisa M. Bodnar, Alison D. Gernand, Janet M. Catov); Department of Obstetrics, Gynecology, and Reproductive Sciences, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania (Lisa M. Bodnar, Janet M. Catov, Hyagriv N. Simhan); Magee-Womens Research Institute, Pittsburgh, Pennsylvania (Lisa M. Bodnar, Janet M. Catov, Hyagriv N. Simhan); Center for Perinatal Research, The Research Institute at Nationwide Children's Hospital, Ohio State University College of Medicine, Columbus, Ohio (Mark A. Klebanoff); Department of Pediatrics, Ohio State University College of Medicine, Columbus, Ohio (Mark A. Klebanoff); Department of Pediatrics, McGill University, Montreal, Quebec, Canada (Robert W. Platt); Department of Epidemiology and Biostatistics, McGill University, Montreal, Quebec, Canada (Robert W. Platt); and Department of Pathology, University of Pittsburgh School of Medicine and Magee-Womens Hospital, Pittsburgh, Pennsylvania (W. Tony Parks).
This work was supported by the National Institutes of Health (grant HD 056999 to L.M.B). R.W.P. is a Chercheur-National (National Research Scholar) of the Fonds de la Recherche en Santé du Quebéc and a member of the McGill University Health Centre Research Institute, which receives core funding from the Fonds de la Recherche en Santé du Quebéc.
We thank Dr. Michael Holick for providing laboratory support, as well as Drs. Timothy Lash and Matthew Fox for supplying the statistical code needed to implement the sensitivity analysis.
Conflict of interest: none declared.
REFERENCES
- 1.March of Dimes; Partnership for Maternal, Newborn, and Child Health; Save the Children; World Health Organization. Born Too Soon: The Global Action Report on Preterm Birth. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 2.Martin JA, Osterman MJK, Sutton PD. Are Preterm Births on the Decline in the United States? Recent Data from the National Vital Statistics System. Hyattsville, MD: National Center for Health Statistics; 2010. National Center for Health Statistics data brief no. 39. [PubMed] [Google Scholar]
- 3.Institute of Medicine. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: National Academy of Sciences; 2006. [Google Scholar]
- 4.Aghajafari F, Nagulesapillai T, Ronksley PE, et al. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ. 2013;346:f1169. doi: 10.1136/bmj.f1169. [DOI] [PubMed] [Google Scholar]
- 5.Liu N, Kaplan AT, Low J, et al. Vitamin D induces innate antibacterial responses in human trophoblasts via an intracrine pathway. Biol Reprod. 2009;80(3):398–406. doi: 10.1095/biolreprod.108.073577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans KN, Nguyen L, Chan J, et al. Effects of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on cytokine production by human decidual cells. Biol Reprod. 2006;75(6):816–822. doi: 10.1095/biolreprod.106.054056. [DOI] [PubMed] [Google Scholar]
- 7.Liu NQ, Kaplan AT, Lagishetty V, et al. Vitamin D and the regulation of placental inflammation. J Immunol. 2011;186(10):5968–5974. doi: 10.4049/jimmunol.1003332. [DOI] [PubMed] [Google Scholar]
- 8.Bodnar LM, Simhan HN, Powers RW, et al. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr. 2007;137(2):447–452. doi: 10.1093/jn/137.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bodnar LM, Simhan HN. Vitamin D may be a link to black-white disparities in adverse birth outcomes. Obstet Gynecol Surv. 2010;65(4):273–284. doi: 10.1097/OGX.0b013e3181dbc55b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niswander K. The Collaborative Perinatal Study of the National Institute of Neurological Diseases and Stroke: The Women and Their Pregnancies. Philadelphia, PA: WB Saunders; 1972. [Google Scholar]
- 11.Hardy JB. The Collaborative Perinatal Project: lessons and legacy. Ann Epidemiol. 2003;13(5):303–311. doi: 10.1016/s1047-2797(02)00479-9. [DOI] [PubMed] [Google Scholar]
- 12.Cologne J, Preston DL, Imai K, et al. Conventional case-cohort design and analysis for studies of interaction. Int J Epidemiol. 2012;41(4):1174–1186. doi: 10.1093/ije/dys102. [DOI] [PubMed] [Google Scholar]
- 13.Blondel B, Morin I, Platt RW, et al. Algorithms for combining menstrual and ultrasound estimates of gestational age: consequences for rates of preterm and postterm birth. BJOG. 2002;109(6):718–720. doi: 10.1111/j.1471-0528.2002.01068.x. [DOI] [PubMed] [Google Scholar]
- 14.Platt RW, Abrahamowicz M, Kramer MS, et al. Detecting and eliminating erroneous gestational ages: a normal mixture model. Stat Med. 2001;20(23):3491–3503. doi: 10.1002/sim.1095. [DOI] [PubMed] [Google Scholar]
- 15.Secher NJ, Hansen PK, Lenstrup C, et al. Birthweight-for-gestational age charts based on early ultrasound estimation of gestational age. Br J Obstet Gynaecol. 1986;93(2):128–134. doi: 10.1111/j.1471-0528.1986.tb07877.x. [DOI] [PubMed] [Google Scholar]
- 16.Kelly R, Holzman C, Senagore P, et al. Placental vascular pathology findings and pathways to preterm delivery. Am J Epidemiol. 2009;170(2):148–158. doi: 10.1093/aje/kwp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gernand AD, Bodnar LM, Klebanoff MA, et al. Maternal serum 25-hydroxyvitamin D and placental vascular pathology in a multicenter US cohort. Am J Clin Nutr. 2013;98(2):383–388. doi: 10.3945/ajcn.112.055426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ananth CV, Oyelese Y, Prasad V, et al. Evidence of placental abruption as a chronic process: associations with vaginal bleeding early in pregnancy and placental lesions. Eur J Obstet Gynecol Reprod Biol. 2006;128(1-2):15–21. doi: 10.1016/j.ejogrb.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Williams MC, O'Brien WF, Nelson RN, et al. Histologic chorioamnionitis is associated with fetal growth restriction in term and preterm infants. Am J Obstet Gynecol. 2000;183(5):1094–1099. doi: 10.1067/mob.2000.108866. [DOI] [PubMed] [Google Scholar]
- 20.Holick MF, Siris ES, Binkley N, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90(6):3215–3224. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 21.Zerwekh JE. The measurement of vitamin D: analytical aspects. Ann Clin Biochem. 2004;41(Pt 4):272–281. doi: 10.1258/0004563041201464. [DOI] [PubMed] [Google Scholar]
- 22.Bodnar LM, Catov JM, Wisner KL, et al. Racial and seasonal differences in 25-hydroxyvitamin D detected in maternal sera frozen for over 40 years. Br J Nutr. 2009;101(2):278–284. doi: 10.1017/S0007114508981460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academy Press; 2010. [Google Scholar]
- 24.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 25.Myrianthopoulos NC, French KS. An application of the U.S. Bureau of the Census socioeconomic index to a large, diversified patient population. Soc Sci Med. 1968;2(3):283–299. doi: 10.1016/0037-7856(68)90004-8. [DOI] [PubMed] [Google Scholar]
- 26.Rothman KJ. The estimation of synergy or antagonism. Am J Epidemiol. 1976;103(5):506–511. doi: 10.1093/oxfordjournals.aje.a112252. [DOI] [PubMed] [Google Scholar]
- 27.Hernan MA, Hernandez-Diaz S, Werler MM, et al. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. 2002;155:176–184. doi: 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- 28.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freedman DM, Cahoon EK, Rajaraman P, et al. Sunlight and other determinants of circulating 25-hydroxyvitamin D levels in black and white participants in a nationwide US study. Am J Epidemiol. 2013;177(2):180–192. doi: 10.1093/aje/kws223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Dam RM, Snijder MB, Dekker JM, et al. Potentially modifiable determinants of vitamin D status in an older population in the Netherlands: the Hoorn Study. Am J Clin Nutr. 2007;85(3):755–761. doi: 10.1093/ajcn/85.3.755. [DOI] [PubMed] [Google Scholar]
- 31.Hypponen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr. 2007;85(3):860–868. doi: 10.1093/ajcn/85.3.860. [DOI] [PubMed] [Google Scholar]
- 32.Imhoff-Kunsch B, Briggs V, Goldenberg T, et al. Effect of n-3 long-chain polyunsaturated fatty acid intake during pregnancy on maternal, infant, and child health outcomes: a systematic review. Paediatr Perinat Epidemiol. 2012;26(suppl 1):91–107. doi: 10.1111/j.1365-3016.2012.01292.x. [DOI] [PubMed] [Google Scholar]
- 33.Jukic AM, Evenson KR, Daniels JL, et al. A prospective study of the association between vigorous physical activity during pregnancy and length of gestation and birthweight. Matern Child Health J. 2012;16(5):1031–1044. doi: 10.1007/s10995-011-0831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gelfand JM, Cree BA, McElroy J, et al. Vitamin D in African Americans with multiple sclerosis. Neurology. 2011;76(21):1824–1830. doi: 10.1212/WNL.0b013e31821cccf5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dressler WW. Social class, skin color, and arterial blood pressure in two societies. Ethn Dis. 1991;1(1):60–77. [PubMed] [Google Scholar]
- 36.Keith VM, Herring C. Skin tone and stratification in the black community. Am J Sociol. 1991;97(3):760–778. [Google Scholar]
- 37.Gravlee CC, Dressler WW, Bernard HR. Skin color, social classification, and blood pressure in southeastern Puerto Rico. Am J Public Health. 2005;95(12):2191–2197. doi: 10.2105/AJPH.2005.065615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaufman JS, Cooper RS, McGee DL. Socioeconomic status and health in blacks and whites: the problem of residual confounding and the resiliency of race. Epidemiology. 1997;8(6):621–628. [PubMed] [Google Scholar]
- 39.Lash TL, Fink AK. Semi-automated sensitivity analysis to assess systematic errors in observational data. Epidemiology. 2003;14(4):451–458. doi: 10.1097/01.EDE.0000071419.41011.cf. [DOI] [PubMed] [Google Scholar]
- 40.Hollis BW, Wagner CL. Vitamin D and pregnancy: skeletal effects, nonskeletal effects, and birth outcomes. Calcif Tissue Int. 2013;92(2):128–139. doi: 10.1007/s00223-012-9607-4. [DOI] [PubMed] [Google Scholar]
- 41.Hollis BW, Johnson D, Hulsey TC, et al. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011;26(10):2341–2357. doi: 10.1002/jbmr.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bodnar LM, Rouse DJ, Momirova V, et al. Maternal 25-hydroxyvitamin D and preterm birth in twin gestations. Obstet Gynecol. 2013;122(1):91–98. doi: 10.1097/AOG.0b013e3182941d9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morley R, Carlin JB, Pasco JA, et al. Maternal 25-hydroxyvitamin D and parathyroid hormone concentrations and offspring birth size. J Clin Endocrinol Metab. 2006;91(3):906–912. doi: 10.1210/jc.2005-1479. [DOI] [PubMed] [Google Scholar]
- 44.Thorp JM, Camargo CA, McGee PL, et al. Vitamin D status and recurrent preterm birth: a nested case-control study in high-risk women. BJOG. 2012;119(13):1617–1623. doi: 10.1111/j.1471-0528.2012.03495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shand AW, Nassar N, Von Dadelszen P, et al. Maternal vitamin D status in pregnancy and adverse pregnancy outcomes in a group at high risk for pre-eclampsia. BJOG. 2010;117(13):1593–1598. doi: 10.1111/j.1471-0528.2010.02742.x. [DOI] [PubMed] [Google Scholar]
- 46.Mehta S, Hunter DJ, Mugusi FM, et al. Perinatal outcomes, including mother-to-child transmission of HIV, and child mortality and their association with maternal vitamin D status in Tanzania. J Infect Dis. 2009;200(7):1022–1030. doi: 10.1086/605699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bodnar LM, Krohn MA, Simhan HN. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. J Nutr. 2009;139(6):1157–1161. doi: 10.3945/jn.108.103168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunlop AL, Taylor RN, Tangpricha V, et al. Maternal vitamin D, folate, and polyunsaturated fatty acid status and bacterial vaginosis during pregnancy. Infect Dis Obstet Gynecol. 2011;2011 doi: 10.1155/2011/216217. ( doi:10.1155/2011/216217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klebanoff MA, Hillier SL, Nugent RP, et al. Is bacterial vaginosis a stronger risk factor for preterm birth when it is diagnosed earlier in gestation? Am J Obstet Gynecol. 2005;192(2):470–477. doi: 10.1016/j.ajog.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 50.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D–mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 51.Liu NQ, Hewison M. Vitamin D, the placenta and pregnancy. Arch Biochem Biophys. 2012;523(1):37–47. doi: 10.1016/j.abb.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 52.Thota C, Farmer T, Garfield RE, et al. Vitamin D elicits anti-inflammatory response, inhibits contractile-associated proteins, and modulates toll-like receptors in human myometrial cells. Reprod Sci. 2013;20(4):463–475. doi: 10.1177/1933719112459225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bell NH, Greene A, Epstein S, et al. Evidence for alteration of the vitamin D–endocrine system in blacks. J Clin Invest. 1985;76(2):470–473. doi: 10.1172/JCI111995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 55.Looker AC, Pfeiffer CM, Lacher DA, et al. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr. 2008;88(6):1519–1527. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson DD, Wagner CL, Hulsey TC, et al. Vitamin D deficiency and insufficiency is common during pregnancy. Am J Perinatol. 2011;28(1):7–12. doi: 10.1055/s-0030-1262505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.