Abstract
Studies have suggested that exposure to ultraviolet (UV) light may increase risk of herpes simplex virus (HSV) recurrence. Between 1993 and 1997, the Herpetic Eye Disease Study (HEDS) randomized 703 participants with ocular HSV to receipt of acyclovir or placebo for prevention of ocular HSV recurrence. Of these, 308 HEDS participants (48% female and 85% white; median age, 49 years) were included in a nested study of exposures thought to cause recurrence and were followed for up to 15 months. We matched weekly UV index values from the National Oceanic and Atmospheric Administration to each participant's study center and used marginal structural Cox models to account for time-varying psychological stress and contact lens use and selection bias from dropout. There were 44 recurrences of ocular HSV, yielding an incidence of 4.3 events per 1,000 person-weeks. Weighted hazard ratios comparing persons with ≥8 hours of time outdoors to those with less exposure were 0.84 (95% confidence interval (CI): 0.27, 2.63) and 3.10 (95% CI: 1.14, 8.48) for weeks with a UV index of <4 and ≥4, respectively (ratio of hazard ratios = 3.68, 95% CI: 0.43, 31.4). Though results were imprecise, when the UV index was higher (i.e., ≥4), spending 8 or more hours per week outdoors was associated with increased risk of ocular HSV recurrence.
Keywords: cohort studies, herpes simplex virus, recurrence, sunlight, ultraviolet light, UV index
Approximately 70% of the US population is seropositive for herpes simplex virus (HSV) type 1 or 2 (1). After initial infection, HSV becomes latent in neuronal cell bodies, including those affecting the eye, but it can be reactivated, resulting in recurring eruptive lesions. Ocular HSV lesions can cause corneal scarring, and recurrent ocular HSV infections are a leading cause of vision loss (2, 3). Antiviral treatment is available for ocular HSV disease; in more severe cases, physicians may recommend prophylactic treatment to prevent recurrences (4, 5). However, prophylaxis may not be entirely effective, making it essential to understand and intervene with regard to the causes of ocular recurrence.
Exposure to ultraviolet (UV) B light has been used in animal models to induce recurrences of HSV infection (6, 7) and to induce recurrences of orofacial HSV in humans (8, 9). However, the existing literature regarding the association between season (10–16) or sunlight exposure (17, 18) and ocular HSV recurrence remains inadequate to inform preventive interventions on limiting exposure to UV light. Previous studies have largely based measures of UV exposure on self-reports (17), typically reports of the number of hours spent outdoors each week; however, this measure does not account for seasonal and weather-related variation in UV levels.
Investigators in the Herpetic Eye Disease Study (HEDS) prospectively followed participants with ocular HSV for 15 months and found that participants exposed to more than 21 hours of sunlight per week experienced 1.93 times the rate of ocular HSV recurrence as those exposed to ≤21 hours, although results were imprecise, with a nearly 10-fold wide confidence interval (95% confidence interval (CI): 0.68, 5.49) (17). The HEDS analysis did not adequately control for confounding by time-varying factors, such as psychological stress. Psychological stress might be a time-varying confounder because psychological stress 1) may be an independent predictor of recurrence (19) and 2) may be affected by prior UV exposure (20–22). Moreover, there may be feedback between psychological stress and UV exposure given that the number of hours spent outdoors each week may be associated with prior level of psychological stress. Simply using time-updated reports of sunlight exposure and including confounders in standard regression models does not allow for possible feedback between time-varying UV exposure and confounder levels (23). If there are time-varying confounders that are affected by prior sunlight exposure, then standard analytical methods (e.g., restriction, stratification, regression) fail to estimate the total (i.e., direct and indirect) effect of UV exposure on the risk of HSV recurrence. Regression adjustment blocks effects of UV exposure mediated through psychological stress, a possible time-varying confounder, as well as induces possible collider-conditioning bias (24). Marginal structural models can be used to obtain asymptotically consistent estimates of the total effect of UV exposure on ocular HSV recurrence in the presence of time-varying confounding (25–27).
We used weekly prospective data on self-reported hours of sunlight exposure, UV index measurements from the National Oceanic and Atmospheric Administration, physician-documented HSV recurrences, and marginal structural models to estimate the total effect of unprotected UV exposure on ocular HSV recurrence.
MATERIALS AND METHODS
Herpetic Eye Disease Study
The HEDS enrolled 703 persons in a placebo-controlled randomized trial assessing the prophylactic effect of 500 mg of acyclovir administered orally twice daily on ocular HSV recurrence (28). Participants were immunocompetent persons aged 12 years or older who had a documented history of ocular HSV recurrence during the prior year, with no active disease in the month prior to study entry. They were enrolled at 74 US clinical sites between 1992 and 1996. Of the 703 eligible participants, 308 volunteered to enroll in a nested cohort study to assess potential triggers of ocular HSV recurrence (17). Participants in this nested cohort study filled out weekly reports of the past week's exposures, including unprotected time spent outdoors and psychological stress. To avoid the recall bias shown to be present in this cohort (29) and decrease misreports, we excluded from this analysis weekly forms that had been completed more than 2 days before the end of the week or more than 3 days after the end of the week (1,850 forms) or that were missing the date of completion (320 forms). Participants were followed from study entry to the first documented recurrence of ocular HSV or were censored at the end of study follow-up (15 months) or at the third week of missing data, regardless of whether weekly reports were resumed (i.e., dropout).
The parent study protocol and informed consent forms were approved by the institutional review boards at the participating sites, and all participants provided written informed consent. Our analysis protocol was reviewed by the University of North Carolina Biomedical Institutional Review Board.
Ascertainment of HSV recurrences
Recurrence of ocular HSV was ascertained by a study-certified ophthalmologist using slit-lamp biomicroscopy at clinical examinations performed during months 1, 3, 6, 9, 12, 13, and 15 after randomization, and at additional times when participants reported new ocular symptoms. Dates of recurrence were recorded, and analyses were conducted for the first recurrence of ocular HSV after enrollment.
Assessment of sunlight exposure, covariates, and UV index
Weekly forms completed by participants were used to measure self-reported number of hours spent outdoors each week. Participants responded to the question, “In the past week, approximately how many total hours did you spend outdoors unprotected by a brimmed hat or other protective clothing?” Response categories were ≤7 hours, 8–14 hours, 15–21 hours, 22–28 hours, and ≥29 hours.
Data on age (in years), gender, race/ethnicity (self-reported; included in this study as white or other), study region, and numbers of self-reported prior ocular and nonocular HSV recurrences were collected at the first study visit. Weekly reports included information about use of contact lenses, regular use of glasses or sunglasses, and psychological stress. Psychological stress was recorded by the participants weekly using a global stress scale (30), where participants responded on a 7-point Likert scale to the question, “How stressed did you feel in the past week?” (1 = not at all stressed, 7 = extremely stressed). Because study personnel collected data at the first trial visit, there were no missing data for time-fixed variables. When information on contact lens use (21% missing), psychological stress (25% missing), and time spent outdoors (24% missing) was missing at baseline, these variables were set to studywide median values; in the weeks following baseline, the previous week's covariate and exposure data were carried forward for a maximum of 3 weeks when weekly reports were missing. Of the 9,855 weeks after baseline included in the study, 20% of information on contact lens use was carried forward, 21% of information on psychological stress was carried forward, and 20% of information on time spent outdoors was carried forward.
Daily UV index data for at least 1 city per state became publicly available through the National Oceanic and Atmospheric Administration beginning in January 1994. UV indices represent UV exposure predicted by a computer model that relates atmospheric ozone levels to UV incidence on the ground, forecasted cloud coverage, and elevation (31). Participants were assigned weekly averaged UV index values corresponding to the city closest to the clinical site they visited. For study dates prior to January 1994 (8% of weeks), the UV index was multiply imputed (32, 33) 30 times, assuming that the UV index values were missing at random given the month of the year, daily values for precipitation, minimum temperature, maximum temperature, average temperature, and cloud cover, and ocular recurrences (34).
Statistical analysis
Time spent outdoors was dichotomized into 2 groups—≤7 hours per week and ≥8 hours per week—because of the relatively small number of ocular recurrences (for the full distribution, see Web Table 1, available at http://aje.oxfordjournals.org/). According to World Health Organization reporting guidelines (35), UV index values of ≤2, 3–5, 6–7, 8–10, and ≥11 are considered to represent low, moderate, high, very high, and extreme risk, respectively. Protective clothing, including long sleeves, sunglasses, and a hat, is recommended for days when the UV index is 3 or higher, and sunscreen is recommended for days when the UV index is 6 or higher (31). The median UV index during the week prior to an ocular HSV recurrence was 4; we dichotomized the UV index at 4 (<4 vs. ≥4) to enhance precision.
Absolute incidence differences were estimated using additive Poisson regression (36). Hazard ratios were estimated using Cox proportional hazards models (37) with time in the study as the time scale and Efron's approximation for tied event times (38). Inferences from all 30 multiple imputations were combined using Rubin's multiple imputation formula (32). Wald-type 95% confidence intervals were estimated using the standard large-sample approximation for variance in the crude models and the robust variance for weighted models (27). We tested the proportional hazards assumption by computing the slope and variance of the Schoenfeld residuals and combining these values over the 30 imputations using Rubin's multiple imputation formula (32). A Wald test of the slope being equal to 0 (proportional hazards) was considered significant at the P < 0.10 level.
Observed data were weighted by the product of stabilized inverse probability-of-exposure-and-dropout weights to account for confounding and selection bias by measured characteristics. Dichotomized measures of hours spent outdoors and dropout were modeled using standard pooled logistic models (39). Candidate confounders included age, gender, race/ethnicity, number of prior ocular recurrences, number of prior nonocular recurrences, psychological stress, use of glasses or sunglasses, and use of contact lenses. Randomization assignment was not considered as a confounder because it was not thought to be associated with time spent outdoors. Not included as confounders in the final weight model were race/ethnicity, number of prior recurrences of ocular HSV, number of prior recurrences of nonocular HSV, and use of glasses or sunglasses, because addition of these variables did not alter the final effect estimate.
Time-fixed covariates included in the final model were age and gender. Age at baseline was included in the model using restricted quadratic splines with 3 knots, at 39, 52, and 65 years (40). Time-varying covariates included in the final model were psychological stress (lagged 1 week), UV index (not lagged), contact lens use (lagged 1 week), time spent outdoors (lagged 1, 2, and 3 weeks), and interactions between UV index and lagged outdoor exposures. Weights were stabilized by history of hours spent outdoors in each of the previous 3 weeks.
The resultant weights had a mean of 1.11 (standard deviation, 2.33), with a range of 0.01–184.15 (Web Table 2). Inverse-probability-of-exposure weights trimmed at 0.1 and 10 yielded weights with a mean of 1.01 (standard deviation, 0.67). Results using the trimmed weights are reported here (41); use of the untrimmed weights produced similar results (shown in Web Table 3). All analyses were conducted using SAS 9.3 (SAS Institute Inc., Cary, North Carolina).
RESULTS
Table 1 presents data collected at study entry for 308 participants in the nested substudy, who were followed for a median of 25 weeks. At study entry, participants had a median age of 49 years; 48% were female, and 85% reported their race as white. Compared with the entire population (n = 308), those who had an ocular HSV recurrence were slightly older and less likely to have self-reported nonocular recurrences prior to study entry.
Table 1.
Baseline Characteristics of All Participants and Participants Who Experienced a Recurrence of Ocular Herpes Simplex Virus, Herpetic Eye Disease Study, 1993–1997
| Characteristic | All Participants (n = 308) |
Participants With Ocular HSV Recurrence (n = 44) |
||||
|---|---|---|---|---|---|---|
| No. | % | Median (IQR) | No. | % | Median (IQR) | |
| Age at randomization, years | 49 (37–63) | 54 (38–65) | ||||
| Female gender | 147 | 48 | 22 | 50 | ||
| Race/ethnicity | ||||||
| White | 263 | 85 | 41 | 93 | ||
| African-American | 20 | 6 | 1 | 2 | ||
| Othera | 25 | 8 | 2 | 5 | ||
| Acyclovir treatment group | 155 | 50 | 23 | 52 | ||
| Study center location | ||||||
| New Orleans, Louisiana | 33 | 11 | 5 | 11 | ||
| Houston, Texas | 21 | 7 | 2 | 5 | ||
| San Francisco, California | 61 | 20 | 5 | 11 | ||
| Atlanta, Georgia | 66 | 21 | 10 | 23 | ||
| Chicago, Illinois | 44 | 14 | 8 | 18 | ||
| Milwaukee, Wisconsin | 37 | 12 | 6 | 14 | ||
| Philadelphia, Pennsylvania | 21 | 7 | 6 | 14 | ||
| New York, New York | 25 | 8 | 2 | 5 | ||
| No. of prior HSV recurrencesb | ||||||
| Ocular | 4 (1–5) | 3 (2–6) | ||||
| Nonocular | 5 (0–11) | 0 (0–9) | ||||
Abbreviations: HSV, herpes simplex virus; IQR, interquartile range.
a Includes persons who reported their race/ethnicity as Asian (2 cases, 18 in total) or Hispanic (no cases, 7 in total).
b Numbers of prior recurrences of HSV were self-reported at study enrollment.
Table 2 presents data averaged over 10,163 ocular HSV recurrence-free person-weeks of follow-up. In most of the person-weeks, observed participants reported spending ≤7 unprotected hours outdoors. Use of glasses and sunglasses was frequent, with 80% of participants reporting using them on a regular basis. There were few differences between the weeks in which participants reported spending 7 or fewer hours outdoors and those in which they reported spending 8 or more hours outdoors. In total, there were 44 recurrences of ocular HSV, yielding an incidence rate of 4.3 events per 1,000 person-weeks. Of the 308 participants, 159 (52%) were censored after more than 3 weeks of missing reports (Web Figure 1).
Table 2.
Distribution of Person-Weeks During the Entire Follow-up Period and According to Self-reported Sun Exposure, Herpetic Eye Disease Study, 1993–1997
| Reported Sun Exposure, hours/week |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Total Follow-up (10,163 PW) |
≤7 (7,169 PW) |
≥8 (2,994 PW) |
||||||
| No. | % | Median (IQR) | No. | % | Median (IQR) | No. | % | Median (IQR) | |
| Age at randomization, years | 52 (39–65) | 52 (40–66) | 49 (36–63) | ||||||
| Female gender | 4,822 | 47 | 3,616 | 50 | 1,206 | 40 | |||
| Race/ethnicity | |||||||||
| White | 8,933 | 88 | 6,350 | 89 | 2,583 | 86 | |||
| African-American | 565 | 6 | 382 | 5 | 183 | 6 | |||
| Othera | 665 | 6 | 437 | 6 | 228 | 8 | |||
| Acyclovir treatment group | 5,337 | 53 | 3,708 | 52 | 1,629 | 54 | |||
| Study center location | |||||||||
| New Orleans, Louisiana | 955 | 9 | 655 | 9 | 300 | 10 | |||
| Houston, Texas | 755 | 7 | 616 | 9 | 139 | 5 | |||
| San Francisco, California | 1,929 | 19 | 1,253 | 17 | 676 | 23 | |||
| Atlanta, Georgia | 1,878 | 18 | 1,383 | 19 | 495 | 17 | |||
| Chicago, Illinois | 1,560 | 15 | 1,046 | 15 | 514 | 17 | |||
| Milwaukee, Wisconsin | 1,525 | 15 | 1,062 | 15 | 463 | 15 | |||
| Philadelphia, Pennsylvania | 697 | 7 | 517 | 7 | 180 | 6 | |||
| New York, New York | 864 | 9 | 637 | 9 | 227 | 8 | |||
| Current use of contact lensesb | 1,024 | 10 | 787 | 11 | 237 | 8 | |||
| Use of glasses/sunglasses while outsideb | 8,037 | 79 | 5,631 | 79 | 2,406 | 80 | |||
| Overall stress levelb,c | |||||||||
| 1 (lowest) | 2,525 | 25 | 1,696 | 24 | 829 | 28 | |||
| 2 | 2,073 | 20 | 1,424 | 20 | 649 | 22 | |||
| 3 | 2,140 | 21 | 1,539 | 21 | 601 | 20 | |||
| 4 | 1,667 | 16 | 1,210 | 17 | 457 | 15 | |||
| 5 | 1,005 | 10 | 750 | 10 | 255 | 9 | |||
| 6 | 493 | 5 | 350 | 5 | 143 | 5 | |||
| 7 (highest) | 260 | 3 | 200 | 3 | 60 | 2 | |||
| Ultraviolet light index | 4.5 (2.4–6.5) | 3.9 (2.1–6.2) | 5.5 (3.3–7.0) | ||||||
| Time spent outdoors, hours/weekb | |||||||||
| ≤7 | 7,169 | 71 | 7,169 | 100 | 0 | 0 | |||
| 8–14 | 1,742 | 17 | 0 | 0 | 1,742 | 58 | |||
| 15–21 | 668 | 7 | 0 | 0 | 668 | 22 | |||
| 22–28 | 265 | 3 | 0 | 0 | 265 | 9 | |||
| ≥29 | 319 | 3 | 0 | 0 | 319 | 11 | |||
Abbreviations: IQR, interquartile range; PW, person-weeks.
a Includes persons who reported their race/ethnicity as Asian (384 in total, 236 for ≤7 hours/week, and 148 for ≥8 hours/week) or Hispanic (281 in total, 201 for ≤7 hours/week, and 80 for ≥8 hours/week).
b Before data were carried forward, the following amounts of data were missing (percentage of weeks prior to recurrence, dropout, or end of study period): for use of contact lenses, 20.3%; for use of glasses/sunglasses, 26.7%; for overall stress, 21.5%; and for time spent outdoors, 20.1%.
c Stress was measured by asking, “In the past week, how stressed have you felt overall?” Participants could answer on a 7-point Likert scale.
From our crude additive Poisson model, when comparing weeks with ≥8 hours outdoors with weeks with ≤7 hours outdoors, we estimated an incidence difference of −0.50 cases per 1,000 person-years (95% CI: −2.01, 1.01) during weeks with a UV index less than 4 and 3.73 cases per 1,000 person-years (95% CI: 2.98, 4.48) during weeks with a UV index of 4 or higher.
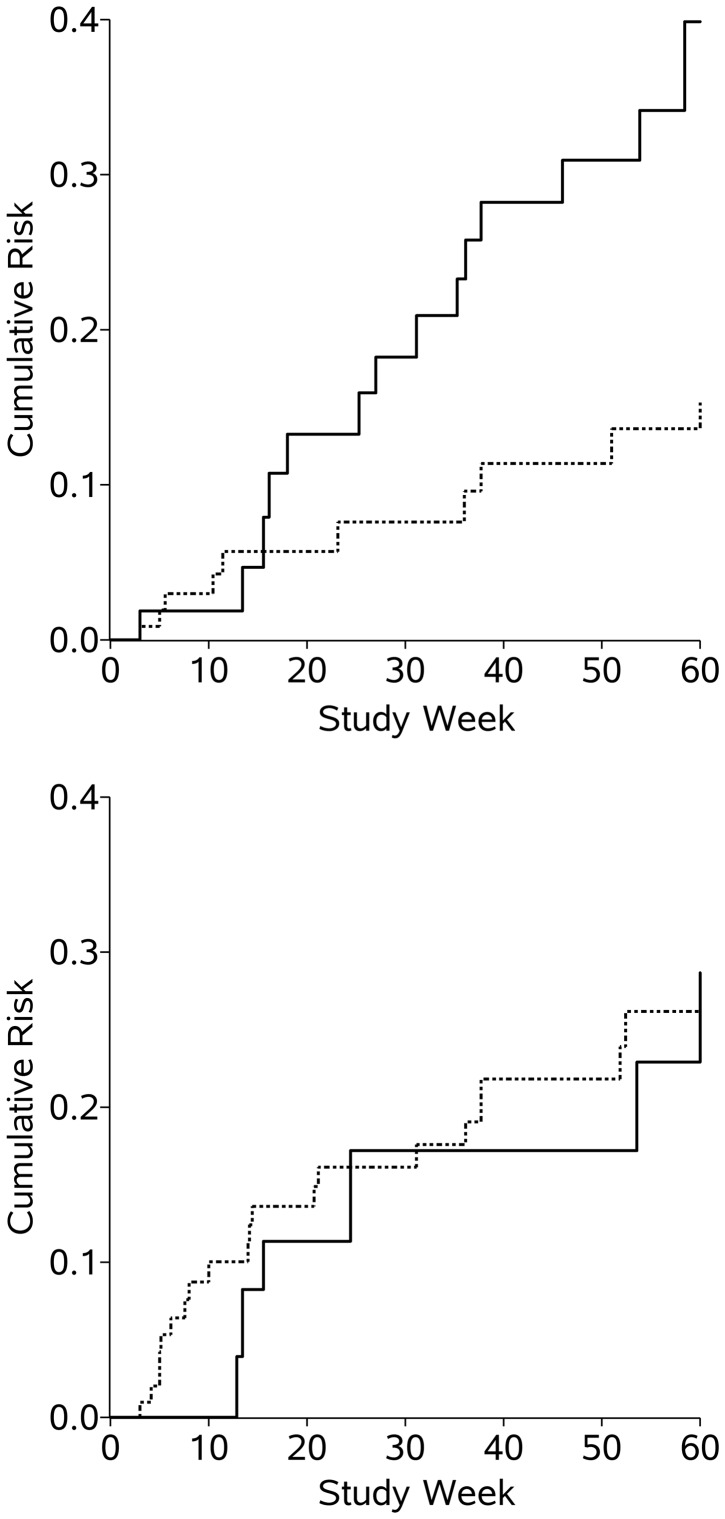
The weighted Kaplan-Meier curves for ocular HSV recurrence according to UV index, pooled over all 30 imputations (equivalent to taking the mean value), are shown in Figure 1 (crude Kaplan-Meier curves are shown in Web Figure 2). For the weighted analysis, in the weeks where the UV index was less than 4, the incidence of ocular HSV recurrence at 60 weeks was 23% for persons exposed to ≤7 hours outdoors and 22% for persons exposed to ≥8 hours outdoors. In the weeks where the UV index was 4 or higher, the incidence of ocular HSV recurrence at 60 weeks was 13% for those exposed to ≤7 hours outdoors and 46% for those exposed to ≥8 hours.
Figure 1.
Weighted Kaplan-Meier estimates of the cumulative risk of ocular herpes simplex virus recurrence among 308 participants in the Herpetic Eye Disease Study during person-weeks with an ultraviolet index value of ≥4 (top) or <4 (bottom), 1993–1997. Results were pooled over 30 imputations. Solid line, high sunlight exposure (≥8 hours/week); dashed line, low sunlight exposure (≤7 hours/week).
Table 3 shows estimated hazard ratios for ocular HSV recurrence due to sunlight exposure. Within levels of UV index, we estimated crude hazard ratios of 0.81 (95% CI: 0.27, 2.41) and 2.42 (95% CI: 1.00, 5.84) for weeks with a UV index of <4 and ≥4, respectively (ratio of hazard ratios = 2.99, 95% CI: 0.42, 21.4). Hazard ratios from a marginal structural Cox model that accounted for potential confounding by time-varying psychological stress and contact lens use and for baseline age and gender were 0.84 (95% CI: 0.27, 2.63) and 3.10 (95% CI: 1.14, 8.48), comparing ≥8 hours spent outdoors with ≤7 hours, for weeks with UV indices of <4 and ≥4, respectively (ratio of hazard ratios = 3.68, 95% CI: 0.43, 31.4).
Table 3.
Estimated Effect of Sunlight and Ultraviolet Light Exposure (UV Index) on Time to Recurrence of Ocular Herpes Simplex Virus Among 308 Participants in the Herpetic Eye Disease Study, 1993–1997
| Model and Sun Exposure, hours/week | No. of Events | Person- Weeks of Follow-up | Hazard Ratio | 95% Confidence Intervala |
|---|---|---|---|---|
| Crude Model | ||||
| Total | ||||
| ≤7 | 27 | 7,169 | 1 | |
| ≥8 | 17 | 2,994 | 1.46 | 0.79, 2.68 |
| UV index <4 | ||||
| ≤7 | 18 | 3,619 | 1 | |
| ≥8 | 4 | 949 | 0.81 | 0.27, 2.41 |
| UV index ≥4 | ||||
| ≤7 | 9 | 3,550 | 1 | |
| ≥8 | 13 | 2,045 | 2.42 | 1.00, 5.84 |
| Weighted Modelb | ||||
| Total | ||||
| ≤7 | 27 | 7,169 | 1 | |
| ≥8 | 17 | 2,994 | 1.85 | 0.89, 3.88 |
| UV index <4 | ||||
| ≤7 | 18 | 3,619 | 1 | |
| ≥8 | 4 | 949 | 0.84 | 0.27, 2.63 |
| UV index ≥4 | ||||
| ≤7 | 9 | 3,550 | 1 | |
| ≥8 | 13 | 2,045 | 3.10 | 1.14, 8.48 |
Abbreviation: UV, ultraviolet.
a 95% confidence intervals for results stratified by UV index were calculated using robust methods to account for variance between imputations.
b The weighted model accounted for time-varying psychological stress and use of contact lenses and for baseline age and gender.
The test of proportional hazards yielded P values of 0.50 and 0.12 for the crude results and 0.47 and 0.10 for the inverse-probability-of-treatment-and-censoring-weighted model for UV indices of <4 and ≥4, respectively. The hazard ratio for ocular HSV recurrence weakened over time. For instance, between randomization and 29 weeks, the marginal structural model hazard ratios for ocular HSV recurrence were 1.01 (95% CI: 0.14, 7.38) and 6.49 (95% CI: 1.40, 30.1) for weeks with UV indices of <4 and ≥4, respectively, while the hazard ratios for ocular HSV recurrence between 30 and 65 weeks were 0.78 (95% CI: 0.20, 3.12) and 1.41 (95% CI: 0.36, 5.52) for weeks with UV indices of <4 and ≥4, respectively.
When restricted to the 155 participants who were randomized to receive acyclovir treatment (who experienced 23 events), similar patterns of crude hazard ratios comparing ≥8 hours outdoors with ≤7 hours outdoors were observed. The crude hazard ratio for weeks with a UV index less than 4 was 0.74 (95% CI: 0.16, 3.36), and for weeks with a UV index of 4 or higher, it was 5.07 (95% CI: 1.34, 19.2). In the weighted model, results for the same groups were 1.10 (95% CI: 0.24, 5.02) and 7.57 (95% CI: 1.91, 30.1), respectively. Among the 153 persons who were randomized to receive placebo, results for the same groups from the crude and weighted models were 0.85 (95% CI: 0.16, 4.41) and 0.97 (95% CI: 0.26, 4.07), respectively, and 0.65 (95% CI: 0.11, 3.74) and 0.86 (95% CI: 0.18, 4.16), respectively.
DISCUSSION
Because the HEDS analysis did not appropriately adjust for time-varying psychological stress or contact lens use, we expected to observe a different hazard ratio for ocular HSV recurrence attributable to sunlight exposure. While marginal structural models were indicated since we suspected time-varying confounding, we found little evidence of time-varying confounding, as shown by the similar effect estimates from the crude and weighted models.
Similarly, we expected to see a stronger relationship between sunlight exposure and recurrence when UV index values were higher and when we accounted for effect modification by UV exposure. We did find a stronger estimate for the effect of sunlight exposure on ocular HSV recurrence when the UV index was ≥4, but our results were imprecise.
There are many ways that UV exposure is thought to impact HSV recurrence; we review 2 possible mechanisms here. The first pathway is depression of immune response due to UV exposure. UV radiation has been shown to suppress HSV antigen presentation in epidermal cells (42) and lead to the reduction of type 1 cytokine release (43), an important part of immunological control for viruses such as HSV. Garssen et al. (44) extrapolated from an animal model that exposure to 100 minutes of sunlight around noon on a clear summer day in a southern Mediterranean country would lead to a 50% suppression in the T-cell response to a microbe. This localized immunosuppression may allow sufficient viral replication to cause a recurrence. The second pathway by which UV radiation may affect recurrence is directly through HSV reactivation. Cell repair, through the c-Jun and c-Fos transcription factors, activates the HSV transcription promoter (infected cell polypeptide 0), leading to HSV transcription and reactivation (45). Additionally, these repair pathways circumvent the activity of HSV latency-associated transcript, which prevents infected neurons from undergoing apoptosis (46), in turn reactivating HSV. Given the proposed mechanisms for interaction of UV light with HSV recurrence, it becomes clear that looking for seasonal trends in recurrence or relating recurrences to time spent outdoors would probably not capture an individual's exposure. While our assessment of UV exposure was more specific than that of previous studies, other methods (e.g., a UV dosimeter) would more finely delineate exposure.
There were limitations to this research. We assumed no unmeasured confounding and no informative censoring by unmeasured factors; if either of these assumptions were not met, our hazard ratios would be biased. In particular, psychological stress is a difficult quantity to reliably measure and may not have been well captured in this study. Second, the results from this nested cohort study may not be generalizable to a target population of persons with ocular HSV disease, who may differ in composition from trial population samples (47, 48), which are often highly selective. Third, 51% of the participants were censored after not completing 3 weekly logs in a row. Sensitivity analysis for time-varying missing data could be undertaken but was beyond the scope of this study. Fourth, while use of a fuller account of the covariate histories, a less restrictive functional form for age and time in the study, and a broader set of covariates (e.g., region, systemic illness, acyclovir treatment) did not appreciably alter our results, there could have been model misspecification. Fifth, because biological samples were not collected in HEDS, we were unable to assess the effect of UV exposure on viral shedding, an important component of HSV transmission. Sixth, we assumed positivity (49), that is, a nonzero probability of exposure at every level of the observed confounders in the population.
Last, we assumed consistency and its practical implication of irrelevance of exposure variation (in this case, time spent outdoors) (50). This assumption was the most problematic here. Participants may have experienced their time spent outdoors differently (e.g., time spent outside in the early morning hours might be different from that spent during the afternoon). Additionally, an intervention designed to restrict persons who usually spend ≥8 hours per week outdoors to ≤7 hours per week would have to mimic the distribution of exposures found in the ≤7-hour group in order to produce an effect estimate similar to ours (51). However, we improved upon previous work by including UV index data to decrease unquantified exposure variation.
There were several strengths of this research. Recurrences of ocular HSV were well documented and were confirmed by study-trained physicians. The use of prospective data helped ensure the proper temporal order between exposures, confounders, and ocular HSV recurrences. Exclusion of weekly reports completed tardily minimized recall bias (29). The use of time-updated reports reduced bias due to measurement error (52). Use of modern methods that allow feedback offered us an opportunity to disentangle the relationship between UV light and stress. Randomization to a certain number of hours spent outdoors may be difficult to implement and would likely have low compliance. Without randomized evidence, a thorough analysis of prospective observational data with repeated measures provides the best estimates of etiological effects.
Findings from this and further research could guide clinicians in recommending behavioral interventions for persons with ocular HSV. Physicians could recommend restriction of a patient's time outdoors during peak hours of UV exposure on days with a high UV index. Another possible route for prevention of recurrence would be the use of UV-blocking sunglasses—although many of the participants in this study did wear some type of glasses, so perhaps a brimmed hat or glasses with particularly strong UV protection should be considered. Our results, though imprecise, do not suggest an effect of acyclovir on UV-induced recurrences; however, further research on this relationship is warranted.
In conclusion, we found an association between increased time spent outdoors and the risk of ocular HSV recurrence when the UV index was relatively high. Further research is needed both to replicate these results and to explore more finely the UV exposure associated with increased risk of HSV recurrence.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Christina Ludema, Stephen R. Cole, Charles Poole, Jennifer S. Smith, Victor J. Schoenbach); and Cullen Eye Institute, Department of Ophthalmology, Baylor College of Medicine, Houston, Texas (Kirk R. Wilhelmus).
This work was supported in part through National Institutes of Health grant R21EY021478.
We thank Dr. Roy W. Beck, Dr. Bryan Shepherd, Dr. Lauren Cain, and Chris Fuhrmann for their expert advice.
Conflict of interest: none declared.
REFERENCES
- 1.Xu F, Schillinger JA, Sternberg MR, et al. Seroprevalence and coinfection with herpes simplex virus type 1 and type 2 in the United States, 1988–1994. J Infect Dis. 2002;185(8):1019–1024. doi: 10.1086/340041. [DOI] [PubMed] [Google Scholar]
- 2.Liesegang TJ, Melton LJ, Daly PJ, et al. Epidemiology of ocular herpes simplex: incidence in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989;107(8):1155–1159. doi: 10.1001/archopht.1989.01070020221029. [DOI] [PubMed] [Google Scholar]
- 3.Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012;57(5):448–462. doi: 10.1016/j.survophthal.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toma HS, Murina AT, Areaux RG, Jr, et al. Ocular HSV-1 latency, reactivation and recurrent disease. Semin Ophthalmol. 2008;23(4):249–273. doi: 10.1080/08820530802111085. [DOI] [PubMed] [Google Scholar]
- 5.Pepose JS, Keadle TL, Morrison LA. Ocular herpes simplex: changing epidemiology, emerging disease patterns, and the potential of vaccine prevention and therapy. Am J Ophthalmol. 2006;141(3):547–557. doi: 10.1016/j.ajo.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Norval M, el-Ghorr AA. UV radiation and mouse models of herpes simplex virus infection. Photochem Photobiol. 1996;64(2):242–245. doi: 10.1111/j.1751-1097.1996.tb02452.x. [DOI] [PubMed] [Google Scholar]
- 7.Laycock KA, Lee SF, Brady RH, et al. Characterization of a murine model of recurrent herpes simplex viral keratitis induced by ultraviolet B radiation. Invest Ophthalmol Vis Sci. 1991;32(10):2741–2746. [PubMed] [Google Scholar]
- 8.Spruance SL, Kriesel JD, Evans TG, et al. Susceptibility to herpes labialis following multiple experimental exposures to ultraviolet radiation. Antiviral Res. 1995;28(1):57–67. doi: 10.1016/0166-3542(95)00038-n. [DOI] [PubMed] [Google Scholar]
- 9.Rooney JF, Bryson Y, Mannix ML, et al. Prevention of ultraviolet-light-induced herpes labialis by sunscreen. Lancet. 1991;338(8780):1419–1422. doi: 10.1016/0140-6736(91)92723-f. [DOI] [PubMed] [Google Scholar]
- 10.Bell DM, Holman RC, Pavan-Langston D. Herpes simplex keratitis: epidemiologic aspects. Ann Ophthalmol. 1982;14(5):421–422. 424. [PubMed] [Google Scholar]
- 11.Brandt BM, Mandleblatt J, Asbell PA. Risk factors for herpes simplex-induced keratitis: a case-control study. Ann Ophthalmol. 1994;26(1):12–16. [PubMed] [Google Scholar]
- 12.Gamus D, Romano A, Sucher E, et al. Herpetic eye attacks: variability of circannual rhythms. Br J Ophthalmol. 1995;79(1):50–53. doi: 10.1136/bjo.79.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liesegang TJ. Epidemiology of ocular herpes simplex: natural history in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989;107(8):1160–1165. doi: 10.1001/archopht.1989.01070020226030. [DOI] [PubMed] [Google Scholar]
- 14.Mortensen KK, Sjolie AK. Keratitis dendritica: an epidemiological investigation. Acta Ophthalmol (Copenh) 1979;57(5):750–754. doi: 10.1111/j.1755-3768.1979.tb01840.x. [DOI] [PubMed] [Google Scholar]
- 15.Norn MS. Dendritic (herpetic) keratitis. I. Incidence–seasonal variations–recurrence rate–visual impairment–therapy. Acta Ophthalmol (Copenh) 1970;48(1):91–107. doi: 10.1111/j.1755-3768.1970.tb06577.x. [DOI] [PubMed] [Google Scholar]
- 16.Uchio E, Hatano H, Ohno S. Altering clinical features of recurrent herpes simplex virus-induced keratitis. Ann Ophthalmol. 1993;25(7):271–276. [PubMed] [Google Scholar]
- 17.Herpetic Eye Disease Study Group. Psychological stress and other potential triggers for recurrences of herpes simplex virus eye infections. Arch Ophthalmol. 2000;118(12):1617–1625. doi: 10.1001/archopht.118.12.1617. [DOI] [PubMed] [Google Scholar]
- 18.Wilhelmus KR, Todaro LA. Effect of sunlight on recurrent herpetic keratitis [abstract] Invest Ophthalmol Vis Sci. 1992;33(4):790. [Google Scholar]
- 19.Chida Y, Mao X. Does psychosocial stress predict symptomatic herpes simplex virus recurrence? A meta-analytic investigation on prospective studies. Brain Behav Immun. 2009;23(7):917–925. doi: 10.1016/j.bbi.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Bertone-Johnson ER. Vitamin D and the occurrence of depression: causal association or circumstantial evidence? Nutr Rev. 2009;67(8):481–492. doi: 10.1111/j.1753-4887.2009.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ulrich R, Simons R, Losito B, et al. Stress recovery during exposure to natural and urban environments. J Environ Psychol. 1991;11(3):201–230. [Google Scholar]
- 22.Keller MC, Fredrickson BL, Ybarra O, et al. A warm heart and a clear head: the contingent effects of weather on mood and cognition. Psychol Sci. 2005;16(9):724–731. doi: 10.1111/j.1467-9280.2005.01602.x. [DOI] [PubMed] [Google Scholar]
- 23.Howe CJ, Sander PM, Plankey MW, et al. Effects of time-varying exposures adjusting for time-varying confounders: the case of alcohol consumption and risk of incident human immunodeficiency virus infection. Int J Public Health. 2010;55(3):227–228. doi: 10.1007/s00038-010-0120-0. [DOI] [PubMed] [Google Scholar]
- 24.Greenland S. Quantifying biases in causal models: classical confounding vs collider-stratification bias. Epidemiology. 2003;14(3):300–306. [PubMed] [Google Scholar]
- 25.Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the joint causal effect of nonrandomized treatments. J Am Stat Assoc. 2001;96(454):440–448. [Google Scholar]
- 26.Cole SR, Hernán MA, Robins JM, et al. Effect of highly active antiretroviral therapy on time to acquired immunodeficiency syndrome or death using marginal structural models. Am J Epidemiol. 2003;158(7):687–694. doi: 10.1093/aje/kwg206. [DOI] [PubMed] [Google Scholar]
- 27.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Herpetic Eye Disease Study Group. Acyclovir for the prevention of recurrent herpes simplex virus eye disease. N Engl J Med. 1998;339(5):300–306. doi: 10.1056/NEJM199807303390503. [DOI] [PubMed] [Google Scholar]
- 29.Kip KE, Cohen F, Cole SR, et al. Recall bias in a prospective cohort study of acute time-varying exposures: example from the Herpetic Eye Disease Study. J Clin Epidemiol. 2001;54(5):482–487. doi: 10.1016/s0895-4356(00)00310-3. [DOI] [PubMed] [Google Scholar]
- 30.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 31.Long C, Miller A, Lee H, et al. Ultraviolet index forecasts issued by the National Weather Service. Bull Am Meteorol Soc. 1996;77(4):729–748. [Google Scholar]
- 32.Rubin DB. Inference and missing data. Biometrika. 1976;63(3):581–592. [Google Scholar]
- 33.Schafer JL. Analysis of Incomplete Multivariate Data. New York, NY: Chapman & Hall, Inc; 1997. [Google Scholar]
- 34.Moons KG, Donders RA, Stijnen T, et al. Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol. 2006;59(10):1092–1101. doi: 10.1016/j.jclinepi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. What Is the UV Index (UVI)? Geneva, Switzerland: World Health Organization; 2013. (http://www.who.int/uv/intersunprogramme/activities/uv_index/en/ ). (Accessed October 7, 2013) [Google Scholar]
- 36.Boshuizen HC, Feskens EJM. Fitting additive Poisson models. Epidemiol Perspect Innov. 2010;7:4. doi: 10.1186/1742-5573-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cox DR. Regression models and life-tables [with discussion] J R Stat Soc Ser B (Methodol) 1972;34(2):187–220. [Google Scholar]
- 38.Efron B. The efficiency of Cox's likelihood function for censored data. J Am Stat Assoc. 1977;72(359):557–565. [Google Scholar]
- 39.Abbott RD. Logistic regression in survival analysis. Am J Epidemiol. 1985;121(3):465–471. doi: 10.1093/oxfordjournals.aje.a114019. [DOI] [PubMed] [Google Scholar]
- 40.Howe CJ, Cole SR, Westreich DJ, et al. Splines for trend analysis and continuous confounder control. Epidemiology. 2011;22(6):874–875. doi: 10.1097/EDE.0b013e31823029dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Molen RG, Out-Luiting C, Claas FH, et al. Ultraviolet-B radiation induces modulation of antigen presentation of herpes simplex virus by human epidermal cells. Hum Immunol. 2001;62(6):589–597. doi: 10.1016/s0198-8859(01)00248-8. [DOI] [PubMed] [Google Scholar]
- 43.Norval M. The effect of ultraviolet radiation on human viral infections. Photochem Photobiol. 2006;82(6):1495–1504. doi: 10.1562/2006-07-28-IR-987. [DOI] [PubMed] [Google Scholar]
- 44.Garssen J, Goettsch W, de Gruijl F, et al. Risk assessment of UVB effects on resistance to infectious diseases. Photochem Photobiol. 1996;64(2):269–274. doi: 10.1111/j.1751-1097.1996.tb02457.x. [DOI] [PubMed] [Google Scholar]
- 45.Loiacono CM, Taus NS, Mitchell WJ. The herpes simplex virus type 1 ICP0 promoter is activated by viral reactivation stimuli in trigeminal ganglia neurons of transgenic mice. J Neurovirol. 2003;9(3):336–345. doi: 10.1080/13550280390201047. [DOI] [PubMed] [Google Scholar]
- 46.Henderson G, Peng W, Jin L, et al. Regulation of caspase 8- and caspase 9-induced apoptosis by the herpes simplex virus type 1 latency-associated transcript. J Neurovirol. 2002;8(suppl 2):103–111. doi: 10.1080/13550280290101085. [DOI] [PubMed] [Google Scholar]
- 47.Cole SR, Stuart EA. Generalizing evidence from randomized clinical trials to target populations. The ACTG 320 Trial. Am J Epidemiol. 2010;172(1):107–115. doi: 10.1093/aje/kwq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greenhouse JB, Kaizar EE, Kelleher K, et al. Generalizing from clinical trial data: a case study. The risk of suicidality among pediatric antidepressant users. Stat Med. 2008;27(11):1801–1813. doi: 10.1002/sim.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Westreich D, Cole SR. Invited commentary: positivity in practice. Am J Epidemiol. 2010;171(6):674–677. doi: 10.1093/aje/kwp436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hernán MA, VanderWeele TJ. Compound treatments and transportability of causal inference. Epidemiology. 2011;22(3):368–377. doi: 10.1097/EDE.0b013e3182109296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.VanderWeele TJ, Hernán MA. Causal inference under multiple versions of treatment. J Causal Inference. 2013;1(1):1–20. doi: 10.1515/jci-2012-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149(6):531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.