Abstract
We investigated associations between short-term exposure to air pollution and central augmentation index and augmentation pressure, correlates of arterial stiffness, in a cohort of elderly men in the Boston, Massachusetts, metropolitan area. This longitudinal analysis included 370 participants from the Veterans Affairs Normative Aging Study with up to 2 visits between 2007 and 2011 (n = 445). Augmentation index (as %) and augmentation pressure (in mmHg) were measured at each visit by using radial artery applanation tonometry for pulse wave analysis and modeled in a mixed effects regression model as continuous functions of moving averages of air pollution exposures (over 4 hours and 1, 3, 7, and 14 days). The results suggest that short-term changes in air pollution were associated with augmentation index and augmentation pressure at several moving averages. Interquartile range (IQR) increases in 3-day average exposure to particles with aerodynamic diameter less than 2.5 μm (3.6-μg/m3 IQR increase) and sulfate (1.4-μg/m3 IQR increase) and 1-day average exposure to particle number counts (8,741-counts/cm3 IQR increase) were associated with augmentation index values that were 0.8% (95% confidence interval (CI): 0.2, 1.4), 0.6% (95% CI: 0.1, 1.2), and 1.7% (95% CI: 0.4, 2.9) higher, respectively. Overall, the findings were similar for augmentation pressure. The findings support the hypothesis that exposure to air pollution may affect vascular function.
Keywords: air pollution, particulate matter, pulse wave analysis
The associations between short-term exposure to particles with aerodynamic diameter less than 2.5 μm (PM2.5) and cardiovascular morbidity and mortality are well documented (1). In recent years, more emphasis has been placed on identifying the underlying mechanisms of the observed associations, for which there is moderate evidence from observational and experimental studies to suggest the role of vascular function (1). Short-term exposures during the first 30 minutes and up to 6 days for PM2.5 and its components including sulfate and black carbon have been shown to be associated with decreased brachial artery diameter and decreased brachial artery flow–mediated dilation in healthy and diabetic adults (2–6). Exposures to PM2.5 averaged over 5 and 30 days were also associated with higher systolic blood pressure in healthy and diabetic adults (7, 8).
Another component of vascular function, arterial stiffness, may also be affected by short-term exposure to particulate matter. Stiffening of the arteries is a process associated with aging and cardiovascular risk factors. Arterial stiffness measured as the carotid-femoral pulse wave velocity, which is the “gold standard” for arterial stiffness assessment (9), is associated with risk of cardiovascular death (10). A correlate of arterial stiffness and measure of wave reflection, the augmentation index (AIx), is also associated with increased risk of cardiovascular disease and death (11, 12). Risk factors observed in association with AIx include age (13), sex (14), race (15), body weight (16), smoking (17, 18), and alcohol consumption (19).
Few studies have examined whether arterial stiffness increases after short-term exposure to air pollution. AIx values have been shown to increase in welders after acute exposure to PM2.5 (20) and in healthy men exposed to diesel exhaust in a chamber-controlled setting (21). In a prospective panel study of adults with type 2 diabetes mellitus, both large- and small-artery elasticity indices decreased in association with PM2.5 exposure with a lag of 3 days (4).
A large, cross-sectional, community-based study consisting of hypertensive patients and normotensive controls did not find any association between AIx and short-term exposure to particles with aerodynamic diameter less than 10 μm (PM10) but identified a positive association restricted to men between PM10 exposure averaged over 5 days and augmentation pressure (AP) (22); no association was observed between PM10 exposure and carotid-femoral pulse wave velocity. PM10 was the only air pollutant examined by the study investigators, and it is not known if short-term exposure to ambient PM2.5 and its components and gaseous air pollutants are associated with AIx in community- or population-based settings.
The objective of this study was to evaluate associations between short-term changes in air pollution and correlates of arterial stiffness (AIx and AP), in a community-based cohort of elderly men in the Boston, Massachusetts, metropolitan area. We analyzed the following air pollutants: PM2.5, black carbon, sulfate, particle number counts (PNCs), nitrogen dioxide, and ozone. Many of the study participants presented with hypertension, coronary heart disease, or diabetes at the time of examination. Therefore, we evaluated whether these conditions modified the observed associations.
MATERIALS AND METHODS
Study population and design
Participants included in this analysis were enrolled in the Normative Aging Study, an ongoing longitudinal study of aging established by the US Department of Veterans Affairs in 1963, details of which have been published previously (23). Briefly, the Normative Aging Study is a closed cohort of 2,280 male volunteers from the greater Boston, Massachusetts, area, aged 21–80 years at study entry, who enrolled after an initial health screening determined that they were free of known chronic medical conditions. Participants have been reevaluated every 3–5 years by using detailed, onsite physical examinations and questionnaires. The present study was approved by the human research committees of Brigham and Women's Hospital (Boston, Massachusetts) and the Veterans Affairs Boston Healthcare System, and written informed consent was obtained from subjects prior to participation. Eligibility for this study required continued participation as of the time when pulse wave analysis (PWA) measurements began. From July 2007 through August 2011, PWA measurements were collected at each visit. From the 432 study participants who were followed-up during this period, we collected a total of 481 PWA measurements from 397 participants. This analysis included a maximum of 445 PWA measurements from 370 participants, depending on the number of measurements available for the air pollutant of interest. For the purpose of this analysis, we will refer to the first visit with PWA measurement as the baseline visit. Refer to the Web Appendix (Web Figure 1, available at http://aje.oxfordjournals.org/) for a more detailed description of the participation and inclusion criteria in this analysis.
Air pollution and meteorology data
Ambient particulate pollutant concentrations were monitored at our Harvard Air Pollution Supersite located near downtown Boston, Massachusetts, 1 km from the Veterans Affairs medical center. Measurements included PM2.5, sulfate, black carbon, and PNCs. We measured hourly PNCs (0.007- to 3-μm counts/cm3) with a condensation particle counter, model 3022A (TSI Inc., Shoreview, Minnesota). PNCs are primarily influenced by freshly generated particles from local traffic (24). Through 2009, we measured hourly PM2.5 concentrations with a tapered element oscillation microbalance, model 1400A (Rupprecht & Patashnick, East Greenbush, New York), after which the beta attenuation monitor 1020 (Met One Instruments, Inc., Grants Pass, Oregon) was used. Hourly black carbon concentrations were measured using an aethalometer, model AE-16 (Magee Scientific Corp., Berkeley, California). Black carbon is associated with traffic emissions, especially those related to diesel fuel combustion. Daily sulfate concentrations were calculated from elemental sulfur, measured by x-ray fluorescence analysis of the PM2.5 filter samples. On days when sulfate measurements were not available, we calculated daily sulfate concentrations from a sulfate particulate analyzer, model 5020 (Thermo Electron Corp., Franklin, Massachusetts). Sulfate particles are formed through the oxidation of sulfur dioxide emitted primarily by coal- and oil-burning power plants and can be transported regionally over long distances (e.g., hundreds of kilometers) (25). We obtained hourly ozone and nitrogen dioxide concentration data (in ppm) from local state monitors within greater Boston, and we estimated average hourly concentrations (see Web Appendix for a detailed description of nitrogen dioxide and ozone monitoring and exposure estimation). Hourly temperature and humidity data were obtained from the Logan International Airport (Boston, Massachusetts) weather station (12 km from the Veterans Affairs medical center). Pollutant sampling, processing of samples, analysis, and reporting were conducted according to standard operating procedures (26). At least 75% of all hourly measurements were needed to estimate daily PNCs and the daily concentrations of PM2.5, black carbon, nitrogen dioxide, and ozone, and we considered moving averages based on the short-term exposure windows of 4 hours, 24 hours, and 3, 7, and 14 days preceding each participant's examination. Hourly sulfate measurements were not available; thus, the 4-hour moving average exposure for sulfate could not be estimated.
Augmentation index and pressure
AIx and AP values were derived from PWA, conducted with a SphygmoCor Px Pulse Wave Analysis System, model SCOR-Px (Atcor Medical Pty. Ltd., Sydney, Australia). The PWA measurements were performed in a temperature-controlled room by 1 of 3 trained study personnel; 1 of the trained personnel performed approximately 90% of all PWA measurements, including those from repeat visits. After 5 minutes of rest in a seated position, each participant extended the dominant arm onto a flat surface, ensuring that the elbow was at heart level. Applanation tonometry of the radial artery, guided by visual inspection of the pulse pressure waveform and by a built-in quality score of the homogeneity of the observed waveforms, was conducted to record 10 seconds of 20 sequential peripheral pulse waveforms. The peripheral waveforms were transformed into corresponding central aortic waveforms via a previously validated transfer function (27). Pulse pressure, AP, and AIx values were derived from the aortic pressure wave. The AIx is defined as the difference between the first and second systolic peak pressure (AP in mmHg) divided by the pulse pressure and expressed as a percentage as follows: AIx (as %) = [AP / pulse pressure] × 100. Larger values indicate a higher pulse wave velocity and earlier return of the reflected wave, which is caused by increased arterial stiffness or vascular resistance. AIx values were also normalized for a heart rate of 75 bpm because AIx is influenced by heart rate in a linear and inverse fashion (28). The PWA device simultaneously measures heart rate, and the system software provides heart rate–adjusted AIx and AP values.
Statistical analysis
All statistical analyses were carried out using SAS, version 9.2, software (SAS Institute, Inc., Cary, North Carolina) and R software (R Foundation for Statistical Computing, Vienna, Austria). We used linear mixed-effects regression models with random participant-specific intercepts accounting for the correlation of repeated measures (29) to model AIx (as %) and AP (in mmHg) as a continuous function of moving averages (of 4 hours, 24 hours, and 3, 7, and 14 days) of PM2.5, black carbon, PNCs, sulfate, nitrogen dioxide, and ozone. Estimates are given per interquartile range of the pollutant for the specific moving average. We fit each moving average exposure of each pollutant 1 at a time in an established fixed confounder model that was adjusted for age, body mass index (weight (kg)/height (m)2), high density lipoprotein cholesterol level (in mg/dL), years of education, race (black or white), alcohol intake (≥2 or <2 drinks/day), smoking status (current/former smoker or never smoker), cumulative pack-years smoked, diabetes status (physician diagnosed diabetes or fasting blood glucose greater than 126 mg/mL), seasonality, weekday of examination, and 3-day moving average of absolute temperature (in °C) and relative humidity (as %). These covariates were chosen a priori as potential confounders or important determinants of AIx and AP. After demonstrating the best model fit for AIx and AP in a preliminary analysis, the 3-day moving average of absolute temperature and relative humidity was selected for adjustment. Presence of hypertension, coronary heart disease, and diabetes at each visit was assessed by review of the subject′ medical records, and these factors were evaluated as potential modifiers of the association between air pollution and AIx and AP by using interaction terms.
To evaluate whether the association between each pollutant and each outcome was nonlinear, we fit generalized additive mixed models with penalized splines for the pollutant variable. The generalized cross-validation criterion of the model was used to determine the degrees of freedom of the spline for the nonlinear term, and we compared the Akaike information criterion to assess model fit between models with the linear and nonlinear terms. For all models, the linear term for the pollutant variable was the better fit.
Of the 370 participants who continued to follow up in 2007, when PWA measurements began, only 75 (20.3%) had participated in a subsequent follow-up visit with repeated measurements of AIx and AP by the end of the study period. Healthier men may be more likely to participate in a subsequent follow-up visit, so we used stabilized inverse probability weights to correct for this potential survival bias in all models (30) (see the Web Appendix for additional description of stabilized inverse probability weights).
Secondary analyses
All linear mixed-effects regression models were restricted to participants with repeat visits. Because approximately 80% of the study participants did not have a repeat visit with PWA measurements in this analysis, we also conducted a baseline cross-sectional analysis of AIx and AP using multiple linear regression, excluding the available second visits from participants. To assess whether colder or warmer room temperature may influence the association between ambient air pollution and AIx and AP, we additionally adjusted for room temperature. Beginning in July 2007, hourly PM10 concentrations were measured concurrently with PM2.5 using 2 beta attenuation monitors. Taking the difference between PM10 and PM2.5 concentrations, we estimated the coarse fraction (PM2.5-10) concentrations, and evaluated whether 4-hour, 24-hour, and 3-, 7-, and 14-day moving average PM2.5-10 was associated with AIx and AP.
RESULTS
Characteristics of the participants at the baseline visit are presented in Table 1. The mean age of participants was 78.0 years, and the majority were either current or former smokers and diagnosed with hypertension. Of the 75 participants who had a repeat visit, we observed a strong correlation between the first and second measurements of AIx (intraclass correlation coefficient = 0.72); a moderate correlation was observed between first and second AP measurements (intraclass correlation coefficient = 0.65) (with 0–0.2, 0.3–0.4, 0.5–0.7, 0.7–0.8, and >0.8 indicating poor, fair, moderate, strong, and very strong correlations, respectively). A very strong correlation was observed between AIx and AP among all visits (Pearson correlation coefficient = 0.84).
Table 1.
Baseline Characteristics of Study Participants (n = 370) in the Veterans Affairs Normative Aging Study, Boston, Massachusetts, July 2007–August 2011
| Characteristic | No. | % | Mean (SD) |
|---|---|---|---|
| AIx normalized at 75 bpm, % | 21.4 (7.4) | ||
| AP normalized at 75 bpm, mmHg | 10.5 (5.3) | ||
| Age, years | 78.0 (6.2) | ||
| Race | |||
| Black | 10 | 2.7 | |
| White | 360 | 97.3 | |
| Body mass indexa | 27.8 (4.1) | ||
| High-density lipoprotein, mg/dL | 48.6 (12.8) | ||
| Years of education | 15.1 (2.9) | ||
| Alcohol consumption, drinks/day | |||
| ≥2 | 63 | 17.0 | |
| <2 | 307 | 83.0 | |
| Smoking status | |||
| Former | 243 | 65.7 | |
| Current | 7 | 1.9 | |
| Never | 120 | 32.4 | |
| Cumulative smoking, pack-yearsb | 26.3 (21.2) | ||
| Diabetes mellitusc | 80 | 21.6 | |
| Hypertension | 293 | 79.2 | |
| Coronary heart disease | 142 | 38.4 | |
| Participated in follow-up visit | 75 | 20.3 |
Abbreviations: AIx, augmentation index; AP, augmentation pressure; SD, standard deviation.
a Weight (kg)/height (m)2.
b Among current and former smokers; median and interquartile range were 20.0 and 30.0, respectively.
c Diabetes mellitus was defined as having physician diagnosis of diabetes mellitus or fasting blood glucose level greater than 125 mg/dL.
Air pollution and meteorology distributions over the study period are reported in Table 2. The daily concentrations of PM2.5 during the study period were always below the US daily fine particle standard (35 μg/m3), ranging from 1.1 to 34.3 μg/m3. The yearly average PM2.5 concentrations steadily decreased from 10.8 μg/m3 (standard deviation, 5.8) in 2007 to 7.5 μg/m3 (standard deviation, 3.1) in 2011, and all yearly averages were below the US yearly fine particle standard, which was recently lowered to 12 μg/m3. A strong correlation was observed between PM2.5 and sulfate, and fair to moderate correlations were observed between PM2.5 and black carbon and nitrogen dioxide. PM2.5 was poorly correlated with PNC. Fair to moderate correlations were also observed between black carbon, nitrogen dioxide, and sulfate.
Table 2.
Summary Statistics and Pearson Correlation Coefficients of 24-Hour Mean Air Pollutant Concentrations and Meteorological Variables in the Veterans Affairs Normative Aging Study, Boston, Massachusetts, July 2007–August 2011
| Exposure | Summary Statistics |
r |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Mean (SD) | Median (IQR) | PM2.5 | Black Carbon | PNC | NO2 | O3 | SO42− | Temperature | Relative Humidity | |
| PM2.5, μg/m3 | 1,508 | 8.6 (4.7) | 7.3 (5.7, 10.2) | 1.00 | 0.65 | −0.06 | 0.37 | 0.24 | 0.79 | 0.29 | 0.16 |
| Black carbon, μg/m3 | 1,503 | 0.7 (0.3) | 0.6 (0.5, 0.8) | 1.00 | 0.20 | 0.65 | −0.20 | 0.48 | 0.13 | 0.28 | |
| PNC, counts/cm3 | 1,424 | 13,160 (5,577) | 13,233.4 (8,568, 16,401) | 1.00 | 0.48 | −0.45 | −0.26 | −0.66 | −0.18 | ||
| Nitrogen dioxide, ppm | 1,512 | 0.02 (0.01) | 0.02 (0.01, 0.02) | 1.00 | −0.37 | 0.23 | −0.36 | −0.04 | |||
| Ozone, ppm | 1,512 | 0.03 (0.01) | 0.03 (0.02, 0.03) | 1.00 | 0.34 | 0.42 | −0.19 | ||||
| Sulfate, μg/m3 | 1,100 | 2.3 (1.9) | 1.7 (1.0, 2.6) | 1.00 | 0.32 | 0.17 | |||||
| Temperature, °C | 1,511 | 13.3 (8.9) | 14.0 (6.0, 20.6) | 1.00 | 0.14 | ||||||
| Relative humidity, % | 1,511 | 66.8 (15.0) | 65.9 (55.3, 78.5) | 1.00 | |||||||
Abbreviations: IQR, interquartile range; PM2.5, particles with aerodynamic diameter <2.5 μm; PNC, particle number count; SD, standard deviation.
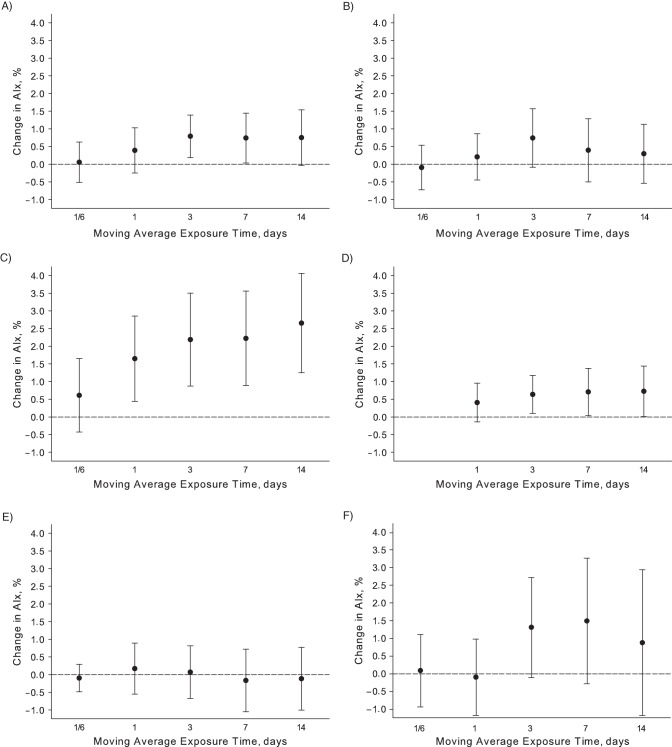
Statistically significant (P < 0.05) positive associations were observed between exposure to PM2.5, PNCs, and sulfate, and AIx (Figure 1) for several moving averages, beginning at 24 hours for PNCs and 3 days for PM2.5 and sulfate. An interquartile range increase in 3-day average pollutant exposure was associated with a 0.8% higher AIx (95% confidence interval (CI): 0.2, 1.4) for a 3.6-μg/m3 increase in PM2.5, and a 0.6% (95% CI: 0.1, 1.2) higher AIx for a 1.4-μg/m3 increase in sulfate. The observed associations for PM2.5 and sulfate exposures averaged over 7 and 14 days were of similar magnitudes. Among the air pollutants examined in this analysis, the largest changes in AIx were observed in association with PNC averaged over 24 hours and 3, 7, and 14 days; an interquartile range increase in 3-day average exposure of PNC was associated with a 2.2% (95% CI: 0.9, 3.5) higher AIx for a 7,874-counts/cm3 increase in PNC. Associations of marginal statistical significance (P < 0.10) were observed between AIx and 3-day moving average exposure of black carbon and ozone. Interquartile range increases of 0.3 μg/m3 and 0.013 ppm for 3-day moving averages of black carbon and ozone, respectively, were associated with 0.7% (95% CI: −0.1, 1.6) and 1.3% (95% CI: −0.1, 2.7) higher AIx. No associations were observed between nitrogen dioxide and AIx. Associations between air pollutants and AIx were not considerably different when stable inverse probability weights were not applied (Web Appendix, Web Table 1).
Figure 1.
Adjusted associations between augmentation index (AIx) and 1 interquartile range increase in 4 hours, 24 hours, and 3, 7, and 14 days moving average exposure of A) fine particulate matter ≤2.5 µm in aerodynamic diameter, B) black carbon, C) particle number counts, D) sulfate, E) nitrogen dioxide, and F) ozone in the Veterans Affairs Normative Aging Study, Boston, Massachusetts, 2007–2011.
Similar to the findings presented for AIx, statistically significant positive associations were observed between exposure to PM2.5, PNCs, and sulfate, and AP (Web Appendix, Web Figure 2) for several moving averages. An interquartile range increase in 3-day average pollutant exposure was associated with 0.6-mmHg (95% CI: 0.2, 1.0) and 1.2-mmHg (95% CI: 0.2, 2.0) increases in AP for 3.6-μg/m3 and 7,874-counts/cm3 increases in PM2.5 and PNC, respectively. An interquartile range increase in 14-day average exposures was associated with a 0.6-mmHg (95% CI: 0.6, 1.1) increase in AP for a 1.4-μg/m3 increase in sulfate.
The estimated associations of PM2.5, PNC, and sulfate with AIx and AP, as presented in Figure 1 and in Web Figure 2 of the Web Appendix, were not considerably different between those participants with and without hypertension, coronary heart disease, and diabetes, with no consistent pattern of effect modification (data not shown). Generally, the 95% confidence intervals of the estimated associations present or absent of the condition of interest widely overlapped each other.
Secondary analyses
After we restricted the longitudinal analysis to participants with follow-up visits, stronger positive associations for each air pollutant moving average and AIx were generally observed, except the 4-hour and 24-hour moving average exposures of ozone (Web Appendix, Web Table 2). Similar findings were observed for the associations between PNC and AIx and AP from the baseline cross-sectional analysis (Web Appendix, Web Table 3), but weak, nonsignificant associations were observed for PM2.5 and sulfate. Additional adjustment for room temperature resulted in very little change in the associations between air pollutant moving averages and AIx (Web Appendix, Web Table 4). Finally, no associations were observed between PM2.5-10 and AIx (Web Appendix, Web Figure 3).
DISCUSSION
Our results suggest that short-term changes in air pollution are associated with correlates of arterial stiffness, as characterized by AIx and AP. A 1.7% or higher AIx was observed after an interquartile range increase in 1-day and up to 14-day moving average of PNC. Smaller increments in AIx were observed in association with moving averages of PM2.5 and sulfate. Overall, the findings were similar for AP. The associations were strengthened after restricting the analysis to participants with repeat visits. When the analysis was restricted to baseline visits, associations of similar magnitude were observed for PNC, however, no associations were observed for PM2.5 and sulfate.
Whereas earlier studies of healthy participants exposed to diesel exhaust (20) and welders exposed to PM2.5 (21) reported increases in AIx after very short-term exposure, the associations between air pollutants and AIx in our study were observed at longer lags. In the former study, in which 12 participants were exposed to filtered air or to diesel exhaust (∼350 μg/m3) for 1 hour, the mean AIx was 7.8% higher comparing 10 minutes postexposure (0.8%, 95% CI: −5.5, 7.1) to filtered air (−7.0%, 95% CI: −11.5, −2.5). In the latter study, exposure to welding fumes during 6 hours of welding was associated with a 2.8% (95% CI: −1.4, 7.0) higher AIx immediately postexposure compared with preexposure and during exposure; a smaller increment of 1.0% (95% CI: −0.3, 2.4) in AIx was observed for a 2.6-μg/m3 interquartile range increase in PM2.5.
A previous large cross-sectional community-based study in Greece consisting of 1,222 participants observed a significant association between PM10 concentration and higher AP (2.0 mmHg (95% CI: 0.56, 3.39) per 43.4 μg/m3), but did not observe an association between AIx and PM10 (11). However, the authors of that study were not able to investigate PM2.5 or its components, which may also be relevant in examination of AIx, because these pollutants have been shown to be associated with decreased brachial artery diameter and decreased brachial artery flow–mediated dilation in healthy and diabetic adults (2–6).
Investigators from the Multi-Ethnic Study of Atherosclerosis did not observe any association between long-term exposure to PM10 or PM2.5 and measures of arterial elasticity and compliance (31). The Atherosclerosis Risk in Young Adults Study, based in the Netherlands, reported that a 25-μg/m3 increase in long-term exposure to nitrogen dioxide was associated with 4.1% and 37.6% higher carotid-femoral pulse wave velocity and AIx, respectively (32). Future studies should evaluate and compare the impact of long- and short-term exposure to air pollutants on changes in augmentation index.
Although similar findings were observed for PNC in the baseline cross-sectional and repeated analyses, weak, nonsignificant associations were observed for PM2.5 and sulfate in the baseline cross-sectional analysis. The discrepancy of the estimated associations between the models suggests that the within-individual variation of AIx and AP may be considerably greater than the between-individual variation. Another possible explanation is that loss to follow-up prior to the collection of PWA measurements may have led to a healthier selection of participants resulting in a downward bias for the associations between air pollutants and AIx and AP toward the null.
The biological mechanisms for the observed associations between air pollution and AIx and AP are not fully understood. Impaired endothelial function, characterized as reduced brachial artery flow–mediated dilation, has been shown to be associated with measures of arterial stiffness including pulse wave velocity and AIx (33, 34). Short-term exposure to ambient particulate matter has also been shown to be associated with reduced brachial artery flow–mediated dilation (3, 4, 6). Therefore, it may be hypothesized that the observed associations between short-term changes in air pollution and AIx and AP are partially explained by endothelial dysfunction. Additionally, AIx is measure of wave reflection and considered a negative effect of arterial stiffening rather than a direct measure of arterial stiffness (e.g., carotid-femoral pulse wave velocity) (35). In future studies, it will be important to evaluate more direct measures of arterial stiffness in association with these exposures to confirm or refute our findings. AIx is associated with cardiovascular events and mortality, and a meta-analysis has shown that a 10% increase in AIx corresponds with risk increases of 31.8% and 34.8% for cardiovascular events and total mortality, respectively (11). The changes in AIx observed in this analysis were much smaller and likely do not correspond with cardiovascular health risk of similar magnitude, particularly in healthy participants.
This study has a number of strengths, including prospective design, methods to address selection bias, and adjustment for multiple confounders, but there are several limitations that should be considered. First, AIx and AP are noninvasive indirect correlates of aortic arterial stiffness, for which a validated transfer function was applied to transform radial pressure waveforms into aortic pressure waveforms (16). Application of the transfer function may result in measurement error of the true measurement of aortic arterial stiffness, but measurement errors of AIx and AP are likely independent of air pollutant exposure measurements assigned to participants in this study. We hypothesize that any measurement errors of AIx and AP would result in a downward bias and underestimation of the true associations between air pollution and AIx and AP.
Exposure measurement error is also a potential source of bias because we relied on a central monitoring site for measurement of ambient particulate concentrations, which may not be representative of where the participants resided. An exposure validation study of 25 individuals living in Boston, Massachusetts, (36) compared home indoor and outdoor concentrations of PM2.5 and sulfate with ambient concentrations as measured from the central monitoring site used in the present study and observed that ambient PM2.5 and sulfate were both significantly associated with home indoor and outdoor concentrations. Measurement error from using a single site in this study will result in primarily Berkson-type measurement error (37); therefore, the standard errors but not the estimated associations may be biased. Lastly, the study population consists of predominantly elderly white men, so our results may not be generalizable to the general population if the associations between air pollutants and AIx are modified by sex, age, and race and or ethnicity, factors that have shown to be associated with AIx (13–15).
In conclusion, short-term exposures to air pollution were associated with correlates of arterial stiffness in this study sample of predominantly elderly white men. The findings support the hypothesis for the role of vascular function in the cardiovascular health effects associated with air pollution.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Exposure, Epidemiology, and Risk Program, Department of Environmental Health, Harvard School of Public Health, Boston, Massachusetts (Amar J. Mehta, Antonella Zanobetti, Petros Koutrakis, Joel Schwartz); Cardiovascular Epidemiology Research Unit, Beth Israel Deaconess Medical Center, Boston, Massachusetts (Murray A. Mittleman); Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Murray A. Mittleman); Department of Medicine, Harvard Medical School, Boston, Massachusetts (Murray A. Mittleman); Normative Aging Study, Veterans Affairs Boston Healthcare System, Boston, Massachusetts (David Sparrow, Pantel Vokonas); Channing Laboratory, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts (David Sparrow); and Department of Medicine, Boston University School of Medicine, Boston, Massachusetts (David Sparrow, Pantel Vokonas).
This work was supported by the National Institute of Environmental Health Sciences (grants ES014663-01A2 and P01 ES09825) and the US Environmental Protection Agency (grants R832416, R827353, and R83241601). The Normative Aging Study is supported by the Cooperative Studies Program/Epidemiology Research and Information Center of the US Department of Veterans Affairs and is a component of the Massachusetts Veterans Epidemiology Research and Information Center, Boston, Massachusetts. Support was also provided by the National Institutes of Health (grants R01-AG002287, R01-AG018436, and R29-AG007465). This research was also supported by a Veterans Affairs Research Career Scientist award to D.S.
This article's contents are solely the responsibility of the grantee and do not necessarily represent the official views of the US Environmental Protection Agency. Further, the US Environmental Protection Agency does not endorse the purchase of any of the commercial products or services mentioned in the article.
Conflict of interest: none declared.
REFERENCES
- 1.Brook RD, Rajagopalan S, Pope CA, 3rd, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 2.Brook RD, Brook JR, Urch B, et al. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002;105(13):1534–1536. doi: 10.1161/01.cir.0000013838.94747.64. [DOI] [PubMed] [Google Scholar]
- 3.O'Neill MS, Veves A, Zanobetti A, et al. Diabetes enhances vulnerability to particulate air pollution–associated impairment in vascular reactivity and endothelial function. Circulation. 2005;111(22):2913–2920. doi: 10.1161/CIRCULATIONAHA.104.517110. [DOI] [PubMed] [Google Scholar]
- 4.Schneider A, Neas L, Herbst MC, et al. Endothelial dysfunction: associations with exposure to ambient fine particles in diabetic individuals. Environ Health Perspect. 2008;116(12):1666–1674. doi: 10.1289/ehp.11666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rundell KW, Hoffman JR, Caviston R, et al. Inhalation of ultrafine and fine particulate matter disrupts systemic vascular function. Inhal Toxicol. 2007;19(2):133–140. doi: 10.1080/08958370601051727. [DOI] [PubMed] [Google Scholar]
- 6.Dales R, Liu L, Szyszkowicz M, et al. Particulate air pollution and vascular reactivity: the Bus Stop Study. Int Arch Occup Environ Health. 2007;81(2):159–164. doi: 10.1007/s00420-007-0199-7. [DOI] [PubMed] [Google Scholar]
- 7.Auchincloss AH, Diez Roux AV, Dvonch JT, et al. Associations between recent exposure to ambient fine particulate matter and blood pressure in the Multiethnic Study of Atherosclerosis (MESA) Environ Health Perspect. 2008;116(4):486–491. doi: 10.1289/ehp.10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann B, Luttmann-Gibson H, Cohen A, et al. Opposing effects of particle pollution, ozone, and ambient temperature on arterial blood pressure. Environ Health Perspect. 2012;120(2):241–246. doi: 10.1289/ehp.1103647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30(3):445–448. doi: 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
- 10.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37(5):1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 11.Vlachopoulos C, Aznaouridis K, O'Rourke MF, et al. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31(15):1865–1871. doi: 10.1093/eurheartj/ehq024. [DOI] [PubMed] [Google Scholar]
- 12.Weber T, Auer J, O'Rourke MF, et al. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004;109(2):184–189. doi: 10.1161/01.CIR.0000105767.94169.E3. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell GF, Parise H, Benjamin EJ, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43(6):1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 14.Segers P, Rietzschel ER, De Buyzere ML, et al. Noninvasive (input) impedance, pulse wave velocity, and wave reflection in healthy middle-aged men and women. Hypertension. 2007;49(6):1248–1255. doi: 10.1161/HYPERTENSIONAHA.106.085480. [DOI] [PubMed] [Google Scholar]
- 15.Chirinos JA, Kips JG, Roman MJ, et al. Ethnic differences in arterial wave reflections and normative equations for augmentation index. Hypertension. 2011;57(6):1108–1116. doi: 10.1161/HYPERTENSIONAHA.110.166348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chirinos JA, Rietzschel ER, De Buyzere ML, et al. Arterial load and ventricular-arterial coupling: physiologic relations with body size and effect of obesity. Hypertension. 2009;54(3):558–566. doi: 10.1161/HYPERTENSIONAHA.109.131870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahmud A, Feely J. Effect of smoking on arterial stiffness and pulse pressure amplification. Hypertension. 2003;41(1):183–187. doi: 10.1161/01.hyp.0000047464.66901.60. [DOI] [PubMed] [Google Scholar]
- 18.Mahmud A, Feely J. Effects of passive smoking on blood pressure and aortic pressure waveform in healthy young adults—influence of gender. Br J Clin Pharmacol. 2004;57(1):37–43. doi: 10.1046/j.1365-2125.2003.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Trijp MJ, Beulens JW, Bos WJ, et al. Alcohol consumption and augmentation index in healthy young men: the ARYA Study. Am J Hypertens. 2005;18(6):792–796. doi: 10.1016/j.amjhyper.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Lundbäck M, Mills NL, Lucking A, et al. Experimental exposure to diesel exhaust increases arterial stiffness in man. Part Fibre Toxicol. 2009;6:7. doi: 10.1186/1743-8977-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang SC, Eisen EA, Cavallari JM, et al. Acute changes in vascular function among welders exposed to metal-rich particulate matter. Epidemiology. 2008;19(2):217–225. doi: 10.1097/EDE.0b013e31816334dc. [DOI] [PubMed] [Google Scholar]
- 22.Adamopoulos D, Vyssoulis G, Karpanou E, et al. Environmental determinants of blood pressure, arterial stiffness, and central hemodynamics. J Hypertens. 2010;28(5):903–909. doi: 10.1097/hjh.0b013e3283369f67. [DOI] [PubMed] [Google Scholar]
- 23.Bell B, Rose C, Damon A. The Normative Aging Study: an interdisciplinary and longitudinal study of health and aging. Int J Aging Hum Dev. 1972;3(1):5–17. [Google Scholar]
- 24.Thurston GD, Spengler JD. A quantitative assessment of source contributions to inhalable particulate matter pollution in metropolitan Boston. Atmos Environ. 1985;19(1):9–25. [Google Scholar]
- 25.Morawska L, Zhang JJ. Combustion sources of particles. 1. Health relevance and source signatures. Chemosphere. 2002;49(9):1045–1058. doi: 10.1016/s0045-6535(02)00241-2. [DOI] [PubMed] [Google Scholar]
- 26.Dockery DW, Luttmann-Gibson H, Rich DQ, et al. Particulate air pollution and nonfatal cardiac events. Part II. Association of air pollution with confirmed arrhythmias recorded by implanted defibrillators. Res Rep Health Eff Inst. 2005;124:83–126. [PubMed] [Google Scholar]
- 27.Chen CH, Nevo E, Fetics B, et al. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation. 1997;95(7):1827–1836. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson IB, Mohammad NH, Tyrrell S, et al. Heart rate dependency of pulse pressure amplification and arterial stiffness. Am J Hypertens. 2002;15(1 Pt 1):24–30. doi: 10.1016/s0895-7061(01)02252-x. [DOI] [PubMed] [Google Scholar]
- 29.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken, NJ: Wiley-Interscience; 2004. [Google Scholar]
- 30.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Neill MS, Diez-Roux AV, Auchincloss AH, et al. Long-term exposure to airborne particles and arterial stiffness: the Multi-Ethnic Study of Atherosclerosis (MESA) Environ Health Perspect. 2011;119(6):844–851. doi: 10.1289/ehp.0901524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lenters V, Uiterwaal CS, Beelen R, et al. Long-term exposure to air pollution and vascular damage in young adults. Epidemiology. 2010;21(4):512–520. doi: 10.1097/EDE.0b013e3181dec3a7. [DOI] [PubMed] [Google Scholar]
- 33.McEniery CM, Wallace S, Mackenzie IS, et al. Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension. 2006;48(4):602–608. doi: 10.1161/01.HYP.0000239206.64270.5f. [DOI] [PubMed] [Google Scholar]
- 34.Soga J, Nakamura S, Nishioka K, et al. Relationship between augmentation index and flow-mediated vasodilation in the brachial artery. Hypertens Res. 2008;31(7):1293–1298. doi: 10.1291/hypres.31.1293. [DOI] [PubMed] [Google Scholar]
- 35.O'Rourke MF, Adji A. An updated clinical primer on large artery mechanics: implications of pulse waveform analysis and arterial tonometry. Curr Opin Cardiol. 2005;20(4):275–281. doi: 10.1097/01.hco.0000166595.44711.6f. [DOI] [PubMed] [Google Scholar]
- 36.Brown KW, Sarnat JA, Suh HH, et al. Ambient site, home outdoor and home indoor particulate concentrations as proxies of personal exposures. J Environ Monit. 2008;10(9):1041–1051. doi: 10.1039/b805991h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeger SL, Thomas D, Dominici F, et al. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect. 2000;108(5):419–426. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.