Abstract
The pH on the frustule of individual cells of the marine centric diatoms Coscinodiscus granii and Coscinodiscus wailesii (Bacillariophyceae) was measured with pH microsensors in culture media with increasing pH values of 8.04, 8.14, and 8.22, respectively. In 85–96% of the C.granii cells the pH on the frustule was up to 0.4 units higher than that of the medium, reaching a maximum pH 8.95. Only in 2–3% the surface pH exceeded that of the medium by up to 0.7 pH units. These results strongly suggest that diatoms in batch cultures differ, at least temporarily, in their individual photosynthetic activities. Infection experiments with the parasitoid nanoflagellate Pirsonia diadema (Stramenopile) showed that flagellates failed to infect when the culture pH was 8.8 and above. pH measurements on freshly infected C. granii showed that the prevalence of infection was higher in tendency on diatoms with low surface pH. Application of these results to parasitoid-diatom interactions in natural waters suggests that within phytoplankton populations a strong photosynthetic activity might prevent diatom cells temporarily from infection by pH-sensitive parasitoids.
Introduction
Most photosynthetically active diatoms take up CO2 (carbon dioxide), whereas others can also take up HCO3− (bicarbonate) (Tortell et al. 1997; Matsuda et al. 2001). Photosynthetic removal of CO2 causes an increase of pH of the boundary layer of the medium. Thus, phytoplankton mass developments, either in culture or in the field, may cause a general increase of pH. In phytoplankton batch cultures, pH values of 9.5 and above, can be reached in the stationary phase (Goldman et al. 1982; Taguchi et al. 1987). Natural seawater has an average pH of approximately 8.0–8.2 but during phytoplankton blooms in the German Bight, North Sea, it can increase to pH 8.7 (Pegler and Kempe 1988).
It is generally assumed that all cells in unialgal diatom cultures are more or less identical in their physiological properties. This assumption may not necessarily hold true as was indicated by infection experiments in which the marine parasitoid nanoflagellate Pirsonia diadema Kühn (Stramenopile) infected, and eventually consumed, the large diatoms Coscinodiscus granii and Coscinodiscus wailesii (Bacillariophyceae). Pirsonia clearly showed interspecific selectivity between host species and intraspecific selectivity within unialgal host cultures (Kühn 1998). Laboratory experiments indicated that the photosynthetic activity of individual C. wailesii cells might affect their susceptibility to infection (Kühn 1998). While in cultures kept in light some cells seemed to be most attractive for further infections, about 1% of the diatoms remained uninfected for some time. In darkness, all cells were infected equally. It was suggested that photosynthesis enhances the formation of individual physiological properties of diatoms leading to intraspecific variability of susceptibility.
Pirsonia infecting Coscinodiscus gradually ingest diatom cell contents. A feeding flagellate will divide approximately 6 h after attachment for the first time, without having severely affected the internal structure of the diatom protoplast. Multiple infections, however, will kill a diatom within a few hours. If the photosynthetic activity of individual diatoms influences the infection behaviour of Pirsonia flagellates, the external pH on the frustule should be an indicator for their susceptibility to infections. Microsensors for pH and oxygen have successfully been used to determine variations of pH and oxygen saturation on the frustule and the plasma membrane of individual C. wailesii cells during light and dark shifts (Kühn and Raven 2008).
Generally, Pirsonia flagellates are attracted by intact C. wailesii (Kühn 1998) but rapidly avoid manually damaged crushed cells (unpublished). We assumed that this escape behaviour was caused by the acidic vacuolar sap. Therefore, it was also of interest to test if pH microsensors could be used to measure the acidity of the vacuole.
In the present study we investigated (a) the effect of pH values in Coscinodiscus cultures on the ability of P. diadema to successfully infect host cells, (b) the pH on the surface (frustule) of individual photosynthesising diatoms with the aid of pH microsensors, (c) potential effects of surface pH on the ability of the parasitoid to infect successfully and (d) the pH of the diatom vacuole.
Materials and methods
Cultures
Cultures of the marine diatom C. granii Gough and C. wailesii Gran and Angst were established from cells isolated off List/Sylt in the North Sea, German Bight. Cultures were maintained in modified F/2 (Guillard and Ryther 1962) at 15°C on a 16:8 L:D cycle. The salinity was adjusted to 30 PSU. Microsensor experiments were carried out at room temperature (21–22°C). Cell diameters were in the range of 300–400 μm. As in natural populations, diatom division was not synchronised. Pirsonia diadema was isolated in 1993 from the same site and cultivated according to Kühn et al. (1996). Infection experiments were carried out under non-turbulent conditions, i.e. culture vessels remained stationary and were only shaken cautiously before sampling. Previous experiments had shown that that infection dynamics differed between Coscinodiscus cultures maintained under turbulent conditions (rolling tanks) and those maintained under non-turbulent conditions (Kühn and Hoffmann 1999). It was necessary to count live samples to distinguish between infected and uninfected diatoms (preservation with Lugol’s solution led to the detachment of Pirsonia flagellates from the diatoms). This limited the feasible number of replicates.
pH microelectrodes
pH was measured with pH liquid ion exchange (LIX) microelectrodes (de Beer et al. 1997) in combination with a calomel reference electrode (Radiometer 401, Denmark), connected to a high-impedance mV meter (Mascom, Germany). The tip diameter of the microelectrodes was 6–10 μm. Electrodes were calibrated at room temperature in standard pH buffers (pH 7 and 9.26) (Mettler Toledo), and the signals were recorded on strip chart recorder (Görz, Germany). pH sensors responded within milliseconds.
Experimental set up for microsensor measurements
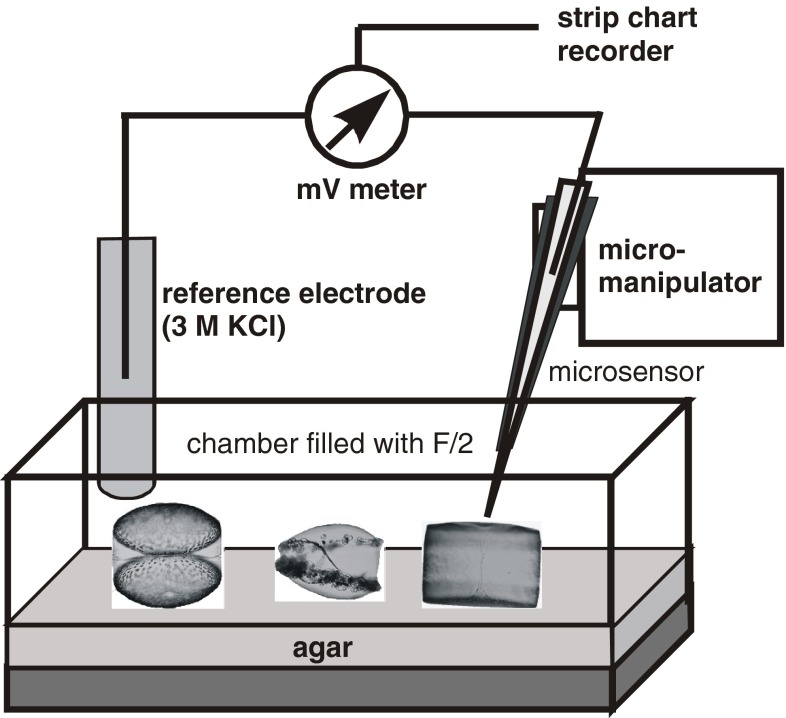
Experiments were carried out in a small Plexiglass chamber filled with F/2 or artificial seawater. Since Coscinodiscus spp. cells tended to sink down, the bottom of the chamber was covered with a ∼5-mm thick layer of agar (1% prepared from F/2 with pH according to the experiment). This was the best approximation to simulate a natural environment where cells are surrounded by seawater. The agar served at the same time as a protection against damage of the microsensor tip. Microsensors were positioned by a manually operated micromanipulator (Märtzhäuser, Germany) (Fig. 1). The position at the diatom surface was determined by observation of the microsensor under a dissection microscope. Inclination of microsensor was 10°–20° to diatom surface. Light source was a fiber optic halogen lamp (Schott KL-1500, Germany), and quantum irradiance (400–700 nm) was measured with a quantum scalar irradiance meter (Biospherical Instruments, QSL101, USA). Experiments were carried out with an irradiance of 160–170 μmol photons m−2 s−1.
Fig. 1.
Experimental set-up of microsensor experiments. Diatoms were placed in small chambers on agar. Photographs show a “double” cell of Coscinodiscus granii (left), a “single” C. granii cell infected by Pirsonia diadema (middle) and C. wailesii (right)
pH development in culture medium and Coscinodiscus wailesii cultures (Experiment 1)
For pH measurements in culture medium, 150 ml of F/2 were filled in 250 ml Erlenmeyer flasks and covered with aluminium foil. The starting volume of diatom batch cultures was 700 ml in 1000 ml Erlenmeyer flasks. In all flasks, the initial pH values were adjusted with 0.1–1 N HCl or NaOH to pH 6.8, 7.2, 7.6, 8.0, 8.7 and 9.5. The pH was determined daily the same time (±1 h). Ten ml were removed every day from the culture for cell counts.
Infection experiments with Pirsonia diadema and Coscinodiscus granii (Experiment 2)
Experiments were carried out in 78-ml Volume Disposable Tissue Culture Flasks with 35 ml C. granii culture (initial diatom density ∼28 cells ml−1), and the initial pH was adjusted to 8.0. On five successive days, diatom culture media were inoculated with one C. granii cell equally infected by P. diadema. Ten replicates were used for each day. In the following days, infected cultures were counted daily at the same time (±1 h), and the pH was measured. The numbers of infected and uninfected cells were determined directly in culture flasks using a self-constructed grid.
pH on diatom frustules (Experiment 3)
Measurements started 20–30 min after transferring diatoms into the experimental chamber to allow cells to adjust to their new environment. The pH of the F/2 medium varied between 7.98 and 8.29. For analysis, measurements in medium with pH 7.98–8.09, 8.10–8.19 and 8.20–8.29 were pooled together. Since the pH at the cell surfaces depended on the irradiance (manuscript in preparation) experiments were carried out at one light intensity only (160–170 μmol photons m−2 s−1).
pH on frustules of infected cells vs. non-infected cells (Experiment 4)
Coscinodiscus granii cultures were infected in Erlenmeyer flasks with varying inoculate of P. diadema flagellates. The pH of the medium was between 7.98 and 8.26. For analysis, measurements in medium with pH 7 98–8.09, 8.10–8.19 and 8.20–8.29 were pooled together.
Approximately 1 h after the attachment of a Pirsonia flagellate to Coscinodiscus the diatom showed first signs of infection, i.e. chloroplasts began to slowly accumulate around the infection site. The internal structure of the diatom remained intact for several hours if only one flagellate was feeding but was strongly affected within a short time (>2 h) when several flagellates were consuming cell contents. To ensure the firm attachment of flagellates to diatoms, infected cultures were transferred approximately 1 h after incubation into the experimental chamber. Surface pH was determined within 2–3 h after inoculating Coscinodiscus cultures with Pirsonia to avoid that photosynthetic activity was affected by progressive infection. It was only distinguished between the infected and uninfected diatoms and the number of attached flagellates was not considered.
Vacuolar pH in Coscinodiscus wailesii (Experiment 5)
Coscinodiscus wailesii cells had a diameter of ∼400 μm. For measurements, the microsensor was adjusted at the depression in the valve centre before the cells were rapidly impaled (when the microsensor was pressed down on the valve edge the cells often slipped away).
Results
Experiment 1: pH in F/2 culture medium and C. wailesii cultures
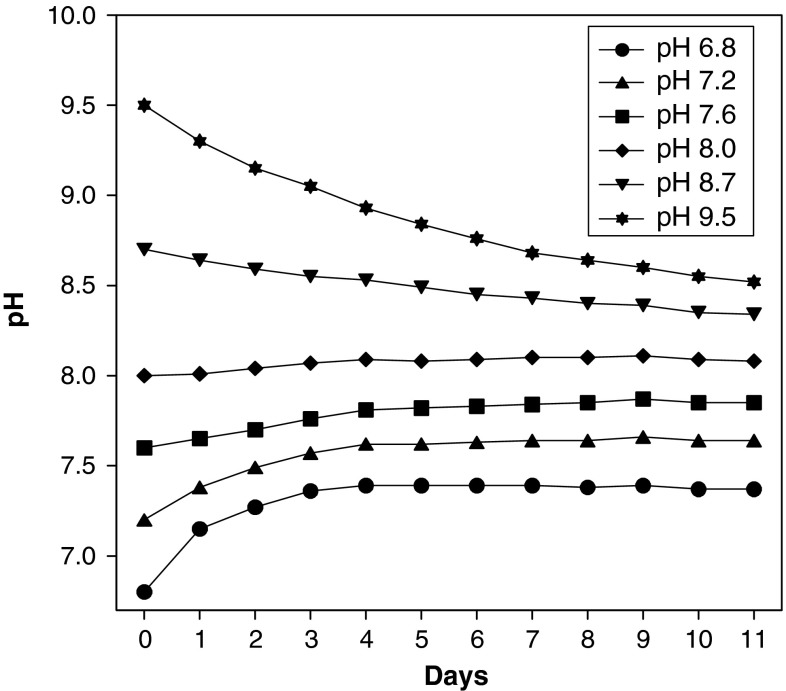
In culture medium (without diatoms) with an initial pH of 9.5 the pH decreased within 11 days more or less continuously by about one unit to pH 8.5, whereas in that with an initial pH of 6.8 the pH increased within the first 4 days to pH 7.4 and then remained constant at this value (Fig. 2).
Fig. 2.
Development of pH in seawater adjusted to initial pH values varying from 6.8 to 9.5
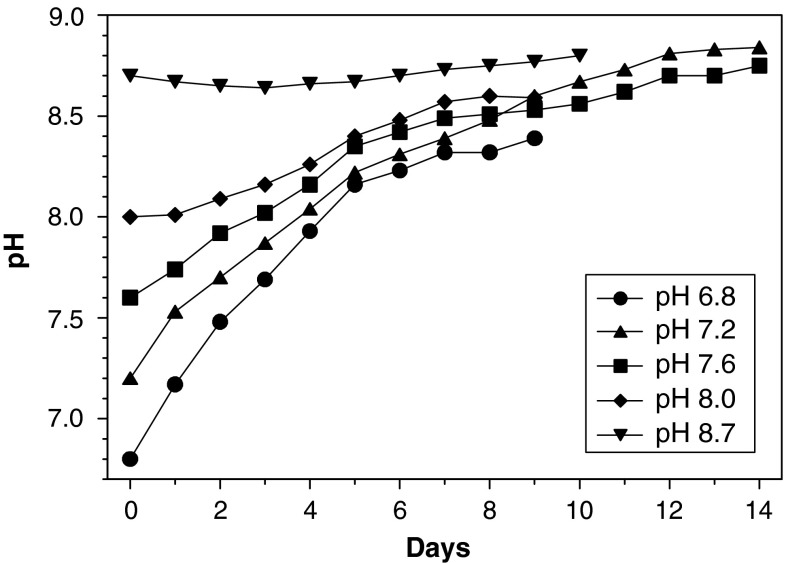
In C. wailesii cultures that had been adjusted to an initial pH of 9.5 all cells died within the first 2 days. When the initial pH was 8.7, the diatoms survived and the pH remained at ∼pH 8.7 until the culture was discontinued after 11 days (Fig. 3). In the other cultures starting from pH 8 and lower, the maximum value of pH 8.8 was reached after up to 14 days. In contrast, in similar experiments the maximum pH in cultures of the marine diatoms Thalassiosira punctigera was 9.1 (not shown).
Fig. 3.
Development of pH in Coscinodiscus wailesii cultures adjusted to initial pH values varying from 6.8 to 8.7
Experiment 2: Infection of C. granii by Pirsonia
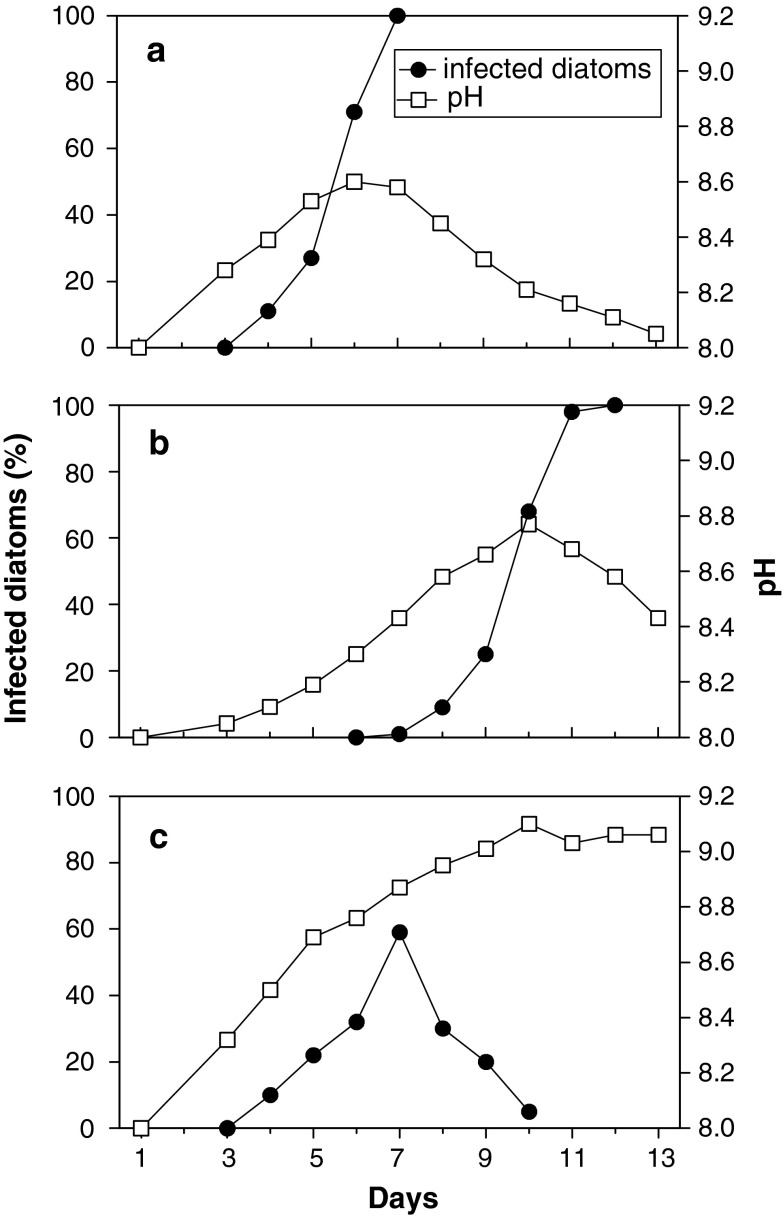
The infection dynamics depended on the pH in the experimental cultures at the time when the P. diadema was added. pH changes caused by infection were principally the same in all cultures but differed too much in their temporal development to be treated statistically. Therefore, three replicates (out of ten) are depicted to demonstrate the interaction of infection rates and pH changes. A C. granii culture with an initial pH 8.0 was inoculated on day three with P. diadema when the pH had risen to 8.3 (Fig. 4a). On day six, the pH had increased to 8.6, and 70% of the diatoms were infected. The following day, all diatoms were infected and the pH decreased again. The flagellates also successfully infected all remaining diatoms when the pH had risen to almost 8.8 (Fig. 4b).
Fig. 4.
Coscinodiscus granii batch cultures infected by Pirsonia diadema: development of pH (open square) and percentage of infected diatoms (filled circle) in single replica. Diatom cultures were inoculated with P. diadema on Day 3 (a, c) and Day 4 (b)
In comparison, at an ambient pH of 8.9, P. diadema could not infect anymore although 59% of the C. granii cells had already been infected before (Fig. 4c). In this case, the prevalence of infection decreased rapidly, whereas the pH in the diatom culture increased to a maximum pH of 9.1.
When P. diadema flagellates were added to C. granii and or C. wailesii cultures with a pH above 8.8 the flagellates did not infect the diatoms (n > 10; not shown). Obviously, a critical value between pH 8.8 and 8.9 existed, above which the flagellates were not infective any more.
Experiment 3: pH at diatom frustules
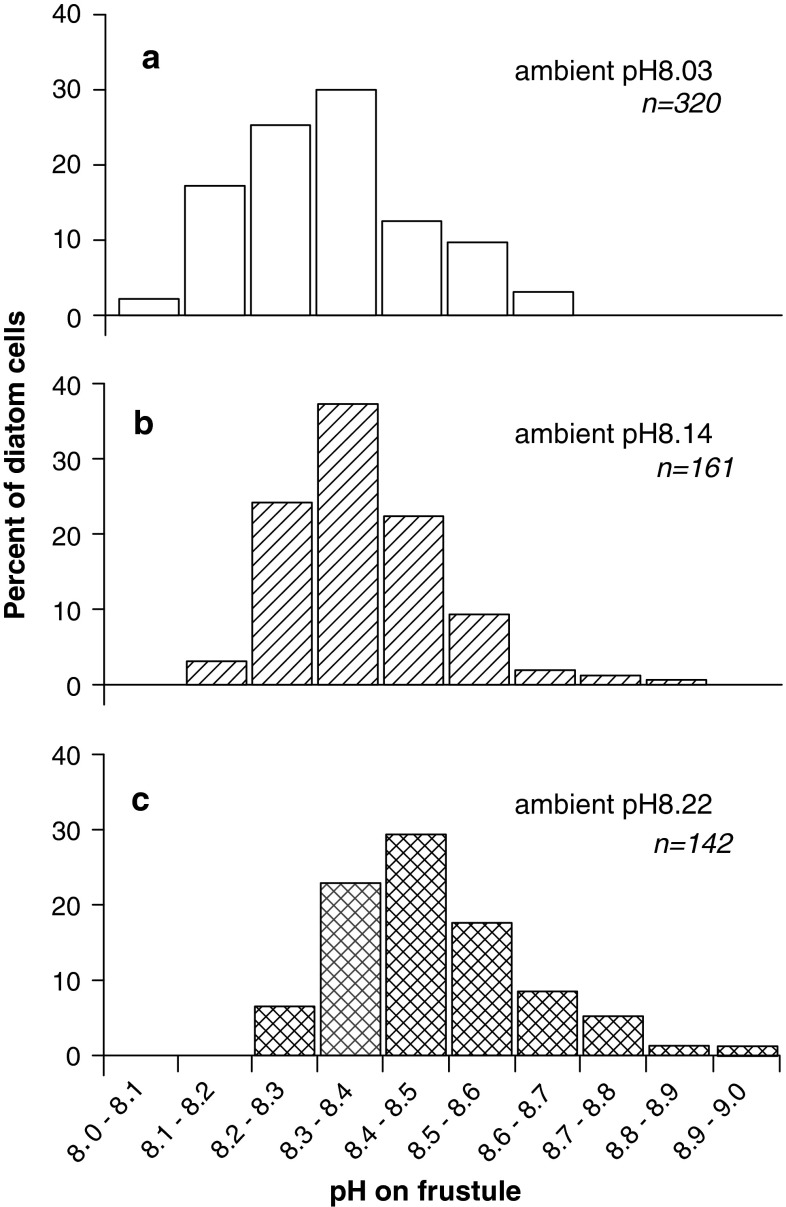
In C. granii cultures, the distribution of pH values on the frustule showed at average ambient pH values of 8.03, 8.14 and 8.22 a normal distribution.
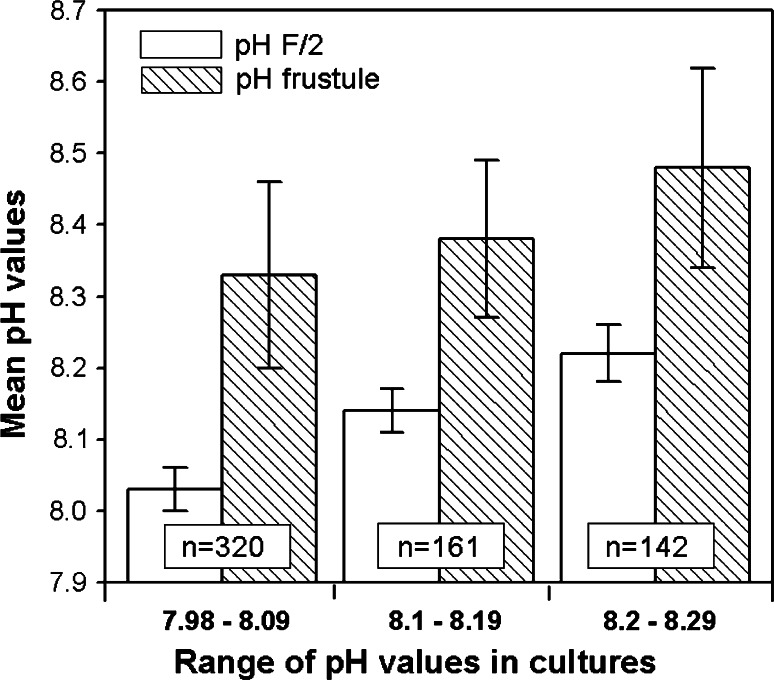
In 85–96% of the C. granii cells the pH on the frustule was up to 0.4 units higher than that of the ambient medium (Figs 5a–c). In 2–3% of the cells their pH values at the frustule exceeded that of the ambient F/2 by up to 0.7 pH units, and reached a maximum pH of 8.95. Although all diatoms appeared to be healthy, in 2–7% the surface pH was not higher than that of the culture medium. On average, the pH on the frustule was 0.24–0.3 pH units higher than in the culture medium (Fig. 6).
Fig. 5.
Surface pH of Coscinodiscus granii cells at different pH values of ambient seawater, measured with pH microsensors
Fig. 6.
The pH of the F/2 medium varied between 7.98 and 8.29. Measurements at the ranges pH 7.98–8.09, 8.10–8.19 and 8.20–8.29 were grouped together. Mean values (±SD) of ambient seawater (F/2) pH and corresponding pH on the frustule of Coscinodiscus granii are given
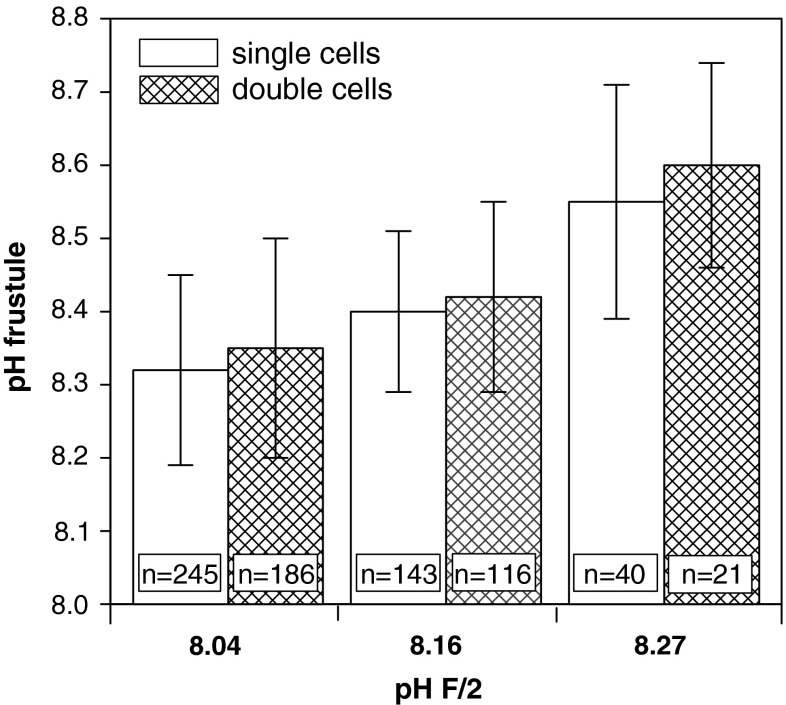
Dividing Coscinodiscus appear as “double” cells before the daughter cells eventually separate. The surface pH values of these not-yet-divided cells were slightly higher (but not significantly) than those of “single” cells by up to 0.05 units (Fig. 7).
Fig. 7.
pH on frustules of Coscinodiscus granii single cells and dividing (“double”) cells at different pH of F/2. The number of measurements (n) is given at the base of the bar
Experiment 4: pH on frustules of infected Coscinodiscus granii cells versus non-infected cells
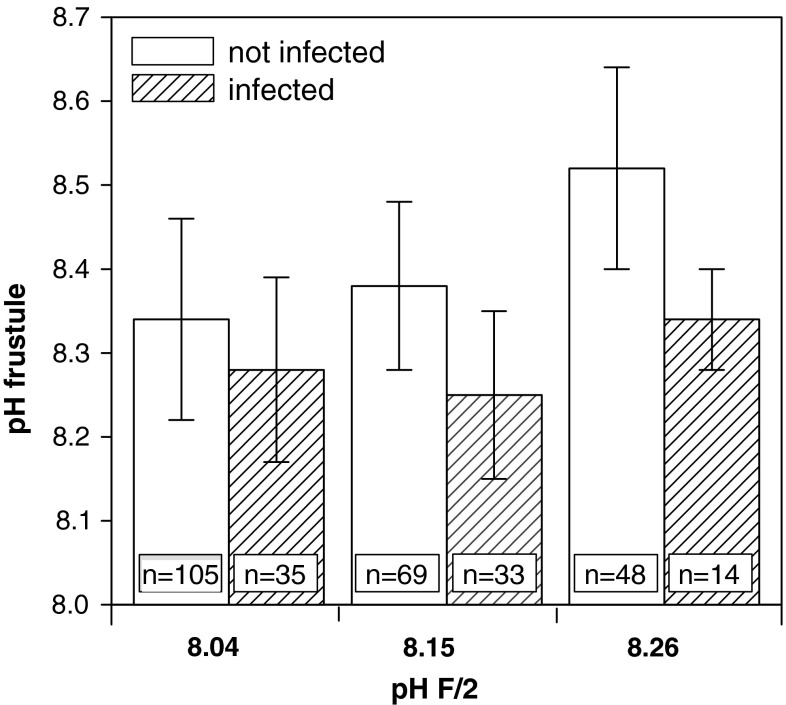
The pH of uninfected C. granii was 0.24–0.30 pH units higher than of the ambient seawater. The pH on the frustule of uninfected diatoms was always higher than that of infected cells (statistically not significant): the difference increased from 0.06 (F/2: pH 8.04) to 0.18 pH units (pH F/2: 8.26) (Fig. 8). The differences indicate a trend that with higher ambient pH Pirsonia preferred host diatoms with low surface pH. Advanced feeding led to progressive reduction of surface pH (not shown). As pH was measured shortly after successful infection, feeding flagellates had not yet severely affected the diatoms cytoplasmic structure or taken up extensive amounts of cell contents.
Fig. 8.
pH on frustule of infected and non-infected Coscinodiscus granii cells at different pH of F/2. The number of measurements (n) is given at the base of the bar
Experiment 5: pH measurements of vacuole sap
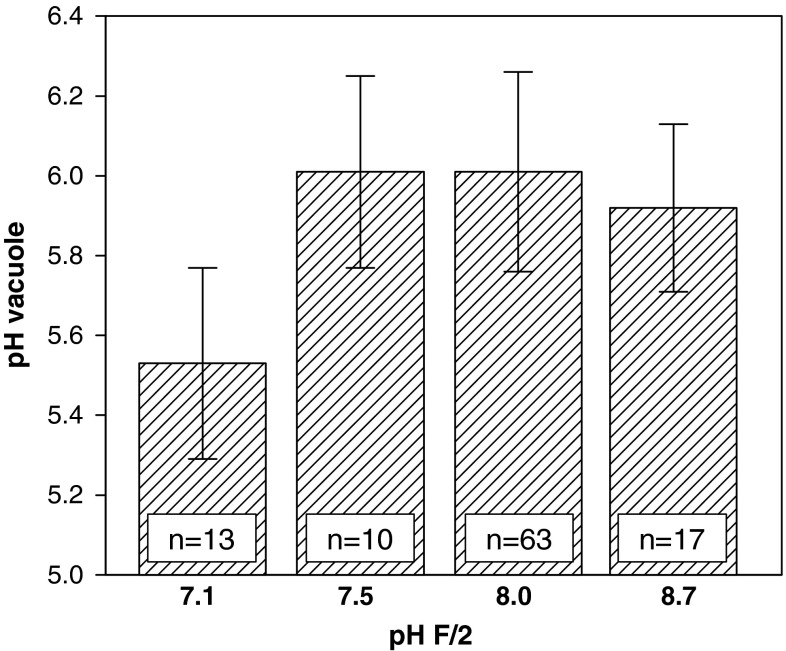
When the pH microsensor was forced into the vacuole of C. wailesii, generally the protoplast collapsed very rapidly. At an ambient F/2 pH between 7.5 and 8.7, the mean vacuolar pH was 5.9–6.0, and the lowest values between pH 5.44 and 5.69 (Fig. 9). Only at an average ambient pH of 7.1, the average vacuolar pH of 5.5 was lower (lowest value pH 5.24).
Fig. 9.
Microsensor measurements of vacuolar pH of Coscinodiscus wailesii at different ambient pH
Discussion
pH tolerance and dynamics
To investigate the response of microalgae upon changes in their physico-chemical environment most commonly batch cultures are used. It is assumed that cells growing in the same culture have more or less the same physiological properties. In batch cultures of C. wailesii, the pH increased over time and reached a maximum of approximately pH 8.8, whereas C. granii cultures tolerated a maximum pH of 9.1. When C. wailesii were transferred into culture medium with a slightly higher pH value of 9.5, all cells died within 1–2 days. This indicates that the maximum pH reached in a Coscinodiscus culture is the maximum pH tolerated by this species. In cultures of the antarctic diatom Chaetoceros gracile the maximum pH was 10.3 (Plettner, pers. com.) which corresponds to pH values as high as 9.9 in antarctic sea ice brine (Gleitz et al. 1995). This confirms the data compiled by Hansen (2002) that the maximum pH values tolerated by diatoms and other algal groups are species specific.
For P. diadema apparently a critical ∼pH 8.8 exists, above which the flagellates do not survive. Since the pH of natural seawater will not exceed this value, even during phytoplankton mass developments, this pH limitation will not affect the viability of P. diadema under natural conditions.
When in the experimental cultures all Coscinodiscus had been killed by Pirsonia and no photosynthetic activity remained, the pH of the medium decreased as expected. However, in seawater adjusted to an initial pH of 8.7, the pH decreased over 11 days slowly by 0.4 pH units, whereas in infected cultures the pH decreased more rapidly, i.e. 0.4 pH units within 4 days.
Vacuolar pH
Previous experiments had shown that P. diadema are chemoperceptive, i.e. flagellates attracted to host cells, were able to distinguish between host species, and preferred already infected diatoms over uninfected cells (Kühn 1997, 1998). A new observation was that flagellates rapidly swam away when C. wailesii were crushed manually. This strong negative chemosensory response indicated that P. diadema could also sense hydrogen ion concentrations. When a microsensor was pushed into the vacuole of C. wailesii this led to a mechanical destruction of the protoplast within a more or less undamaged frustule (e.g. Kühn and Brownlee 2005). Nonetheless, cytoplasm (including vacuole content) was rapidly mixed with culture medium. Since the pH of the culture medium was higher than the pH of the vacuole, the pH measured inside the frustule will tend to overestimate the vacuolar pH. At an ambient pH of 7.1, the lowest vacuolar pH measured was pH 5.2. In contrast, at an ambient pH 8.7, the lowest value for the vacuolar pH was 5.6. These results correspond with the results of Kesseler (1967), who used indicator paper and estimated the pH of C. wailesii cell sap to be around 5. This vacuolar pH of ∼5 is relatively high compared to e.g. pH <1 in the vacuole of the marine macroalga Desmarestia viridae (McClintock et al. 1982). pH microsensors cannot be used to determine the vacuole in algae with high turgor: when the microsensor was pressed into the vacuole of Chara sp. (Characeae), the pH sensitive LIX-matrix at the microsensor tip was very rapidly pushed back into the shaft by the cell turgor (not shown). However, this has never occurred in Coscinodiscus, which confirms observations by Kühn and Brownlee (2005) that the turgor of Coscinodiscus is low.
Effect of pH on infection
Since P. diadema did not survive pH above 8.8, it appears reasonable that flagellates should respond with a negative chemokinetic response to high pH. The pH microsensor experiments showed that at natural pH conditions of pH 8.0–8.3, the pH at the frustule of most Coscinodiscus cells was approximately up to 0.4 units higher than the ambient seawater. Only in 2–3% of the C. granii cells the pH on the frustule exceeded that of the seawater by up to 0.7 pH units, and reached maximum values of pH 8.95. If P. diadema actively avoids high pH, obviously these individual diatoms should not get infected. Previous experiments had shown that about 1% of C. granii cells remained uninfected by P. diadema for some time (Kühn 1998), which corresponds with the results of the present study. Apparently, very high pH values on the frustule deter Pirsonia from infecting. Our experiments also showed that flagellates preferred diatoms with low surface pH. Interestingly, the pH difference between infected and uninfected cells increased with the pH increase of seawater. This supports the hypothesis that P. diadema selects host diatoms according to their photosynthetic activities. During phytoplankton blooms the pH of natural seawater can increase to pH 8.7. In this case, the pH on the frustule of Coscinodiscus easily may reach pH 8.9 (we measured maximum values of pH 9.5; publication in preparation). These cells will, at least temporarily, be avoided by Pirsonia.
Richardson and Stolzenbach (1995) showed by means of a chemical reaction (extracellular reaction of oxidised manganese with the dye leukoberbelin blue) that individual phytoplankton cells changed the pH of their microenvironment by photosynthesis. They also found out that the size of the phycosphere depended on the cell size. A pH increase up to 8.6 has been found in the microenvironment of foraminifera due to the photosynthetic activity of their symbiotic diatoms (Köhler-Rink and Kühl 2000).
It is general knowledge that phytoplankton cells release amino acids and carbohydrates and thus produce extracellular microenvironments or phycospheres (Hellebust 1974; Fogg 1983). Coscinodiscus has been reported to contain high internal concentrations of amino acids, which are released as well as carbohydrates (Admiraal 1984; Martin-Jézéquel et al. 1988; Malej and Harris 1993). If the exudation of individual Coscinodiscus cells depends on their photosynthetic activity, a high surface pH on cells could correlate with an increased excretion of organic substances noxious to P. diadema.
Concept of individuality of diatom cells
It is generally assumed that diatom populations are a homogenous assemblage of identical cells. Laboratory experiments on the intraspecific selectivity of the parasitoid P. diadema, however, indicated that host diatoms temporarily differed in their individual physiological properties and hence the susceptibility to infections (Kühn 1997, 1998). Coates and Park (2002) reported that, for reasons unknown, some specimens of the mixotrophic dinoflagellate host Karlodinium micrum were apparently resistant to infection by the parasitic dinoflagellate Amoebophrya. In the field, encounters between parasitoids and potential host are always encounters between individual organisms, and the current state of one partner may decide about the survival of the other. For example, fully fed or starved parasitoids may not infect diatoms. The “fitness” of individual diatom cells, however, is more difficult to assess.
In natural populations (and most laboratory cultures) diatom growth is not synchronous so that most cells are at different stages of the cell cycle. So far, there is no indication on how the life cycle may affect the physiological properties of individual cells. Chisholm et al. (1980) reported a large non-genetic variability in generation times in clonal diatom populations and concluded “that population growth rates do not reflect the experience of the individual cells in the population”. The perception of “individualism” among unialgal cultures was supported by the observation of Du Preetz and Bate (1992) that some individual cells of the diatom Anaulus discus were capable of surviving prolonged darkness, whereas other cells died. Based on differences in the integrity of the plasma membrane Veldhuis et al. (2001) demonstrated a considerable intraspecific variation in cell viability in diatom populations. Differential sinking of cells in diatom cultures was reported by Brzezinski and Nelson (1988).
Here we have demonstrated that the pH on the frustule of individual C. granii cells differed by up to 0.7 units (mean ambient pH 8.0–8.2), which strongly indicates variable photosynthetic activities. Maximum surface pH values were found in 1–3% of the diatom cells which is concordant with the percentage of cells reported to be temporarily resistant to infections (Kühn 1998). Photosynthetic oscillations were observed in less than 1% of the C. wailesii cells examined (Kühn and Raven 2008). It was suggested that in diatom populations the photosynthetic, physiological and metabolic properties of individual cells differed, at least temporarily.
Even subtle differences between individual phytoplankton cells could determine whether they become prey to heterotrophic organisms or not. Discriminant chemoperceptive feeding behaviour in several protozoans was reviewed by Verity (1991). Jacobson and Anderson (1996) stated that, generally, feeding behaviour studies of heterotrophic organisms, such as dinoflagellates, tend to neglect the physiological condition of the food organism.
Conclusions
This study showed for the first time that unicellular diatoms in a batch culture differ in their individual photosynthetic activities, as indicated by individual pH values on their surfaces in the light. Monitoring the pH on the frustule of individual diatoms for several days could reveal if photosynthetic activities depend on different stages in their cell division cycles. Apparently, increased pH values on Coscinodiscus frustules prevent the cells to be infected by Pirsonia. In regard to their attractiveness for parasitoids or predators small differences in the competitive fitness could determine whether individual microalgae survive or not. It remains to be investigated if cells of chain-forming diatoms, which divide synchronously, are homogenous in their physiological properties.
References
- Admiraal W. The ecology of estuarine sediment-inhabiting diatoms. Prog Phycol Res. 1984;3:269–322. [Google Scholar]
- Brzezinski MA, Nelson DM. Differential cell sinking as a factor influencing diatom species competition for limiting nutrients. J Exp Mar Biol Ecol. 1988;119:179–200. doi: 10.1016/0022-0981(88)90192-X. [DOI] [Google Scholar]
- Chisholm SW, Morel FMM, Slocum WS. Phasing and distribution of cell division cycles in marine diatoms. In: Falkowsky PG, editor. Primary productivity of the sea. New York: Plenum Publishing Corp; 1980. pp. 281–299. [Google Scholar]
- Coats DW, Park MG. Parasitism of photosynthetic dinoflagellates by three strains of Amoebophrya (Dinophyta): parasite survival, infectivity, generation time, and host specificity. J Phycol. 2002;38:520–528. doi: 10.1046/j.1529-8817.2002.t01-1-01200.x. [DOI] [Google Scholar]
- de Beer D, Glud A, Epping E, Kühl M. A fast responding CO2 microelectrode for profiling in sediments, microbial mats and biofilms. Limnol Oceanogr. 1997;42:1590–1600. [Google Scholar]
- Du Preez DR, Bate BC. Dark survival of the surf diatom Anaulus australis Drebes et Schulz. Bot Mar. 1992;35:315–319. [Google Scholar]
- Fogg GE. The ecological significance of extracellular products of phytoplankton photosynthesis. Bot Mar. 1983;26:3–14. [Google Scholar]
- Gleitz M, van der Loeff MR, Thomas DN, Dieckmann GS, Millero FJ. Comparison of summer and winter inorganic carbon, oxygen and nutrient concentrations in Antarctic sea ice brine. Mar Chem. 1995;1284:81–91. doi: 10.1016/0304-4203(95)00053-T. [DOI] [Google Scholar]
- Goldman JC, Azov Y, Riley CB, Dennett MR. The effect of pH in intensive microalgal cultures. I. Biomass regulation. J Exp Mar Biol Ecol. 1982;57:1–3. doi: 10.1016/0022-0981(82)90140-X. [DOI] [Google Scholar]
- Guillard RRL, Ryther JH. Studies of marine planktonic diatoms. Can J Microbiol. 1962;8:229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- Hansen PJ. Effect of high pH on the growth and survival of marine phytoplankton: implications for species succession. Aquat Microbial Ecol. 2002;28:279–288. doi: 10.3354/ame028279. [DOI] [Google Scholar]
- Hellebust JA. Extracellular products. In: Steward ND, editor. Algal physiology and biochemistry. Berkeley: University of California Press; 1974. pp. 838–863. [Google Scholar]
- Jacobson DM, Anderson DM. Widespread phagocytosis of ciliates and other protists by marine mixotrophic and heterotrophic thecate dinoflagellates. J Phycol. 1996;32:279–285. doi: 10.1111/j.0022-3646.1996.00279.x. [DOI] [Google Scholar]
- Kesseler H. Untersuchungen über die chemische Zusammensetzung des Zellsaftes der Diatomee Coscinodiscuswailesii (Bacillariophyceae, Centrales) Helgoländer wiss Meeresunters. 1967;16:262–270. doi: 10.1007/BF01611710. [DOI] [Google Scholar]
- Köhler-Rink S, Kühl M. Microsensor studies of photosynthesis and respiration in larger foraminifera. I. The physico-chemical microenvironment of Marginopora vertebralis, Amphistegina lobifera, and Amphisorus hemprichii. Mar Biol. 2000;137:473–486. doi: 10.1007/s002270000335. [DOI] [Google Scholar]
- Kühn SF. Infection of Coscinodiscus spp. by the parasitoid nanoflagellate Pirsonia diadema: I. Behavioural studies on the infection process. J Plankton Res. 1997;19:791–804. doi: 10.1093/plankt/19.7.791. [DOI] [Google Scholar]
- Kühn SF. Infection of Coscinodiscus spp. by the parasitoid nanoflagellate Pirsonia diadema: II. Selective infection behaviour for host species and individual host cells. J Plankton Res. 1998;20:443–454. doi: 10.1093/plankt/20.3.443. [DOI] [Google Scholar]
- Kühn SF, Hofmann M. Infection of Coscinodiscus spp. by the parasitoid nanoflagellate Pirsonia diadema: III. Effects of turbulence on the incidence of infection. J Plankton Res. 1999;21:2323–2340. doi: 10.1093/plankt/21.12.2323. [DOI] [Google Scholar]
- Kühn SF, Brownlee C. Membrane organisation and dynamics in the marine diatom Coscinodiscus wailesii (Bacillariophyceae) Bot Mar. 2005;48:297–305. doi: 10.1515/BOT.2005.039. [DOI] [Google Scholar]
- Kühn SF, Raven JA. Photosynthetic oscillation in individual cells of the marine diatom Coscinodiscus wailesii (Bacillariophyceae) revealed by microsensor measurements. Photosynth Res. 2008;95:37–44. doi: 10.1007/s11120-007-9221-x. [DOI] [PubMed] [Google Scholar]
- Kühn SF, Drebes G, Schnepf E. Five new species of the nanoflagellate Pirsonia in the German Bight, North Sea, feeding on planktic diatoms. Helgoländer Meeresunters. 1996;50:205–222. doi: 10.1007/BF02367152. [DOI] [Google Scholar]
- Malej A, Harris RP. Inhibition of copepod grazing by diatom exudates: a factor in the development of mucus aggregates? Mar Ecol Prog Ser. 1993;96:33–42. doi: 10.3354/meps096033. [DOI] [Google Scholar]
- Martin-Jézéquel V, Poulet SA, Harris RP, Moal J, Samain JF. Interspecific and intraspecific composition and variation of free amino acids in marine phytoplankton. Mar Ecol Prog Ser. 1988;44:303–313. doi: 10.3354/meps044303. [DOI] [Google Scholar]
- Matsuda Y, Hara T, Colman B. Regulation of the induction of bicarbonate uptake by dissolved CO2 in the marine diatom Phaedactylum tricornutum. Plant Cell Environ. 2001;24:611–620. doi: 10.1046/j.1365-3040.2001.00702.x. [DOI] [Google Scholar]
- McClintock M, Higinbotham N, Uribe EG, Cleland E. Active, irreversible accumulation of extreme levels of H2SO4 in the brown alga, Desmarestia. Plant Physiol. 1982;70:771–774. doi: 10.1104/pp.70.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegler K, Kempe S. The carbonate system of the North Sea: Determination of alkalinity and TCO2 and calculation of PCO2 and Sical (Spring 1986) Mitt geol-paläont Inst Univ Hamb. 1988;65:35–87. [Google Scholar]
- Richardson LL, Stolzenbach KD. Phytoplankton cell size and the development of microenvironments. FEMS Microbiol Ecol. 1995;16:185–192. doi: 10.1111/j.1574-6941.1995.tb00282.x. [DOI] [Google Scholar]
- Taguchi S, Hirata JA, Laws EA. Silicate deficiency and lipid synthesis of marine diatoms. J Phycol. 1987;23:260–267. doi: 10.1111/j.1529-8817.1987.tb04133.x. [DOI] [Google Scholar]
- Tortell PD, Reinfelder JR, Morel FMM. Active uptake of bicarbonate by diatoms. Nature. 1997;390:243–244. doi: 10.1038/36765. [DOI] [PubMed] [Google Scholar]
- Veldhuis MJW, Kraay GW, Timmermans KR. Cell death in phytoplankton: correlation between changes in membrane permeability, photosynthetic activity, pigmentation and growth. Eur J Phycol. 2001;36:167–177. doi: 10.1080/09670260110001735318. [DOI] [Google Scholar]
- Verity PG. Feeding in planktonic protozoans: Evidence for non-random acquisition of prey. J Protozool. 1991;38:69–76. [Google Scholar]