Abstract
Deep brain stimulation (DBS) is effective in reducing motor symptoms in Parkinson’s disease (PD). However, objective methods for quantifying its efficacy are lacking. We present a principal component (PC) -based tracking method for quantifying the effects of DBS in PD by using EMG and acceleration measurements. Ten parameters capturing PD characteristic signal features were initially extracted from isometric EMG and acceleration recordings. Using a PC approach, the original parameters were transformed into a smaller number of PCs. Finally, the effects of DBS were quantified by examining the PCs in a low-dimensional feature space. The EMG and acceleration data from 13 PD patients with DBS on and off, and 13 healthy age-matched controls were used for analysis. Clinical evaluation of patients showed that their motor symptoms were effectively reduced with DBS. The analysis results showed that the signal characteristics of 12 patients were more similar to those of the healthy controls with DBS on than with DBS off. These observations indicate that the PC-based tracking method can be used to objectively quantify the effects of DBS on the neuromuscular function of PD patients. Further studies are suggested to estimate the clinical sensitivity of the method to different types of PD.
Index Terms: Deep brain stimulation (DBS), nonlinear methods, Parkinson’s disease (PD), principal components (PCs), surface electromyography (EMG)
I. Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by four primary symptoms: resting tremor, rigidity, bradykinesia and postural instability. Because there is currently no definitive test for PD, the diagnosis is based on the presence of clinical symptoms and the response to antiparkinsonian medications [1]. The most established scale for assessing disability and impairment in PD is the Unified Parkinson’s Disease Rating Scale (UPDRS) [2], which is based on subjective clinical evaluation of symptoms. A need therefore exists to objectively quantify PD characteristics in order to improve the diagnosis, define disease subtypes, monitor disease progression and demonstrate treatment efficacy [3], [4].
Deep brain stimulation (DBS) is one method for PD therapy that uses high frequency pulses to stimulate the subthalamic nucleus and associated brain regions. Although the mechanisms of DBS action are unclear, correct electrode placement and stimulation programming may improve motor symptoms (i.e. reduce tremor, rigidity and bradykinesia) and allow for a reduction in antiparkinsonian medication doses [5]. To date, however, DBS stimulation parameters are set by subjective evaluation of symptoms, and no physiological-based quantitative measures are used to optimize the efficacy of DBS in reducing motor disorders [6].
Surface electromyography (EMG) and kinematic measurements enable the objective quantification of neuromuscular function and movement, and may therefore be used for quantifying the effects of DBS, antiparkinsonian medication or other treatments. Previous EMG-based studies have shown that the EMG characteristics of patients with PD may change due to DBS and antiparkinsonian medication in at least three ways. First, the dominant tremor frequency in the EMG spectrum is increased with DBS and medication [7], [8]. Secondly, the coherence between EMG and acceleration (indicating tremor) is reduced with DBS and medication during a resting condition (without mental or physical stressors) and with backward counting [8], [9]. Thirdly, the duration and amplitude of the first agonist burst is increased and the number of agonist bursts is reduced with DBS and medication during rapid point-to-point movements of elbow [10] and ankle [11]. However, even the combination of DBS and medication cannot normalize EMG burst characteristics [11].
Previous kinematic studies have shown that the motor function of patients with PD may change due to DBS and antiparkinsonian medication also in at least three ways. First, the amplitude and regularity of tremor are decreased during quiet and cognitive tasks [7], [8], [9]. Secondly, the movement speed is increased during rapid point-to-point movements of the elbow [10] and ankle [11]. And thirdly, the latency of movements is decreased in choice reaction time tasks [12]. According to [7], DBS is more effective than medication in reducing tremor amplitude. However, even the combination of DBS and medication cannot normalize the speed of movement [10], [11].
Previous studies have indicated that nonlinear and morphological methods of EMG and acceleration analysis in combination with a principal component (PC) approach are potentially useful for discriminating patients with PD from healthy persons [13], [14], [15], [16]. This results from the fact that the EMGs of patients with PD differ from the EMGs of healthy persons, showing spiky and recurring structures and the acceleration recordings showing regularity. However, it has not been tested whether similar methods of nonlinear dynamics and the PC-based approach are capable of quantifying the effects of DBS objectively.
We present a PC-based tracking method for quantifying the effects of DBS in PD by using EMG and kinematic analysis. Ten parameters capturing PD characteristic signal features were initially extracted from isometric EMG and acceleration recordings. These signal features were parameters of nonlinear dynamics, the coherence between EMG and acceleration, and the amplitude of acceleration. Using the PC approach, the original parameters were transformed into a smaller number of parameters i.e. PCs. Finally, the effects of DBS were quantified by examining the PCs in a low-dimensional feature space. The EMG and acceleration data from 13 PD patients with DBS on and off, and 13 healthy age-matched controls were used for analysis. The effects of DBS on elbow flexion and extension movements were analyzed separately.
The hypothesis of this study was that the developed method is capable of quantifying objectively the effects of DBS on patients with PD. In that case, the measurement characteristics of patients are more similar to the measurement characteristics of the healthy controls with DBS on than with DBS off.
II. EMG and acceleration measurements
Twenty-six subjects participated in this study after giving their informed consent: 13 patients with PD (4 females and 9 males, age 67 ± 8 years) and 13 healthy age-matched controls (7 females and 6 males, age 65 ± 8 years). The study was approved by the IRB Boards of the Beth Israel Deaconess Medical Center, University of Massachusetts and Kuopio University Hospital. The PD patients were treated with DBS and they were off-medication (medications are listed in Table I) during the measurements. The exact stimulation parameters were depending upon the patient and they were not registered individually during the measurements. In all patients, the stimulation frequency was between 130 – 185 Hz, the pulse width between 60–90 μsec, the amplitude between 2 -4 V and the electrode polarity monopolar or dipolar depending upon the patient. For the patients, the EMG and acceleration measurements and the UPDRS examination were performed twice: with DBS on (stimulator was turned on) and with DBS off (stimulator was turned off). The total UPDRS motor score of them was 33 ± 9 with DBS on and 52 ± 12 with DBS off. The controls were measured once. The clinical characteristics of the patients are provided in Table I.
TABLE I.
Clinical characteristics of patients
| Patient no. | Age | Gender | UPDRS off | UPDRS on | Medications | Co-morbidities |
|---|---|---|---|---|---|---|
| 1 | 70 | F | 56 | 43 | Amantadine HCl, Clozapine, Diazepam, Ex- elon, Fludrocortisone Acetate, Mirtazapine, Namenda, Senna Plus, Seroquel, Sinemet | none |
| 2 | 70 | F | 64 | 48 | Carbidopa-Levodopa, Amantadine HCl, Simvastatin | chronic obstructive pulmonary disease |
| 3 | 82 | F | 59 | 40 | Zelapar, Mirapex, Azilect | none |
| 4 | 71 | M | 34 | 14 | Sinemet, Lipitor, Metformin, Glipizide, Lisinopril, Aspirin, Wellbutrin | diabetes, angioplasty in 2000 |
| 5 | 61 | M | 71 | 42 | Sinemet, Sinemet CR, Omeprazole, Aspirin, Lasix | none |
| 6 | 61 | M | 38 | 31 | Mirapex, Sinemet, Famotidine | hypertension |
| 7 | 66 | F | 47 | 28 | Sinemet, Azilect, Baclofen, Amitriptyline, Evista, Glycolax | none |
| 8 | 63 | M | 57 | 33 | Sinemet, Sinemet CR, Tasmar, Metoprolol, Pantprozol, Simvastatin | none |
| 9 | 50 | M | 43 | 34 | Zelapar, Sinemet, Mirapex, Trazadone, Tas- mar, Metformin, Simvastatin, Glimepiride, Nexium, Lisinopril, Advair, Metolazone, Clonazepam | diabetes |
| 10 | 59 | M | 43 | 24 | Carbidopa-Levodopa, Clonazepam, Detrol LA, Imipramine HCl | diabetes |
| 11 | 63 | M | 44 | 30 | Sinemet, Simvastatin | none |
| 12 | 64 | M | 62 | 38 | Azilect, Carbidopa-Levodopa, CoQ10, Is- radipine, MiraLax, Naproxen, Ropinirole HCl, Strattera, Tasmar | hypertension |
| 13 | 67 | M | 43 | 30 | Sinemet, Sinemet CR, Simvastatin, Lev- odopa | none |
| Mean ± SD | 67±8 | 52 ± 12 | 33 ± 9 |
Two types of muscle contraction were examined: isometric contraction of biceps brachii (BB) muscles (isometric task) and elbow flexion-extension movements (dynamic task). During the isometric task, subjects were asked to hold their elbows at a 90° angle with their palms up. The isometric contraction lasted for 30 seconds. During the dynamic task, subjects were asked to perform elbow flexion and extension movements in vertical direction with full-range of motion in two-second cycles with their palms up. The movement was not restricted and the actual durations of the movement cycles were checked afterwards by using the measured acceleration data. It was observed that most of the patients could not reach the asked movement speed with DBS off. The number of flexion-extension cycles was between 8 – 15. Additional weights were not used in either tasks, as earlier studies have indicated that PD related EMG signal features are most significant during unloaded conditions [16].
During both tasks, surface EMGs were measured continuously from BB muscles and tri-axial accelerations of forearms simultaneously from the palmar side of subject’s wrists. EMG and acceleration measurements were performed by using ME6000 -biosignal monitor (Mega Electronics Ltd., Kuopio, Finland) with 1000 Hz sampling frequency. EMG registration was performed by using disposable Ag/AgCl electrodes (Medicotest, model M-00-S, Ølstykke, Denmark). The electrodes were attached bilaterally over the belly of BB muscles with 3 cm inter-electrode spacing (center to center) in accordance with previous studies [13], [14], [15], [17]. The reference electrodes were placed 6 – 7 cm laterally from the recording electrodes. Raw EMG signals were analogically band-pass filtered with an anti-aliasing filter (Butterworth, band-pass 1 – 500 Hz) and amplified (differential amplifier, CMRR > 130 dB, total gain 1000, noise < 1 μV). The acceleration registration was performed by using triaxial accelerometers (Mega Electronics Ltd., range ±10 g). The signals were analogue-to-digital converted (14-bit AD converter) and stored in a PC for later analysis.
III. Signal analysis
It has been shown previously that both the isometric and the dynamic EMG and acceleration measurements can be used for discriminating between PD patients and healthy persons [14], [15]. It depends on the studied patient group, which one of them works better in characterizing PD related features in the measurements. In this study, the main focus in the analysis was to introduce a method for quantifying the effects of DBS by using isometric EMG and acceleration recordings. The analysis steps for the isometric task are described in section III-A. The dynamic EMG and acceleration recordings were analyzed for demonstration as described in section III-B.
A. Analysis of isometric task
For analysis, fifteen seconds long segments of EMG and acceleration were chosen from the middle of the isometric task. One isometric contraction was measured and analyzed for each control and two isometric contractions for each patient (one with DBS on and one with DBS off). The EMG recordings were visually inspected and did not contain peaked artifacts. These peaked artifacts could be caused by involuntary movements that are present when the forearm is not supported on a surface. The resultant of the three acceleration components was used in the analysis. The low-frequency trends were removed from both signals by using the smoothness priors method, which is a time-varying high pass filter (see [18] for details). The high-pass cut-off frequencies were 10 Hz for EMG and 2 Hz for acceleration. In addition to trend removal, EMG signals of all subjects were low-pass filtered by using a ninth-order Butter worth low-pass filter with 110 Hz cut-off frequency. This low-pass filtering removed the DBS artifact (basically in the frequency band 130–185 Hz) and its harmonics from the signals.
1) EMG and acceleration features
Five signal features were extracted from EMG and acceleration measurements of both sides of the body (resulting in 10 features). These features were:
correlation dimension of EMG (D2,r and D2,1)
recurrence rate of EMG (%RECr and %REC1)
root mean square amplitude of acceleration (RMSr and RMS1)
sample entropy of acceleration (SampEnr and SampEn1)
coherence between EMG and acceleration (Cohr and Coh1)
The sides of the body are denoted here with r (right) and l (left).
Two parameters of nonlinear dynamics were extracted from EMG signals: the correlation dimension and the recurrence rate. %REC was calculated as described in [14] and [19]. As a result, %REC is a scalar parameter that measures the percentage of recurring structures in a time series. Correlation dimension was calculated as described in [14] and [20]. D2 is a scalar parameter that measures the complexity of a time series. Patients with PD have been previously connected with higher %REC (more recurring structures in EMG) and lower D2 (less complex EMG) than healthy persons [14].
Two of the parameters were extracted from acceleration signals: the root mean square amplitude and the sample entropy. RMS measures the amplitude of tremor and SampEn its complexity. SampEn is a method of nonlinear dynamics and it measures the negative natural logarithm of the conditional probability that two sequences in a time series that are similar for m points are similar for m+1 points (see [21] for details). Patients with PD have been previously connected with higher RMS (higher amplitude of tremor) and lower SampEn (more regular tremor) than healthy persons [14].
Coherence between EMG and acceleration was calculated to measure similarities in their power spectra. It was estimated with Welch’s averaged periodogram method (window length 2048 ms and 75 % overlap). The coherence area above 0.99 confidence limit (in the frequency band 0–50 Hz) was used for further analysis.
2) Principal component approach
The ten signal features (detailed in section III-A1) and the principal component approach [22] were used for analysis. PC approach was used for two things: 1) to transform the original possibly correlated variables into uncorrelated variables and 2) to reduce the number of variables while keeping as much information as possible about the original variables.
First, the ten calculated signal parameters were sequentially placed and normalized (to zero mean and unit SD of controls) to form feature vectors for all subjects. One feature vector was formed for each control and two feature vectors for each patient: one with DBS on and one with DBS off. The dimension of the feature vectors was then reduced by applying the PC approach. In that approach, the feature vectors were decomposed into weighted sums of orthogonal basis vectors where the scalar weights were called the principal components. These PCs were the new uncorrelated features.
The basis vectors were chosen here by using the feature vectors of healthy controls. With this choice, we scaled the features of healthy controls inside one SD (the healthy control group formed the normal group for later comparison). For solving the basis vectors, we formed a feature matrix that contained the feature vectors of healthy controls in its columns. Then, we calculated the correlation matrix of the feature matrix and solved the eigenvectors from it. Five eigenvectors corresponding to the five largest eigenvalues were chosen as the basis vectors. These five eigenvectors contributed to 95 % of the total variation in the feature vectors of all controls. Thus, each feature vector could be quite accurately modeled as a weighted sum of these five eigenvectors. Finally, we solved the PCs in the least squares sense for all controls and patients with DBS on and off.
The best PCs to differentiate between DBS on- and off-states and between controls and patients were chosen for further analysis. These PCs are presented in a two-dimensional feature space and compared between DBS on- and off-states and between healthy controls and patients. According to the hypothesis of this study, in the feature space the patients will be closer to the group of healthy controls with DBS on than with DBS off.
B. Analysis of dynamic task
The EMG and acceleration signals, which were measured during elbow flexion - extension movements, were filtered in the same way as the recordings from the isometric task. Eight flexion and extension phases of movement were chosen for analysis, as this was the minimum number of flexion-extension cycles completed by all subjects. Two parameters were extracted from the dynamic recordings for demonstration: the recurrence rate of EMG and the sample entropy of acceleration. These parameters were selected from a larger set of variables that have been used previously for characterizing PD related features in the dynamic EMG and acceleration recordings [15]. They were calculated for the flexion and extension phases of movement as described in [15] and denoted here as %RECf, %RECe, SampEnf and SampEne (f stands for flexion and e for extension). In addition, the EMG and acceleration recordings were compared between DBS on- and off-states by visually examining signal morphology.
IV. Results
A. Clinical UPDRS motor scores
In all patients, the total UPDRS motor score was lower with DBS on than with DBS off (see Table III for details). However, the reduction rate was highly individual. The rest tremor scores decreased for most (10/13) of the patients and the rigidity scores for all patients in either side of the body. The clinical scores describing disabilities in hand movements decreased for all patients in either side of the body.
TABLE III.
Total updrs motor scores, resting tremor scores and distances from the center of healthy controls (Dist) with dbs on and off.
| Patient no. | UPDRS off | UPDRS on | Right tremor off | Right tremor on | Left tremor off | Left tremor on | Dist off | Dist on |
|---|---|---|---|---|---|---|---|---|
| 1 | 56 | 43 | 2 | 2 | 2 | 1 | 26 | 25 |
| 2 | 64 | 48 | 2 | 1 | 3 | 2 | 32 | 12 |
| 3 | 59 | 40 | 2 | 1 | 2 | 2 | 7 | 5 |
| 4 | 34 | 14 | 1 | 0 | 3 | 2 | 180 | 30 |
| 5 | 71 | 42 | 4 | 1 | 3 | 0 | 289 | 4 |
| 6 | 38 | 31 | 2 | 1 | 1 | 0 | 5 | 12 |
| 7 | 47 | 28 | 1 | 0 | 1 | 0 | 6 | 2 |
| 8 | 57 | 33 | 2 | 0 | 1 | 0 | 6 | 4 |
| 9 | 43 | 34 | 1 | 1 | 1 | 1 | 13 | 11 |
| 10 | 43 | 24 | 1 | 0 | 0 | 0 | 11 | 10 |
| 11 | 44 | 30 | 0 | 0 | 0 | 0 | 6 | 5 |
| 12 | 62 | 38 | 1 | 0 | 2 | 0 | 5 | 4 |
| 13 | 43 | 30 | 0 | 0 | 0 | 0 | 5 | 3 |
B. Measurements and signal features of isometric task
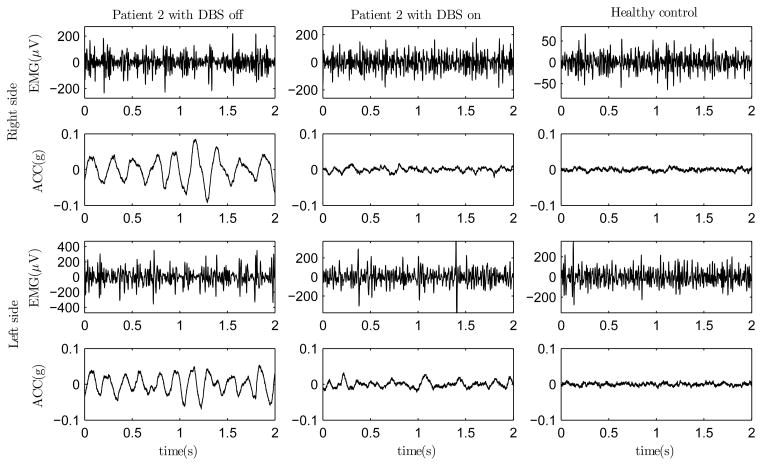
The EMG and ACC measurements during the isometric task for one healthy control and one PD patient with DBS on and off are presented in Fig. 1. One can observe that the EMGs of the patient differ from the EMGs of the healthy control by containing recurring EMG bursts. These bursts are likely due to motor unit synchronization in PD [23] and they are more apparent with DBS off than with DBS on. The accelerations of the patient appear to contain more regular oscillation as compared to the control. This oscillation is due to tremor in PD and the amplitude and regularity of it are higher with DBS off than with DBS on.
Fig. 1.
EMG and acceleration recordings of one PD patient with DBS off (left) and on (middle) and one healthy control (right) during the isometric contraction of BB muscles.
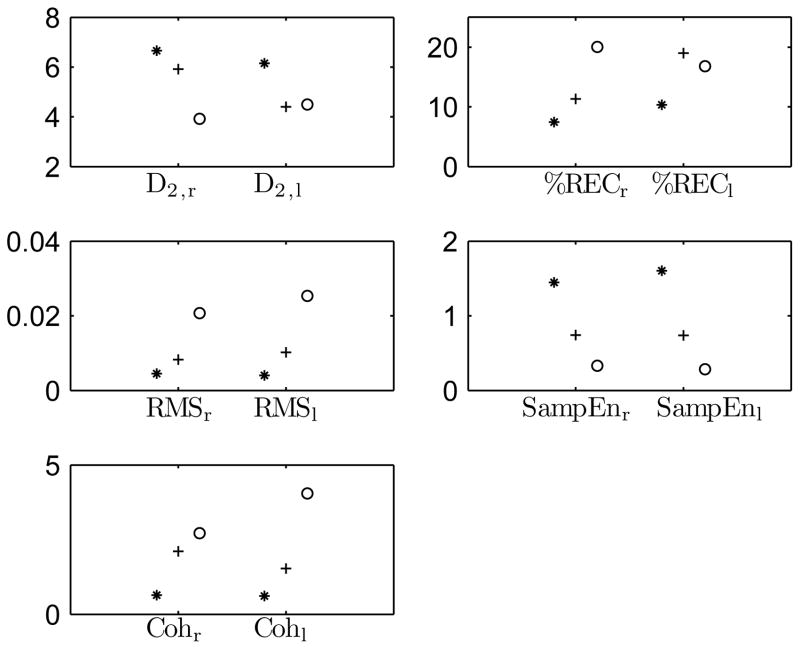
Ten features were extracted from the EMG and acceleration measurements of all subjects as described in section III-A1. The feature values for the one control and the one patient with DBS on and off are presented in Fig. 2. One can observe that the healthy control has lower values in %REC, RMS and Coh, and higher values in D2 and SampEn. Taken together, these observations indicate that the EMG and acceleration signals are more complex, and the amplitude of acceleration and the coherence between EMG and acceleration are lower for the control.
Fig. 2.
EMG and acceleration feature values for one healthy control (asterisk) and one PD patient with DBS on (plus) and off (circle).
For the patient, D2,r and SampEn are higher, and %RECr, RMS and Coh are lower with DBS on than with DBS off. That is, the DBS off -state is characterized with more regular signals, higher amplitude of acceleration and higher coherence. In the EMG signal from the left BB, the differences between the DBS on- and off-state are not as pronounced. However, the differences in the acceleration features between the DBS on- and off-state are clear in both the right and the left side arm.
The mean±SD values of the signal features for the control group and for the patient group with DBS on and off are presented in Table II. One can observe that the results in Table II support the observations made from Fig. 2. The standard deviations of the feature values are high for the patient group especially with DBS off. This results from the fact that the patient group was very heterogeneous. Therefore, it was reasonable to study the patient measurements individually instead of focusing on group comparisons.
TABLE II.
Feature values (mean±SD) for healthy controls and for pd patients with dbs on and off.
| Signal feature | Controls | PD patients with DBS on | PD patients with DBS off |
|---|---|---|---|
| D2 r | 6.9±0.8 | 6.1±1.0 | 5.4±1.6 |
| D2,1 | 6.7±0.9 | 5.7±1.4 | 5.4±2.1 |
| %RECr | 5.7±3.4 | 9.2±5.1 | 14.9±13.6 |
| %REC1 | 6.8±3.9 | 12.7±7.6 | 15.3±14.9 |
| RMSr · 103 | 0.4±0.1 | 0.8±0.4 | 2.5±4.6 |
| RMS1 · 103 | 0.4±0.1 | 1.0±0.9 | 6.0±12.4 |
| SampEnr | 1.3±0.1 | 0.9±0.3 | 0.7±0.3 |
| SampEn1 | 1.4±0.2 | 1.0±0.4 | 0.8±0.4 |
| Cohr | 0.6±0.3 | 1.3±0.7 | 1.5±1.4 |
| Coh1 | 0.7±0.5 | 1.4±0.8 | 2.2±1.5 |
C. Principal components
The PCs were solved for all subjects as described in section III-A2 by using the solved eigenvectors. It was observed that the first (PC1) and the third PC (PC3) work best in discriminating between the DBS on- and off-states, and between the patients and the healthy controls. The first eigenvector is the best mean square fit for the feature vectors of healthy subjects. Therefore, PC1 (i.e. the weight of the first eigenvector) describes the amplitude of EMG and acceleration features in relation to the mean of healthy subjects. By visually inspecting the morphology of the third eigenvector, we could recognize, that PC3 emphasizes differences between right and left side variables. In fact, the unilateral onset and persistent asymmetry of symptoms support the diagnosis of PD in relation to other similar diseases [1].
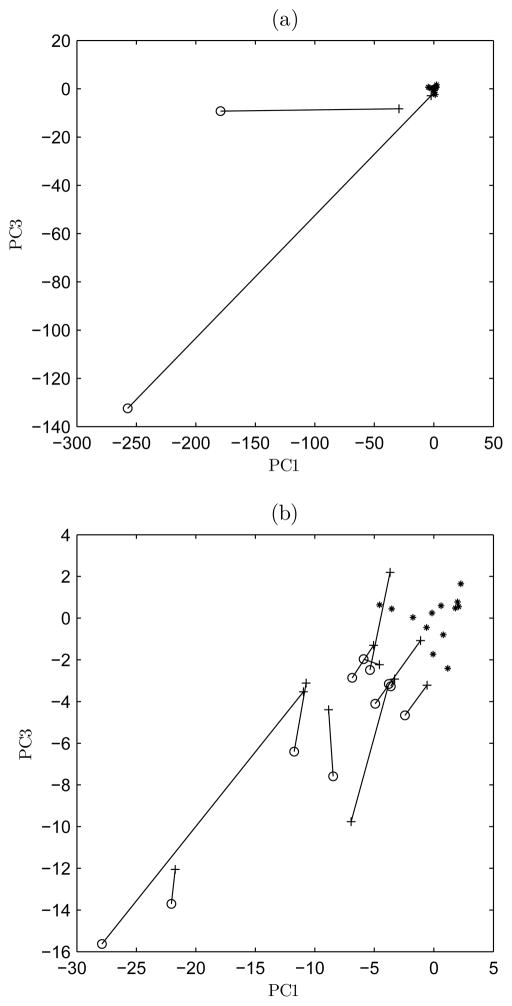
The third PCs with respect to the first PCs of 13 healthy controls and 13 patients with DBS on and off are presented in Fig. 3. The PC values of 12 patients are closer to the center of healthy controls (point 0,0) in the feature space with DBS on than with DBS off. That is, the EMG and acceleration feature values of them get closer to the controls and the side differences get smaller when the stimulator is on. The distances between the DBS on- and off-states in the feature space are highly individual. The distances to the center of healthy controls are detailed in Table III.
Fig. 3.
The third PCs with respect to the first PCs of 13 healthy controls (asterisk) and 13 PD patients with DBS on (plus) and off (circle). The patients were divided into two figures, but the controls are the same in both figures. (a) Patients 4, 5 and controls. (b) The remaining patients and controls. The DBS on and off-states of each patient are connected with a line.
For the two patients (patients 4 and 5 in Table III) in Fig. 3(a), the distances between the DBS off- and on-states are large. Correspondingly, the reductions in their total UPDRS motor scores due to DBS are strong (59 % and 41 %). DBS seems to relieve their motor disorders effectively. Especially tremor is relieved for patient 5 and especially bradykinesia for patient 4. In Fig. 3(b), the EMG and acceleration features of other 11 patients get closer to controls when the stimulator is on. There are four patients (patients 7, 8, 12 and 13) that get near the controls with DBS on. For them, the resting tremor disappears with DBS on. In the same figure, there is one patient (patient 6) that is further from controls with DBS on than with DBS off. The patient is characterized with tremor (acceleration signal) that has higher amplitude and regularity with DBS on than with DBS off. In addition, the EMG recordings of the patient are more complex (lower %REC and higher D2) with DBS off than with DBS on. In clinical examination, both the total UPDRS motor score and the tremor score of the patient were defined higher with DBS off than with DBS on. For this patient, the EMG and acceleration results contradict the clinical assessment.
D. Dynamic task
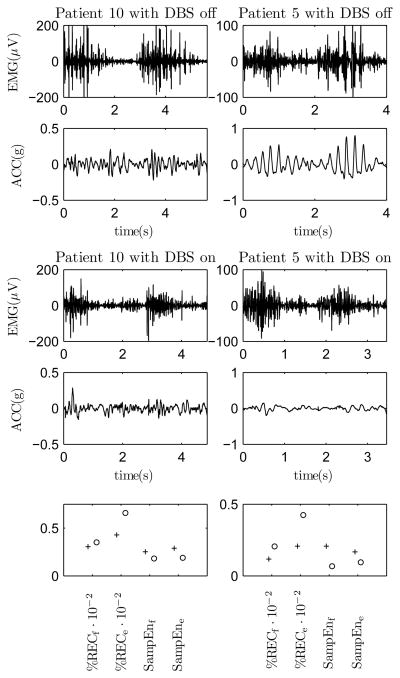
We analyzed elbow flexion and extension movements as described in section III-B. It was observed that there were DBS induced changes in the EMG and acceleration recordings for a subgroup of patients (patients that have bigger problems in performing movement tasks) but not for all patients. The EMG and acceleration measurements during two elbow flexion - extension movements for two of these patients (patients 5 and 10) with DBS on and off are presented in Fig. 4. Note that the acceleration recordings were high-pass filtered with 2 Hz cut-off frequency. In addition, the calculated recurrence rates of EMG and the sample entropies of acceleration in flexion and in extension are presented for these two patients in the same figure.
Fig. 4.
The EMG and acceleration measurements during two elbow flexion-extension movements for two PD patients with DBS on and off. The recurrence rates of EMG and the sample entropies of acceleration for the flexion and extension phases of movement with DBS on (plus) and off (circle) in bottom figures.
It is observed in Fig. 4 that the EMG recordings of both patients differ between the DBS on- and off-states by containing more recurring EMG bursts and the acceleration recordings by containing more regular oscillation with DBS off. It is also observed in Fig. 4 that the recurrence rates of EMG are lower and the sample entropies higher with DBS on for these two patients. The oscillation in acceleration is likely due to action tremor and rigidity in PD. The mean±SD values of the variables for the healthy control group were: %RECe = 18±7, %RECf = 12±7, SampEne = 0.28±0.05 and SampEnf = 0.40 ± 0.07. For the two patients in Fig. 4, the parameter values are closer to the control mean values with DBS on than off.
In the dynamic task, there were five patients (patients 4, 6, 7, 9 and 12) that did not show decrease in %REC or increase in SampEn with DBS on. These patients were characterized with lower hand movement scores in the clinical UPDRS. That is, they could better perform hand movement tasks (such as finger tapping and open-close movements of hands) in the clinical examination.
V. Discussion
We presented a PC-based tracking method for quantifying the effects of DBS in PD by using surface EMG and acceleration measurements. The method was tested with EMG and acceleration data from 13 PD patients with DBS on and off, and 13 healthy age-matched controls.
The results of the isometric task showed that the signal characteristics of 12 patients were more similar to the signal characteristics of the healthy controls with DBS on than with DBS off. Observed differences in the EMG and acceleration signals between patients and controls, and between DBS on- and off-states suggested three things. First, the EMG measurements of patients changed into more complex and contained less recurring structures due to DBS. These recurring structures are likely due to motor unit synchronization in muscles, which is characteristic for PD [23]. Effects of DBS have not been studied earlier by analyzing nonlinear EMG features. Secondly, the amplitude and regularity of acceleration measurements and the coherence between EMG and acceleration reduced due to DBS. These findings refer to reduction in tremor and are consistent with earlier studies [7], [8], [9]. And thirdly, the side differences between left and right side variables reduced with DBS. The asymmetry of symptoms is characteristic for PD [1].
The distances between the DBS on- and off -states in the feature space were highly individual. Likewise, the improvements in clinical scores were highly individual. However, strong changes in the total UPDRS motor score did not always result in strong changes in the analyzed PCs and vice versa. This may be due to the fact that the total UPDRS motor score [2] is a complicated score that consists of a large number of subscores. These subscores are defined for different areas of the body in different movement conditions. In this study, we analyzed only BB muscle and arm movements. According to the results, hardly any of the patients reached the controls in the feature space with DBS on. In fact, previous studies have shown that the analyzed EMG and acceleration features are potentially useful for discriminating between PD patients and healthy persons regardless of treatment with medication [14], [15]. The one patient, to whom we measured higher amplitude and more regular tremor with DBS on than with DBS off, was clearly further from the healthy controls with DBS on. In the clinical examination, his tremor score was defined higher with DBS off than with DBS on. This shows that the acceleration measurement can provide different information about the tremor than the clinical eye of a doctor.
It was observed that the PC-based tracking method was more sensitive to PD with associated tremor. The correlation between the rest tremor scores and the distance to healthy controls in the feature space was significant (Spearman correlation, p < 0.05). Because tremor occurs in 80–90 % of PD cases [24] and is effectively reduced with DBS [5], our analysis method may be suitable for many patients. However, if the patient’s main symptom is bradykinesia, the effects of DBS may not be observed by analyzing EMG and acceleration recordings from isometric tasks. In that case, the analysis of dynamic tasks may prove more sensitive to the effects of DBS on the patient’s motor function.
The results of the dynamic task indicated that there were differences in the signal complexity between the DBS on- and off-states for a subgroup of patients. Therefore, we should not ignore the relevance of the dynamic analysis when quantifying the effects of DBS in the future. In this study, the dynamic EMG and acceleration recordings could not differentiate between the DBS on- and off-states as clearly as the isometric recordings. In the dynamic task, there were five patients that did not show PD characteristic increase in the signal complexity with DBS on. These patients had milder problems in the hand movements also in the clinical examination. One reason for not so clear differences between the DBS on- and off-states may be the fact, that the movement of the forearm was not restricted in this study. That is, the subjects were asked to perform movements in two-second cycles (one second for flexion and one second for extension), but most of the patients could not reach the asked movement speed with DBS off. Therefore, the variability between different flexion-extension cycles was high and the comparison between the DBS on-and off-states was difficult. Another explaining factor is that aging itself causes higher EMG burst activities [25] and loss of acceleration complexity [26] during movement. Therefore, the signal features associated with aging may have mixed with the signal features associated with PD.
One essential thing to remember in the analysis biosignal measurements from patients with DBS is the removal of DBS artifact because its filtering may affect the calculated signal parameters. In the case of EMG, the DBS artifact has often been removed by low-pass filtering the rectified signal with a low (20–60 Hz) cut-off frequency [6], [8], [9], [10], [11]. In this study, we wanted to keep the effects of filtering on the EMG signals as minimal as possible. We tried several filtering techniques of notch filtering and low-pass filtering. By comparing the original EMG signals with the filtered EMG signals and the original EMG spectra with the spectra of the filtered EMG, we observed that the used filtering technique worked best in removing the DBS artifact from this data without disturbing the EMG signal morphology. The filtering was performed in the same way for all subjects (controls and patients with DBS on and off) in order to get comparable results. The low-pass cut-off frequency 110 Hz was the highest value that removed the stimulation artifact from the measurements of all subjects.
When discriminating between subjects or between different states of the subjects (such as between DBS on and off) on the basis of biosignal measurements there are often many signal parameters that can capture essential features in the signals. Each of the parameters describes one feature (e.g. the amplitude, complexity, etc.) in the measurements. It could be possible to examine the statistics of single parameters. However, often a combination of several parameters works better in discrimination and a dimension reduction technique, such as the PC approach, can be used to capture the essential and ignore the irrelevant information in the combination of variables. It must be noted that prior to the clinical use of the presented method we should train the PC-based method with a larger number of subjects and derive PD related indexes from the PCs. For example, one possible index could measure the asymmetry of neuromuscular function in PD patients. In clinical use, we could then use the known PD related indexes for quantifying objectively the effects of DBS treatment in PD.
As a conclusion, isometric EMG and acceleration measurements are potentially useful for quantifying the effects of DBS on the neuromuscular function of PD patients. These measurements in combination with the PC-based tracking method can be used to quantify the effects of DBS objectively, cost-effectively and non-invasively. In further studies, the presented approach could be tested in helping the adjustment of DBS settings. In addition, the sensitivity of the presented method to different types of PD should be estimated more carefully in further clinical studies.
Acknowledgments
The project was supported by Grant Number UL1 RR025758 -Harvard Clinical and Translational Science Center, from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. This work was supported also by the Tekes under Project 905/07, and by the Academy of Finland under Project 123579 and Project 126873.
Biographies

Saara M. Rissanen received the M.Sc. degree in physics from the University of Kuopio, Kuopio, Finland, in 2003.
Since 2003, she has been working as a researcher in the Department of Applied Physics, University of Eastern Finland, and also in the Department of Physical and Rehabilitation Medicine, Kuopio University Hospital. Her current research interests include novel methods of EMG analysis and Parkinson’s disease.

Markku Kankaanpää received the Licenciate of Medicine (M.D.) and Doctor of Medical Sciences (D.M.S.) degrees from the University of Kuopio, Kuopio, Finland, in 2000.
In 2006, he specialized in Physical Medicine and Rehabilitation and was appointed with associate professorship in 2009. Currently he is the Head of the Department of Physical Medicine and Rehabilitation, Tampere University Hospital, Tampere, Finland. His research interests include lumbar disorders, especially active rehabilitation, motor control, muscle physiology, and myoelectric alterations related to low back and neck pain, and also Parkinson’s disease.

Mika P. Tarvainen received the M.Sc. and Ph.D. degrees from the University of Kuopio, Kuopio, Finland, in 1999 and 2004, respectively.
Since 1999, he has been working as a researcher in the Department of Applied Physics, University of Eastern Finland. He is currently a Senior Researcher in the Biosignal Analysis and Medical Imaging Group. His research areas include biomedical signal analysis methods and their applications in modeling of human physiology. Dr. Tarvainen is the Secretary of Finnish Society for Medical Physics and Medical Engineering.

Vera Novak M.D., Ph.D. graduated from Charles University and Czech Academy of Sciences.
She is the Director of the Syncope and Falls in the Elderly Program at Beth Israel Deaconess Medical Center, an Associate Professor of Medicine at Harvard Medical School, Boston, MA and an Adjunct Professor of Mathematics at NCS. Dr. Novak studies effects of age-related disorders on biological systems regulation.
Peter Novak M.D. Ph.D.
He is an Assistant Professor in Neurology at the Department of Neurology, University of Massachusetts and the Director of the Autonomic Laboratory. Dr. Novak is board certified in Neurology and Psychiatry and trained in autonomic nervous system and movement disorders subspecialties. His primary interests are clinical autonomic nervous system, Parkinson’s disease and Multiple system atrophy.

Kun Hu obtained his Ph.D. degree in statistical physics from Boston University in 2005, and then received a 2-year postdoctoral training in human physiology at Harvard Medical School.
From 2007 to 2009, he was an Instructor of Medicine at Beth Israel Deaconess Medical Center. Since 2009 he has been an Associate Physiologist at Brigham & Women’s Hospital. His research interests include behavioral, cardiovascular and circadian regulations in health and disease.
Dr. Hu is member of American Academy of Sleep Medicine, and member of Sleep Research Society Research Committee.

Brad Manor received an undergraduate degree in kinesiology from The University of Toledo in 2002, a Master’s degree in biomechanics from The University of Toledo in 2004, and a doctoral degree in biomechanics from Louisiana State University in 2008. He then completed a postdoctoral fellowship in gerontology at Harvard Medical School in 2010.
He is currently employed as an Instructor in Medicine at the Beth Israel Deaconess Medical Center and Harvard Medical School in Boston, MA, USA, and his research interests include the effects of aging and rehabilitation on the neuromuscular control of human locomotion and balance.
Dr. Manor is a member of the International Society of Biomechanics, the Organization for Human Brain Mapping, and the Gerontological Society of America.

Olavi Airaksinen received the Licenciate of Medicine (M.D.) and Doctor of Medical Sciences (D.M.S.) degrees from the University of Kuopio, Kuopio, Finland, in 1979 and 1991, respectively.
In 1986 he specialized in Physical Medicine and Rehabilitation and was appointed with associate professorship in 1993. Currently he is the Head of the Department of Physical Medicine and Rehabilitation, Kuopio University Hospital. His research interests include low back and neck pain, active rehabilitation, motor control, muscle physiology, and myoelectric alterations related to low back and neck pain, and also to Parkinson’s disease.

Pasi A. Karjalainen received the Ph.D. degree from the University of Kuopio, Kuopio, Finland, in 1997.
Since 1998, he has been the Head of the research group for Biosignal Analysis and Medical Imaging. He is currently a Professor in the Department of Applied Physics, University of Eastern Finland. His research interests include biomedical signal analysis and medical imaging applications. Most of his work concerns the application of Bayesian and regularization methods in event related biosignal analysis. Clinical applications of his work include the use of human motion analysis in neurorehabilitation.
Contributor Information
Saara M. Rissanen, Email: saara.rissanen@uef.fi, Department of Applied Physics, University of Eastern Finland, FI-70211 Kuopio, Finland and also with the Department of Physical and Rehabilitation Medicine, Kuopio University Hospital, FI-70211 Kuopio, Finland
Markku Kankaanpää, Department of Physical Medicine and Rehabilitation, Tampere University Hospital, FI-33521 Tampere, Finland.
Mika P. Tarvainen, Department of Applied Physics, University of Eastern Finland, FI-70211 Kuopio, Finland
Vera Novak, Division of Gerontology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02215, USA.
Peter Novak, Division of Gerontology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02215, USA.
Kun Hu, Division of Sleep Medicine, The Brigham and Women’s Hospital and Harvard Medical School, Boston, MA 02115, USA.
Brad Manor, Division of Gerontology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02215, USA.
Olavi Airaksinen, Department of Physical and Rehabilitation Medicine, Kuopio University Hospital, FI-70211 Kuopio, Finland.
Pasi A. Karjalainen, Department of Applied Physics, University of Eastern Finland, FI-70211 Kuopio, Finland
References
- 1.Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 2.Fahn S, Elton RL. The Unified Parkinsons Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent developments in Parkinsons disease. Florham Park, N.J: Macmillan Healthcare Information; 1987. pp. 153–63. [Google Scholar]
- 3.Antoniades CA, Barker RA. The search for biomarkers in Parkinsons disease: a critical review. Expert Rev. 2008;8(12):1841–1852. doi: 10.1586/14737175.8.12.1841. [DOI] [PubMed] [Google Scholar]
- 4.Morgan JC, Mehta SH, Sethi KD. Biomarkers in Parkinsons disease. Curr Neurol Neurosci Rep. 2010;10:423–430. doi: 10.1007/s11910-010-0144-0. [DOI] [PubMed] [Google Scholar]
- 5.Benabid AL, Chabardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol. 2009;8:67–81. doi: 10.1016/S1474-4422(08)70291-6. [DOI] [PubMed] [Google Scholar]
- 6.Levin J, Krafczyk S, Valkovic P, Eggert T, Claassen J, Bötzel K. Objective measurement of muscle rigidity in Parkinsonian patients treated with subthalamic stimulation. Mov Disord. 2009;24(1):57–63. doi: 10.1002/mds.22291. [DOI] [PubMed] [Google Scholar]
- 7.Blahak C, Wöhrle JC, Capelle HH, Bäzner H, Grips E, Weigel R, Hennerici MG, Krauss JK. Tremor reduction by subthalamic nucleus stimulation and medication in advanced Parkinson’s disease. J Neurol. 2007;254:169–178. doi: 10.1007/s00415-006-0305-x. [DOI] [PubMed] [Google Scholar]
- 8.Sturman MM, Vaillancourt DE, Metman LV, Bakay RAE, Corcos DM. Effects of subthalamic nucleus stimulation and medication on resting and postural tremor in Parkinson’s disease. Brain. 2004;127:2131–2143. doi: 10.1093/brain/awh237. [DOI] [PubMed] [Google Scholar]
- 9.Sturman MM, Vaillancourt DE, Metman LV, Sierens DK, Bakay RAE, Corcos DM. Deep brain stimulation and medication for Parkinsonian tremor during secondary tasks. Mov Disord. 2007;22(8):1157–1163. doi: 10.1002/mds.21518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaillancourt DE, Prodoehl J, Metman LV, Bakay RA, Corcos DM. Effects of deep brain stimulation and medication on bradykinesia and muscle activation in Parkinson’s disease. Brain. 2004;127:491–504. doi: 10.1093/brain/awh057. [DOI] [PubMed] [Google Scholar]
- 11.Vaillancourt DE, Prodoehl J, Sturman MM, Bakay RAE, Metman LV, Corcos DM. Effects of deep brain stimulation and medication on strength, bradykinesia, and electromyographic patterns of the ankle joint in Parkinson’s disease. Mov Disord. 2006;21:50–58. doi: 10.1002/mds.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumru H, Summerfield C, Vallderiola F, Valls-Solé J. Effects of subthalamic nucleus stimulation on characteristics of EMG activitiy underlying reaction time in Parkinson’s disease. Mov Disord. 2003;19(1) doi: 10.1002/mds.10638. [DOI] [PubMed] [Google Scholar]
- 13.Rissanen S, Kankaanpää M, Tarvainen MP, Nuutinen J, Tarkka IM, Airaksinen O, Karjalainen PA. Analysis of surface EMG signal morphology in Parkinson’s disease. Physiol Meas. 2007;28:1507–1521. doi: 10.1088/0967-3334/28/12/005. [DOI] [PubMed] [Google Scholar]
- 14.Rissanen SM, Kankaanpää M, Meigal A, Tarvainen MP, Nuutinen J, Tarkka IM, Airaksinen O, Karjalainen PA. Surface EMG and acceleration signals in Parkinson’s disease: feature extraction and cluster analysis. Med Biol Eng Comput. 2008;46(9):849–858. doi: 10.1007/s11517-008-0369-0. [DOI] [PubMed] [Google Scholar]
- 15.Rissanen SM, Kankaanpää M, Tarvainen MP, Meigal A, Nuutinen J, Tarkka IM, Airaksinen O, Karjalainen PA. Analysis of dynamic voluntary muscle contractions in Parkinson’s disease. IEEE Trans Biomed Eng. 2009;56(9):2280–2288. doi: 10.1109/TBME.2009.2023795. [DOI] [PubMed] [Google Scholar]
- 16.Meigal AI, Rissanen S, Tarvainen MP, Karjalainen PA, Iudina-Vassel IA, Airaksinen O, Kankaanpää M. Novel parameters of surface EMG in patients with Parkinson’s disease and healthy young and old controls. J Electromyogr Kinesiol. 2009;19(l. 3):e206–e213. doi: 10.1016/j.jelekin.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Farfán FD, Politti JC, Felice CJ. Evaluation of EMG processing techniques using information theory. BioMed Eng Online. 2010;9(72) doi: 10.1186/1475-925X-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarvainen MP, Rantaaho PO, Karjalainen PA. An advanced detrending method with application to HRV analysis. IEEE Trans Biomed Eng. 2002;49(2):172–175. doi: 10.1109/10.979357. [DOI] [PubMed] [Google Scholar]
- 19.Clancy EA, Farina D, Filligoi G. Single-channel techniques for information extraction from the surface EMG signal. In: Merletti R, Parker P, editors. Electromyography: Physiology, Engineering, and Noninvasive Applications. USA: Wiley-IEEE Press; 2004. pp. 133–168. [Google Scholar]
- 20.Kuusela TA, Jartti TT, Tahvanainen KUO, Kaila TJ. Nonlinear methods of biosignal analysis in assessing terbutaline-induced heart rate and blood pressure changes. Am J Physiol Heart Circ Physiol. 2002;282:H773–H781. doi: 10.1152/ajpheart.00559.2001. [DOI] [PubMed] [Google Scholar]
- 21.Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol. 2000;278:H2039–H2049. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- 22.Jolliffe IT. Principal Component Analysis. Springer-Verlag; 1986. [Google Scholar]
- 23.Fattorini L, Felici F, Filligoi GC, Traballesi M, Farina D. Influence of high motor unit synchronization levels on non-linear and spectral variables of the surface EMG. J Neurosci Meth. 2005;143:133–139. doi: 10.1016/j.jneumeth.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Grosset DG, Grosset KA, Okun MS, Fernandez HH. Parkinson’s Disease: Clinician’s Desk Reference. Manson Publishing Ltd; London: 2009. [Google Scholar]
- 25.Harwood B, Edwards DL, Jakobi JM. Age- and sex-related differences in muscle activation for a discrete functional task. Eur J Appl Physiol. 2008;103:677–686. doi: 10.1007/s00421-008-0765-z. [DOI] [PubMed] [Google Scholar]
- 26.Hong SL, James EG, Newell KM. Coupling and irregularity in the aging motor system: Tremor and movement. Neurosci Lett. 2008;433:119–124. doi: 10.1016/j.neulet.2007.12.056. [DOI] [PubMed] [Google Scholar]