Abstract
Obliterative bronchiolitis (OB) post lung transplantation involves IL-17 regulated autoimmunity to type V collagen and alloimmunity, which could be enhanced by complement activation. However, the specific role of complement activation in lung allograft pathology, IL-17 production, and OB are unknown. The current study examines the role of complement activation in OB. Complement regulatory protein (CRP) (CD55, CD46, Crry/CD46) expression was down regulated in human and murine OB; and C3a, a marker of complement activation, was up regulated locally. IL-17 differentially suppressed Crry expression in airway epithelial cells in vitro. Neutralizing IL-17 recovered CRP expression in murine lung allografts and decreased local C3a production. Exogenous C3a enhanced IL-17 production from alloantigen or autoantigen (type V collagen) reactive lymphocytes. Systemically neutralizing C5 abrogated the development of OB, reduced acute rejection severity, lowered systemic and local levels of C3a and C5a, recovered CRP expression, and diminished systemic IL-17 and IL-6 levels. These data indicated that OB induction is in part complement dependent due to IL-17 mediated down regulation of CRPs on airway epithelium. C3a and IL-17 are part of a feed forward loop that may enhance CRP down regulation, suggesting that complement blockade could be a therapeutic strategy for OB.
Introduction
Obliterative bronchiolitis (OB), characterized by fibrosis of terminal airways (1), remains the limiting factor in long-term survival of lung transplants and is synonymous with chronic rejection. Conversely, primary graft dysfunction (PGD) is an acute clinical condition characterized by hypoxemia and diffuse pulmonary infiltrates within 72 hours of transplantation. PGD is the leading cause of early morbidity and mortality after transplantation and may predispose to OB (2-3). Despite their clear importance to lung transplantation, there are no effective long term treatments for PGD or OB as the immune mechanisms that cause allograft dysfunction remain elusive.
Our laboratory reported that immune responses to type V collagen [col(V)] are key risk factors for PGD (4) and OB post lung transplantation (5). We have demonstrated that passive transfer of anti-col(V) antibodies to rat lung isograft recipients results in PGD-like histology and physiology (6). Injury to airway epithelial cells in this model was due to complement-dependent cytotoxicity (CDC) and correlated with expression of col(V) on epithelial cells (6). Although native and transplanted lung epithelium express col(V), anti-col(V) antibody mediated injury was limited to the transplanted lung, suggesting differential complement regulation in the transplanted lung compared to native lung.
Complement regulatory proteins prevent the activation of the complement cascade by inhibiting the formation or activity of complement cascade components. In humans, CD55 (decay accelerating factor, DAF) accelerates the decay of classical and alternative pathway C3 and C5 convertases, preventing the amplification of the cascade (7). DAF is present on the membranes of virtually all blood cells (8-9), vascular endothelium (10) and epithelial cells (11-12). CD46 (membrane cofactor protein, MCP) is also a human CRP and acts in the cleavage of C3b and C4b, preventing the formation of the C3 and C5 convertases (13). Together, DAF and CD46 prevent the destruction of autologous cells by activating complement via inhibiting and degrading the convertases. Complement receptor-1-related protein y (Crry) protein is the rodent orthologue of CD46. Crry is of importance in rodent complement regulation as it has both CD55 and CD46 functions (14). Rodents also express DAF, but Crry is more efficient than DAF at C3 convertase inhibition, and therefore, Crry is of greater physiologic importance than CD55 in regulating local complement activation in rodents (15).
IL-17a is key to the development of PGD (5, 16-17), and as PGD is important for development of OB, IL-17a is also a major mediator of OB (18). PGD incidence is an independent risk factor for the development of OB (19-20). Recent studies demonstrate complement related functions of IL-17a. For example, CD55 expression is down regulated during clinical asthma (21). In addition, recent reports indicate a crucial role for IL-17a in asthma pathogenesis and that IL-17a is induced by C3a in a model of allergic airway inflammation (22-23). These data are consistent with a study showing C3a induced IL-17a production in kidney transplant recipients (24). Collectively, these studies suggest interplay between IL-17a, lung expression of complement regulatory proteins, complement activation, and inflammatory pulmonary diseases, but they have not been studied in the setting of lung transplantation. Therefore, we hypothesized that complement activation occurring in transplanted lungs is in part due to local down regulation of CRPs. In addition, we hypothesize that IL-17a is related to this process and subsequent complement activation that culminate in OB development.
Materials and Methods
Animals
C57BL/6 and C57BL/10 mice (25-30 g, Harlan, Indianapolis, IN) were used for orthotopic left lung transplantation. Animals were housed in the Laboratory Animal Resource Center at Indiana University School of Medicine according to institutional guidelines. All studies were approved by the Indiana University School of Medicine Institutional Care and Use Committee.
Human Lung Tissues
Lung transplant tissues were obtained from explanted lungs harvested at that time for re-transplantation due to refractory OB. COPD or idiopathic pulmonary fibrosis were the initial indications for transplantation. Tissues were either fixed in formalin or snap frozen. The former and latter tissues were H&E stained to confirm the presence of OB. RNA and protein were extracted from frozen tissues with histologically proven OB and utilized for PCR and western blotting, respectively. Normal lung tissues were obtained from lobectomy tissues collected at the time of lung cancer resection.
Murine lung transplantation and complement inhibition
The orthotopic transplantation of left lungs was performed as previously described (18). Histological examination of lungs after 21 days, as described previously (18), identified those recipients with OB. Some mice were treated with an IL-17a:Fc fusion protein (Ad IL-17a Fc) as previously described (18). To study the effect of C5 inhibition, other lung transplant recipients were treated with anti-mouse C5 mAb (BB5.1mG1, 40 mg/kg) (Alexion, Chesire, CT) (25-26), which blocks the generation of C5a and C5b-9, and 40mg/kg of an isotype-matched irrelevant control mAb (r135.8) (Alexion) were purified from ascites. Subcutaneous injection of both reagents were performed at two days, 30 minutes before surgery and three times a week for three weeks after transplantation (eleven total injections preoperatively). All transplants were harvested on day 21 post transplantation. Hemolytic complement activity was determined as reported (27-28) using the same plasma samples assayed for C3a and C5a levels at day 21 post transplantation. Preliminary studies confirmed that BB5.1 completely abrogated hemolytic complement activity.
Bronchoalveolar Lavage (BAL) Fluid Samples
Human BAL samples were obtained as described and clinical characterization of the human samples has been reported (29). Mouse BAL fluid samples were obtained as previously reported (18).
Immunohistochemistry
Staining was performed on 4 μm tissue sections as previously described (30). In brief, sections obtained from paraffin-embedded, formalin-fixed lungs underwent antigen retrieval treatment, followed by peroxide and protein blocks (1× Power Block; Biogenex, San Ramon, CA). Sections were then incubated with the following primary antibodies; rabbit anti-mouse Crry (1:200), rabbit anti-mouse CD55 (1:200), rabbit anti-human CD46 (1:150), and rabbit anti-human CD55 (1:150) (all Santa Cruz Biotechnology, Santa Cruz, CA). Then sections were stained using a sensitive avidin-streptavidin-DAB peroxidase kit (Biogenex) according to the manufacturer's instructions. Sections were counterstained with hematoxylin. Quantification of CD55 and CD46 in human tissues was conducted using the Aperio Scanscope Imaging System. In brief, the slides were imaged using the Aperio Scancscoe CS and normal and OB bronchioles were identified. The Positive Pixel Count algorithm (Apeio Scanscope software) was used to quantify the amount of a specific stain present in a scanned slide image. A color range was specified (range of hues and saturation), as were three intensity ranges (weak, positive, and strong). For pixels which satisfied the color specification, the algorithm counted the number and intensity-sum in each intensity range, along with three additional quantities: average intensity, ratio of strong/total number, and average intensity of weak positive pixels. Default input parameters were pre-configured for brown color intensity fraction of positive to quantification in the three intensity ranges (220-175, 175-100, and 100-0). Pixels which were stained, but did not fall into the positive-color specification were considered negative stained pixels. These pixels were counted as well, so the fraction of positive to total stained pixels was determined. The algorithm was applied to an image by using ImageScope software which allows selection of an image Region of Analysis, specify the input parameters, run the algorithm and view and save the algorithm results. Computer-assisted morphometric analysis of digital images was done suing the Aperio software that came with the Aperio Digital Imaging System. Specific algorithms were used for the positive pixel analysis. Bronchioles comprised approximately 15% of the entire lung section analyzed.
C3a and C5a ELISA
C3a was quantitated in human BAL fluid samples using the human C3a ELISA Kit (BD Biosciences, Franklin Lakes, NJ) according to manufacturer's instructions. Human BAL samples were obtained from lung transplant recipients and were previously described in detail (29). Mouse C3a was assessed from BAL fluids by standard ELISA. The native protein (Cat. 558618), capture antibody (I-87-1162), and biotinylated detection antibody (I87-419) were obtained from BD Pharmingen. C5a ELISA was conducted with a commercial ELISA kit per manufacturer's protocol (R&D Systems, Minneapolis, MN).
Generation of col(V)-reactive lymphocytes
C57BL/6 mice were immunized by injecting 200 μg of col(V) emulsified in 200 μl of complete Freund's adjuvant (CFA); into the base of the tail. To boost the initial immunization, mice received a second injection of 200 μg of col(V) emulsified in 200 μl of incomplete Freund's adjuvant (IFA), 10 days after the initial injection of CFA (16). Ten days after boosting, mice were sacrificed, draining (inguinal) lymph nodes harvested and individual lymph node lymphocytes isolated as reported (6). Lymph node cells were resuspended in 1% sterile PBS prior to co-culture in mixed leukocyte reactions.
Western Blotting
Immunoblotting of soluble protein from airway epithelial cell (RLE-6TN, ATCC, Manassas, VA) cultures incubated with IL-17a, IL-6, or IL-10 (eBiosceinces, San Diego, CA) for varying time periods. Cytokine concentrations utilized were determined by preliminary studies. Cell cultures were performed as described (31). Primary antibodies for western blotting included CD55 (I-97, Santa Cruz Biotechnology, Santa Cruz, CA), Crry (M-180, Santa Cruz), and glyceraldehyde dehydrogenase (Santa Cruz).
Real Time Polymerase Chain Reaction
Total RNA from mouse lung samples was isolated as previously described (18). Reverse transcription and real-time PCR were performed as described (18). Primer pairs for IL-17a and β-actin primers are published (18). Primer pairs for Crry, CD55 (Human and mouse), and CD46 were purchased: [Mouse Crry: Mm00785297_s1 Taqman Primer Applied Biosystems, Mouse CD55(DAF1): Mm00438377_m1 Taqman Primer Applied Biosystems, Human CD55:Hs00892618_m1 Taqman Primer Applied Biosystems, Human CD46: Hs00611256_m1 Taqman Primer Applied Biosystems]. Relative quantity of target was assessed by 2−ΔΔt method, using β-actin Ct for normalization.
Mixed Lymphocyte Reaction
Splenic CD3+ T lymphocytes were isolated from C57BL/6 or C57BL/10 mice, or from C57BL/6 mice 21 days after lung transplantation as previously described (31). Col(V)-reactive T cells (CD3+) were also isolated from C57BL/6 mice immunized with col(V) in complete Freund's adjuvant as reported above. Alloantigen or col(V)-reactive T cells were co-cultured with irradiated T cell depleted splenocytes isolated from normal C57BL/6 mice in mixed leukocyte reactions as reported in the presence or absence of exogenous C3a (10 ng/ml, R&D Systems, Minneapolis, MN). Supernatants were collected for cytokine profiles after incubating for 72 hours.
Cytometric Bead Array
Cell free samples of culture medium or heparinized serum were assessed for cytokines by the Mouse Th1, Th2, Th17 CBA kit (BD Biosciences) as described, and analyzed by BD Cytometric Bead Array software, version 1.3 per manufacturer's instruction.
Statistical Analyses
Data are expressed as mean ± SEM. Analysis was by either 2-way ANOVA with paired or non-parametric t-test using Prism 4 (GraphPad Software, San Diego, CA). Significance was determined by p <0.05.
Results
Complement regulatory protein expression
Previous results from this laboratory showing complement-mediated cytotoxicity to airway epithelium in lung isografts after passive transfer of col(v) antibodies (6) led to the question why complement is being activated in the transplanted lung and not native lungs. To assess if complement regulators were down regulated on epithelial cells, the targets of early and late immune-mediated injury post transplantation, we utilized immunohistochemistry to detect CD55 and CD46 in lung tissues from normal humans and patients with lung transplant-associated OB. We also assessed CD55 and Crry, the murine homologue of CD46, expression in the lungs of orthotopically transplanted mice early post transplant and in OB lesions.
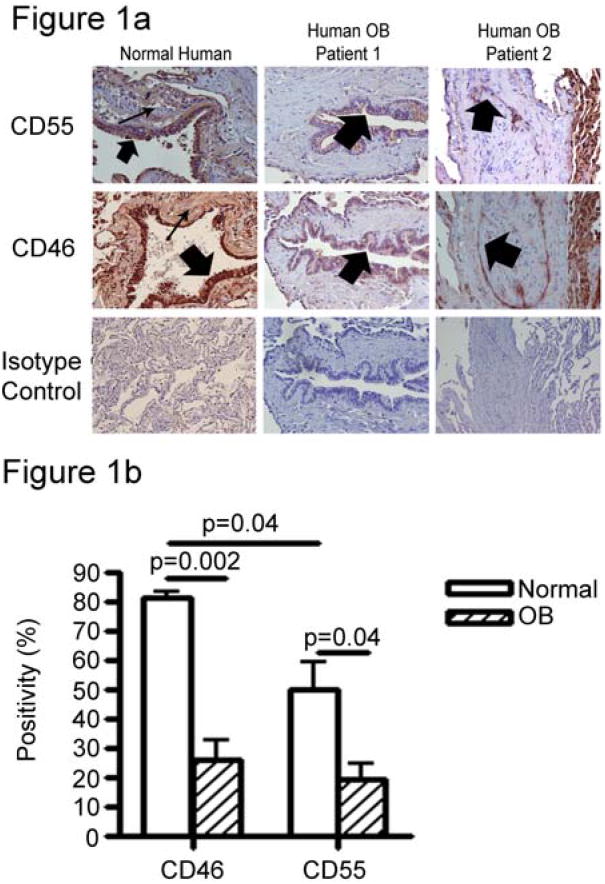
In normal human tissue, CD55 and CD46 were highly expressed on the epithelial cells of the large and small airways (Figure 1, wide arrows). These two CRPs were also expressed, to varying degrees, in the interstitial tissue of the lung (narrow arrows). However, CRP staining was seen less intensely on the vascular endothelium (data not shown). Lung tissue from patients diagnosed with clinical and histologic OB was also evaluated for CRP expression. One patient, (patient 1 in Figure 1A) showed constriction of airways without fibrotic growth into the airways or loss of epithelium. Lung tissue from this patient showed an intact epithelial layer on small airways, but CD55 and CD46 staining was nearly undetected in the epithelium or interstitium (Figure 1A). A second patient diagnosed with OB (patient 2 Figure 1A) showed obliteration of the airways, with partial loss of the epithelial cell layer. This patient also showed reduced levels of CD55 and CD46 staining throughout the lesion, including the airway epithelium, indicating that OB is characterized by low levels of CRP expression on airway epithelium. In two additional patients (including those shown in Figure 1A) quantitative immunohistochemistry was utilized to compare the expression of bronchiolar CD46 and CD55 in normal lungs and allografts with OB. Notably, expression of CD46 and CD55 were significantly less in bronchioles of OB lungs (Figure 1B).
Figure 1. Epithelial cell CRP expression is diminished in human OB.
A. Complement regulatory proteins expression was assessed by immunohistochemistry on 4 μm lung sections from normal and OB specimens. In humans, sections of normal lung show discrete CD55 and CD46 staining in airway epithelial cells (wide arrows) and in the interstitium (narrow arrows). However, lung sections from patients with OB (OB1 and OB2), airway epithelial cell staining of both CD55 and CD46 is severely down regulated. Two different OB patients are represented, patient 1 showing intact airway epithelium and patient 2 showing fibrotic plugs in the airway with a loss of epithelial cell integrity. Photomicrographs are representative of two normal human samples and six human OB patients. Magnification = 400×. B. The bar graph below the photomicrographs shows quantitation of the immunohistochemical images described above. Data shown are mean + SEM of four normal lungs and four lung transplants with histologically proven OB. (CD46: normal compared to OB, p<0.001; CD55 normal compared to OB, p<0.036).
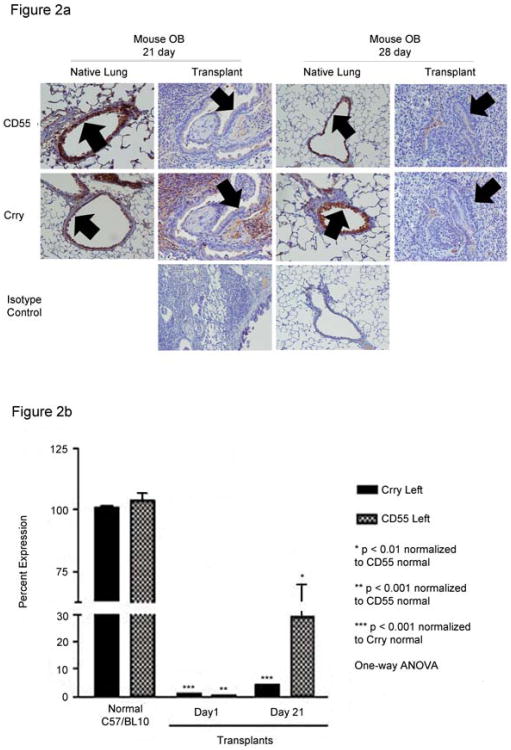
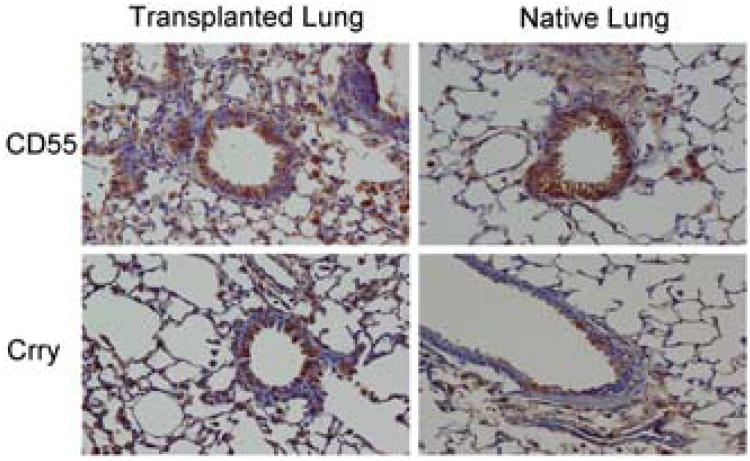
Orthotopic mouse lung transplants that developed OB were assessed for CD55 and Crry expression at 21 days and 28 days after transplantation (Figure 2A). Our prior studies confirm that OB occurs in approximately 50% of mice at 21 or 28 days post transplant (32). Mice diagnosed with OB 21 days post surgery had low levels of CD55 and Crry expression on the airway epithelium particularly within OB lesions (Figure 2A, wide arrows). CD55 staining in the interstitium was also low, although there was variable staining of Crry in the interstitium of some of these mice. In contrast, the native lung from 21 day OB mice had robust staining of both CD55 and Crry, indicating that the pathology was local and restricted to the transplanted lung. For both the transplanted and native lungs, animals with OB diagnosed at 28 days showed similar staining patterns to those of the 21 day animals (Figure 2A). To investigate the time to loss of CRP expression, we determined CD55 and Crry transcript expression at 1 day and 21 days post transplantation. Quantitative PCR confirmed significantly lower levels of transcripts for CD55 and Crry in allograft compared to normal C57BL/10 (donor) lungs at day 1 post transplantation, and that these changes persist at 21 days post transplantation (Figure 2B).
Figure 2. Airway epithelial cell CRP expression in mouse lung transplant recipients.
A. Recipient C57BL/6 mice developed OB after transplantation of left lungs from C57BL/10 donors. CD55 and Crry immunostaining in the transplanted lungs were low in airway epithelial cells at both 21 and 28 days post transplantation. Some interstitial staining of Crry was maintained in the 21 day animals. Both CD55 and Crry are apparent in airway epithelium (wide arrows) and in the parenchymal tissue of the native lungs at both time points and is similar to staining intensities and patterns from normal humans (Panel A) and from normal mice (data not shown). Epithelial expression of Crry and CD55 were markedly diminished in OB lesions of transplanted lungs (wide arrows). Isotype controls were carried out for either transplanted or native lungs from each time point as staining of both lungs were performed at the same time. Images are representative of 5-8 animals per condition. Magnification = 400×. B. Quantitative PCR showing diminished transcripts for CD55 and Crry 1 day and 21 days post murine lung transplantation as compared to lungs from normal C57BL/10 mice. Data shown are mean ± S.E.M. of three individual transplant experiments and three normal lungs.
Complement activation
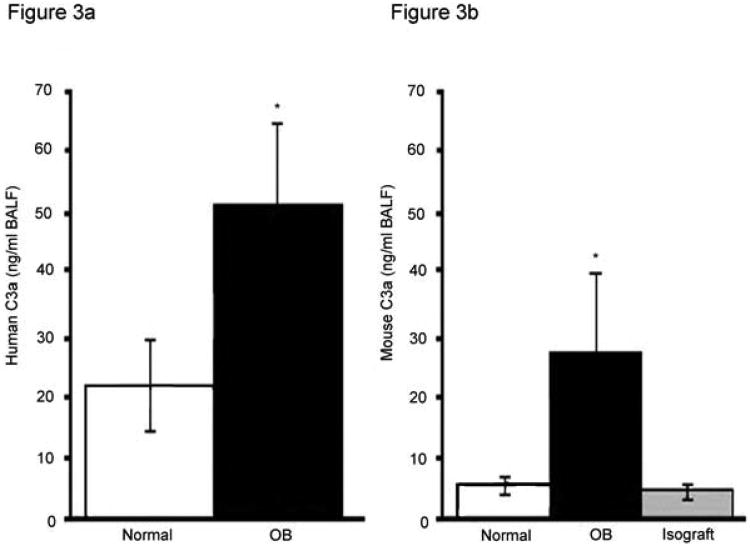
Low levels of CD55, CD46 and Crry on airway epithelium during OB in humans and mice suggested that complement might be activated in the lung. ELISA assay showed that normal human lung BAL fluid samples contained little C3a (Figure 3A). In contrast, patients with histologically proven OB showed a 2.5 fold higher (p<0.05) C3a level in BAL fluid. Normal mice also had low levels of C3a in BAL fluid by ELISA (Figure 3B). C57BL/6 mice receiving C57BL/6 isografts had similar low levels of C3a. In contrast, C3a levels were more than 5 fold higher in the BAL fluid from the lungs of C57BL/10 lungs transplanted into C57BL/6 mice during OB compared to normal or isograft mice (Figure 3B, p<0.05). Therefore, low levels of CRP expression on airway epithelium correlate with increased complement activation.
Figure 3. Humans and mice with OB have increased C3a in BAL fluids.
The complement split product, C3a was assessed in BAL fluids from humans and mice. BAL fluids were obtained under standard protocols and were performed identically for normal and OB samples. A) Normal patients (n=10) showed significantly lower C3a levels as compared to patients with OB (n=6), *p<0.05 vs. normal. B) Mice with allografted or isografted lungs were lavaged. Unoperated mice of the same sex, strain and age (normals) were also lavaged in the same manner. These normal mice (n=5) had significantly lower levels of BAL fluids C3a as compared to mice with OB (n=14). Isografted mice (n= 10) had C3a levels similar to those of normal mice * p<0.05 vs. normal or isograft).
IL-17 down regulates CRP expression
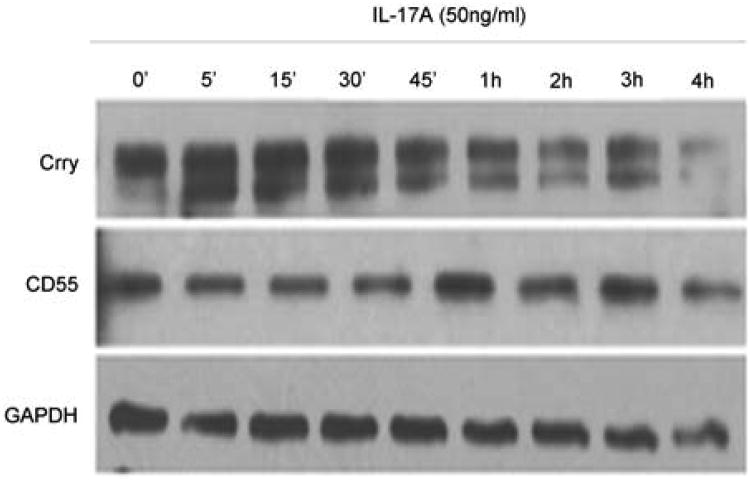
CRPs are down-regulated early in the course of rejection following transplantation, with reduction in Crry and CD55 transcripts noted within one day post transplantation in mice (Figure 2) at a time when IL-17a lung transcripts and protein were also increased (data not shown, manuscript in preparation). Notably, Crry and CD55 were also down regulated at day 28, the time when OB occurred; and our prior study reported that OB is IL-17 dependent (18). These data suggested that IL-17a levels and CRP expression might be linked and was further investigated by incubation of cultured rat airway epithelial cells with IL-17a or IL-6, a potent inducer of IL-17, and assessing CD55 and Crry by western blotting. IL-17a (50 ng/ml) induced dose-dependent reductions in Crry, but not CD55 on airway epithelial cells within 6 hours (Figure 4). IL-6 did not affect expression of CD55 or Crry at any time point (data not shown).
Figure 4. IL-17a mediates down-regulation of Crry in cultured rat airway epithelial cells.
RLE-6TN cells were cultured to 70% confluency and then treated with 50 ng/ml IL-17a for various times. Soluble protein was separated, transferred to a solid support and probed for Crry, CD55, and GAPDH as an internal protein loading control. Crry isoform levels decreased significantly by 6 hr. CD55 was also unaffected by IL-17a treatment. Data are representative of 4 experiments with the same results.
C3a stimulates alloantigen and col(V)-induced IL-17a production
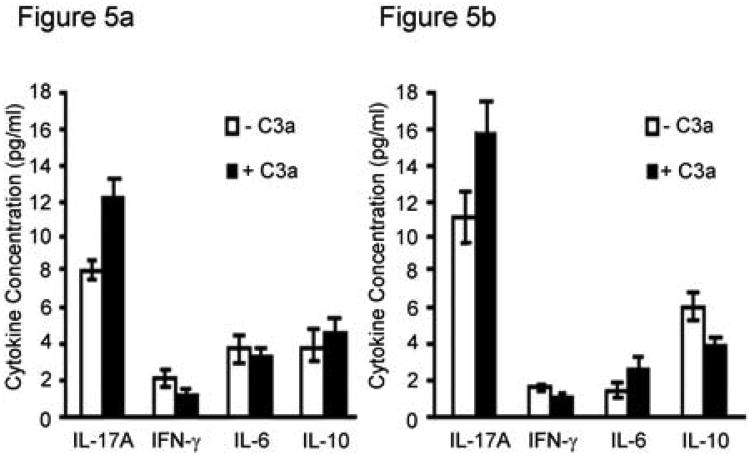
The results of the airway epithelial cell experiments suggested that IL-17a controls CRP expression, and in turn, complement activation in the lung after transplantation. We asked if a possible feed forward loop exists, wherein complement activation, in the form of C3a (22), could affect IL-17a production. Exogenous C3a induced significantly more IL-17 production from alloantigen-primed T cells (CD3+) in co-culture with allogeneic antigen presenting cells (C57BL/6-derived T cell-depleted splenocytes) (Figure 5A). In contrast, addition of C3a reduced IFN-γ production, but had no significant effect on IL-10, IL-6, or IL-2 (Figure 5A). We have reported that immune responses to the autoantigen col(V) have key roles in the rejection response, including OB, and are IL-17 dependent. Therefore, we also determined if C3a increased col(V)-induced IL-17 production from col(V)-reactive T cells. Indeed, exogenous col(V) significantly increased IL-17 levels, yet decreased IL-10 production from col(V)-reactive CD3+ T cells (C57BL/6) in co-culture with C57BL/6 T cell-depleted splenocytes as a source of antigen presenting cells (Figure 5B).
Figure 5. C3a effects on cytokine production and lymphocyte proliferation.
A. CD3+ splenic T lymphocytes (3 × 105) derived from C57BL/6 that received lung allografts from C57BL/10 mice were incubated with T cell-depleted splenocytes from C57BL/10 mice as a source of antigen presenting cells (3 × 105) in the presence and absence of C3a (10 ng/ml). B. Pure CD3+ T cells from col(V)-immunized mice (C57BL/6, 3 × 105) were incubated with T cell-depleted splenocytes from C57BL/6 mice as a source of antigen presenting cells (3 × 105) in the presence and absence of C3a (10 ng/ml). Conditioned medium was assessed for cytokines by cytometric bead array after 72 hour incubation. Levels of cytokines from wells of T lymphocytes alone or antigen presenting cells alone were below the level of detection. Values represent averages ± S.D. of three independent experiments.
IL-17a blockade effects on CRP expression, IL-17a, and complement activation
Previous results from this laboratory showed that neutralizing IL-17a using an adenoviral construct to over express the IL-17 receptor (IL-17RFc) in mice undergoing orthotopic lung transplantation abrogated OB development (18). Using tissues from these animals, we asked whether neutralizing IL-17 would restore CRP expression and reduce C3a levels in the animals that did not experience OB by 21 days. In contrast to Figure 2 showing diminished CD55 and Crry expression in the transplanted lung relative to native lung, neutralizing IL-17 resulted in comparable expression of these proteins in native and transplanted lungs (Figure 6). Luciferase adenoviral construct control animals showed no effect of the vector on CRP staining (data not shown).
Figure 6. IL-17a blockade restores CRP expression and reduces C3a and Il-17a levels.
In the mouse model of OB development after lung transplantation, some transplanted mice were treated with an Ad IL-17 Fc vector. Animals were assessed 21 days after transplantation, as this time point was common for OB development in this model (18). A) Lung sections were assessed for CD55 and Crry expression. Transplanted lung in Ad IL-17a Fc-treated animals showed significantly increased epithelial levels of both Crry and CD55 in airway epithelium as compared to mice with OB (Figure 2). Transplanted lungs showed similar staining patterns and staining intensity as native lungs. A luciferase control vector indicated that target signals were not altered by the vector used to deliver the Fc fusion protein. Micrographs are representative of at least three mice. Photomicrographs are representative of four Ad IL-17a Fc-treated mice and two control vector-treated mice, counterstained with hematoxylin. Magnification = 400×. B) In Fc fusion protein-treated animals, the IL-17a mRNA levels in the lung were significantly lower than in animals that were treated with a control vector. Values are averages ± S.D. of four Ad IL-17a Fc-treated mice and two control vector-treated mice, p=0.0313.
Blockade of IL-17 activity was further investigated by comparing BAL fluid C3a levels in Ad IL-17a Fc-treated animals and allografted animals that did not have OB (Table 1). BAL fluid from the transplanted lungs of the treated mice had low levels of C3a, similar to those of normal mice. Since these animals did not develop OB, the low levels of C3a might reflect robust transplant health. However, allografted mice that received the control vector and did not develop OB had high levels of BAL fluid C3a (p<0.05). C3a in untreated animals without OB was similar to those with OB (Table 1), which agrees with previous results showing that while only 50% of allografted mice develop OB, all showed acute rejection and increases in IL-17 (18). These data suggest that lower C3a levels were due to IL-17 blockade.
Tables 1. BAL fluid levels of C3a in mice post-lung transplant.
| Condition | BAL fluid C3a (ng/ml) ± SEM | p value vs. Normal |
|---|---|---|
| Normal | 5.25 ± 2.9* | |
| Allograft, OB† | 27.10 ± 14.2‡ | 0.0443‡‡ |
| Allograft, Ad IL-17 Fc-treated§ | 6.28 ± 3.1‖ | 0.2172 |
| Allograft, without OB** | 21.83 ± 7.3†† | 0.0076‡‡ |
n= 5 mice
OB diagnosed by histology 21 or 28 days post-lung transplant
n= 14 mice
Assessed 21 days after transplant
n= 4 mice
Assessed 21, 28, or 35 days after transplant, OB not apparent by histological analysis
n= 10 mice
statistically significant
Effect of complement inhibition on OB development
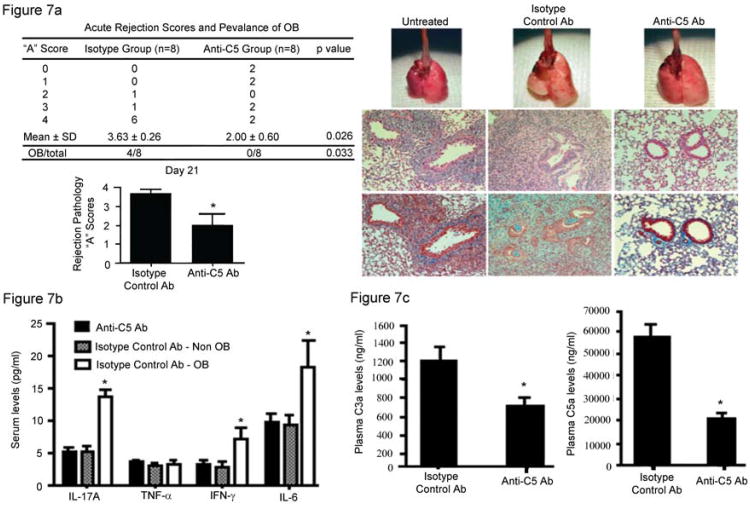
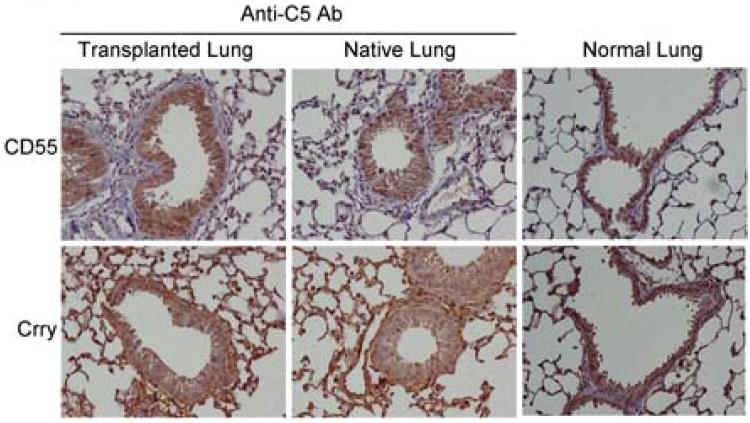
Data showing decreased expression of CD55 and Crry, with associated increased complement activation during OB that is associated with increased IL-17 suggested that complement inhibition may prevent OB and diminish systemic IL-17 levels. To address this question, C5 was inhibited systemically using an anti-C5 antibody (BB5.1) as described in Methods. Acute rejection scores, the presence or absence of OB, and systemic cytokine levels were determined. In addition, serum levels of C3a and C5a were measured. Notably, C5 inhibition abrogated the development of OB 21 days post transplantation, whereas OB occurred in 50% of control antibody-treated mice as expected (Figure 7A). Additionally, acute rejection scores (“A” scores) were reduced significantly in anti-C5 antibody-treated mice (Figure 7A). Whereas mice that develop OB also had higher systemic IL-17a, IL-6, and IFN-γ levels, inhibiting C5 resulted in significantly lower levels of each cytokine (Figure 7B). C5 inhibition resulted in significantly lower levels of C3a and C5a in plasma (Figure 7C). Since C5 inhibition down regulated IL-17, shown to decrease Crry and CD55 in vitro (Figure 4), we next determined if C5 blockade recovered CRP expression in vivo. Notably, immunostaining for Crry and CD55 protein expression was recovered in mice treated with the anti-C5 inhibitor compared to that observed in normal (non-transplanted) lungs (Figure 8). Total complement inhibition by BB5.1 antibodies were confirmed by quantifying complement hemolytic activity in BB5.1 compared to control mice as reported in Methods.
Figure 7. C5 blockade prevents OB, down regulates acute rejections and results in lower plasma levels of C3a and C5a following murine orthotopic lung transplantation.
The left lungs C57BL/10 mice were transplanted into C57BL/6 mice. Some mice were treated with anti-C5 antibody, or control antibody as described in Methods. Twenty-one days post transplantation the lungs were harvested and scored for rejection pathology using standard criteria. C3a and C5a plasma levels were determined. A. Rejection scores and histopathology. Fifty percent of control antibody treated mice developed OB, whereas none of the anti-C5 treated mice did. Anti-C5 treated mice also revealed lower acute rejection scores (also shown in the bar graph, “A” scores, p<0.026). Histopathology shows development of OB in control antibody treated mice plus severe acute rejection, whereas less severe acute rejection and absence of OB were noted in anti-C5 treated mice. The lower histologic sections are trichrome stains to show connective tissue deposition. Histologic scores represent the mean ± S.E.M. of 8 mice in each group. Histology representative of eight mice in each group (400× magnification). B. Plasma cytokine levels. Levels of each cytokine were measured as reported in Methods. The groups included those that received isoytpe control antibody and are divided into those that developed OB (n=4) and those that did not develop OB (n=4). The other group received anti-C5 antibodies (BB5.1) (n=8). Data shown are the mean ± S.E.M., *p<0.05 compared to mice that received anti-C5 treatment and did not develop OB, or that received the isotype control antibody that also did not develop OB. C. Plasma levels of C3a and C5a were determined at 21 days post transplantation in each group. Data represent the mean ± S.E.M. of eight mice in each group (p<0.0029 for C3a, and p<0.00007 for C5a).
Figure 8. Neutralizing C5 recovers airway epithelial cell CRP expression in mouse lung transplant recipients.
C57BL/6 that were recipients of orthotopic C57BL/10 lung grafts were treated with anti-C5 (BB5.1) or isotype control antibodies as reported in Methods. Twenty-one days post transplantation the lungs were harvested and immunostained for CD55 and Crry as reported. Data are representative of eight mice treated with anti-C5 inhibitor (BB5.1) and five normal C57BL/10 lungs. 400× magnification.
Discussion
This series of experiments shows that the CRPs, CD55 and Crry (CD46), are down regulated during OB in both humans and mice. At the same time, BAL fluid C3a is increased in both species. Importantly, IL-17a mediates down-regulation of Crry in cultured airway epithelial cells in vitro. Neutralizing IL-17a rescued Crry and CD55 expression in vivo and was associated with lower local levels of C3a. Finally, blocking C5 not only down regulated acute rejection and abrogated OB, it also reduced systemic IL-17 levels and local and systemic concentrations of C3a and C5a; and recovered Crry and CD55 expression. Data showing that C3a induces IL-17 in autoimmune and alloimmune environments and that IL-17a down regulates the expression of epithelial cell-derived Crry suggests a feed forward loop of IL-17 induced down regulation of CRPs and complement activation.
To the best of our knowledge, this is the first study is to demonstrate the integrated roles of IL-17a and complement in autoimmunity and alloimmunity of chronic lung transplant rejection. Some studies have noted the importance of IL-17a in both autoimmune and alloimmune mediated rejection (5, 18, 33-34), while others have noted that complement may be activated during rejection pathology (35-37), but the data shown here suggests a mechanism by which the innate and adaptive immune systems are interconnected in the development of OB.
IL-17a mediated down regulation of Crry (Figure 4) suggests that pre-existing inflammatory or autoimmune conditions in the recipient, especially with exposure of col(V), can predispose to early rejection, agreeing with the results of Iwata et al. (6). Furthermore, Crry is reported to be more dominant than CD55 in local complement regulation (15). Therefore, data showing that IL-17 is linked to down regulating CD55, but not Crry, in vitro may have great physiologic significance. However, in vivo it appears that IL-17 may regulate both CD55 and Crry expression suggesting differential effects of IL-17 in vivo compared to in vitro. Alternatively, IL-17 may induce other pathways that regulate CD55/Crry/CD46 expression.
The results of the present study suggest a feed forward loop in which IL-17a suppresses CRP expression, leading to complement activation and production of C3a. This stimulates more IL-17a production and possible further CRP down-regulation. CRP loss may be due to increased production of IL-17a. It is also notable that C5 blockade down regulated systemic production of IL-17 and IL-6, and this was associated with recovery of Crry and CD55 in the transplanted lung. An unexpected finding was how rapidly Crry and CD55 were down regulated post transplant which occurred within 24 hours post transplantation. This time frame corresponds to IL-17a induced down regulation in vitro as shown in Figure 4. It is also notable that when we assessed the time frame of systemic IL-17a production in the C57BL/10→C57BL/6 lung transplant model we observed IL-17a and IL-6 allograft lung transcripts were up regulated significantly at 24 hours post transplant (H. Suzuki and D.S. Wilkes, manuscript in preparation). The rapidity of this response suggests that IL-17a is induced during ischemia reperfusion injury in the transplanted lung. Indeed, Sharma et al reported robust IL-17 production from δγ-T cells within three hours of ischemia reperfusion injury in the lung (38). It is interesting to speculate that IL-17a or IL-6 directly have this effect or via induction of other cytokines that act in a paracrine fashion to block transcript expression. Alternatively, each cytokine or both could induce expression of matrix metalloproteases (MMPs) known to cleave Crry or CD55 from the cell surface (39). However, the specific molecular mechanism of IL-17 mediated down regulation of Crry and CD55 is unknown. Furthermore, while the loss of Crry and CD55 is rapid in lungs transplanted into normal recipients as shown in the mouse model, it is interesting to speculate that pre-transplant conditions such as idiopathic pulmonary fibrosis that is associated with systemic IL-17 activity, may only accelerate CRP loss. While these events could account for early CRP loss, data showing that Crry and CD55 are down regulated during OB suggests chronic dysregulation of CRP expression post lung transplantation. The mechanisms for chronic loss could also be due to MMPs that are known to be up regulated post lung transplantation (40-41), or perhaps mediated by chronic airway hypoxia known to occur post lung transplantation which can also enhance complement activation (42-44). Indeed, lung transplantation airway hypoxia has been implicated in fibrosis that could culminate in OB(43). While intriguing, the technical limitations of bronchial artery re-anastomosis in both mice and humans precludes our ability to directly answer this question. However, performing retrograde flush of the bronchial arteries at the time of donor harvest has been shown to decrease cytokines that play key roles in inflammation and immunity (45). Such an approach may prevent immune events associated with OB.
While antibodies have key roles in activating complement, recent evidence from Murakami et al. (46) suggests that Th17 development and IL-17a mediated complement activation can be initiated in the absence of a humoral immune response. In an IL-6- and IL-17 dependent model of arthritis, microbleeding into joint followed by transfer of Th17 polarized T cells were sufficient to stimulate a complement-dependent fulminant autoimmune arthritis. Based on data shown in the current study, it is interesting to speculate that complement activation in the arthritis model was in part induced by IL-17 and/or IL-6 mediated down regulation of CRPs on synovial tissues. This could account for IL-17 mediated damage associated with MHC class II alleles for which tissue-specific causative antigens cannot be identified (47). Alternatively, any pre-existing condition which increases IL-17 levels, such as ischemia reperfusion injury (38) or idiopathic pulmonary fibrosis (48), could down regulate CRPs on airway epithelium of newly transplanted donor lung as described above.
Importantly, these published reports taken together with the present data strongly suggest that any IL-17 mediated inflammatory process, in the presence of activated T cells, is sufficient to initiate the complement activation and production of C3a. What is more, this initiation and amplification of the feed forward loop may occur strictly in response to, and generate, a cellular immune response, as shown by the effects of C3a on cytokine production (Figure 5). Humoral immunity, in the form of anti-HLA antibodies or autoantibodies, may not be essential to the initiation or propagation of the loop, and this idea is currently under investigation. Alternatively, constitutive, low-level activation of the complement cascade via the alternative pathway could be amplified by IL-17a-mediated CRP down regulation and thereby initiate the feed forward loop.
However, our results could also support a role for antibodies in amplification of this loop. Complement mediated damage, whether induced by autoantibodies or anti-HLA antibodies, or infections could lead to exposure of interstitial col(V) on airway epithelial cells, thereby inducing an autoimmune response, more complement activation and IL-17a production. Indeed, our preclinical and patient studies, and those from other investigators clearly show a role for both alloimmune and autoimmune pathologies in OB (5, 33, 49). C3a induction of IL-17 in alloreactive and col(V)-reactive lymphocytes cytokine production support the concept of a feed forward loop of complement activation and IL-17a levels and further reductions in CRP expression that culminate in graft destruction.
In summary, the pre-clinical and clinical data in the current study suggest a key role for complement activation in OB pathogenesis. While the results of complement inhibition in ischemia reperfusion injury post lung transplantation have been promising, the current studies suggest a need for clinical trials in complement inhibition for the treatment of OB.
Acknowledgments
This study was funded by National Institutes of Health grants HL067177, HL096845, and P01AI084853 to DSW. This study also funded by The National Heart, Lung, and Blood Institute RO1 grant HL109288 to RV and RO1 grant HL109310 to RS.
References
- 1.Weigt SS, Wallace WD, Derhovanessian A, Saggar R, Lynch JP, Belperio JA. Chronic allograft rejection: epidemiology, diagnosis, pathogenesis, and treatment. Semin Respir Crit Care Med. 31:189–207. doi: 10.1055/s-0030-1249116. [DOI] [PubMed] [Google Scholar]
- 2.Christie JD, Bavaria JE, Palevsky HI, Litzky L, Blumenthal NP, Kaiser LR, Kotloff RM. Primary graft failure following lung transplantation. Chest. 1998;114:51–60. doi: 10.1378/chest.114.1.51. [DOI] [PubMed] [Google Scholar]
- 3.Khan SU, Salloum J, O'Donovan PB, Mascha EJ, Mehta AC, Matthay MA, Arroliga AC. Acute pulmonary edema after lung transplantation: the pulmonary reimplantation response. Chest. 1999;116:187–194. doi: 10.1378/chest.116.1.187. [DOI] [PubMed] [Google Scholar]
- 4.Bobadilla JL, Love RB, Jankowska-Gan E, Xu Q, Haynes LD, Braun RK, Hayney MS, Munoz del Rio A, Meyer K, Greenspan DS, Torrealba J, Heidler KM, Cummings OW, Iwata T, Brand D, Presson R, Burlingham WJ, Wilkes DS. Th-17, monokines, collagen type V, and primary graft dysfunction in lung transplantation. Am J Respir Crit Care Med. 2008;177:660–668. doi: 10.1164/rccm.200612-1901OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, Meyer KC, Hayney MS, Braun RK, Greenspan DS, Gopalakrishnan B, Cai J, Brand DD, Yoshida S, Cummings OW, Wilkes DS. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwata T, Philipovskiy A, Fisher AJ, Presson RG, Jr, Chiyo M, Lee J, Mickler E, Smith GN, Petrache I, Brand DB, Burlingham WJ, Gopalakrishnan B, Greenspan DS, Christie JD, Wilkes DS. Anti-type V collagen humoral immunity in lung transplant primary graft dysfunction. Journal of immunology. 2008;181:5738–5747. doi: 10.4049/jimmunol.181.8.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medof ME, Kinoshita T, Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984;160:1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinoshita T, Medof ME, Silber R, Nussenzweig V. Distribution of decay-accelerating factor in the peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria. J Exp Med. 1985;162:75–92. doi: 10.1084/jem.162.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicholson-Weller A, March JP, Rosen CE, Spicer DB, Austen KF. Surface membrane expression by human blood leukocytes and platelets of decay-accelerating factor, a regulatory protein of the complement system. Blood. 1985;65:1237–1244. [PubMed] [Google Scholar]
- 10.Asch AS, Kinoshita T, Jaffe EA, Nussenzweig V. Decay-accelerating factor is present on cultured human umbilical vein endothelial cells. J Exp Med. 1986;163:221–226. doi: 10.1084/jem.163.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medof ME, Walter EI, Rutgers JL, Knowles DM, Nussenzweig V. Identification of the complement decay-accelerating factor (DAF) on epithelium and glandular cells and in body fluids. J Exp Med. 1987;165:848–864. doi: 10.1084/jem.165.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Amouri IS, Bani-Ahmad M, Tang-Feldman Y, Lin F, Ko C, Pomeroy C, Oakley OR. Increased morbidity and mortality in murine cytomegalovirus-infected mice following allogeneic bone marrow transplant is associated with reduced surface decay accelerating factor expression. Clin Exp Immunol. 162:379–391. doi: 10.1111/j.1365-2249.2010.04241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu GH, Holers VM, Seya T, Ballard L, Atkinson JP. Identification of a third component of complement-binding glycoprotein of human platelets. J Clin Invest. 1986;78:494–501. doi: 10.1172/JCI112601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim YU, Kinoshita T, Molina H, Hourcade D, Seya T, Wagner LM, Holers VM. Mouse complement regulatory protein Crry/p65 uses the specific mechanisms of both human decay-accelerating factor and membrane cofactor protein. J Exp Med. 1995;181:151–159. doi: 10.1084/jem.181.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miwa T, Zhou L, Hilliard B, Molina H, Song WC. Crry, but not CD59 and DAF, is indispensable for murine erythrocyte protection in vivo from spontaneous complement attack. Blood. 2002;99:3707–3716. doi: 10.1182/blood.v99.10.3707. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida S, Haque A, Mizobuchi T, Iwata T, Chiyo M, Webb TJ, Baldridge LA, Heidler KM, Cummings OW, Fujisawa T, Blum JS, Brand DD, Wilkes DS. Anti-type V collagen lymphocytes that express IL-17 and IL-23 induce rejection pathology in fresh and well-healed lung transplants. Am J Transplant. 2006;6:724–735. doi: 10.1111/j.1600-6143.2006.01236.x. [DOI] [PubMed] [Google Scholar]
- 17.Braun RK, Molitor-Dart M, Wigfield C, Xiang Z, Fain SB, Jankowska-Gan E, Seroogy CM, Burlingham WJ, Wilkes DS, Brand DD, Torrealba J, Love RB. Transfer of tolerance to collagen type V suppresses T-helper-cell-17 lymphocyte-mediated acute lung transplant rejection. Transplantation. 2009;88:1341–1348. doi: 10.1097/TP.0b013e3181bcde7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan L, Benson HL, Vittal R, Mickler EA, Presson R, Fisher AJ, Cummings OW, Heidler KM, Keller MR, Burlingham WJ, Wilkes DS. Neutralizing IL-17 prevents obliterative bronchiolitis in murine orthotopic lung transplantation. Am J Transplant. 11:911–922. doi: 10.1111/j.1600-6143.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bharat A, Kuo E, Steward N, Aloush A, Hachem R, Trulock EP, Patterson GA, Meyers BF, Mohanakumar T. Immunological link between primary graft dysfunction and chronic lung allograft rejection. Ann Thorac Surg. 2008;86:189–195. doi: 10.1016/j.athoracsur.2008.03.073. discussion 196-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daud SA, Yusen RD, Meyers BF, Chakinala MM, Walter MJ, Aloush AA, Patterson GA, Trulock EP, Hachem RR. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175:507–513. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 21.Agrawal A, Sinha A, Ahmad T, Aich J, Singh P, Sharma A, Ghosh B. Maladaptation of critical cellular functions in asthma: bioinformatic analysis. Physiol Genomics. 2009;40:1–7. doi: 10.1152/physiolgenomics.00141.2009. [DOI] [PubMed] [Google Scholar]
- 22.Lajoie S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, Dienger K, Budelsky AL, Wills-Karp M. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol. 11:928–935. doi: 10.1038/ni.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X, Wang Y, Bernstein X, Li JT, Atabai K, Huang X, Sheppard D. IL-17A produced by alphabeta T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nature medicine. 2012;18:547–554. doi: 10.1038/nm.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loverre A, Tataranni T, Castellano G, Divella C, Battaglia M, Ditonno P, Corcelli M, Mangino M, Gesualdo L, Schena FP, Grandaliano G. IL-17 Expression by Tubular Epithelial Cells in Renal Transplant Recipients with Acute Antibody-Mediated Rejection. Am J Transplant. 11:1248–1259. doi: 10.1111/j.1600-6143.2011.03529.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Hu Q, Madri JA, Rollins SA, Chodera A, Matis LA. Amelioration of lupus-like autoimmune disease in NZB/WF1 mice after treatment with a blocking monoclonal antibody specific for complement component C5. Proc Natl Acad Sci U S A. 1996;93:8563–8568. doi: 10.1073/pnas.93.16.8563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng T, Hao L, Madri JA, Su X, Elias JA, Stahl GL, Squinto S, Wang Y. Role of C5 in the development of airway inflammation, airway hyperresponsiveness, and ongoing airway response. J Clin Invest. 2005;115:1590–1600. doi: 10.1172/JCI22906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, Arp J, Liu W, Faas SJ, Jiang J, Gies DR, Ramcharran S, Garcia B, Zhong R, Rother RP. Inhibition of terminal complement components in presensitized transplant recipients prevents antibody-mediated rejection leading to long-term graft survival and accommodation. J Immunol. 2007;179:4451–4463. doi: 10.4049/jimmunol.179.7.4451. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Rollins SA, Gao Z, Garcia B, Zhang Z, Xing J, Li L, Kellersmann R, Matis LA, Zhong R. Complement inhibition with an anti-C5 monoclonal antibody prevents hyperacute rejection in a xenograft heart transplantation model. Transplantation. 1999;68:1643–1651. doi: 10.1097/00007890-199912150-00007. [DOI] [PubMed] [Google Scholar]
- 29.Smith GN, Jr, Mickler EA, Payne KK, Lee J, Duncan M, Reynolds J, Foresman B, Wilkes DS. Lung transplant metalloproteinase levels are elevated prior to bronchiolitis obliterans syndrome. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7:1856–1861. doi: 10.1111/j.1600-6143.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- 30.Vittal R, Fan L, Greenspan DS, Mickler EA, Gopalakrishnan B, Gu H, Benson HL, Zhang C, Burlingham W, Cummings OW, Wilkes DS. IL-17 induces type V collagen overexpression and EMT via TGF-beta-dependent pathways in obliterative bronchiolitis. Am J Physiol Lung Cell Mol Physiol. 2013;304:L401–414. doi: 10.1152/ajplung.00080.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong L, Webb TJ, Wilkes DS. Dendritic cell-T cell interactions: CD8 alpha alpha expressed on dendritic cells regulates T cell proliferation. Immunol Lett. 2007;108:174–178. doi: 10.1016/j.imlet.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan L, Benson HL, Vittal R, Mickler EA, Presson R, Fisher AJ, Cummings OW, Heidler KM, Keller MR, Burlingham WJ, Wilkes DS. Neutralizing IL-17 prevents obliterative bronchiolitis in murine orthotopic lung transplantation. Am J Transplant. 2011;11:911–922. doi: 10.1111/j.1600-6143.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bharat A, Saini D, Steward N, Hachem R, Trulock EP, Patterson GA, Meyers BF, Mohanakumar T. Antibodies to self-antigens predispose to primary lung allograft dysfunction and chronic rejection. Ann Thorac Surg. 2010;90:1094–1101. doi: 10.1016/j.athoracsur.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vanaudenaerde BM, De Vleeschauwer SI, Vos R, Meyts I, Bullens DM, Reynders V, Wuyts WA, Van Raemdonck DE, Dupont LJ, Verleden GM. The role of the IL23/IL17 axis in bronchiolitis obliterans syndrome after lung transplantation. Am J Transplant. 2008;8:1911–1920. doi: 10.1111/j.1600-6143.2008.02321.x. [DOI] [PubMed] [Google Scholar]
- 35.Magro CM, Abbas AE, Seilstad K, Pope-Harman AL, Nadasdy T, Ross P., Jr C3d and the septal microvasculature as a predictor of chronic lung allograft dysfunction. Hum Immunol. 2006;67:274–283. doi: 10.1016/j.humimm.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Nakashima S, Qian Z, Rahimi S, Wasowska BA, Baldwin WM., 3rd Membrane attack complex contributes to destruction of vascular integrity in acute lung allograft rejection. J Immunol. 2002;169:4620–4627. doi: 10.4049/jimmunol.169.8.4620. [DOI] [PubMed] [Google Scholar]
- 37.Wallace WD, Reed EF, Ross D, Lassman CR, Fishbein MC. C4d staining of pulmonary allograft biopsies: an immunoperoxidase study. J Heart Lung Transplant. 2005;24:1565–1570. doi: 10.1016/j.healun.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 38.Sharma AK, LaPar DJ, Zhao Y, Li L, Lau CL, Kron IL, Iwakura Y, Okusa MD, Laubach VE. Natural killer T cell-derived IL-17 mediates lung ischemia-reperfusion injury. American journal of respiratory and critical care medicine. 2011;183:1539–1549. doi: 10.1164/rccm.201007-1173OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morgan J, Spendlove I, Durrant LG. The role of CD55 in protecting the tumour environment from complement attack. Tissue antigens. 2002;60:213–223. doi: 10.1034/j.1399-0039.2002.600303.x. [DOI] [PubMed] [Google Scholar]
- 40.Iwata T, Chiyo M, Yoshida S, Smith GN, Jr, Mickler EA, Presson R, Jr, Fisher AJ, Brand DD, Cummings OW, Wilkes DS. Lung transplant ischemia reperfusion injury: metalloprotease inhibition down-regulates exposure of type V collagen, growth-related oncogene-induced neutrophil chemotaxis, and tumor necrosis factor-alpha expression. Transplantation. 2008;85:417–426. doi: 10.1097/TP.0b013e31815e91b6. [DOI] [PubMed] [Google Scholar]
- 41.Sato M, Hirayama S, Lara-Guerra H, Anraku M, Waddell TK, Liu M, Keshavjee S. MMP-dependent migration of extrapulmonary myofibroblast progenitors contributing to posttransplant airway fibrosis in the lung. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9:1027–1036. doi: 10.1111/j.1600-6143.2009.02605.x. [DOI] [PubMed] [Google Scholar]
- 42.Khan MA, Jiang X, Dhillon G, Beilke J, Holers VM, Atkinson C, Tomlinson S, Nicolls MR. CD4+ T cells and complement independently mediate graft ischemia in the rejection of mouse orthotopic tracheal transplants. Circulation research. 2011;109:1290–1301. doi: 10.1161/CIRCRESAHA.111.250167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dhillon GS, Zamora MR, Roos JE, Sheahan D, Sista RR, Van der Starre P, Weill D, Nicolls MR. Lung transplant airway hypoxia: a diathesis to fibrosis? American journal of respiratory and critical care medicine. 2010;182:230–236. doi: 10.1164/rccm.200910-1573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang X, Khan MA, Tian W, Beilke J, Natarajan R, Kosek J, Yoder MC, Semenza GL, Nicolls MR. Adenovirus-mediated HIF-1alpha gene transfer promotes repair of mouse airway allograft microvasculature and attenuates chronic rejection. The Journal of clinical investigation. 2011;121:2336–2349. doi: 10.1172/JCI46192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roe DW, Fehrenbacher JW, Niemeier MR, Zieger M, Labarrere C, Wilkes DS. Lung preservation: pulmonary flush route affects bronchial mucosal temperature and expression of IFN-gamma and Gro in regional lymph nodes. Am J Transplant. 2005;5:995–1001. doi: 10.1111/j.1600-6143.2005.00789.x. [DOI] [PubMed] [Google Scholar]
- 46.Murakami M, Okuyama Y, Ogura H, Asano S, Arima Y, Tsuruoka M, Harada M, Kanamoto M, Sawa Y, Iwakura Y, Takatsu K, Kamimura D, Hirano T. Local microbleeding facilitates IL-6- and IL-17-dependent arthritis in the absence of tissue antigen recognition by activated T cells. J Exp Med. 208:103–114. doi: 10.1084/jem.20100900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skapenko A, Leipe J, Lipsky PE, Schulze-Koops H. The role of the T cell in autoimmune inflammation. Arthritis Res Ther. 2005;7(2):S4–14. doi: 10.1186/ar1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, Cheever AW, Wynn TA. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med. 207:535–552. doi: 10.1084/jem.20092121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saini D, Weber J, Ramachandran S, Phelan D, Tiriveedhi V, Liu M, Steward N, Aloush A, Hachem R, Trulock E, Meyers B, Patterson GA, Mohanakumar T. Alloimmunity-induced autoimmunity as a potential mechanism in the pathogenesis of chronic rejection of human lung allografts. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2011;30:624–631. doi: 10.1016/j.healun.2011.01.708. [DOI] [PMC free article] [PubMed] [Google Scholar]