The inability to grow normal or premalignant human mammary epithelial cells as in vivo explants has thwarted research biologists for the past 30 years (1–4). Although human breast cancer cells can be passaged in vivo as a xenograft or in vitro as cell cultures, this capability does not extend to normal or premalignant human mammary cells, with a few rare exceptions (5). Previous studies have shown that normal and premalignant human mammary epithelial cells can be maintained as a xenograft, but expansive growth has been extremely rare (1–4). The future of preventing the onset and progression of breast cancer lies in elucidating the biology of premalignant development. This cannot be accomplished without appropriate in vivo models and the methodology to establish such models. Although rodent models are extremely valuable for understanding the salient biological and molecular changes critical for premalignant and malignant development, the best models still cannot replicate entirely the human situation. There are several reasons for this problem, but one of the most important is the fundamental difference in the microenvironment between the commonly used models, i.e., rodent and human. The mouse and rat mammary glands comprise primarily an adipose stroma with epithelial parenchyma; the human gland is composed of a fibrous–adipose stroma with epithelial parenchyma. In this issue of PNAS, Kuperwasser et al. (6) describe a method that allows the expansion of normal human breast epithelium as a xenograft in immunocompromised mice. The procedure is reproducible and, with a modest learning curve, is technically available to most any laboratory. The procedure is relatively inexpensive and theoretically applicable to premalignant breast epithelium, as well. The promise the method brings to the study of normal and premalignant breast disease is enormous. Researchers now have the means to develop transplantable cell lines of normal and premalignant human breast epithelium to test chemopreventive agents in an environment that resembles that from which human cancer arises.
The success of the method rests on two fundamental pillars of mammary gland biology, one well established and one newly recognized. The well established pillar is the ability to transplant mammary epithelium into its orthopic site, the mammary fat pad. This procedure was established by DeOme et al. in 1959 (7) and has been used extensively in modern mammary studies (8). The second pillar is the recognition that the mammary stroma has an instructive role in mammary epithelial cell function (9–12), with the added recognition that human stroma is fundamentally different from mouse stroma. Using these two premises, Kuperwasser et al. (6) established a procedure to humanize the mouse stroma and provide an appropriate microenvironment to grow normal human epithelium. In a series of experiments, they demonstrate that normal mammary epithelium will grow and expand in the appropriately prepared mouse stroma, and that these epithelial cells undergo normal morphogenesis and functional differentiation. Furthermore, this procedure is reproducible with a high rate of success.
Normal mammary epithelium will grow and expand in the prepared mouse stroma.
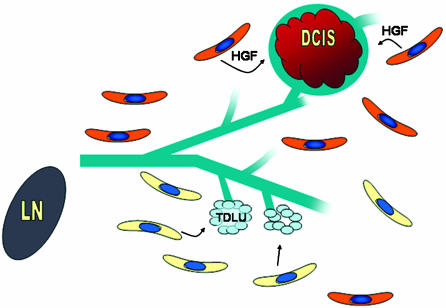
Perhaps the most astonishing result was the demonstration that stroma (fibroblasts) genetically modified to function in an atypical fashion [here, the overexpression of either hepatocyte growth factor or type β1 transforming growth factor (TGF-β1) promoted the outgrowth of premalignant and malignant epithelial cells from a cell preparation of morphologically normal mammary epithelium]. In contrast, normal human fibroblasts allowed only the growth of normal mammary epithelium from the same donor material (Fig. 1). This result has enormous implications for understanding the progression of human premalignancy, because it stresses the importance of a determinative role for stroma. There have been several results in recent years that support the concept that stromal cells in a tumor field are atypical and that this atypical stroma can influence the expression of premalignant and malignant cells (11, 12). In a recent study, Bhowmick et al. (12) demonstrated that conditional inactivation of the TGF-βII receptor gene in fibroblasts leads to epithelial proliferation in prostate. The epithelial proliferation resembled prostate intraepithelial neoplasia. In their study, the loss of TGF-β signaling in fibroblasts was accompanied by activation of hepatocyte growth factor signaling. The combination of the loss of a growth inhibitory pathway with the activation of a growth-promoting pathway provided a local environment that promoted epithelial proliferation of apparently normal cells.
Fig. 1.
An idealized view of epithelial–stromal interactions based on the results of Kuperwasser et al. (6). “Normal” fibroblasts (yellow) support growth and normal morphogenesis of human mammary epithelial cells as a xenograft. Ducts and terminal ductal lobular units (TDLU) are a normal morphogenic signature. Fibroblasts (orange) engineered to express the hepatocyte growth factor (HGF) at high levels promote the outgrowth of premalignant mammary epithelium, ductal carcinoma in situ (DCIS). In this model, fibroblasts can dictate the morphogenic pathway of mammary epithelial cells (drawing courtesy of Anne Shepard, Baylor College of Medicine).
The importance of stroma as a determinative factor in the development of breast cancer and in epithelial cancer in general has gained a wide and appreciative audience. For breast cancer, the pioneering work of Emerman and Pitelka (9) in 1977 set the stage for the illuminating and paradigm-shifting experiments of Bissel et al. (10). Bissel et al. (10) have argued long and eloquently for the determinative role of stroma function for both normal mammary gland morphogenesis and tumor development. They have been in the forefront of defining the signaling mechanisms underlying the function of stroma in regulating epithelial cell function. Kuperwasser et al. (6) have taken a giant step and have shown in vivo the tremendous influence of stromal fibroblasts for human breast morphogenesis and premalignant progression. The results of Kuperwasser et al. (6) open up multiple avenues of research and lift the dark cloud overhanging innovative and productive research using normal and premalignant human breast epithelium. In the foreseeable future, researchers will be able to develop transplantable cell lines of normal and premalignant human breast epithelia to test the effect of chemopreventive agents on growth and progression and to use these stable cell populations to examine the molecular and genetic basis for premalignant progression. Experiments that have been only dreams are now possible because of the innovative and pioneering experiments of this research team.
See companion article on page 4966.
References
- 1.Outzen, H. C. & Custer, R. P. (1975) J. Natl. Cancer Inst. 55, 1461–1463. [DOI] [PubMed] [Google Scholar]
- 2.McManus, J. J. & Welsch, C. W. (1980) Cancer 45, 2160–2165. [DOI] [PubMed] [Google Scholar]
- 3.Mahta, R. R., Graves, J. M., Hart, G. D., Shitkaitis, A. & Das Gupta, T. K. (1993) Breast Cancer Res. Treat. 25, 65–71. [DOI] [PubMed] [Google Scholar]
- 4.Yang, J., Guzman, R. C., Poprikolov, N., Bandyopadhyay, G. K., Christov, K., Collins, G. & Nandi, S. (1994) Cancer Lett. 81, 117–127. [DOI] [PubMed] [Google Scholar]
- 5.Dawson, P. J., Wolman, S. R., Tait, L., Heppner, G. H. & Miller, F. R. (1996) Am. J. Pathol. 148, 313–319. [PMC free article] [PubMed] [Google Scholar]
- 6.Kuperwasser, C., Chavarria, T., Wu, M., Magrane, G., Gray, J. W., Carey, L., Richardson, A. & Weinberg, R. A. (2004) Proc. Natl. Acad. Sci. USA 101, 4966–4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeOme, K. B., Faulkin, L. J., Jr., Bern, H. A. & Blair, P. E. (1959) Cancer Res. 19, 515–520. [PubMed] [Google Scholar]
- 8.Medina, D. (1996) J. Mammary Gland Biol. Neoplasia 1, 5–19. [DOI] [PubMed] [Google Scholar]
- 9.Emerman, J. T. & Pitelka, D. R. (1977) In Vitro 13, 316–328. [DOI] [PubMed] [Google Scholar]
- 10.Bissell, M. J., Rizki, A. & Mian, I. S. (2003) Curr. Opin. Cell Biol. 15, 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parmar, H., Young, P., Emerman, J. T., Neve, R. M., Dairkee, S. & Cumha, G. R. (2002) Endocrinology 143, 4886–4896. [DOI] [PubMed] [Google Scholar]
- 12.Bhowmick, N. A., Chytil, A., Plieth, D., Gorska, H. E., Dumont, N., Shappell, S., Washington, M. K., Neilson, E. G. & Moses, H. L. (2004) Science 303, 848–851. [DOI] [PubMed] [Google Scholar]