Abstract
T cell infiltration of melanoma is associated with enhanced clinical efficacy and is a desirable endpoint of immunotherapeutic vaccination. Infiltration is regulated, in part, by chemokine receptors and selectin ligands on the surface of tumor-specific lymphocytes. Therefore, we investigated the expression of two homing molecules – CXCR3 and CLA – on vaccine-induced CD8 T cells, in the context of a clinical trial of a melanoma-specific peptide vaccine. Both CXCR3 and CLA have been associated with T cell infiltration of melanoma. We demonstrate that a single subcutaneous/intradermal administration of peptide vaccine in Montanide adjuvant induces tumor-specific CD8 T cells that are predominantly positive for CXCR3, with a subpopulation of CXCR3+CLA+ cells. Addition of GM-CSF significantly enhances CXCR3 expression and increases the proportion of CLA-expressing cells. Concurrent with CXCR3 and CLA expression, vaccine-induced CD8 cells express high levels of Tbet, IFN-γ, and IL-12Rβ1. Collectively, these studies demonstrate that peptide vaccination in adjuvant induces CD8 T cells with a phenotype that may support infiltration of melanoma.
Keywords: CXCR3, CLA, IL-12R, peptide vaccines, adjuvant, GM-CSF, melanoma
Introduction
T cell infiltration of melanoma is required, yet still poorly achieved, for effective immune-mediated tumor control of this aggressive and recalcitrant cancer. T cell extravasation from circulation and infiltration to tissue is a multi-step process that is mediated, in part, by lymphocyte-expressed chemokine receptors (CCRs) which recognize chemokines displayed on the luminal surface of vascular endothelium(1,2). The CXC chemokine receptor 3 (CXCR3) is expressed by Th1-skewed CD4 helper T cells(3,4) and Tc1-type CD8 effector T cells(5). CXCR3 mediates chemotaxis upon binding its chemokine ligands: CXCL9 (monokine induced by gamma-interferon, MIG)(6), CXCL10 (interferon-induced protein of 10kDa, IP-10)(7), and CXCL11 (interferon-inducible T cell alpha chemokine, I-TAC)(8). These ligands are strongly induced by type II interferon (IFN-γ), and to a lesser extent by IFN-α/β and tumor necrosis factor-alpha (TNF-α)(9). Strong induction of CXCL9-11 in sites of interferon-driven type-1 inflammation lead to the accumulation of Th1 helper cells and Tc1-skewed CTL(10).
Recent studies have demonstrated the relevance of CXCR3 and its chemokines for the effective immune-mediated control of melanoma. Expression of CXCR3 by tumor-specific CD8 T cells has been associated with increased survival in patients with advanced metastatic melanoma(11), suggesting that CXCR3 is a critical mediator of T cell chemotaxis to the tumor microenvironment. Accordingly, the presence of CXCL9 and CXCL10 in primary or metastatic melanomas is associated with robust T cell infiltrates(12,13). Thus, the induction of CXCR3+ tumor antigen-specific CD8 cells is thought to be a desirable outcome of active immunotherapy.
CXCR3 is absent from naïve T cells but upregulated by dendritic cell (DC)-induced activation of human T cells in vitro(3) and murine T cells in vivo(14). Using in vitro assay systems, CXCR3 is expressed within 48-72h following activation of CD8 T cells. Na and colleagues have also reported that adding GM-CSF to intradermal/subcutaneous peptide vaccines significantly enhanced CXCR3 expression on CD4+ T cells specific for the neoantigen keyhole limpet hemocyanin (KLH), suggesting that CXCR3 expression on vaccine-activated T cells can be modulated by addition of cytokine to the vaccine microenvironment(15). However, it remains unknown whether peptide vaccination and adjuvant can induce or increase CXCR3 expression by CD8 T cells that recognize and target endogenous melanocyte differentiation protein (MDP)-derived antigens or cancer-testis antigens.
Molecules other than CCRs are also important for T cell targeting of inflamed or neoplastic tissues, and recent studies have highlighted the importance of cutaneous lymphocyte antigen (CLA) in the infiltration of melanoma lesions(16). CLA is an inducible carbohydrate modification of P-selectin glycoprotein ligand-1 (PSGL-1)(17) that facilitates binding of T cells to E-selectin, an adhesion molecule expressed on vascular endothelium in inflamed skin(18). E-selectin was reported to be expressed by tumor-infiltrating vasculature in a majority of examined dermal malignant melanomas(19), although largely absent from metastases(20). CLA is expressed on T cells following antigen-specific activation in peripheral lymphoid tissues(21), and CLA expression has been linked to T cell activation and expression of CXCR3 and IL-12R(22). Most melanoma-specific active immunotherapies are delivered by subcutaneous and/or intradermal injection, resulting in antigen presentation in skin draining lymph nodes (LN), yet it is unknown whether peptide vaccination induces CLA-expressing T cells.
We hypothesized that subcutaneous/intradermal vaccination with peptide antigens in adjuvant may induce – and that GM-CSF may enhance – the expression of CXCR3, CLA, and IL-12R by antigen-specific CD8 T cells. As the binding partners of CXCR3 and CLA may be present or inducible in melanoma-associated vasculature, the expression of CXCR3 and CLA may define the capacity of vaccine-induced T cells to efficiently infiltrate tumors. In the present study, we evaluated CXCR3 and CLA expression on human tumor-specific CD8 cells isolated from patients following the administration of a multi-peptide vaccine and Montanide ISA-51, in the presence or absence of GM-CSF(23).
Materials and Methods
Vaccination and collection of patient samples
T cells analyzed in this study were collected from patients with advanced (stage III or IV) melanoma who had been vaccinated in an experimental phase II melanoma peptide vaccine trial, which has been reported (University of Virginia trial Mel43(23)). The clinical trial was approved by the University of Virginia Human Investigations Committee/Institutional Review Board (HIC#10524) and the FDA (BB-IND #9847), and was registered at clinicaltrials.gov (NCT00089193).
For primary analyses, patients received a vaccine comprising 12 melanoma peptides restricted by HLA-A1, HLA-A2, or HLA-A3 as previously described(23): A1 peptides: DAEKSDICTDEY (Tyrosinase 240-251, which has a substitution of S for C at residue 244), SSDVIPIGTY (Tyrosinase 146-156), EADPTGHSY (MAGE-A1 161-169), and EVDPIGHLY (MAGE-A3168-176); A2 peptides: YMDGTMSQV (Tyrosinase 369-377D), IMDQVPFSV (gp100 209-217, 209-2M), YLEPGPVTA (gp100 280-288), and GLYDGMEHL (MAGE-A10 254-262); and A3 peptides: ALLAVGATK (gp100 17-25), LIYRRRLMK (gp100 614-622), SLFRAVITK (MAGE-A1 96-104), and ASGPGGGAPR (NY-ESO-1 53-62). The tetanus helper peptide used was AQYIKANSKFIGITEL. This combination of 12 MHC class I–restricted peptides plus 1 class II–restricted peptide is called MELITAC 12.1. Each vaccine was 2mL of a stable water-in-oil emulsion consisting of 100μg of each of the 12 MHC class I–restricted peptides, 190μg of the tetanus helper peptide, and 1mL Montanide ISA-51 adjuvant (Seppic, Inc; Paris, France). For some patients, the emulsion also contained 110μg GM-CSF (Berlex, now Genzyme). The full emulsion was administered to one extremity, with half of the dose administered subcutaneously and half administered intradermally. Vaccines were administered on days 1, 8, 15, 29, 36, and 43 and then at 3, 6, 9, and 12 months.
To obtain PBMCs, blood (80 to 100 mL) was drawn before treatment and one week after each vaccination. Lymphocytes were isolated using Ficoll gradient centrifugation and cryopreserved in 10% dimethyl sulfoxide/90% serum.
Flow Cytometric Staining and Analyses
Enumeration of antigen-specific T-cell responses was done using tetrameric HLA class I/peptide reagents (tetramers; Beckman Coulter), as described. HLA-A2 tetramers used were MAGE-A10254-262 (GLYDGMEHL), gp100209-217 (IMDQVPFSV), gp100280-288 (YLEPGPVTA), and Tyrosinase369-377D (YMDGTMSQV); A3 tetramers used were gp10017-25 (ALLAVGATK) and gp100614-622 (LIYRRRLMK). Tetramers for the other six peptides were not studied: two do not recognize functional antigen-reactive T cells (DAEKSDICTDEY and SLFRAVITK), one could not be synthesized (ASGPGGGAPR), two others were not used because of the low rates of T-cell response previously observed in ELIspot assays (EADPTGHSY and SSDVIPIGTY), and one worked but was not evaluated (EVDPIGHLY).
Samples for homing receptor analysis were selected based on previous demonstration of immunogenic response to at least one antigen, as assessed by ELIspot assays(23). After thawing, PBMCs were enriched for CD8+ cells (Miltenyi Biotec), washed twice, suspended in PBS/2% FCS (Sigma-Aldrich), and incubated with tetramer for 15 min. at room temperature before adding a mixture of anti-CD8 (clone: SFCI21Thy2D3, Beckman Coulter), anti-CD45RO (clone UCHL1, BD Biosciences), and anti-CLA (clone HECA-452, BD Biosciences). Some samples were then stained for surface 12Rβ1 (clone 69319, R&D Systems). For intracellular staining, following tetramer staining, T cells were washed and fixed with 1% paraformaldehyde in PBS at room temperature for 20 min(24). Intracellular CXCR3 was assessed using anti-CXCR3 (clone 1C6, BD Biosciences); intracellular Tbet protein was assessed using a monoclonal antibody (1μg/106 cells, eBioscience clone 4B10); and intracellular IFN-γ protein was assessed using a monoclonal antibody (1μg/106 cells, eBioscience clone 4S.B3). For all experiments, data were acquired on a Becton-Dickinson LSRFortessa and analyzed with FlowJo (Treestar) software.
Statistics
The Student’s t test or Mann-Whitney U test was used to determine whether there were statistically-significant differences in the percentage of chemokine receptor-positive cells in pre- and post-vaccine samples. Statistical analyses were performed using MiniTab 16 Software.
Results
CXCR3 and CLA expression is increased on tumor antigen-specific CD8 T cells ex vivo
To assess whether peptide vaccination induces the expression of selected tissue homing markers, we evaluated PBMC samples of 24 patients who had received repeated immunizations with 12 class I-restricted MDP- and cancer testis antigen-derived peptides and a class II-restricted tetanus peptide (MELITAC 12.1) either with (n = 12) or without (n = 12) GM-CSF(23). Cryopreserved patient-derived PBMC were thawed then enriched for CD8 cells, which were then stained with a pooled set of HLA-A2-restricted melanoma antigen/MHC tetramers and mAbs for homing markers. As CXCR3 is internalized upon ligand binding and may not be available for surface staining(25), we used a modified protocol of Dimopoulos(24) for intracellular (i.c.) staining of CXCR3 (representative data, Supplemental Fig. 2).
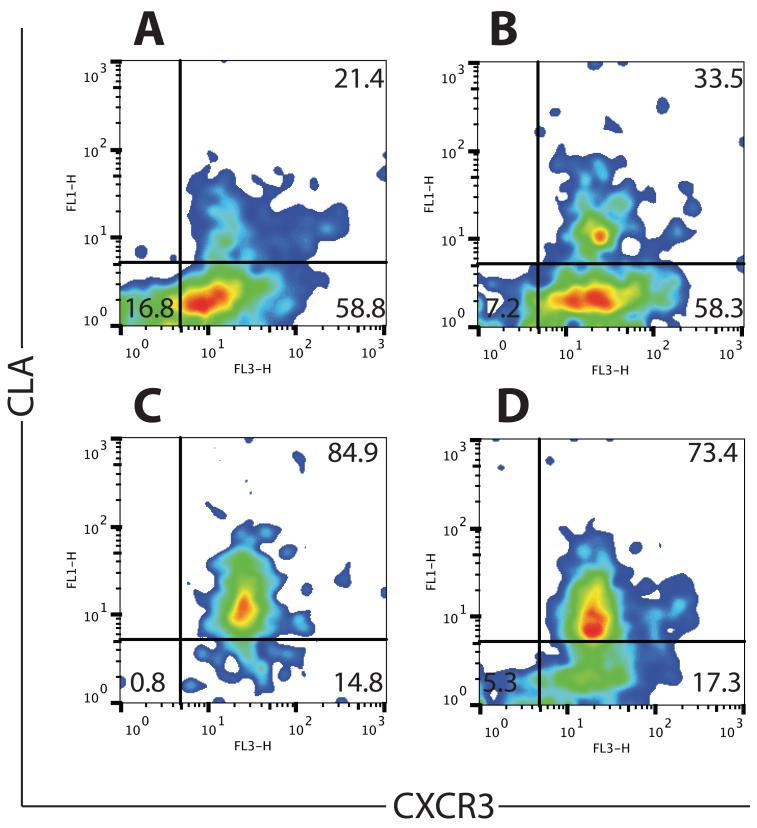
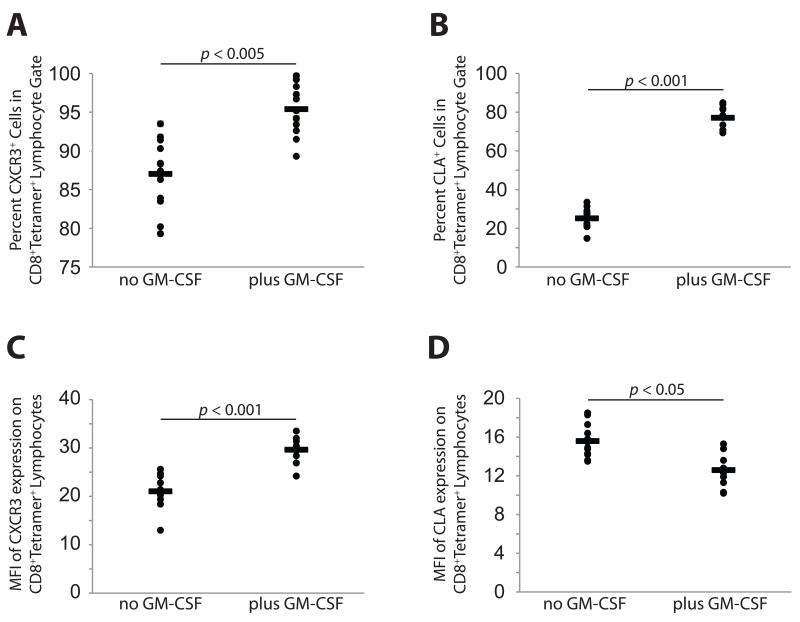
Following a single vaccination with MELITAC 12.1, the majority of tetramer-positive CD8 cells (87.0% ± 4.5) expressed i.c. CXCR3, and a subset (25.1% ± 5.2) expressed surface CLA (representative data from four patients in Fig. 1). Our data for CXCR3, assessed by intracellular staining, suggests a higher proportion of CXCR3-expressing cells following vaccination than previously estimated using surface staining in similar patient isolates(26). Addition of GM-CSF to the vaccine modestly but significantly (p < 0.005) increased the proportion of tetramer-positive cells expressing CXCR3 (95.4% ± 3.2, Fig. 2A) and significantly (p < 0.001) enhanced the proportion of cells expressing CLA (77.1% ± 5.4, Fig. 2B), but vaccination did not induce, and GM-CSF did not enhance (p > 0.05), CXCR3 or CLA expression in non antigen-specific (tetramer-negative) cells (Supplemental Fig. 3).
Fig. 1. Peptide vaccination with MELITAC 12.1 in Montanide adjuvant induces CXCR3 and CLA expression in tetramer-positive CD8+ T cells.
Representative flow cytometry of patient-derived peripheral blood samples. Each panel represents an individual patient. PBMC were collected one week after a single vaccination with MELITAC 12.1, either with (A, B) or without (C, D) GM-CSF. All populations are gated on CD8+ CD45RO+ tetramer+ cells and shows i.c. CXCR3 and surface CLA staining. Each panel is from an individual patient.
Fig. 2. Peptide vaccine enhances the proportion of tetramer-positive CD8 T cells expressing CXCR3 and CLA.
Percentage of tetramer-positive CD45RO+ CD8+ cells expressing CXCR3 and CLA. A). CXCR3 expression in tetramer-positive CD8 cells, with and without GM-CSF in vaccine. B). CLA expression in tetramer-positive CD8 cells, with and without GM-CSF in vaccine. C). Expression levels (mean fluorescence intensity) of CXCR3 on tetramer-positive CD8 cells, with and without GM-CSF in vaccine. D). Expression levels (mean fluorescence intensity) of CLA on tetramer-positive CD8 cells, with and without GM-CSF in vaccine.
All CD8 cells expressing CLA co-expressed CXCR3. Interestingly, the mean fluorescence intensity (MFI) of CXCR3 expression was significantly (p < 0.001) higher in GM-CSF-treated cells (Fig. 2C), whereas CLA expression was modestly but significantly (p < 0.05) lower. Therefore, a peptide vaccine composed of multiple class I-restricted antigens and delivered subcutaneously and intradermally in Montanide adjuvant can induce tumor-specific CD8 T cell populations that express the pleotropic homing receptor CXCR3 and the skin-homing glycoprotein CLA, and the addition of GM-CSF enhances the expression of homing receptors on vaccine-induced CD8 cells.
Vaccination with peptide in adjuvant induces the expression of T-bet (TBX21) and IFN-γ in tetramer-positive CD8 cells
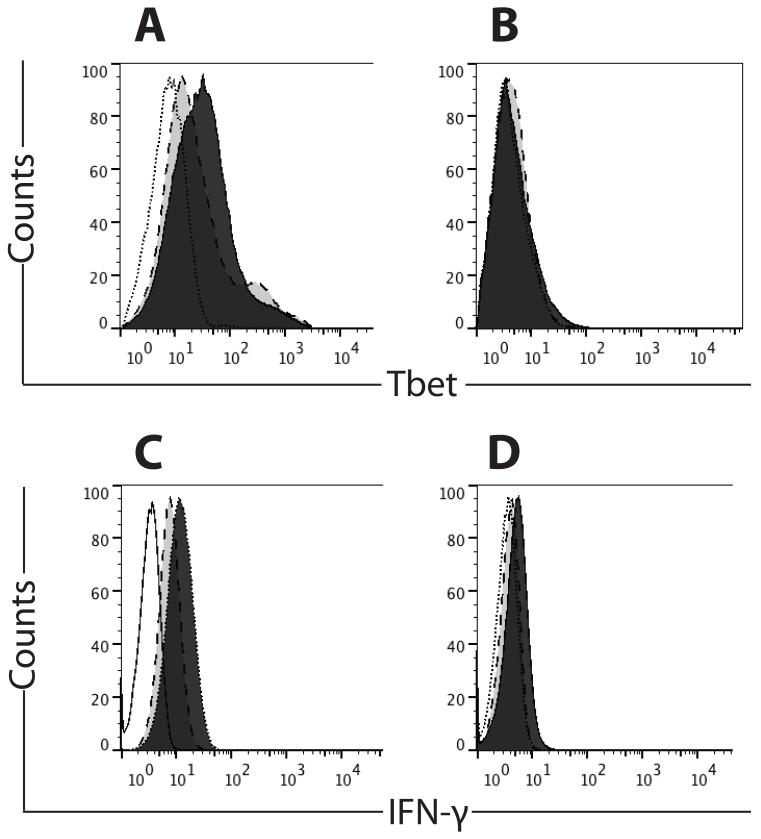
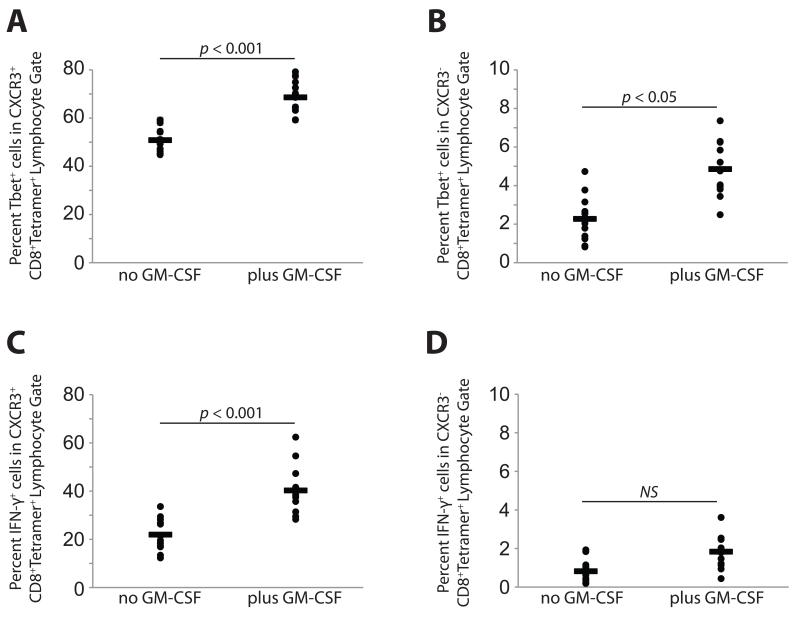
Because CXCR3 expression is regulated by T-box transcription factor T-bet (TBX21)(27), we assessed intracellular T-bet following vaccination with MELITAC 12.1, either with or without GM-CSF. Tbet staining was observed in the majority (>50%) of tetramer-positive CXCR3+ CD8 cells (Figs. 3A and 4A) but unchanged relative to pre-vaccine status in tetramer-negative populations (data not shown). Addition of GM-CSF increased (p < 0.001) the proportion of T-bet-expressing cells, consistent with the observed GM-CSF-associated increase in CXCR3 expression (Figs. 3A and 4A). In the CXCR3-negative, tetramer-positive subset of cells, Tbet was observed in only a small proportion of cells (<5%, Figs. 3B and 4B).
Fig. 3. Peptide vaccine and adjuvant enhances Tbet (TBX21) and IFN-γ production in CXCR3+, but not CXCR3−, tetramer-positive CD8 T cells.
Open histogram, unstained control; light gray histogram, no GM-CSF in vaccine; dark gray histogram, GM-CSF in vaccine. A). Representative Tbet staining in CXCR3+ tetramer-positive cells, with or without GM-CSF in vaccine. B). Representative Tbet staining in CXCR3− tetramer-positive cells, with or without GM-CSF in vaccine. C). Representative IFN-γ staining in CXCR3+ tetramer-positive cells, with or without GM-CSF in vaccine. D). Representative IFN-γ staining in CXCR3− tetramer-positive cells, with or without GM-CSF in vaccine.
Fig. 4. Peptide vaccine and adjuvant enhances the proportion of tetramer-positive CD8 T cells expressing the transcription factor Tbet (TBX21) and IFN-γ in CXCR3+, but not CXCR3−, cells.
A). Tbet expression in CXCR3+ tetramer-positive cells, with or without GM-CSF in vaccine. B). Tbet expression in CXCR3− tetramer-positive cells, with or without GM-CSF in vaccine. C). IFN-γ expression in CXCR3+ tetramer-positive cells, with or without GM-CSF in vaccine. D). IFN-γ expression in CXCR3− tetramer-positive cells, with or without GM-CSF in vaccine.
To assess functionality of vaccine-induced T cells, we measured intracellular IFN-γ. IFN-γ staining was observed in a subset (~22%) of tetramer-positive CXCR3+ CD8 cells (Figs. 3C and 4C), and addition of GM-CSF increased (p < 0.001) the proportion of IFN-γ-expressing cells (>40%, Figs. 3C and 4C). In tetramer-positive, CXCR3-negative cells, IFN-γ staining is largely absent (<2% of cells, regardless of GM-CSF). IFN-γ staining was unchanged by vaccination, regardless of the addition of GM-CSF, in tetramer-negative populations (data not shown).
Expression of CXCR3 and CLA is associated with the expression of IL-12Rα
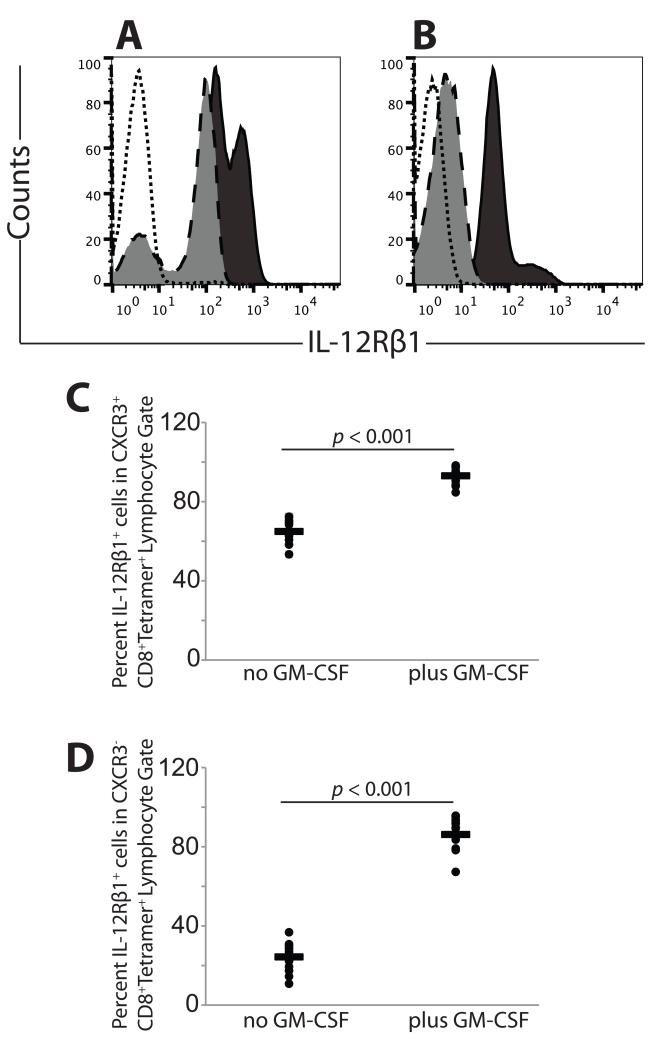
Because CXCR3 and CLA expression are associated with the IL-12 receptor (IL-12R)(22,28), we evaluated CD8+ T cells for expression of IL-12Rβ1. Receptor staining was observed in a subset (~65%) of tetramer-positive CXCR3+ CD8 cells, and addition of GM-CSF to the vaccine increased (p < 0.001) the proportion of IL-12Rβ1-expressing cells (>93%, Figs. 5A and 5C). In the CXCR3-negative, tetramer-positive subpopulation, IL-12Rβ1 staining was detected in a subset (~26%) of cells and significantly (p < 0.001) increased with the addition of GM-CSF (Figs. 5B and 5D). IL-12Rβ1 staining was unchanged by vaccination, regardless of the addition of GM-CSF, in tetramer-negative populations (data not shown). Thus, addition of GM-CSF to vaccination significantly enhanced antigen-specific CD8 T cell expression of IL-12Rβ1, regardless of CXCR3 expression status.
Fig. 5. Peptide vaccine and adjuvant enhances the proportion of tetramer-positive CD8 T cells expressing the transcription factor IL-12Rβ1.
Open histogram, unstained control; light gray histogram, no GM-CSF in vaccine; dark gray histogram, GM-CSF in vaccine. A). Representative IL-12Rβ1 staining in CXCR3+ tetramer-positive cells, with or without GM-CSF in vaccine. B). Representative IL-12Rβ1 staining in CXCR3− tetramer-positive cells, with or without GM-CSF in vaccine. C). IL-12Rβ1 expression in CXCR3+ tetramer-positive cells, with or without GM-CSF in vaccine. D). IL-12Rβ1 expression in CXCR3− tetramer-positive cells, with or without GM-CSF in vaccine.
Multiple vaccinations with peptide in adjuvant increase the proportion of tetramer-positive CD8 cells expressing CXCR3 and CLA
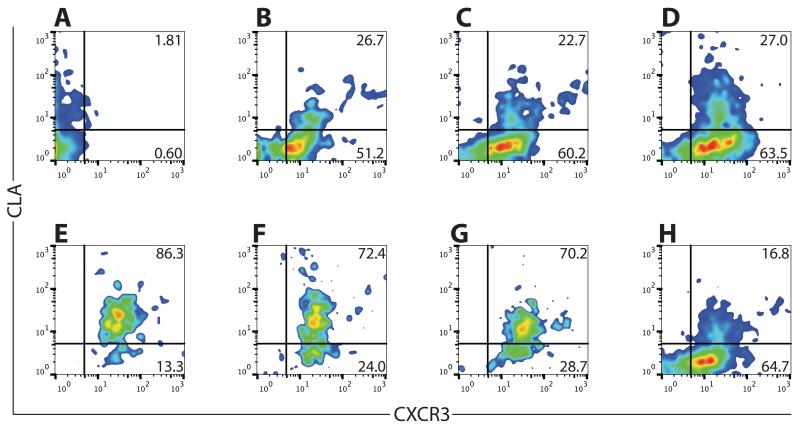
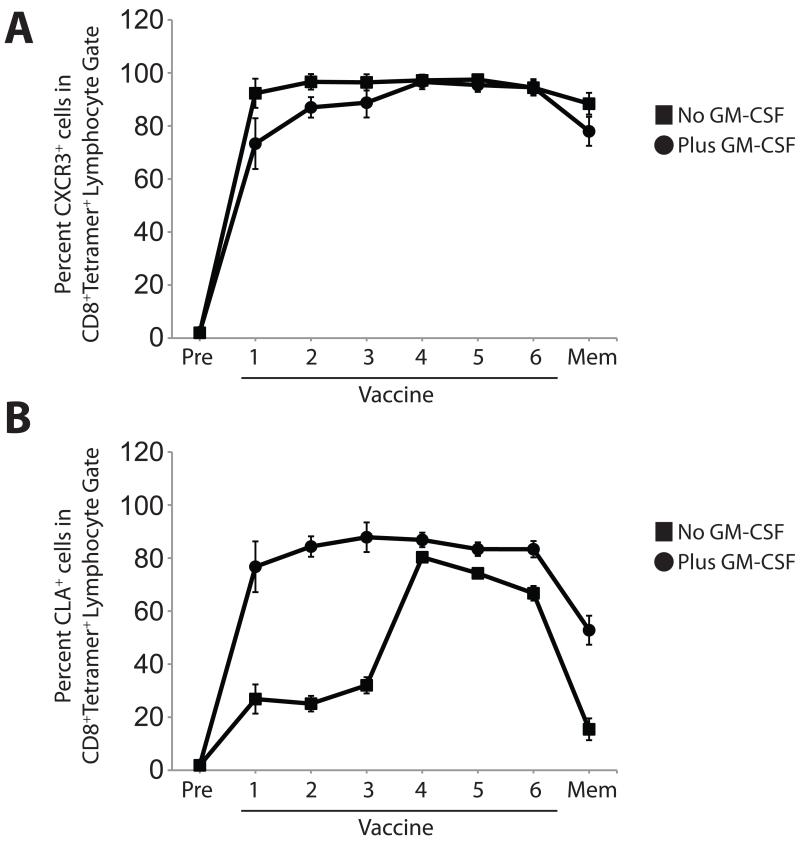
We next assessed whether multiple vaccinations enhanced the proportions of CXCR3- or CLA-expressing, tetramer-positive CD8 cells in circulation. We assessed CXCR3 and CLA expression, as above, before vaccination and after each of six weekly vaccinations, and we assessed memory populations (12 weeks after the final vaccination) (representative data in Fig. 6 A-H). The proportion of tetramer-positive CD8 cells expressing CXCR3 remained relatively stable (92-98%) throughout the course of vaccination and remained high in the memory compartment (>88%, Fig. 7A). Addition of GM-CSF to the vaccine slightly increased the proportion of CXCR3-expressing cells (Fig. 7A). In contrast to the constant pattern of CXCR3 expression, CLA expression varied over the course of multiple vaccines. Without GM-CSF, the proportion of tetramer-positive CD8 cells expressing CLA reached a maximum (~81% expressing CLA, Fig. 7B) after five vaccinations, then modestly decreased in subsequent weeks; CLA expression in the memory compartment was significantly lower (~16%), even though the cells remained predominantly positive for CXCR3. Addition of GM-CSF to the vaccine increased the proportion of cells expressing CLA (Fig. 7B, Supplemental Table I), and CLA expression in memory cells was significantly higher than cells from vaccinated patients without GM-CSF. Thus, sequential peptide vaccination in Montanide adjuvant induces populations of CXCR3- and CLA-expressing tumor antigen-specific cells that persist after the discontinuation of immunization, and the addition of GM-CSF enhances and maintains CLA expression in tumor antigen-specific CD8+ T cells.
Fig. 6. CLA, but not CXCR3, expression fluctuates over the course of peptide vaccination in adjuvant.
Percentage of tetramer-positive CD45RO+ CD8+ cells expressing the indicated homing molecule over the course of vaccination. A). Pre-vaccine. B) One week after 1st vaccine. C). One week after 2nd vaccine. D). One week after 3rd vaccine. E). One week after 4th vaccine. F). One week after 5th vaccine. G). One week after 6th vaccine. H). Twelve weeks after 6th vaccine.
Fig. 7. GM-CSF in vaccine enhanced CLA expression on tetramer-positive CD8 T cells.
Percentage of tetramer-positive CD45RO+ CD8+ cells expressing the indicated homing molecule over the course of vaccination. A). CXCR3 expression over the course of vaccination. B). CLA expression over the course of vaccination.
Discussion
Vaccination with melanoma peptides in Montanide adjuvant is immunogenic(29,30), but the expression of tissue-homing receptors by vaccine-induced CD8+ T cells has not been fully characterized. In the present study, we evaluated whether peptide vaccine in Montanide ISA-51 adjuvant can influence the expression of the chemokine receptor CXCR3 and the glycoprotein ligand CLA on vaccine-activated CD8 T cells. We also investigated whether inclusion of GM-CSF in the vaccine would enhance CXCR3 and CLA expression. Subcutaneous and intradermal administration of class I-restricted tetramer-positive antigens in Montanide ISA-51 induced antigen-specific CD8 cells, and the majority of the vaccine-induced cells expressed both CXCR3 and CLA. A previous report from Na et al(15) demonstrated that addition of GM-CSF to tyrosinase peptide in KLH induced CXCR3 expression by vaccine-induced CD4 T cells(31). We report that the majority of vaccine-induced CD8 cells express CXCR3, based on i.c. staining, and that GM-CSF increases CXCR3 expression by CD8 T cells. Further, we demonstrate that GM-CSF drives early expression of CLA in vaccine-induced CD8 cells.
Consistent with the expression of CXCR3, we observed that vaccine-induced T cells expressed IFN-γ and Tbet. Interestingly, we observed significant expression of IFN-γ and Tbet in cells that were isolated from patient PBMC and directly stained (after cryopreservation), raising the possibility that tetramers are sufficient to cross-link and activate T cells for cytokine production as previously reported in mouse models(32). However, in our studies, an analysis of a subset of samples revealed similar proportions of IFN-γ and Tbet staining in bulk CD8 populations regardless of the inclusion of tetramer reagents in the staining reaction (data not shown). Further, using samples from the same clinical trial, direct ELIspot assessment using individual peptide components of the MELITAC12.1 vaccine demonstrated that 7-50% of patient samples contained IFN-γ-producing CD8 T cells(23). In the present study, we utilized samples that were previously demonstrated to contain vaccine-induced CD8 T cells, based on direct and restimulated ELIspot analyses. The incubation of our T cells with tetramer was also for a short time period (15 min). Thus, we conclude that the observed expression of IFN-γ and Tbet is consistent with previous data and reflects the sustained induction of these factors in antigen-specific CD8 T cells following vaccination with MELITAC12.1.
Although peptide vaccines in Montanide adjuvant drive the expression of CXCR3 and CLA in CD8 cells, the molecular mechanisms remain undefined. Schaefer and colleagues(33) reported that repeated injections with Montanide induce the formation of tertiary lymphoid organs with a Th2-dominant microenvironment, which would not be expected to support the induction or maintenance of CXCR3 expression. However, mature DC were aggregated around superficial vessels and surrounded by lymphocytes. Thus, Montanide may polarize DC to a type I phenotype, which is highly supportive of CXCR3 expression by T cells(34); this is consistent with the observed immunogenicity of peptide/Montanide vaccines, and this possibility is under investigation. Other studies have demonstrated the induction of CLA on CD4 and CD8 T cells by plasmacytoid DCs(35,36), and the possibility that type I DC may induce T cell expression of CLA remains under study.
Addition of GM-CSF to the vaccine significantly increases the expression of CXCR3 and the proportion of CLA-expressing CD8 cells. The molecular and cellular mechanisms of GM-CSF activity remain undefined and are the subject of active investigation. GM-CSF is known to enhance the recruitment of DC to the injection site and may promote DC maturation and LN migration(37), thereby promoting T cell activation and expression of CXCR3 and CLA on tetramer-positive T cells. Vaccine-induced memory cells retain CXCR3 and CLA expression at ~five months after immunization, suggesting that the T cells may have been activated in the presence of IL-12; previous studies demonstrate that CD4 T cells activated by IL-12-producing DC can maintain CXCR3 expression, whereas T cells activated in the absence of IL-12 can express CXCR3 only in a transient manner(38). Montanide and GM-CSF may both promote the activation of DC to produce IL-12 and promote type I T cell responses. This is consistent with the report from Mortirini and colleagues(16), who demonstrated that administration of IL-12 leads to a peripheral burst of CLA-expressing T cells, and consistent with our demonstration that vaccine-induced CD8 cells express surface IL-12R. The specific mechanisms of GM-CSF-mediated upregulation of CXCR3 and CLA remain under investigation.
Limited T cell infiltration of metastatic melanoma remains a major impediment to effective immune therapy(39). A subset of melanomas express CXCR3 ligands(13), and expression of these ligands can be induced by exposure to exogenous interferons(40). We have also observed that intra-tumoral administration of IFN-γ can induce CXCL10 expression in dermal metastases (Mullins and Slingluff, unpublished). Since CXCR3 expression by tumor antigen-specific CD8 cells has been associated with enhanced survival in patients with advanced melanoma(11), the induction and maintenance of CXCR3 expression by vaccine-induced CD8 cells may significantly enhance the anti-tumor efficacy of vaccination. Conversely, recent work from Hailemichael and colleagues(41) suggests that CXCR3+ CD8 cells may be preferentially retained in the Montanide-containing vaccine site, so the consequences of enhanced CXCR3 expression for tumor-specific trafficking remain to be determined.
The utility of CLA expression by tumor Ag-specific CD8 cells in the treatment of metastatic melanoma is debatable. Although vasculature in dermal melanomas often express the CLA binding partner E-selectin(19), vasculature in metastatic melanoma is usually devoid of E-selectin(20). However, the TLR agonist Imiquimod has been shown to induce E-selectin expression in the vasculature of squamous cell tumors(42), leading to the possibility that it will induce E-selectin in melanomas (a possibility that is currently under investigation). Thus, vaccination that drives CLA expression may be useful for the treatment of dermal metastases and potentially other melanomas.
In sum, these data highlight novel phenotypic aspects of CD8 T cells induced by vaccination with peptide antigens in Montanide ISA-51, in the presence or absence of GM-CSF. Induction of CXCR3 and CLA may be useful for enhancing T cell migration to specific tissue compartments, especially in the context of combinatorial therapies to induce specific homing receptor ligands in the tumor microenvironment, and addition of GM-CSF to vaccine increases CXCR3 and CLA expression.
Supplementary Material
Acknowledgments
We appreciate the advice and assistance of Michael Solga and Joanne Lannigan (University of Virginia Cytometry Core Facility), and Alan Bergeron and Jacqueline Channon-Smith (DartLab, the Geisel School of Medicine’s Immune Monitoring and Cytometry Core Facility). Chantel McSkimming (University of Virginia Department of Surgery) provided assistance and advice on the development of tetramer staining protocols.
Support for these studies was provided, in part, by University of Virginia School of Medicine’s Cancer Biology Training Grant (USPHS T32 CA009109 to EC-T); the Geisel School of Medicine at Dartmouth’s Immunology Training Grant (USPHS T32 AI007363 to EC-T); the Joanna M Nicolay Melanoma Research Foundation (Research Scholar Award to EC-T); the Melanoma Research Alliance (Young Investigator Award to DWM); and the USPHS (R21 CA103528 to CLS, and R01 CA134799 to DWM).
Footnotes
Conflicts of Interest: None
Author’s Contributions
EC-T, IMM, and DWM conceived of the study. CLS designed and conducted the clinical trial that generated the patient samples. EC-T, LKK, and LDN acquired data. All authors participated in data analysis, interpretation, and manuscript preparation. All authors agree to the submission of the manuscript.
Works Cited
- 1.Viola A, Molon B, Contento RL. Chemokines: coded messages for T-cell missions. Frontiers in Bioscience. 2008;13:6341–53. doi: 10.2741/3158. [DOI] [PubMed] [Google Scholar]
- 2.Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nature Immunology. 2008;9:970–80. doi: 10.1038/ni.f.213. [DOI] [PubMed] [Google Scholar]
- 3.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. The Journal of Experimental Medicine. 1998;187:875–83. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto J, Adachi Y, Onoue Y, Adachi YS, Okabe Y, Itazawa T, et al. Differential expression of the chemokine receptors by the Th1- and Th2-type effector populations within circulating CD4+ T cells. Journal of Leukocyte Biology. 2000;68:568–74. [PubMed] [Google Scholar]
- 5.Prezzi C, Casciaro M a, Francavilla V, Schiaffella E, Finocchi L, Chircu LV, et al. Virus-specific CD8+ T cells with type 1 or type 2 cytokine profile are related to different disease activity in chronic hepatitis C virus infection. European Journal of Immunology. 2001;31:894–906. doi: 10.1002/1521-4141(200103)31:3<894::aid-immu894>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 6.Liao F, Rabin RL, Yannelli JR, Koniaris LG, Vanguri P, Farber JM. Human Mig chemokine: biochemical and functional characterization. The Journal of Experimental Medicine. 1995;182:1301–14. doi: 10.1084/jem.182.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taub DD, Lloyd AR, Conlon K, Wang JM, Ortaldo JR, Harada A, et al. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. The Journal of Experimental Medicine. 1993;177:1809–14. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole KE, Strick C a, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, et al. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. The Journal of Experimental Medicine. 1998;187:2009–21. doi: 10.1084/jem.187.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunology and Cell Biology. Nature Publishing Group. 2011;89:207–15. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie JH, Nomura N, Lu M, Chen S, Koch GE, Weng Y, et al. Antibody-mediated blockade of the CXCR3 chemokine receptor results in diminished recruitment of T helper 1 cells into sites of inflammation. Journal of Leukocyte Biology. 2003;73:771–80. doi: 10.1189/jlb.1102573. [DOI] [PubMed] [Google Scholar]
- 11.Mullins IM, Slingluff CL, Lee JK, Garbee CF, Shu J, Anderson SG, et al. CXC chemokine receptor 3 expression by activated CD8+ T cells is associated with survival in melanoma patients with stage III disease. Cancer Research. 2004;64:7697–701. doi: 10.1158/0008-5472.CAN-04-2059. [DOI] [PubMed] [Google Scholar]
- 12.Kunz M, Toksoy A, Goebeler M, Engelhardt E, Bröcker E, Gillitzer R. Strong expression of the lymphoattractant C-X-C chemokine Mig is associated with heavy infiltration of T cells in human malignant melanoma. The Journal of Pathology. 1999;189:552–8. doi: 10.1002/(SICI)1096-9896(199912)189:4<552::AID-PATH469>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 13.Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Research. 2009;69:3077–85. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferguson AR, Engelhard VH. CD8 T cells activated in distinct lymphoid organs differentially express adhesion proteins and coexpress multiple chemokine receptors. Journal of Immunology. 2010;184:4079–86. doi: 10.4049/jimmunol.0901903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Na I-K, Keilholz U, Letsch A, Bauer S, Asemissen AM, Nagorsen D, et al. Addition of GM-CSF to a peptide/KLH vaccine results in increased frequencies of CXCR3-expressing KLH-specific T cells. Cancer Immunology, Immunotherapy. 2007;56:391–6. doi: 10.1007/s00262-006-0198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mortarini R, Borri A, Tragni G, Bersani I, Vegetti C, Bajetta E, et al. Peripheral burst of tumor-specific cytotoxic T lymphocytes and infiltration of metastatic lesions by memory CD8+ T cells in melanoma patients receiving interleukin 12. Cancer Research. 2000;60:3559–68. [PubMed] [Google Scholar]
- 17.Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature. 1997;389:978–81. doi: 10.1038/40166. [DOI] [PubMed] [Google Scholar]
- 18.Engelberts I, van Hoof SC, Samyo SK, Buurman WA, van der Linden CJ. Generalized inflammation during peritonitis evidenced by intracutaneous E-selectin expression. Clinical Immunology and Immunopathology. 1992;65:330–4. doi: 10.1016/0090-1229(92)90165-k. [DOI] [PubMed] [Google Scholar]
- 19.Schadendorf D, Heidel J, Gawlik C, Suter L, Czarnetzki BM. Association with clinical outcome of expression of VLA-4 in primary cutaneous malignant melanoma as well as P-selectin and E-selectin on intratumoral vessels. Journal of the National Cancer Institute. 1995;87:366–71. doi: 10.1093/jnci/87.5.366. [DOI] [PubMed] [Google Scholar]
- 20.Weishaupt C, Munoz KN, Buzney E, Kupper TS, Fuhlbrigge RC. T-cell distribution and adhesion receptor expression in metastatic melanoma. Clinical Cancer Research. 2007;13:2549–56. doi: 10.1158/1078-0432.CCR-06-2450. [DOI] [PubMed] [Google Scholar]
- 21.Picker LJ, Treer JR, Ferguson-Darnell B, Collins PA, Bergstresser PR, Terstappen LW. Control of lymphocyte recirculation in man. II. Differential regulation of the cutaneous lymphocyte-associated antigen, a tissue-selective homing receptor for skin-homing T cells. Journal of Immunology. 1993;150:1122–36. [PubMed] [Google Scholar]
- 22.Reddy M, Davis C, Wong J, Prabhakar U. Cutaneous lymphocyte antigen expression on activated lymphocytes and its association with IL-12R (beta1 and beta2), IL-2Ralpha, and CXCR3. Cellular Immunology. 2005;236:131–9. doi: 10.1016/j.cellimm.2005.08.019. A. [DOI] [PubMed] [Google Scholar]
- 23.Slingluff CL, Petroni GR, Olson WC, Smolkin ME, Ross MI, Haas NB, et al. Effect of granulocyte/macrophage colony-stimulating factor on circulating CD8+ and CD4+ T-cell responses to a multipeptide melanoma vaccine: outcome of a multicenter randomized trial. Clinical Cancer Research. 2009;15:7036–44. doi: 10.1158/1078-0432.CCR-09-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dimopoulos N, Jackson HM, Ebert L, Guillaume P, Luescher IF, Ritter G, et al. Combining MHC tetramer and intracellular cytokine staining for CD8+ T cells to reveal antigenic epitopes naturally presented on tumor cells. Journal of Immunological Methods. 2009;340:90–4. doi: 10.1016/j.jim.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 25.Meiser A, Mueller A, Wise EL, McDonagh EM, Petit SJ, Saran N, et al. The chemokine receptor CXCR3 is degraded following internalization and is replenished at the cell surface by de novo synthesis of receptor. Journal of Immunology. 2008;180:6713–24. doi: 10.4049/jimmunol.180.10.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salerno EP, Shea SM, Olson WC, Petroni GR, Smolkin ME, McSkimming C, et al. Activation, dysfunction and retention of T cells in vaccine sites after injection of incomplete Freund’s adjuvant, with or without peptide. Cancer Immunology, Immunotherapy. 2013;62:1149–59. doi: 10.1007/s00262-013-1435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–69. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 28.Reddy M, Wong J, Davis C, Prabhakar U. Co-expression of IL-12 receptors along with CXCR3 and CD25 on activated peripheral blood T lymphocytes. Cellular Immunology. 2005;236:123–30. doi: 10.1016/j.cellimm.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 29.Yamshchikov GV, Barnd DL, Eastham S, Galavotti H, Patterson JW, Deacon DH, et al. Evaluation of peptide vaccine immunogenicity in draining lymph nodes and peripheral blood of melanoma patients. International Journal of Cancer. 2001;92:703–11. doi: 10.1002/1097-0215(20010601)92:5<703::aid-ijc1250>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 30.Chianese-Bullock KA, Irvin WP, Petroni GR, Murphy C, Smolkin M, Olson WC, et al. A multipeptide vaccine is safe and elicits T-cell responses in participants with advanced stage ovarian cancer. Journal of Immunotherapy. 2008;31:420–30. doi: 10.1097/CJI.0b013e31816dad10. [DOI] [PubMed] [Google Scholar]
- 31.Scheibenbogen C, Schadendorf D, Bechrakis NE, Nagorsen D, Hofmann U, Servetopoulou F, et al. Effects of granulocyte-macrophage colony-stimulating factor and foreign helper protein as immunologic adjuvants on the T-cell response to vaccination with tyrosinase peptides. International Journal of Cancer. 2003;104:188–94. doi: 10.1002/ijc.10961. [DOI] [PubMed] [Google Scholar]
- 32.Daniels MA, Jameson SC. Critical role for CD8 in T cell receptor binding and activation by peptide/major histocompatibility complex multimers. The Journal of Experimental Medicine. 2000;191:335–46. doi: 10.1084/jem.191.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaefer JT, Patterson JW, Deacon DH, Smolkin ME, Petroni GR, Jackson EM, et al. Dynamic changes in cellular infiltrates with repeated cutaneous vaccination: a histologic and immunophenotypic analysis. Journal of Translational Medicine. 2010;8:79. doi: 10.1186/1479-5876-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watchmaker PB, Berk E, Muthuswamy R, Mailliard RB, Urban J a, Kirkwood JM, et al. ndependent regulation of chemokine responsiveness and cytolytic function versus CD8+ T cell expansion by dendritic cells. Journal of Immunology. 2010;184:591–7. doi: 10.4049/jimmunol.0902062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koelle DM, Huang J, Hensel MT, McClurkan CL. Innate immune responses to herpes simplex virus type 2 influence skin homing molecule expression by memory CD4+ lymphocytes. Journal of Virology. 2006;80:2863–72. doi: 10.1128/JVI.80.6.2863-2872.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salio M, Cella M, Vermi W, Facchetti F, Palmowski MJ, Smith CL, et al. Plasmacytoid dendritic cells prime IFN-gamma-secreting melanoma-specific CD8 lymphocytes and are found in primary melanoma lesions. European Journal of Immunology. 2003;33:1052–62. doi: 10.1002/eji.200323676. [DOI] [PubMed] [Google Scholar]
- 37.Disis ML, Bernhard H, Shiota FM, Hand SL, Gralow JR, Huseby ES, et al. Granulocyte-macrophage colony-stimulating factor: an effective adjuvant for protein and peptide-based vaccines. Blood. 1996;88:202–10. [PubMed] [Google Scholar]
- 38.Rabin RL, Alston MA, Sircus JC, Knollmann-Ritschel B, Moratz C, Ngo D, et al. CXCR3 is induced early on the pathway of CD4+ T cell differentiation and bridges central and peripheral functions. Journal of Immunology. 2003;171:2812–24. doi: 10.4049/jimmunol.171.6.2812. [DOI] [PubMed] [Google Scholar]
- 39.Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, et al. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Research. 2012;72:1070–80. doi: 10.1158/0008-5472.CAN-11-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dengel LT, Norrod AG, Gregory BL, Clancy-Thompson E, Burdick MD, Strieter RM, et al. Interferons induce CXCR3-cognate chemokine production by human metastatic melanoma. Journal of Immunotherapy. 2010;33:965–74. doi: 10.1097/CJI.0b013e3181fb045d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang X-F, et al. P ersistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nature Medicine. 2013;19:465–72. doi: 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clark RA, Huang SJ, Murphy GF, Mollet IG, Hijnen D, Muthukuru M, et al. Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells. The Journal of Experimental Medicine. 2008;205:2221–34. doi: 10.1084/jem.20071190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.