Abstract
The Shiner Perch (Cymatogaster aggregata Gibbons) exhibits a viviparous reproductive mode and long-term female sperm storage, two biological features that may predispose this fish species for both intense sperm competition and frequent multiple paternity within broods. To test these hypotheses, we used polymorphic microsatellite markers to identify sires and quantify paternal contributions to the progeny arrays of 27 pregnant females from a natural population of C. aggregata. The number of sires per brood ranged from one to eight (mean 4.6), typically with skewed distributions of fertilization success by the fathers but no correlation between sire number and brood size. The extraordinarily high incidences of multiple paternity in this species probably are due in part to high rates of mate encounter, but selection pressures related to the avoidance of maternal–fetal incompatibility may further have promoted the evolution of polyandrous mating behaviors in this female-pregnant species. Our genetic data are consistent with the hypothesis that viviparity, long-term sperm storage, and extreme polyandry are interrelated reproductive phenomena that should promote the evolution of post-copulatory sperm competition and/or cryptic female choice in these fishes.
Introduction
Despite the potential risks and costs associated with copulation, multiple mating by females (polyandry) is a taxonomically widespread phenomenon (Birkhead and Møller 1998; Avise et al. 2002; Griffith et al. 2002; Stockley 2003; Uller and Olsson 2008), the adaptive significance of which is currently under considerable debate (Simmons 2005; Uller and Olsson 2008). Several hypotheses have been advanced to explain the evolution of polyandry, and these are usually divided into direct material benefits and indirect genetic benefits. Direct benefits to polyandrous females include fertilization assurance, nutrient acquisition through courtship, and reduced risk of sexual harassment (Birkhead and Pizzari 2002). Indirect genetic benefits also might arise by any of several routes (Jennions and Petrie 2000; Birkhead and Pizzari 2002). When gametes from two or more males actively vie for fertilizations of ova (Parker 1998; Birkhead and Pizzari 2002), sperm competition should ensure that a polyandrous female’s eggs are fertilized by genetically robust cells that might also enhance offspring viability and/or increase the odds that sons will produce competitive sperm (Curtsinger 1991; Keller and Reeve 1995). Furthermore, when a polyandrous female fertilizes her ova via “cryptic female choice”, the consequence might be an increase in the genetic quality of her offspring and/or a reduced risk of fertilization by genetically incompatible sperm (Zeh and Zeh 1996, 1997; Jennions and Petrie 2000; Tregenza and Wedell 2000; Birkhead and Pizzari 2002). Finally, high genetic diversity among the progeny of a polyandrous female might serve to buffer her brood against unpredictable environmental fluctuations (Yasui 1998, 2001).
Sperm competition and cryptic female choice may be especially important in species in which females can store viable sperm for long periods of time (Birkhead and Møller 1993). A long interval between copulation and fertilization may enhance opportunities for sperm competition as well as facilitate the evolution of mechanisms that might permit a polyandrous female to exercise post-copulatory control over her utilization of sperm from different sexual partners (Birkhead and Møller 1993). Furthermore, reproductive mode can have a critical impact on forces underlying the evolution of polyandry (Zeh and Zeh 2000, 2001). For example, suppose that multiple mating and multiple paternity help a polyandrous female to protect her reproductive investment against the threat of incompatibility between maternal and paternal genomes (Zeh and Zeh 1996, 1997). Then, due to intimate maternal–fetal interactions, such avoidance of genetic incompatibility may be much more important for females in viviparous than in oviparous species (Zeh and Zeh 2001).
Surfperches (Embiotocidae) are viviparous teleost fishes that inhabit coastal waters of the North Pacific (Tarp 1952; DeMartini 1969). Viviparity in the family is highly developed, with progeny being born in an almost adult condition (Baltz 1984). The natural history of the Shiner Perch Cymatogaster aggregata Gibbons, a small embiotocid of coastal waters from southern Alaska to Baja California (DeMartini 1969), has been studied extensively. Shoals of females enter shallow waters in the spring and summer to give birth to broods of large, well-developed embryos (Eigenmann 1892; Turner 1938; Wiebe 1968). Copulations take place soon after parturition and are confined to a short period of time (ca. 2 months after parturition). Both females and males show elaborate courtship and mating behaviors (Hubbs 1917; Wiebe 1968; Shaw and Allen 1977). During each reproductive season, the male’s anal fin develops a complex system of appendages and tubular structures used for sperm transfer (Wiebe 1968). Each male courts only females of his own size group, and intermale aggression is strongly size-assortative (DeMartini 1988). The average testis size is relatively large (Wiebe 1968; DeMartini 1988). Dense aggregations of sperm are introduced into the female reproductive tract via spermatophore packages, which rapidly dissolve and release sperm after copulation (Gardiner 1978a). Inside the female, the sperm are maintained within pockets in the ovarian epithelium (Gardiner 1978b) for about 6 months before ovulation and fertilization take place in December (Eigenmann 1892; Turner 1938; Wiebe 1968). The metabolic systems of the stored sperm are influenced by environmental conditions inside the ovarian lumen (Gardiner 1978c). Fertilization is accomplished by the penetration of sperm through the epithelium of an ovigerous fold covering the mature eggs, and the fertilized eggs are evacuated into the ovarian cavity (Eigenmann 1892; Turner 1938). Sperm are found in the intestinal tract of the developing embryos, and are possibly used as nutrient (Eigenmann 1892). Embryos are retained within the ovarian compartments where they absorb nutritive materials and oxygen from ovarian fluid through modifications that occur in the developing embryos and ovary (Eigenmann 1892; Turner 1938; deVlaming et al. 1983). Brood size is rather small, with a large three-year-old female perhaps giving birth to as many as 30 young, each about 30 mm in length (Eigenmann 1892; Turner 1938). Young-of-the-year males become sexually mature within a short period of time during the first summer (Shaw 1971). Some aspects of sexual dimorphism in various surfperch species, including early maturity as well as reduced growth and survival of males, may be among the evolutionary consequences of selection pressures related to sperm competition and sperm storage (Warner and Harlan 1982).
Long-term sperm storage, mixing of sperm in the ovary, and relatively large testis sizes all suggest a high intensity of sperm competition in C. aggregata. Considering the species’ high potential for sperm competition and its advanced viviparous reproductive mode, we postulated that Shiner Perch might show exceptionally high degrees of multiple paternity. Furthermore, if post-copulatory paternity-biasing mechanisms (sperm competition or cryptic female choice) are at play in this species, we might expect strong evidence for unequal reproductive contributions by different sires of a brood. Previously, multiple paternity was documented in only 42 among 446 surveyed broods (9.4%) in C. aggregata from three populations (Darling et al. 1980), but the limited variability of allozyme markers in that earlier genetic study precluded firmer conclusions about biological parentage. Indeed, highly variable microsatellite loci later were used to uncover much higher rates of multiple paternity in two other surfperch species: Embiotoca jacksoni and E. lateralis (Reisser et al. 2009).
The goal of this study is to assess the potential effect of sperm competition and/or female cryptic choice on patterns of multiple paternity in the viviparous C. aggregata with long-term sperm storage. Specifically, novel microsatellite loci were cloned and characterized from this species and were applied to parentage analysis of field-collected broods. This study allowed us to address three main questions: (a) What are the frequency and degree of multiple paternity in this species? (b) How much reproductive skew exists among the sires of a brood? and c) How do patterns of multiple paternity in this species compare to those of other female-pregnant fishes?
Materials and methods
Sample collection
In March 2010, adult males and gravid females of C. aggregata were collected near a shallow rock jetty at the mouth of Newport Bay, Orange County, California (latitude 33°35′31′′N; longitude 117°52′40′′W). Standard length was measured for each female, and ovaries were dissected and embryos counted. Fin clips of adults and whole embryos were preserved in a saline solution (20% dimethyl sulfoxide, 0.25 EDTA, saturated with NaCl, pH 8.0) (Seutin et al. 1991) for DNA analysis.
Microsatellite development
Microsatellites were isolated from a single specimen of C. aggregata following an enrichment protocol described by Hamilton et al. (1999) and modified by Hauswaldt and Glenn (2003). Primers flanking the microsatellite repeat regions were designed using Primer Premier version 5.00 (PREMIER Biosoft International). Primers were optimized and checked for polymorphisms using a sample of 36 adults. Observed and expected heterozygosities were calculated, and deviations from Hardy–Weinberg equilibrium were examined using an exact test based on a Markov Chain method (Guo and Thompson 1992) for each locus. The presence of genotypic disequilibrium between all pairs of loci was tested using the likelihood ratio test. All analyses were conducted by using Arlequin version 2.0 (Schneider et al. 2000). Expected exclusion probabilities (Dodds et al. 1996) for each locus and across all loci were calculated with GERUD 2.0 (Jones 2005). The presence of null alleles for each locus was checked using Micro-Checker ver. 2.2.3 (Van Oosterhout et al. 2004). A total of six highly polymorphic dinucleotide loci were chosen for this study.
Microsatellite genotyping
Genomic DNA was extracted from fin clips of adults and posterior parts of embryos using a proteinase K digestion and phenol/chloroform/isoamyl extraction procedure (Milligan 1998). Tailed PCR was used to produce fluorescently labeled DNA fragments (Boutin-Ganache et al. 2001). M13 reverse (5′-GGAAACAGCTATGACCAT-3′) was added to the 5′ end of one primer in each pair. An M13 reverse primer that is fluorescently labeled (FAM, HEX, or NED) was included in the PCR, resulting in a labeled product for detection. All loci were amplified separately on a Mastercycler (Eppendorf) in a 10-μl reaction containing about 50 ng genomic DNA, 2 μl of 5× buffer (Promega, Madison, WI), 0.2 mM of each dNTP, 0.2 μM labeled M13 reverse primer and locus specific primer without tail, 0.02 μM locus specific primer with M13 reverse tail, and 0.25 U Taq DNA polymerase (Promega, Madison, WI). Thermal cycling parameters for all amplifications were as follows: 95°C for 3 min, then 35 cycles each at 95°C for 20 s, 52°C for 20 s, and 72° for 30 s, followed by 1 cycle of final elongation at 72°C for 10 min. Amplified products were pooled and diluted 15 to 20-fold, and 1 μl of the pool was mixed with 10 μl of deionized formamide and 0.3 μl of GS500 size standard (Applied Biosystems, Foster City, CA). Samples were denatured for 4 min at 95°C and electrophoresed on an ABI PRISM 3100xl DNA analyzer. Allele scoring was performed using GENEMAPPER software version 4.0 (Applied Biosystems, Foster City, CA). Scoring was repeated for 30 random individuals at all six loci to estimate scoring error (but identical genotypes were obtained for all replicates).
Paternity and statistical analysis
Because our data set exceeded the maximum numbers of sires set by GERUD 2.0 (six), this software was not used for paternity analysis. Paternity inference was conducted using COLONY 2.0, which implements a maximum likelihood method that takes into account maternal information and estimated allele frequencies in the population (Wang 2004; Wang and Santure 2009; Jones and Wang 2010). COLONY evaluates genetic parentage by partitioning offspring into full-sib groups according to likelihood scores assuming Mendelian segregation and no maximum limit on the numbers of contributing parents. The configuration with maximum likelihood is searched by a simulated annealing algorithm. Thus, the number of sires contributing to each female’s broods and the reproductive skew among males could be determined. The degree of reproductive skew was measured by the binomial skew index B, which is based on the observed variance among sires corrected by the expected binomial variance if all sires had an equal probability of contributing to the brood (Nonacs 2000). A value of zero implies a random distribution of offspring among sires, positive values indicate skew, and significant negative values imply an overly equal distribution of offspring. Significance levels of B were estimated by simulation with 10,000 permutations. All of the skew analyses were conducted by SKEW CALCULATOR 2003 (http://www.eeb.ucla.edu/Faculty/Nonacs/shareware.htm). A test for a correlation between brood size and female standard length was performed. To assess whether larger broods were more likely to contain offspring from each of the males with whom a female had mated, a linear regression test for correlation between brood size and the number of sires was performed.
Results
Population characterization
A total of 27 pregnant females and 9 males, with standard lengths ranging from 97 mm to 127 mm, were collected. Average brood size was 12.9 individuals (ranging from 7 to 22 across the 27 broods). Stages of gestation varied among broods, but within a brood there was little range in the stage of embryonic development. Brood size was positively correlated with female standard length (r 2 = 0.65, df = 26, P < 0.001).
Microsatellite markers
All six dinucleotide microsatellite loci were highly variable, displaying from 15 to 28 alleles per locus (Table 1). Observed and expected heterozygosities were high for all loci and ranged from 0.94 to 1.00 (Table 1). No evidence of genotypic disequilibrium between loci was detected, and all loci were in Hardy–Weinberg equilibrium. These loci were extremely informative for parentage analysis, with expected exclusion probabilities (under the one-parent-known model) ranging from 0.79 to 0.91 for each locus and >0.999 for all six loci combined (Table 1). Microchecker did not find any evidence for the presence of null alleles at any locus.
Table 1.
Characteristics of six microsatellite loci in a sample of 36 adult individuals of Shiner Perch
| Locus | Repeat motif | Primer sequences | Size range | Number of alleles | Ho | He | Exclusion probability |
|---|---|---|---|---|---|---|---|
| Cagg15 | (AC)25 |
*F: Fam-TAGCACATTAGGATTCAAAA R: ATTAGAGCAGGGTAGGATTA |
205–263 | 22 | 0.972 | 0.951 | 0.875 |
| Cagg27 | (GT)28 |
*F: Hex-GAAACATAATAAACAGCAGGAT R: GAAGGAGATGAAAGGAACAA |
218–288 | 22 | 0.944 | 0.919 | 0.816 |
| Cagg28 | (AC)19 |
*F: Ned-CATACCGATGTTTAGACAGAA R: ACAAATGGCAGTGAAAGGAG |
251–292 | 22 | 1.000 | 0.949 | 0.870 |
| Cagg29 | (AC)35 |
*F: Ned-ACAAGGACACCTGTCTCAAC R: GAACTAACTCTTCCAGCAAA |
166–258 | 28 | 0.972 | 0.967 | 0.906 |
| Cagg36 | (GT)20 |
*F: Hex-GCAAGTTGGCATGTGATGAG R: GCCTGGCAGATGTGAAAGAG |
104–142 | 15 | 0.944 | 0.910 | 0.794 |
| Cagg50 | (GT)36 |
*F: Fam-CGTGCAGACGGAAATGTGAT R: ACCTGCTTCTTTCAGGGACA |
123–217 | 26 | 0.972 | 0.959 | 0.890 |
* The complete sequence of the primer includes M13 reverse tail (5′-GGAAACAGCTATGACCATG-3′) at its 5′—end. M13 reverse oligo labeled with Fam, Hex, or Ned was used in the PCR reaction
Genetic paternity
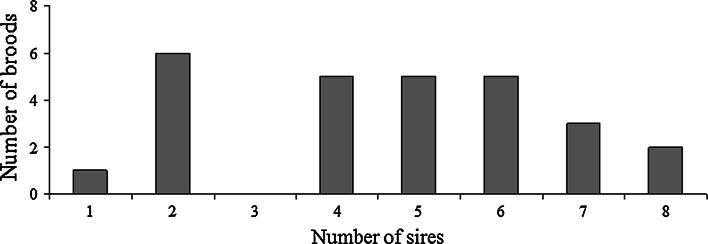
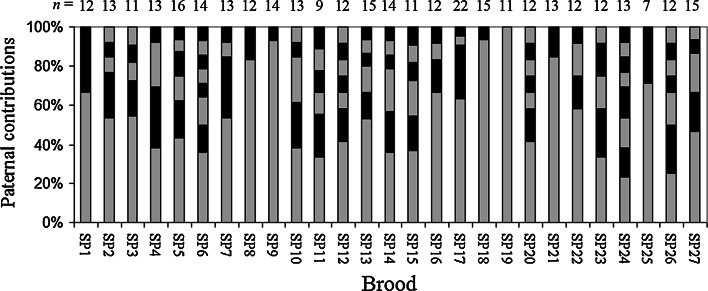
Genotypes at six microsatellite loci were determined for 36 adults and a total of 348 embryos. No null alleles or maternally derived de novo mutations were detected. For each brood, the best (maximum likelihood) inferred configuration consisting of full-sib families is shown in Table 2. Multiple paternity was detected in 26 (96%) of the 27 broods. The number of sires per brood ranged from one to a maximum of eight, with an average of 4.6 sires per brood (Table 2; Fig. 1). Of the 26 multiply sired broods, eight (31%) were significantly skewed from equal paternal contributions (Table 2; Fig. 2). The number of sires per brood was not significantly correlated with brood size (r 2 = 0.007, df = 26, P = 0.66). None of the nine collected males matched any of the genetically inferred sires.
Table 2.
Multiple mating for 27 broods of Shiner Perch from Newport Bay
| Family | Brood size (n) | Standard length (mm) | Number of sires | Sire 1 | Sire 2 | Sire 3 | Sire4 | Sire 5 | Sire 6 | Sire 7 | Sire 8 | B value | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SP01 | 12 | 108 | 2 | 8 | 4 | 0.014 | 0.382 | ||||||
| SP02 | 13 | 112 | 5 | 7 | 3 | 1 | 1 | 1 | 0.099 | 0.035 | |||
| SP03 | 11 | 111 | 5 | 6 | 2 | 1 | 1 | 1 | 0.083 | 0.087 | |||
| SP04 | 13 | 115 | 4 | 5 | 4 | 3 | 1 | −0.006 | 0.511 | ||||
| SP05 | 16 | 114 | 6 | 7 | 3 | 2 | 2 | 1 | 1 | 0.047 | 0.097 | ||
| SP06 | 14 | 111 | 8 | 5 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 0.006 | 0.375 |
| SP07 | 13 | 101 | 4 | 7 | 4 | 1 | 1 | 0.089 | 0.053 | ||||
| SP08 | 12 | 106 | 2 | 10 | 2 | 0.181 | 0.038 | ||||||
| SP09 | 14 | 112 | 2 | 13 | 1 | 0.332 | 0.003 | ||||||
| SP10 | 13 | 111 | 5 | 5 | 3 | 3 | 1 | 1 | 0.005 | 0.408 | |||
| SP11 | 9 | 98 | 6 | 3 | 2 | 1 | 1 | 1 | 1 | −0.049 | 0.910 | ||
| SP12 | 12 | 108 | 7 | 5 | 2 | 1 | 1 | 1 | 1 | 1 | 0.022 | 0.219 | |
| SP13 | 15 | 121 | 6 | 8 | 2 | 2 | 1 | 1 | 1 | 0.111 | 0.008 | ||
| SP14 | 14 | 114 | 6 | 5 | 3 | 3 | 1 | 1 | 1 | 0.009 | 0.387 | ||
| SP15 | 11 | 103 | 6 | 4 | 2 | 2 | 1 | 1 | 1 | −0.019 | 0.623 | ||
| SP16 | 12 | 117 | 4 | 8 | 2 | 1 | 1 | 0.174 | 0.010 | ||||
| SP17 | 22 | 127 | 4 | 14 | 6 | 1 | 1 | 0.199 | 0.000 | ||||
| SP18 | 15 | 115 | 2 | 14 | 1 | 0.342 | 0.001 | ||||||
| SP19 | 11 | 104 | 1 | 11 | NA | NA | |||||||
| SP20 | 12 | 112 | 7 | 5 | 2 | 1 | 1 | 1 | 1 | 1 | 0.022 | 0.211 | |
| SP21 | 13 | 108 | 2 | 11 | 2 | 0.201 | 0.021 | ||||||
| SP22 | 12 | 114 | 4 | 7 | 2 | 2 | 1 | 0.090 | 0.065 | ||||
| SP23 | 12 | 104 | 5 | 4 | 3 | 2 | 2 | 1 | −0.031 | 0.805 | |||
| SP24 | 13 | 113 | 8 | 3 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | −0.044 | 0.981 |
| SP25 | 7 | 97 | 2 | 5 | 2 | 0.020 | 0.458 | ||||||
| SP26 | 12 | 114 | 7 | 3 | 3 | 2 | 1 | 1 | 1 | 1 | −0.036 | 0.847 | |
| SP27 | 15 | 117 | 5 | 7 | 3 | 3 | 1 | 1 | 0.053 | 0.099 |
Brood size (n), standard length, number of sires, and number of offsprings sired by up to eight putative males as calculated by COLONY version 2.0.1.1 (Wang 2004) are provided. The binomial skew index B and corresponding P values are also indicated
Fig. 1.
Microsatellite parentage analysis of 27 broods of Shiner Perch, Cymatogaster aggregata
Fig. 2.
Estimated distribution of paternity within each brood. Gray and black segments of the bars represent the contributions of different sires, n is number of progeny in the brood
Discussion
Our microsatellite analyses demonstrate a high incidence of multiple paternity in C. aggregata. Of the 27 broods analyzed, 26 proved to have been sired by more than one male. The frequency of multiple paternity in C. aggregata (96%) is thus among the highest reported for internally fertilizing vertebrates (Uller and Olsson 2008; Avise and Liu 2010; Soulsbury 2010) and is much higher than the previous estimate (9%) for this species based on allozyme markers that were far less polymorphic (Darling et al. 1980). Microsatellite loci similarly have indicated high rates of multiple paternity (100% for both species) within the broods of two other embiotocid species: the Black Surfperch Embiotoca jacksoni and the Striped Surfperch E. lateralis (Reisser et al. 2009). All of these genetic analyses based on microsatellite markers thus indicate that polyandry must be an extremely common phenomenon in surfperches. Furthermore, we detected an average of 4.6 sires per brood in C. aggregata, a value that is even higher (P < 0.05, one tailed t-test) than the means of about 3.5 sires per brood for both E. jacksoni and E. laterali (Reisser et al. 2009). All of these values place surfperches among the highest-ranked internally fertilizing vertebrate groups in terms of the overall magnitude of genetically documented multiple mating by females (Uller and Olsson 2008; Avise and Liu 2010).
Higher mean number of sires per brood in C. aggregata than in other embiotocids is consistent with more intense sperm competition in C. aggregata, and with the fact that female sperm storage in C. aggregata is temporally longer (ca. 25 weeks; Wiebe 1968) than in E. jacksoni and E. lateralis (courtship behaviors last until 1 month before fertilization of eggs; Froeschke et al. 2007). Because of this prolonged sperm storage, disproportionately greater quantities of sperm may be necessary for paternity assurance in C. aggregata than in E. jacksoni and E. lateralis. Accordingly, testis sizes of C. aggregata average much larger (relative to body size) than those of E. jacksoni and E. lateralis (Wiebe 1968; Froeschke et al. 2007). In C. aggregata, males also mature earlier, grow more slowly, and die younger than females, but not so in E. jacksoni and E. lateralis (Warner and Harlan 1982). All of these differences point toward a higher intensity of sperm competition in C. aggregata than in the other embiotocids (Stockley et al. 1997), which in turn may be related also to this species’ exceptionally high degree of multiple paternity.
The high incidences of multiple paternity in surfperches indicate high levels of female promiscuity. Male embiotocids make no contribution to offspring production except for sperm, so direct benefits to females are unlikely to play an important role. It is still unclear whether the sperm form a significant nutritive source for the developing embryos (Dobbs 1975; Gardiner 1978c). However, if sperm is used as nutrient, the possible nutritive role of sperm may be a mild direct benefit of female multiple mating. Females of C. aggregata actively participate in mating activity, initiating and displaying reproductive behaviors that are similar to males (Shaw and Allen 1977). In E. jacksoni, females appear to exercise the primary selection of mates, because males perform courtship rituals within the full view of many females and the females then choose particular mates (Froeschke et al. 2007). Thus, “convenience polyandry” (Thornhill and Alcock 1983), which is an explanation for species where multiple mating incurs costs to females without obvious benefits, seems not to be a reasonable explanation for the high degree of multiple paternity in surfperches. Instead, multiple paternity in surfperch broods may reflect an evolutionary response to some form of indirect benefits to females of multiple mating.
Zeh and Zeh (2001) have suggested that polyandry for incompatibility avoidance is likely to be of greater importance for viviparous females than for females who lay eggs. Considering the advanced viviparous reproductive mode of surfperches, polyandry for the avoidance of genetic incompatibility may play an important role in the evolution of multiple mating. Embiotocids are conspicuous among viviparous teleosts because of their long gestational period, their minor quantity of yolk and, consequently, the almost complete dependency of their embryos on maternal nutrients supplied during gestation (deVlaming et al. 1983). In addition to nutrient supply, physiological and immunological interactions between fetus and mother are also reported in surfperches (Nakamura et al. 2006, 2009). Such intimate maternal–fetal interactions may make female surfperches particularly vulnerable to genetic incompatibility. Thus, by mating multiple times, a female surfperch may have the potential to utilize post-copulatory mechanisms to avoid the genetic-incompatibility threat. Furthermore, unlike many other marine fish, surfperches lack free-swimming pelagic larvae and both young and adults have low dispersal capabilities, which may increase the risk of mating with close relatives. Thus, the high degree of polyandry in surfperches might also be a mechanism for inbreeding avoidance, which can also be interpreted as a special case of polyandry for the avoidance of genetic incompatibility (Tregenza and Wedell 2000).
Sperm storage by females may also be responsible for the high degree of multiple paternity detected in C. aggregata. Thus, it should be interesting to compare the degree of polyandry in surfperches vis-à-vis oviparous species with prolonged sperm storage. Multiple paternity has been investigated in oviparous species of amphibians (Adams et al. 2005) and reptiles (Pearse et al. 2001; Uller and Olsson 2008) with prolonged sperm storage. However, the levels of multiple paternity in these oviparous species are lower than those observed in surfperches, thus implying that polyandry for incompatibility avoidance may be of greater importance for viviparous than for oviparous females. Considering that female surfperches can store viable sperm for long periods, we propose that the sperm storage phenomenon may have promoted the evolution of post-copulatory mechanisms that in effect enable females to assess their genetic compatibilities with multiple mates.
The key element for this hypothesis relating reproductive mode to genetic benefits is the degree to which embryological development involves maternal–fetal physiological interactions (Zeh and Zeh 2000, 2001). Viviparity (and “ovoviviparity”) is developed to varying degrees in different families of teleost fishes (Wourms 1981). At one extreme are primitively viviparous species (such as members of the Sebastidae) in which the embryos are numerous, eggs have sufficient yolk for embryo nourishment, and offspring are born while still in a very immature state. Intermediate are fishes (such as species in the Poeciliidae) in which fairly large yolk sacs remain available to the embryos but the latter are retained in the ovary until they have reached an advanced stage that is nearly ready for swimming and feeding. At another extreme are members of the Embiotocidae in which the yolk sac is greatly reduced and the young are retained within the ovary until the embryos reach an advanced stage of development. Indeed, male Dwarf Surfperches, Micrometrus minimus, are sexually mature and ready for reproduction at birth (Warner and Harlan 1982). A comparison of the degrees of polyandry in these various teleost families should be useful for testing the genetic benefits/reproductive-mode hypothesis. Indeed, genetic estimates of levels of multiple paternity already have been conducted on natural populations representing several species in these families, and in general, the degree of multiple paternity proved to be higher in surfperches than in members of the Sebastidae and Poeciliidae (reviewed in Avise and Liu 2010). This observation is broadly consistent with predictions of the genetic benefits/reproductive-mode hypothesis.
In addition to indirect genetic benefits to polyandrous females, mate encounter rates may also play a role for the high degree of multiple paternity in surfperches. Mate encounter rates are high in the embiotocid taxa studied because all three species breed in large aggregations (Wiebe 1968; Froeschke et al. 2007), thus increasing the opportunity for mate competition among males and active pre-copulatory female choice. C. aggregata is the most abundant embiotocid species and forms very large aggregations during spawning season (Eigenmann 1892; Wiebe 1968), which could be responsible for the very high incidence of multiple paternity observed. A positive correlation between rates of multiple paternity and rates of mate encounter were reported among natural populations of the poeciliid fish, Heterandria formosa (Soucy and Travis 2003). Furthermore, patterns of multiple paternity across reptilian taxa also indicate that the degree of multiple paternity is generally higher in species with higher frequencies of mate encounters (reviewed in Uller and Olsson 2008). To illustrate the relative role of mate encounter frequency in polyandrous female surfperches, it could be interesting to quantify the degree of multiple paternity in closely related species with low population density, such as the Tule Perch, Hysterocarpus traskii.
In addition to the high degree of multiple paternity in C. aggregata, we detected strongly skewed distributions of fertilization success among the multiple sires of particular single broods, further suggesting that sperm competition and/or cryptic female choice might be important for post-copulatory paternity biasing in this species. Based on a fair-raffle process in sperm competition games (Parker 1990), a positive correlation between brood size and number of sires is anticipated. However, we detected no correlation between number of sires and brood size in C. aggregata, thus further implying that post-copulatory mechanisms inside the female could indeed play an important role in determining paternity. The results from this study differ from that of Darling et al. (1980), in which the polyandrous females appeared to be larger and carried more embryos. The apparent discrepancy between the two studies may merely reflect, however, a difference in the power of the genetic markers used to detect multiple paternity. Reproductive skew among males was also detected in both E. jacksoni and E. lateralis, but, as in C. aggregata, no correlation between brood size and number of sires was evident in these species (Reisser et al. 2009). These strikingly similar genetic results for three surfperch species indicate that cryptic female choice might be important as a post-copulatory mechanism that potentially could bias paternity toward males of high genetic quality. In fact, however, sperm competition and cryptic female choice are not mutually exclusive and are particularly difficult to distinguish (Eberhard 1998; Birkhead and Pizzari 2002). Thus, perhaps we might hypothesize that “female-mediated sperm competition” plays an important role in the post-copulatory paternity biasing in surfperches. To fully address the sperm competition hypothesis, a follow-up experiment is needed. It could be interesting to study the incidence of female multiple mating by analyzing the sperm stored by females and then compare results to those from the analysis of embryos.
Acknowledgments
We thank A. Tatarenkov for technical assistance, A. Tatarenkov, R. Byrne and two anonymous reviewers for their helpful comments. This work was supported by the University of California, Irvine.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Adams EM, Jones AG, Arnold SJ. Multiple paternity in a natural population of a salamander with long-term sperm storage. Mol Ecol. 2005;14:1803–1810. doi: 10.1111/j.1365-294X.2005.02539.x. [DOI] [PubMed] [Google Scholar]
- Avise JC, Liu JX. Multiple mating and its relationship to alternative modes of gestation in male-pregnant versus female-pregnant fish species. Proc Natl Acad Sci USA. 2010;107:18915–18920. doi: 10.1073/pnas.1013786107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avise JC, Jones AG, Walker D, et al. Genetic mating systems and reproductive natural histories of fishes: lessons for ecology and evolution. Annu Rev Genet. 2002;36:19–45. doi: 10.1146/annurev.genet.36.030602.090831. [DOI] [PubMed] [Google Scholar]
- Baltz DM. Life history variation among female surfperches (Perciformes: Embiotocidae) Environ Biol Fish. 1984;10:159–171. doi: 10.1007/BF00001123. [DOI] [Google Scholar]
- Birkhead TR, Møller AP. Sexual selection and the temporal separation of reproductive events: sperm storage data from reptiles, birds and mammals. Biol J Linn Soc. 1993;50:295–311. doi: 10.1111/j.1095-8312.1993.tb00933.x. [DOI] [Google Scholar]
- Birkhead TR, Møller AP. Sperm competition and sexual selection. London: Academic Press; 1998. [Google Scholar]
- Birkhead TR, Pizzari T. Postcopulatory sexual selection. Nat Rev Genet. 2002;3:262–273. doi: 10.1038/nrg774. [DOI] [PubMed] [Google Scholar]
- Boutin-Ganache I, Raposo M, Raymond M, Deschepper CF. M13-tailed primers improve the readability and usability of microsatellite analysis performed with two different allele-sizing methods. Biotechniques. 2001;31:24–28. [PubMed] [Google Scholar]
- Curtsinger JW. Sperm competition and the evolution of multiple mating. Am Nat. 1991;138:93–102. doi: 10.1086/285206. [DOI] [Google Scholar]
- Darling JDS, Noble ML, Shaw E. Reproductive strategies in the surfperches. I. Multiple insemination in natural populations of the shiner perch, Cymatogaster aggregata. Evolution. 1980;34:271–277. doi: 10.2307/2407391. [DOI] [PubMed] [Google Scholar]
- DeMartini EE. A correlative study of the ecology and comparative feeding mechanism morphology of the Embiotocidae (surf-fishes) as evidence of the family’s adaptive radiation into available ecological niches. Wasmann J Biol. 1969;27:177–247. [Google Scholar]
- DeMartini EE. Size-assortative courtship and competition in two Embiotocid fishes. Copeia. 1988;2:336–344. doi: 10.2307/1445873. [DOI] [Google Scholar]
- deVlaming V, Baltz D, Anderson S, Fitzgerald R, Delahunty G, Barkley M. Aspects of embryo nutrition and excretion among viviparous embiotocid teleosts: Potential endocrine involvements. Comp Biochem Physiol A. 1983;76:189–198. doi: 10.1016/0300-9629(83)90313-4. [DOI] [Google Scholar]
- Dobbs GH. Scanning electron microscopy of intraovarian embryos of the viviparous teleost, Micrometrus minimus (Gibbons), (Perciformes: Embiotocidae) J Fish Biol. 1975;7:209–214. doi: 10.1111/j.1095-8649.1975.tb04591.x. [DOI] [Google Scholar]
- Dodds KG, Tate ML, McEwan JC, Crawford AM. Exclusion probabilities for pedigree testing farm animals. Theor Appl Genet. 1996;92:966–975. doi: 10.1007/BF00224036. [DOI] [PubMed] [Google Scholar]
- Eberhard WG. Female roles in sperm competition. In: Birkhead TR, Møller AP, editors. Sperm competition and sexual selection. London: Academic Press; 1998. pp. 91–116. [Google Scholar]
- Eigenmann CH. Cymatogaster aggregatus Gibbons; a contribution to the ontogeny of viviparous fishes. Bull United States Fish Comm. 1892;12:401–478. [Google Scholar]
- Froeschke B, Allen LG, Pondella DF., II Life history and courtship behavior of black perch, Embiotoca jacksoni (Teleostomi: Embiotocidae), from Southern California. Pac Sci. 2007;61:521–531. doi: 10.2984/1534-6188(2007)61[521:LHACBO]2.0.CO;2. [DOI] [Google Scholar]
- Gardiner DM. The origin and fate of spermatophores in the viviparous teleost Cymatogaster aggregata (Perciformes: Embiotocidae) J Morphol. 1978;155:157–172. doi: 10.1002/jmor.1051550203. [DOI] [PubMed] [Google Scholar]
- Gardiner DM. Cyclic changes in fine structure of the epithelium lining of the ovary of the viviparous teleost: Cymatogaster aggregata (Perciformes: Embiotocidae) J Morphol. 1978;156:367–380. doi: 10.1002/jmor.1051560304. [DOI] [PubMed] [Google Scholar]
- Gardiner DM. Utilization of extracellular glucose by spermatozoa of two viviparous fishes. Comp Biochem Physiol A. 1978;59:165–168. doi: 10.1016/0300-9629(78)90200-1. [DOI] [Google Scholar]
- Griffith SC, Owens IP, Thuman KA. Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol Ecol. 2002;11:2195–2212. doi: 10.1046/j.1365-294X.2002.01613.x. [DOI] [PubMed] [Google Scholar]
- Guo SW, Thompson EA. Performing the exact test of Hardy-Weinberg proportion for multiple alleles. Biometrics. 1992;48:361–372. doi: 10.2307/2532296. [DOI] [PubMed] [Google Scholar]
- Hamilton MB, Pincus EL, Di Fiore A, Fleischer RC. Universal linker and ligation procedures for construction of genomic DNA libraries enriched for microsatellites. Biotechniques. 1999;27:500–507. doi: 10.2144/99273st03. [DOI] [PubMed] [Google Scholar]
- Hauswaldt JS, Glenn TC. Microsatellite DNA loci from the Diamondback terrapin (Malaclemys terrapin) Mol Ecol Notes. 2003;3:174–176. doi: 10.1046/j.1471-8286.2003.00388.x. [DOI] [PubMed] [Google Scholar]
- Hubbs CL. The breeding habits of the viviparous perch, Cymatogaster. Copeia. 1917;47:72–74. doi: 10.2307/1435665. [DOI] [Google Scholar]
- Jennions MD, Petrie M. Why do females mate multiply? A review of the genetic benefits. Biol Rev. 2000;75:21–64. doi: 10.1017/S0006323199005423. [DOI] [PubMed] [Google Scholar]
- Jones AG. Gerud 2.0: a computer program for the reconstruction of parental genotypes from half-sib progeny arrays with known or unknown parents. Mol Ecol Notes. 2005;5:708–711. doi: 10.1111/j.1471-8286.2005.01029.x. [DOI] [Google Scholar]
- Jones OR, Wang J. Colony: a program for parentage and sibship inference from multilocus genotype data. Mol Ecol Res. 2010;10:551–555. doi: 10.1111/j.1755-0998.2009.02787.x. [DOI] [PubMed] [Google Scholar]
- Keller L, Reeve HK. Why do females mate with multiple males? The sexually selected sperm hypothesis. Adv Stud Behav. 1995;24:291–315. doi: 10.1016/S0065-3454(08)60397-6. [DOI] [Google Scholar]
- Milligan BG. Total DNA isolation. In: Hoelzel AR, editor. Molecular genetic analysis of populations: a practical approach. Oxford: Oxford University Press; 1998. pp. 28–64. [Google Scholar]
- Nakamura O, Kudo R, Aoki H, Watanabe T. IgM secretion and absorption in the materno-fetal interface of a viviparous teleost, Neoditrema ransonneti (Perciformes; Embiotocidae) Dev Comp Immunol. 2006;30:493–502. doi: 10.1016/j.dci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Nakamura O, Nozawa Y, Saito E, Ikeda D, Tsutsui S. An alpha-1-acid glycoprotein-like protein as a major component of the ovarian cavity fluid of viviparous fish, Neoditrema ransonnetii (Perciformes, Embiotocidae) Comp Biochem Physiol A. 2009;153:222–229. doi: 10.1016/j.cbpa.2009.02.018. [DOI] [PubMed] [Google Scholar]
- Nonacs P. Measuring and using skew in the study of social behavior and evolution. Am Nat. 2000;156:577–589. doi: 10.1086/316995. [DOI] [PubMed] [Google Scholar]
- Parker GA. Sperm competition games—raffles and role. Proc Roy Soc B. 1990;242:120–126. doi: 10.1098/rspb.1990.0114. [DOI] [Google Scholar]
- Parker GA. Sperm competition and the evolution of ejaculates: towards a theory base. In: Birkhead TR, Moller AP, editors. Sperm competition and sexual selection. London: Academic Press; 1998. [Google Scholar]
- Pearse DE, Janzen FJ, Avise JC. Genetic markers substantiate long-term storage and utilization of sperm by female painted turtles. Heredity. 2001;86:378–384. doi: 10.1046/j.1365-2540.2001.00841.x. [DOI] [PubMed] [Google Scholar]
- Reisser CMO, Beldade R, Bernardi G. Multiple paternity and competition in sympatric congeneric reef fishes, Embiotoca jacksoni and E. lateralis. Mol Ecol. 2009;18:1504–1510. doi: 10.1111/j.1365-294X.2009.04123.x. [DOI] [PubMed] [Google Scholar]
- Schneider S, Roessli D, Excoffier L (2000) Arlequin: a software for population genetics data analysis. Ver. 2.0. Genetics and Biometry Laboratory, Department of Anthropology, University of Geneva
- Seutin G, White BN, Boag PT. Preservation of avian blood and tissue samples for DNA analyses. Can J Zool. 1991;69:82–90. doi: 10.1139/z91-013. [DOI] [Google Scholar]
- Shaw E. Evidence of sexual maturation in young adult shiner perch, Cymatogaster aggregata Gibbons (Perciformes, Embiotocidae) Am Mus Novit. 1971;2479:1–10. [Google Scholar]
- Shaw E, Allen J. Reproductive behavior in the female shiner perch, Cymatogaster aggregata. Mar Biol. 1977;40:81–86. doi: 10.1007/BF00390631. [DOI] [Google Scholar]
- Simmons LW. The evolution of polyandry: sperm competition, sperm selection, and offspring viability. Annu Rev Ecol Evol Syst. 2005;36:125–146. doi: 10.1146/annurev.ecolsys.36.102403.112501. [DOI] [Google Scholar]
- Soucy S, Travis J. Multiple paternity and population genetic structure in natural populations of the poeciliid fish, Heterandria formosa. J Evol Biol. 2003;16:1328–1336. doi: 10.1046/j.1420-9101.2003.00608.x. [DOI] [PubMed] [Google Scholar]
- Soulsbury CD. Genetic patterns of paternity and testes size in mammals. Plos One. 2010;5(3):e9581. doi: 10.1371/journal.pone.0009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockley P. Female multiple mating behaviour, early reproductive failure and litter size variation in mammals. Proc Roy Soc B. 2003;270:271–278. doi: 10.1098/rspb.2002.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockley P, Gage MJG, Parker GA, Møller AP. Sperm competition in fishes: the evolution of testis size and ejaculate characteristics. Am Nat. 1997;149:933–954. doi: 10.1086/286031. [DOI] [PubMed] [Google Scholar]
- Tarp FH. A revision of the family Embiotocidae (the surfperches) Fish Bull Calif Dep Fish Game. 1952;88:1–99. [Google Scholar]
- Thornhill R, Alcock J. The evolution of insect mating systems. Cambridge: Harvard University Press; 1983. [Google Scholar]
- Tregenza T, Wedell N. Genetic compatibility, mate choice and patterns of parentage: invited review. Mol Ecol. 2000;9:1013–1027. doi: 10.1046/j.1365-294x.2000.00964.x. [DOI] [PubMed] [Google Scholar]
- Turner CL. Histological and cytological changes in the ovary of Cymatogaster aggregatus during gestation. J Morphol. 1938;62:351–373. doi: 10.1002/jmor.1050620209. [DOI] [Google Scholar]
- Uller T, Olsson M. Multiple paternity in reptiles: patterns and process. Mol Ecol. 2008;17:2566–2580. doi: 10.1111/j.1365-294X.2008.03772.x. [DOI] [PubMed] [Google Scholar]
- Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P. Micro-checker: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes. 2004;4:535–538. doi: 10.1111/j.1471-8286.2004.00684.x. [DOI] [Google Scholar]
- Wang J. Sibship reconstruction from genetic data with typing errors. Genetics. 2004;166:1963–1979. doi: 10.1534/genetics.166.4.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Santure AW. Parentage and sibship inference from multilocus genotype data under polygamy. Genetics. 2009;181:1579–1594. doi: 10.1534/genetics.108.100214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner RR, Harlan RK. Sperm competition and sperm storage as determinants of sexual dimorphism in the dwarf surfperch, Micrometrus minimus. Evolution. 1982;36:44–55. doi: 10.2307/2407965. [DOI] [PubMed] [Google Scholar]
- Wiebe JP. The reproductive cycle of the viviparous seaperch, Cymatogaster aggregata Gibbons. Can J Zool. 1968;46:1221–1234. doi: 10.1139/z68-172. [DOI] [PubMed] [Google Scholar]
- Wourms JP. Viviparity: the maternal-fetal relationship in fishes. Am Zool. 1981;21:473–515. [Google Scholar]
- Yasui Y. The ‘genetic benefits’ of female multiple mating reconsidered. Trends Ecol Evol. 1998;13:246–250. doi: 10.1016/S0169-5347(98)01383-4. [DOI] [PubMed] [Google Scholar]
- Yasui Y. Female multiple mating as a genetic bet-hedging strategy when mate choice criteria are unreliable. Ecol Res. 2001;16:605–616. doi: 10.1046/j.1440-1703.2001.00423.x. [DOI] [Google Scholar]
- Zeh JA, Zeh DW. The evolution of polyandry I: intragenomic conflict and genetic incompatibility. Proc Roy Soc B. 1996;263:1711–1717. doi: 10.1098/rspb.1996.0250. [DOI] [Google Scholar]
- Zeh JA, Zeh DW. The evolution of polyandry II: post-copulatory defenses against genetic incompatibility. Proc Roy Soc B. 1997;264:69–75. doi: 10.1098/rspb.1997.0010. [DOI] [Google Scholar]
- Zeh JA, Zeh DW. Reproductive mode and speciation: the viviparity-driven conflict hypothesis. BioEssays. 2000;22:938–946. doi: 10.1002/1521-1878(200010)22:10<938::AID-BIES9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Zeh JA, Zeh DW. Reproductive mode and the genetic benefits of polyandry. Anim Behav. 2001;61:1051–1063. doi: 10.1006/anbe.2000.1705. [DOI] [Google Scholar]