Abstract
Individuals with atopic dermatitis (AD) immunized with the small pox vaccine, vaccinia virus (VV), are susceptible to eczema vaccinatum (EV), a potentially-fatal disseminated infection. Dysfunction of FoxP3+ regulatory T cells (Treg) has been implicated in the pathogenesis of AD. To test whether Treg-deficiency predisposes to EV, we percutaneously VV-infected FoxP3-deficient (FoxP3KO) mice, which completely lack FoxP3+ Treg. These animals generated both fewer VV-specific CD8+ effector T cells and interferon-γ producing CD8+ T cells than controls, had higher viral loads and exhibited abnormal Th2 polarized responses to the virus. To focus on the consequences of Treg deficiency confined to the skin, we generated mixed CCR4KO FoxP3KO bone marrow (CCR4/FoxP3) chimeras in which skin, but not other tissues or central lymphoid organs, lack Treg. Like FoxP3KO mice, the chimeras had impaired VV-specific effector T cell responses and higher viral loads. Skin cytokine expression was significantly altered in infected chimeras compared to controls. Levels of the antiviral cytokines, type I and II interferons and IL-12, were reduced whereas expression of the proinflammatory cytokines, IL-6, IL-10, TGF-β and IL-23, was increased. Importantly, infection of CCR4/FoxP3 chimeras by a non-cutaneous route (i.p.) induced immune responses comparable to controls. Our findings implicate allergic skin inflammation resulting from local Treg deficiency in the pathogenesis of EV.
Keywords: Viral infection, skin inflammation, regulatory T cells
Introduction
Concerns regarding the potential use of smallpox as a biological weapon have led to resumption of immunization with vaccinia virus (VV). Although immunization is effective, it is associated with significant side effects. The most serious complication, eczema vaccinatum (EV), a localized or potentially-lethal systemic dissemination of the virus, occurs in patients with atopic dermatitis (AD) (1). The basis of enhanced susceptibility to vaccinia virus in AD patients is not well understood. AD is a chronic inflammatory skin disease associated elevated IgE and blood eosinophilia. Acute AD lesions are infiltrated with CD4+ Th2 cells, which secrete predominately IL-4, IL-5 and IL-13 (2). In chronic lesions interferon (IFN)- γ producing Th1 cells dominate (3). Several lines of evidence indicate that Treg dysfunction in the skin underlies AD. Mutations in the Forkhead Box P3 (FOXP3) gene result in the immune dysregulation, polyendocrinopathy, enteropathy X-linked (IPEX) syndrome characterized by severe eczema and allergies (4) and single-nucleotide polymorphisms of FOXP3 have previously been reported to be associated with atopy (5). Diminished CD4+CD25+FoxP3+ Treg cells have been reported in patients with AD and asthma and the number of Treg is inversely correlated with IgE, eosinophilia, and IFN-γ levels (6–8). Mice bearing loss of function FoxP3 mutations display severe dermatitis lymphoproliferation along with allergic airway and gut inflammation, blood and tissue eosinophilia and elevated IgE levels (9–11). Recently it has been shown that tissue-targeted reduction in Treg leads to localized inflammation (12, 13).
We hypothesized that allergic inflammation arising in the setting of Treg deficiency might result in ineffective immune responses to VV delivered via the skin and that this might underlie the pathogenesis of EV. In order to test this hypothesis, we studied VV responses in both FoxP3-deficient (FoxP3KO) mice (11), which lack Treg in all tissues, and in chimeras in which only the skin was deficient in Treg. The chimeras were constructed using a mixture of bone marrow (BM) from chemokine (C-C motif) receptor 4 (CCR4)-deficient and from FoxP3-deficient mice. In these animals the only lymphocytes capable of homing to skin (CCR4+) arise from progenitors of FoxP3-deficient origin resulting in a skin-restricted inflammatory phenotype. Evaluation of the VV response in mice with global or skin-restricted Treg deficiency revealed more intense local and systemic infection, a phenotype reminiscent of EV. We characterize the specific abnormalities in the VV immune responses in these mice that predispose to severe VV infection and show that bypassing the skin altogether, by i.p. injection of the virus, normal responses can be elicited. These findings implicate dysfunction of cutaneous FoxP3+ Treg in VV immunity.
Materials and Methods
Virus source and expansion
The VV Western Reserve strain was obtained from American Type Culture Collection (ATCC) (Manassas, VA; VR-1354) and expanded and titered in CV1 cells (ATCC; CCL-70) by standard procedures (14).
Animals and virus application
FoxP3-deficient mice on a C57BL/6 background were generated as previously described (11). Male CCR4−/− (B6;129P-Ccr4tm1Pwr/J; referred to in the the manuscript as CCR4KO) mice and male Rag1KO mice (B6.129S7-Rag1tm1Mom/J) were purchased from The Jackson Laboratory. All experiments were carried out in accordance with Children’s Hospital policies and procedures and were reviewed by the Institutional Animal Care and Use Committee. For epicutaneous VV-infection (via skin scarification) mice were anesthetized using avertin (2,2,2-tribromoethanol and tertiary amyl alcohol) and 10 μl VV (1 × 107 plaque-forming units (PFU)) were inoculated with 30 superficial scratches in the skin of the shaven back of the mice using a 27 1/2-gauge needle. Alternatively 100 μl VV (2×106 PFU) were injected i.p.. Lesion sizes were analyzed by using NIH Image software (ImageJ).
VV-specific quantitative real-time PCR
DNA was prepared using the Qiagen DNeasy Kit (Qiagen, Valencia, CA) according to the manufacture’s guidelines. Viral genomes were quantified by real-time PCR using primers specific for vaccinia ribonucleotide reductase (Vvl4L) and an ABI 7700 Sequence Detection System (PE Applied Biosystems, Foster City, CA) (15). To quantitatively determine the vaccinia virus genome copies, a standard curve was generated using DNA from purified VV stock with known PFU (determined by plaque assay). Viral copies determined using a standard curve were normalized to the amount of total DNA.
Flow cytometry
Single cell suspensions of splenocytes were prepared 8 days post infection (p.i.). 1×106 splenocytes were stained according to manufacturer’s instructions. Cells were incubated with 5 μl B8R20–27 (TSYKFESV (16)) MHC class I Pro5TM pentamers conjugated to PE (ProImmune, Oxford, UK). Then cell surface staining was performed using APC-conjugated anti-mouse CD8a (Ly-2, clone 53–6.7) (eBioscience, San Diego, CA) and FITC-conjugated anti-mouse CD45R/B220 (RA3-6B2) (BD Biosciences Pharmingen). For intracellular IFN-γ staining, 1×106 splenocytes were incubated in α-MEM complete medium with 10 μM B8R20–27 peptide at 37°C in 5% CO2 for 6 h. To block the secretion of de novo synthesized IFN-γ, GolgiStopTM (BD Biosciences Pharmingen) was added after 1 h. After incubation with 5 μl B8R20–27 MHC class I Pro5TM pentamers conjugated to PE, cells were fixed and permeabilized using BD Cytofix/CytopermTMPlus Kit (BD Biosciences Pharmingen) and stained with APC-conjugated anti-mouse CD8a and FITC-labeled anti-mouse IFN-γ Ab (BD Biosciences Pharmingen). Samples were analyzed on a BD Biosciences FACSCalibur flow cytometer (San Jose, CA). Analysis was performed using CellQuest software (BD Biosciences Pharmingen).
In vitro cytokine synthesis by splenocytes
Single cell suspensions of splenocytes were prepared and stimulated as previously reported (15). Supernatants were collected at 24 h (for IFN-γ), 48 h (for IL-4) and 72 h (for IL-2). Cytokine levels in supernatants were determined by ELISA following the manufacturer’s instructions (BD Bioscience Pharmingen).
Bone Marrow chimeras
Femurs and tibias were flushed with cold sterile PBS. Cells were incubated in hemolytic buffer for 5 min at room temperature, washed and counted. A total of 2×106 cells (1.5×106 derived from FoxP3KO or WT mice and 0.5×106 from CCR4KO mice) were injected retroorbitally into anesthetized and irradiated (2 doses of 450 Rad) Rag1KO mice. Bone marrow reconstitutions were only performed with male mice. Atopic dermatitis-like characteristics of the skin were evident starting 3–6 weeks after reconstitution. BM chimeras were VV-infected between 4–6 weeks after reconstitution.
Tissue histology
Skin and colon were fixed in 10% neutral-buffered formalin, paraffin embedded, cut into 5 μm sections and stained with hematoxylin and eosin. Sections were examined for inflammatory infiltrates using standard light microscopy by an investigator unaware of the identity of individual samples. For quantification, infiltrating cells were counted per 0.01 mm2) of 2 sections per mouse, 5 mice per group.
Enzyme-linked immunosorbent assay
Total IgE levels in the sera were quantified using a sandwich ELISA with paired antibodies from BD Pharmingen (San Diego, CA).
Skin cytokines
Total RNA was isolated from homogenized skin tissue using TRIzol® reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. cDNA was generated with iScript cDNA synthesis kit (Bio-rad Laboratory). Quantitative real-time PCR was done using Taqman® Gene Expression Assay probes, Taqman® PCR master mix and the ABI Prism 7300 sequence detection system, all from Applied Biosystems, Fostercity, CA. Expression of each cytokine transcript was determined relative to a reference gene transcript, GAPDH, calculated as 2^− (Ct, cytokine − Ct, GAPDH).
Statistical analyses
Differences in values between experimental groups were examined for significance with GraphPad Prism® software using unpaired two-tailed Student’s t test. Values are presented as means ± standard error of the mean (SEM).
Results
More severe skin pox lesions in cutaneously VV-infected FoxP3KO mice
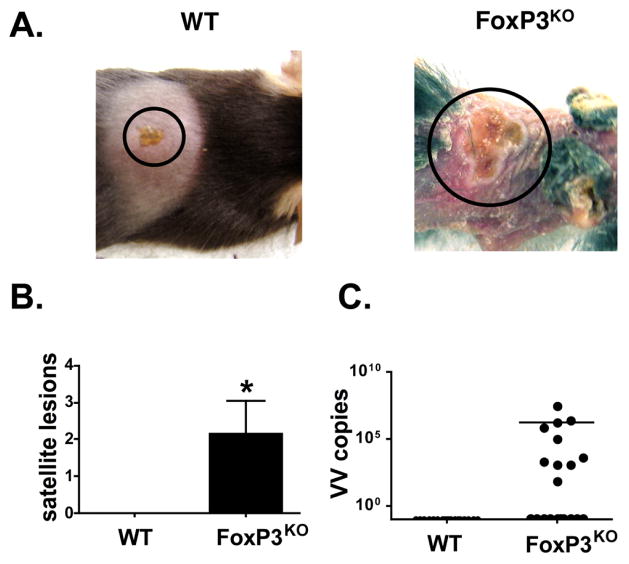
Following skin scarification with VV to mimic smallpox immunization, wild type mice developed confined pox lesions similar to those observed in vaccinated humans starting four days post infection (average lesion area 15.7 mm2 ± 1.5) (Figures S1 and 1A). In contrast, FoxP3KO mice developed both extensive primary lesions (35.0 mm2 ± 4.9) (Figure S1) and numerous satellites in contiguous and distant skin (Figures 1A and B). Similar results were obtained even with a 10-fold lower virus dose (data not shown). Viral load assays confirmed that infection was indeed confined to the inoculation site of WT mice, whereas variable amounts of viral genome (1.5×106 ± 1.3×106 copies/μg DNA) were recovered from contiguous or distant skin in FoxP3KO mice (Figure 1C), a phenotype reminiscent of EV. Primary lesion sizes in FoxP3 KO mice were positively correlated with viral genome numbers contained within the lesions (r2 = 0.934). These observations indicate defective cutaneous clearance of VV in the setting of Treg deficiency.
Figure 1. More intense skin lesions in VV-infected FoxP3KO mice.
(A) Primary lesions 7 days after inoculation of vaccinia virus (VV) via skin scarification. (B) Number of satellite lesions (contiguous and distant from the inoculation site) per mouse (n = 20). (C) Viral genome copy numbers per μg total DNA in skin other than primary lesion (n = 20). *p < 0.05
Absence of VV-specific CD8+ effector T cells in cutaneously-infected FoxP3KO mice
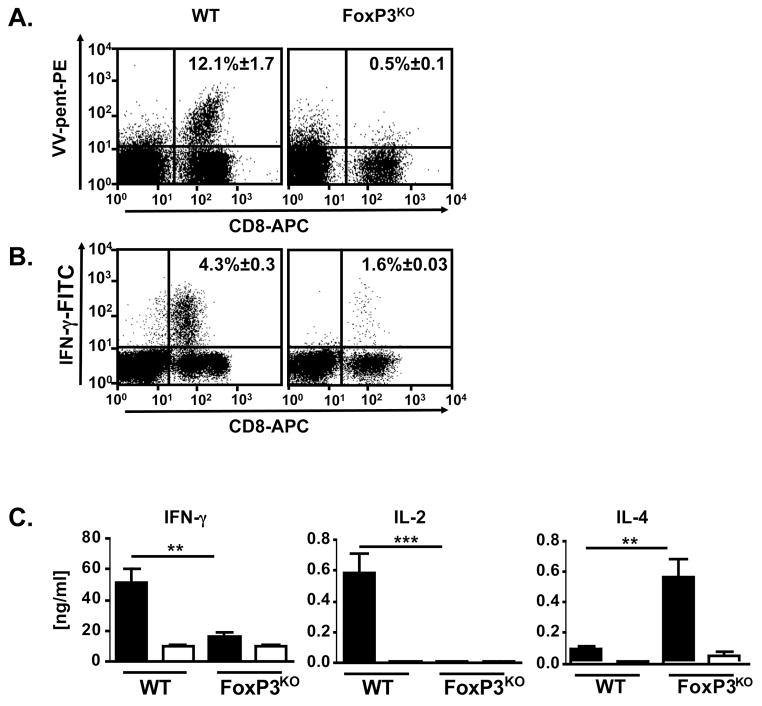
We hypothesized that exposure to virus in the setting of allergic inflammation might fail to induce effective immune responses. In WT mice, cutaneous VV infection elicited a robust virus-specific CD8+ T cell response as measured by pentamer staining (12.1% ± 1.7; Figure 2A). In contrast, FoxP3KO mice failed to generate VV-specific T cells (0.5% ± 0.1; p < 0.0001). Substantial IFN-γ production was evident in VV peptide stimulated CD8+ splenocytes from cutaneously infected WT mice (4.3% ± 0.3), whereas only background IFN-γ staining (1.6% ± 0.3; Figure 2B; p < 0.05) was seen in FoxP3KO mice. This complete absence of VV-induced IFN-γ production was corroborated by a lack of IgG2a responses in the FoxP3KO mice (Figure S2). To investigate whether the impaired immune responses in FoxP3KO mice resulted not only in enhanced cutaneous spread of the virus, but also in greater systemic dissemination, we assayed the presence of viral DNA eight days after inoculation. VV was consistently detectable in the organs of FoxP3KO mice (70–100% positive) while only occasionally recoverable from the organs of WT mice (0–37% positive) (Figure S4). These findings suggest that VV infections established in the setting of skin inflammation might go unchecked by normal adaptive immune defenses.
Figure 2. VV-specific immune responses in FoxP3KO mice.
Splenocytes of cutaneously VV-infected FoxP3KO and WT control mice were analyzed eight days p.i.. Data were compiled of three independent experiments. (A) Splenocytes were stained for CD8, VV-specific TCR (using B8R20–27 MHC class I pentamers) and CD45R/B220 and analyzed by flow cytometry. Representative dot plots are shown (VV infected FoxP3KO mice n = 17, WT n = 8). Uninfected mice did not have any VV-specific CD8+ T cells (Figure S3). (B) Splenocytes were stimulated in vitro with the VV peptide, B8R20–27. Intracellular IFN-γ as well as CD8 and CD4 surface staining were performed and cells were analyzed by flow cytometry gating on CD8+ positive cells. Representative dot plots are shown (n = 7). (C) Cytokine levels in supernatants of splenocytes from VV-infected FoxP3KO and WT control mice stimulated with irradiated VV-infected (black columns) or uninfected (white columns) splenocytes from naïve WT mice. Cytokines were determined by ELISA 24 hours (IFN-γ), 48 hours (IL-4) and 72 hours (IL-2) after stimulation (VV-infected FoxP3KO mice n = 9, all others n = 7). ** p < 0.005, ***p < 0.0005
Impaired Th1 cytokine expression, but enhanced IL-4 production by VV-specific splenocytes from cutaneously VV-infected FoxP3KO mice
The Th1 cytokine, IFN-γ, is critical in VV immunity, driving expansion of virus specific CD8+ T cell populations (17–19) and mediating early control of VV replication (20, 21). In contrast, Th2 cytokines impair VV clearance (22, 23). In this study, WT splenocytes stimulated with VV displayed robust IFN-γ responses (50.2 ng/ml ± 9.6) whereas splenocytes from VV-infected FoxP3KO mice produced background levels (15.6 ng/ml ± 2.4; p < 0.0001) (Figure 2C). IL-2 responses were similarly impaired in FoxP3KO mice. In striking contrast to the diminished production of Th1 cytokines, Th2 responses were amplified in FoxP3KO mice as evidenced by markedly increased production of IL-4 (0.5 ng/ml ± 0.1, compared with 0.1 ng/ml ± 0.0 in WT; p < 0.005). Collectively, these results demonstrate that cutaneous VV infection of FoxP3KO results in impaired Th1 effector immune responses along with a paradoxical induction of Th2 immunity.
Generation and characterization of CCR4/FoxP3 chimeras
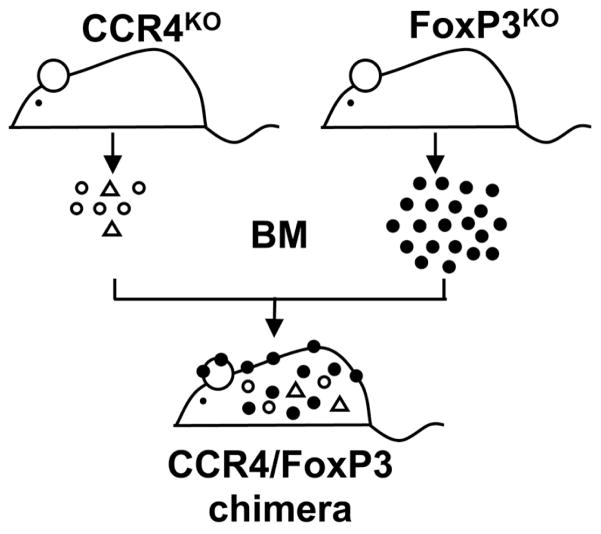
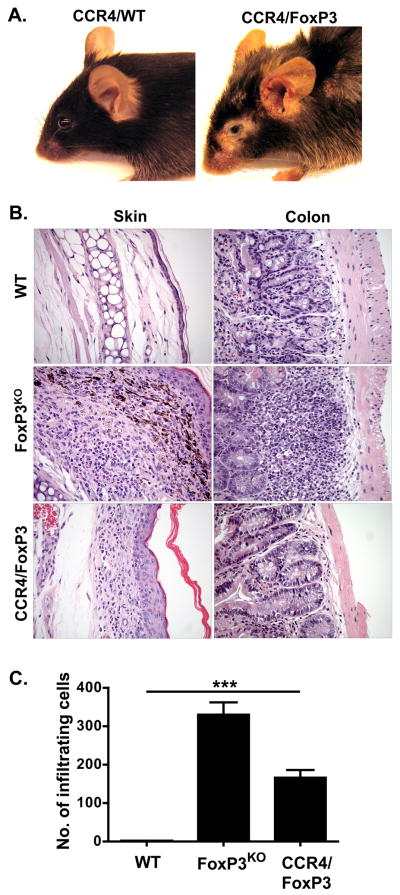
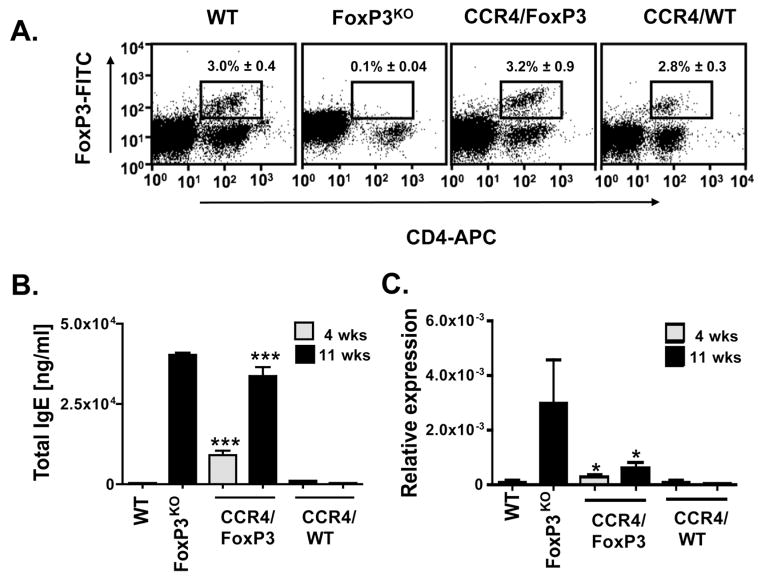
As FoxP3KO mice exhibit multisystem inflammation, studies with these animals did not allow us to distinguish effects of skin inflammation on the EV phenotype from those of generalized immune abnormalities resulting from global FoxP3 deficiency. Therefore additional studies were performed using BM chimeras constructed by infusing mixed marrow from CCR4KO and FoxP3KO mice into Rag1KO in which only FoxP3KO progenitors harbor a normal CCR4 allele critical for skin homing resulting in a Treg deficiency confined to the skin (CCR4/FoxP3 chimeras, see schematic in Figure 3). Control mice received mixed BM from CCR4KO and WT donors (CCR4/WT chimeras). CCR4/FoxP3 chimeras (but not controls) developed patches of inflamed skin eventually spreading to the ears and back with erythema, excoriation, alopecia and crusting (Figure 4A). These lesions were infiltrated with eosinophils and scattered lymphocytes extending from the subcutaneous tissue to the dermal-epidermal interface, similar in appearance but less intense than the infiltrate of FoxP3KO mice (Figure 4B). Quantification of infiltrating cells per 0.01 mm2 showed a significantly higher cutaneous influx in CCR4/FoxP3 chimeras than in control mice (p < 0.0001): 165 ± 20 (CCR4/FoxP3), 331 ± 31 (FoxP3KO) and 2 ± 0.4 (WT) (Figure 4C). As predicted, the chimeras were spared the gastrointestinal inflammation present in FoxP3KO mice. Only FoxP3KO mice and not the chimeras had colonic inflammation with a mixed lymphocytic and eosinophilic infiltrate, crypt regeneration and enterocytes showing reactive changes with nuclear polymorphism. Enlarged spleens (data not shown) lacking CD4+FoxP3+ splenocytes were similarly present in FoxP3KO mice but not evident in the CCR4/FoxP3 chimeras (Figure 5A). Consistent with skin inflammation as a stimulus of IgE production, four weeks after BM reconstitution total IgE levels were significantly increased in CCR4/FoxP3 (p < 0.0001), but not in control chimeras, and reached similar elevations compared to FoxP3KO mice eleven weeks after BM reconstitution (Figure 5B). Significantly higher expression of IL-4 (p < 0.05) was found in the skin of CCR4/FoxP3 chimeras compared to control mice starting four weeks after BM reconstitution (Figure 5C).
Figure 3. Scheme for construction of mixed CCR4/FoxP3 chimeras.
Mixed BM from CCR4KO and FoxP3KO mice (in a ratio of 1:3) is intravenously injected into irradiated Rag1KO mice. Control mice receive mixed BM from CCR4KO and WT mice. Only cells from FoxP3KO mice (black) express the skin homing receptor CCR4 and are capable of migrating to the skin, whereas cells derived from CCR4KO mice (white), including Treg (white triangles) are distributed throughout non-cutaneous sites including central lymphoid organs and the gut.
Figure 4. Characteristics of mixed CCR4/FoxP3 BM chimeras.
(A) A representative CCR4/FoxP3 chimeric mouse (right) displaying skin inflammation, erythema, alopecia and crusting on the eyes, ears and back eleven weeks after BM reconstitution. CCR4/WT chimeras (left) appeared phenotypically normal. (B) Cutaneous inflammation in CCR4/FoxP3 chimeras. Representative hematoxylin- and eosin-stained sections of skin (left column) and colon (right column) from WT (upper panel), FoxP3KO mice (middle panel), and CCR4/FoxP3 chimeras (lower panel) eleven weeks after BM reconstitution. (C) Infiltrating cells per 0.01 mm2 determined by evaluation of two hematoxylin- and eosin-stained sections per mouse (5 mice per group) eleven weeks after BM reconstituton. ***p < 0.0001
Figure 5. Characteristics of CCR4/FoxP3 chimeras.
(A) Splenocytes were stained for FoxP3, CD4 and CD25 and analyzed via flow cytometry eleven weeks after BM reconstitution. Representative dot plots are shown. Numbers indicate the average percentage of FoxP3+ and CD4+ double positive splenocytes ± SEM (n = 3). The average percentages of FoxP3+ CD4+ of total CD4+ cells constituted 19.5% ± 2.2 for WT and 32.5% ± 0.1 for CCR4/FoxP3 chimeras. (B) Serum IgE levels in WT, FoxP3KO mice and CCR4/FoxP3 and CCR4/WT chimeras four weeks (hatched column, n = 31 per group) and eleven weeks (black column, n = 3 per group) after BM reconstitution. (C) IL-4 mRNA measured by quantitative real-time PCR in skin of WT, FoxP3KO, CCR4/FoxP3 and CCR4/WT chimeras four weeks (hatched column, n = 12 per group) and eleven weeks (black column, n = 6 per group) after BM reconstitution. Relative expression of IL-4 is represented in relation to GAPDH. *p < 0.05, ***p < 0.0001
Ineffective immune responses and viral spread in CCR4/FoxP3 chimeras after VV infection
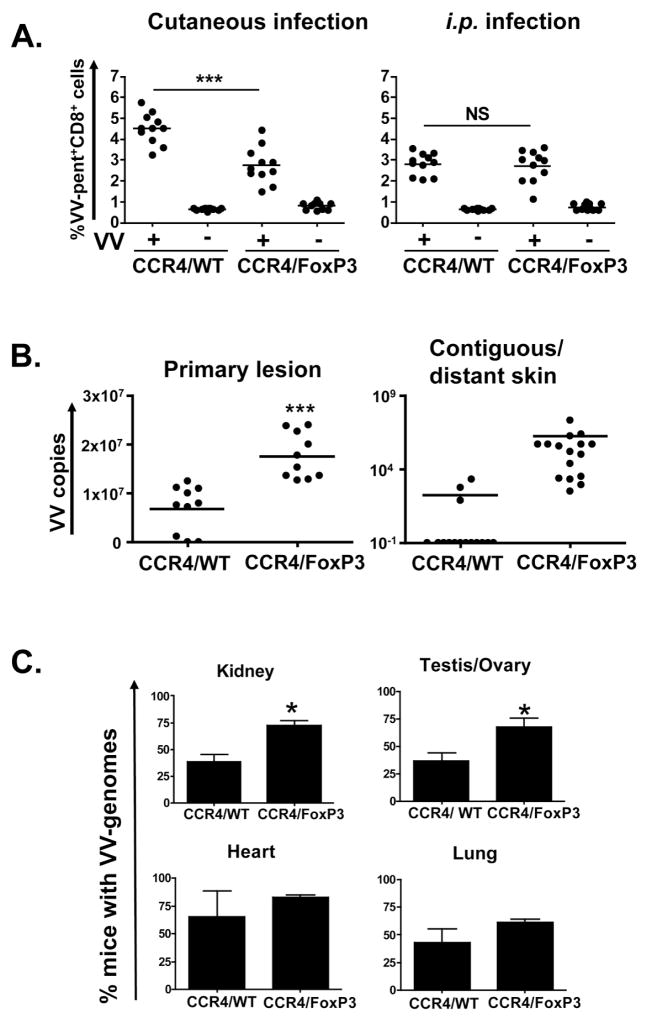
Using the CCR4/FoxP3 chimeras as a model, we tested the effects of skin-specific Treg deficiency on immune responses to VV. CCR4/FoxP3 chimeras generated significantly fewer VV-specific CD8+ T cells (2.8% ± 0.3) compared to control CCR4/WT chimeras (4.5% ± 0.3; p < 0.0001) after cutaneous infection (Figure 6A). In contrast, bypassing the inflamed skin by intraperitoneal VV-infection resulted in similar VV-specific CD8+ T cell responses in CCR4/FoxP3 (2.8% ± 0.3) and CCR4/WT (2.8% ± 0.3) chimeras. The CCR4/FoxP3 chimeras developed significantly larger primary lesions (45.9 mm2 ± 4.1) than control chimeras (17.1 mm2 ± 1.5) (Figure S5). In addition satellite lesions arose at a higher frequency in the CCR4/FoxP3 chimeras (16 lesions per 10 mice) compared with controls (3 lesions per 10 mice) (Figure 6B). Significantly higher viral loads were present in the primary lesions of CCR4/FoxP3 chimeras (1.8×107 ± 0.2×107) than in those of CCR4/WT chimeras (0.7 ×107 ± 0.2×107; p < 0.0001) along with more abundant VV copies in contiguous and distant skin (1.8×106 ± 1.4×106 in CCR4/FoxP3 chimeras compared to 181± 141 in CCR4/WT chimeras) (Figure 6B). In addition, we found significantly higher numbers of CCR4/FoxP3 chimeras with detectable VV in kidney (72.7% ± 3.9) and testis/ovary (70.2% ± 13.1) compared to control CCR4/WT chimeras (Figure 6C). A trend towards higher viral burdens was also suggested for heart and lung. The occasional presence of viral genome copies in the skin and organs of CCR4/WT control chimeras might have resulted from incomplete BM reconstitution with resultant partial immune deficiency in some of these mice.
Figure 6. Vaccinia virus infection of CCR4/FoxP3 chimeras.
CCR4/FoxP3 and CCR4/WT control chimeras were cutaneously or intraperitoneally infected with VV and analyzed eight days p.i.. Data were compiled from three independent experiments. (A) Splenocytes from cutaneously (n = 11) or intraperitoneally (n = 11) infected (+) or uninfected (−) CCR4/FoxP3 and CCR4/WT chimeras were stained for CD8, VV-specific TCR(using B8R20–27 MHC class I pentamers) and for CD45R/B220. (B) Vaccinia virus genome numbers were assessed by quantitative real time PCR for skin from primary lesions and contiguous/distant sites (satellite lesions in CCR4/FoxP3 chimeras) of cutaneously infected chimeras and shown as VV genome copy numbers per μg total DNA (n = 10 per group, 16 samples of contiguous/distant skin were collected from 10 mice). DNA from uninfected controls was assayed to confirm the specificity of quantitative real-time PCR and did not contain any viral genome copies. (C) Vaccinia virus genome numbers were assessed by quantitative real time PCR for kidney, testis/ovary, heart and lung of cutaneously infected chimeras. Data were compiled from three independent experiments (n = 5 per group per experiment). DNA from uninfected controls was assayed to confirm the specificity of quantitative real-time PCR and did not contain any viral genome copies (not shown). *p < 0.05
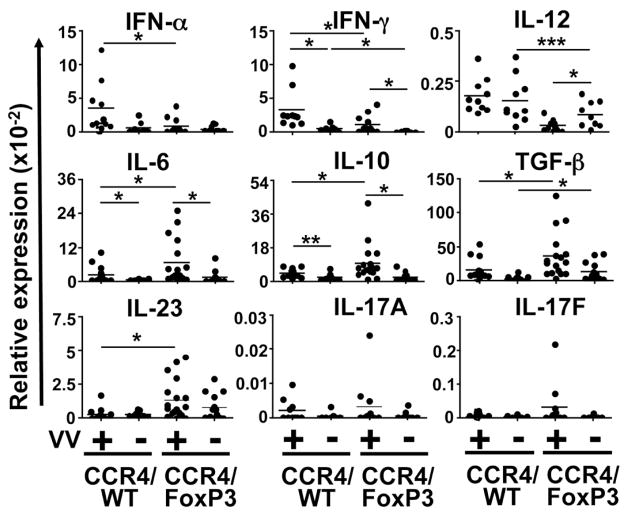
Analysis of cytokine expression in the skin revealed distinct expression patterns for CCR4/FoxP3 vs. CCR4/WT chimeras (Figure 7). Transcripts for type I and II interferons and IL-12, all important in viral immunity, were more strongly expressed in cutaneously infected CCR4/WT control chimeras than in controls. In contrast, mRNA for the proinflammatory mediators IL-6, TGF-β, IL-10 and IL-23 was more abundant in the skin of VV-infected CCR4/FoxP3 chimeras than controls. IFN-γ induction in VV-infected CCR4/FoxP3 chimeras was significantly impaired compared to control chimeras. Although we were not able to detect statistically significant differences in IL-17A and IL-17F expression we observed a trend towards increased levels in CCR4/FoxP3 chimeras. These findings demonstrate impaired local expression of type I interferons and Th1 cytokines in mice lacking skin Treg accompanied by unchecked viral spread from the primary lesion to contiguous skin and viscera.
Figure 7. Expression of cytokines in the skin of CCR4/FoxP3 chimeras after VV infection.
Cytokine expression in the skin contiguous to the primary infection site of VV-infected and uninfected CCR4/FoxP3 and CCR/WT control chimeras were assessed by quantitative real time PCR 8 days p.i. (n = 10–18 per group). *p ≤ 0.05, **p < 0.005, ***p < 0.0001.
Discussion
Recently we and others have used mouse models to investigate the immunological basis underlying eczema vaccinatum. RelB−/− mice (15), epicutaneously OVA-sensitized BALB/c mice (24), mice lacking cathelicidin antimicrobial peptide (CRAMP) (25) and NC/Nga mice bearing eczematous skin lesions (26) all exhibit abnormal VV immunity and viral clearance. However, the cellular immune mechanisms of EV have not been directly addressed.
Several observations implicate important roles for FoxP3 expressing Treg cells in maintenance of normal skin homeostasis and prevention of AD. Verhagen and colleagues have recently reported a relative deficiency of Treg and their cytokine products in AD lesional skin (6). Conflicting data exist regarding associations between the numbers of Treg cells in peripheral blood and AD. Reefer and colleagues as well as Leung’s group observed increased Treg in the peripheral blood of AD patients with numbers linked to disease severity (27, 28), while Orihara reported the opposite, an inverse correlation between peripheral Treg numbers and IgE, eosinophilia, and IFN-γ levels in patients with AD and asthma (7). In mice, FoxP3-deficiency results in severe allergic dysregulation with skin as a primary target (11). In addition to being implicated in skin homeostasis, Treg have been reported to suppress immunity to viral and other infections (29–32). An opposite, protective, role for Treg has recently been suggested in a model of HSV infection (33).
In order to assess whether the absence of FoxP3+ Treg cells contributes to increased susceptibility to VV in AD patients we mimicked smallpox immunization by infecting FoxP3KO mice with VV via skin scarification. Cutaneous infection resulted in viral spread from the inoculation site to contiguous skin and distant satellite lesions in FoxP3KO mice reminiscent of EV. We considered that skin inflammation might impair the induction of protective adaptive immune responses and first focused on the generation of IFN-γ producing virus specific CD8+ effector T cells. IFN-γ has a pivotal role in immune responses to VV. It inhibits VV replication via nitric oxide induction in macrophages (20, 21) and exerts pleiotropic effects on immune cells including activation of dendritic cells and macrophages, induction of MHC class I and II expression (34) and expansion of CD8+ T cells (19). CD8+ T cells play a decisive role in protection against VV (35, 36). In our infection model, introduction of VV via the inflamed skin of FoxP3KO animals was met with a complete lack of the VV-specific IFN-γ producing CD8+ effector cell responses characteristically observed in WT animals suggesting that impaired early adaptive immune responses might contribute to unchecked viral spread. Furthermore, VV-stimulated splenocytes from cutaneously infected FoxP3KO mice failed to produce IFN-γ and IL-2, but produced elevated amounts of IL-4 indicating markedly dysregulated T helper differentiation in these animals following infection.
Our studies using CCR4/FoxP3 chimeras confirm that mice with a FoxP3+ T cell compartment incapable of skin migration spontaneously develop a cutaneous inflammatory disease with red scaly skin, hyperkeratosis, lymphocytic and eosinophilic infiltration and elevated local levels of IL-4, TGF-β and IL-23 and decreased level of IFN-γ as well as high systemic IgE. In addition we have observed an inverse correlation between the ratio of CCR4KO:FoxP3KO cells and the severity and tempo of appearance of skin inflammation. Importantly, the non-skin-homing T cell compartment in these chimeras includes a FoxP3+ CD25+ CD4+ Treg subset and the animals displayed neither splenomegaly nor gut inflammation, consistent with the presence of functional central Treg. Recently it was reported that both epicutaneous and intraperitoneal VV immunization generate T cell-mediated immunity in mice but that immunization via skin scarification is the more immunogenic route (37). Here we show that this is only true when the infection occurs through normal but not through inflamed skin. Epicutaneous, but not intraperitoneal VV infection of CCR4/FoxP3 chimeras resulted in significantly reduced development of VV-specific CD8+ effector T cells compared to control chimeras confirming that infection occurring through Treg-deficient inflamed skin causes impaired adaptive immune responses. As expected, we observed impaired viral clearance in CCR4/FoxP3 chimeras as demonstrated by increased numbers of satellite lesions and higher viral loads in skin and organs compared to controls. Unlike the FoxP3KO mice, which were absolutely incapable of generating VV specific effector T cells after cutaneous infection, the CCR4/FoxP3 chimeras exhibited a partial, albeit markedly impaired, T cell response. We believe this most likely relates both to the much less severe skin inflammation observed in the chimeras and/or to the absence of systemic inflammation. CCR4/FoxP3 chimeras expressed elevated IL-4 and TGF-β levels in the skin. Infection with IL-4 expressing VV has been demonstrated to result in down regulation of Th1 cytokines, impairment of cytolytic activity, and diminishing of viral clearance (22, 38). Furthermore, IL-4 and IL-13 have been reported to enhance VV replication and suppress antimicrobial peptide expression in a Stat6–dependent manner in human skin (39). In addition, mice exhibiting a Th2-bias and elevated IL-4 expression due to T-bet deficiency are more susceptible to primary VV infection (40).
Upon infection, CCR4/FoxP3 chimeras expressed only modest levels of IFN-α and IFN-γ. The critical role of type I interferons in innate immunity and anti viral effects has been established. However, type I interferons are also key players in adaptive immunity to viral infections. They are shown to promote cross-priming after viral infection (41) and effector function of virus-specific CD8+ T cells by activating IFN-γ production in a STAT4 dependent manner (42), enhance the survival of activated T cells (43, 44), and are required for clonal expansion in response to viral infection (45).
Interleukin-12 is a key regulator of cell-mediated immunity through its potent stimulation of IFN-γ production (46). Its cutaneous expression was significantly downregulated in CCR4/FoxP3 chimeras. The enhanced cutaneous expression of IL-23 in CCR4/FoxP3 chimeras mirrors a scenario reported for VV-IL-23 (vaccinia virus expressing IL-23) infection in IL-12/23p40-deficient mice (47). However, VV-IL-23 infected IL-12/23p40-deficient mice expressed high levels of IFN-γ and exhibited no viral spread in complete contrast to our CCR4/FoxP3 chimeras. Taken together with our own data this suggests that the absence of cutaneous IL-12 along with the impaired expression of IFN-α results in the defective IFN-γ response and therefore results in the impaired T effector cell responses in our chimeras.
In contrast to the impaired expression of IL-12 and type I and II interferons, VV infected CCR4/FoxP3 chimeras produced significantly elevated levels of the proinflammatory cytokines IL-6, IL-10, TGF-β and IL-23. Interleukin-10 is highly expressed in AD skin (48) and is known to inhibit Th1-mediated enhancement of CTL activity, NK cell activation and IFN-γ production (49, 50). Combined with IL-4 it has been shown to be an effective inhibitor of VV clearance (23). Interleukin-10 is not only produced by keratinocytes upon VV infection (51) but is also known to be produced by Th9 cells induced by TGF-β and IL-4 (52) and by Th17 cells induced by TGF-β and IL-6 (53) which additionally require IL-23 for expansion and acquiring full effector function (54). All these cytokines are highly expressed in the skin lesions of CCR4/FoxP3 chimeras. We have observed that IL-17A levels promote VV virulence in BALB/c mice with antigen-induced eczematous skin lesions (55) and similar findings have been reported for NC/Nga mice (26). Although we detected only slightly increased levels of IL-17A and IL-17F upon infection this may be an issue with assay sensitivity and the relatively low Th17 responses in C57Bl6 vs. BALB/c mice (56).
Taken together, these data show that Treg deficiency in the skin leads to inflammation and alterations in cytokine expression, which secondarily affect adaptive immune responses to VV. In light of the reported aberrations in Treg function in atopic individuals this suggests that Treg dysfunction may, in part, underlie the pathogenesis of eczema vaccinatum.
Supplementary Material
Acknowledgments
We thank Benjamin Caplan and Diana Kombe for expert technical assistance and Drs. Raif Geha and Michiko Oyoshi for scientific advice.
Footnotes
This project has been funded in whole or in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services under contract number N01 AI40030 (Atopic Dermatitis Vaccinia Network) and by a research grant from the Deutsche Forschungsgemeinschaft FR 2116/1-1 (to EJF).
References
- 1.Engler RJ, Kenner J, Leung DY. Smallpox vaccination: Risk considerations for patients with atopic dermatitis. J Allergy Clin Immunol. 2002;110:357–365. doi: 10.1067/mai.2002.128052. [DOI] [PubMed] [Google Scholar]
- 2.Leung DY, Bhan AK, Schneeberger EE, Geha RS. Characterization of the mononuclear cell infiltrate in atopic dermatitis using monoclonal antibodies. J Allergy Clin Immunol. 1983;71:47–56. doi: 10.1016/0091-6749(83)90546-8. [DOI] [PubMed] [Google Scholar]
- 3.Tsicopoulos A, Hamid Q, Haczku A, Jacobson MR, Durham SR, North J, Barkans J, Corrigan CJ, Meng Q, Moqbel R, et al. Kinetics of cell infiltration and cytokine messenger RNA expression after intradermal challenge with allergen and tuberculin in the same atopic individuals. J Allergy Clin Immunol. 1994;94:764–772. doi: 10.1016/0091-6749(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 4.Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2002;39:537–545. doi: 10.1136/jmg.39.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bottema RW, Kerkhof M, Reijmerink NE, Koppelman GH, Thijs C, Stelma FF, Smit HA, Brunekreef B, van Schayck CP, Postma DS. X-chromosome Forkhead Box P3 polymorphisms associate with atopy in girls in three Dutch birth cohorts. Allergy. 2009 doi: 10.1111/j.1398-9995.2009.02291.x. [DOI] [PubMed] [Google Scholar]
- 6.Verhagen J, Akdis M, Traidl-Hoffmann C, Schmid-Grendelmeier P, Hijnen D, Knol EF, Behrendt H, Blaser K, Akdis CA. Absence of T-regulatory cell expression and function in atopic dermatitis skin. J Allergy Clin Immunol. 2006;117:176–183. doi: 10.1016/j.jaci.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 7.Orihara K, Narita M, Tobe T, Akasawa A, Ohya Y, Matsumoto K, Saito H. Circulating Foxp3+CD4+ cell numbers in atopic patients and healthy control subjects. J Allergy Clin Immunol. 2007;120:960–962. doi: 10.1016/j.jaci.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 8.Ito Y, Adachi Y, Makino T, Higashiyama H, Fuchizawa T, Shimizu T, Miyawaki T. Expansion of FOXP3-positive CD4+CD25+ T cells associated with disease activity in atopic dermatitis. Ann Allergy Asthma Immunol. 2009;103:160–165. doi: 10.1016/S1081-1206(10)60170-6. [DOI] [PubMed] [Google Scholar]
- 9.Schubert LA, Jeffery E, Zhang Y, Ramsdell F, Ziegler SF. Scurfin (FOXP3) acts as a repressor of transcription and regulates T cell activation. J Biol Chem. 2001;276:37672–37679. doi: 10.1074/jbc.M104521200. [DOI] [PubMed] [Google Scholar]
- 10.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 11.Lin W, Truong N, Grossman WJ, Haribhai D, Williams CB, Wang J, Martin MG, Chatila TA. Allergic dysregulation and hyperimmunoglobulinemia E in Foxp3 mutant mice. J Allergy Clin Immunol. 2005;116:1106–1115. doi: 10.1016/j.jaci.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 12.Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY, Campbell DJ. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. 2007;204:1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudda JC, Perdue N, Bachtanian E, Campbell DJ. Foxp3+ regulatory T cells maintain immune homeostasis in the skin. J Exp Med. 2008;205:1559–1565. doi: 10.1084/jem.20072594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Earl PL, CN, Wyatt LS, Moss B, Carroll MW. Preparation of cell cultures and vaccinia virus stocks. Mississauga, Ontario: John Wiley & Sons; 1998. [Google Scholar]
- 15.Freyschmidt EJ, Mathias CB, MacArthur DH, Laouar A, Narasimhaswamy M, Weih F, Oettgen HC. Skin inflammation in RelB(−/−) mice leads to defective immunity and impaired clearance of vaccinia virus. J Allergy Clin Immunol. 2007;119:671–679. doi: 10.1016/j.jaci.2006.12.645. [DOI] [PubMed] [Google Scholar]
- 16.Tscharke DC, Karupiah G, Zhou J, Palmore T, Irvine KR, Haeryfar SM, Williams S, Sidney J, Sette A, Bennink JR, Yewdell JW. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J Exp Med. 2005;201:95–104. doi: 10.1084/jem.20041912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science. 2000;290:1354–1358. doi: 10.1126/science.290.5495.1354. [DOI] [PubMed] [Google Scholar]
- 18.Whitmire JK, Benning N, Whitton JL. Cutting edge: early IFN-gamma signaling directly enhances primary antiviral CD4+ T cell responses. J Immunol. 2005;175:5624–5628. doi: 10.4049/jimmunol.175.9.5624. [DOI] [PubMed] [Google Scholar]
- 19.Whitmire JK, Tan JT, Whitton JL. Interferon-gamma acts directly on CD8+ T cells to increase their abundance during virus infection. J Exp Med. 2005;201:1053–1059. doi: 10.1084/jem.20041463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karupiah G, Xie QW, Buller RM, Nathan C, Duarte C, MacMicking JD. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science. 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 21.Karupiah G, Chen JH, Nathan CF, Mahalingam S, MacMicking JD. Identification of nitric oxide synthase 2 as an innate resistance locus against ectromelia virus infection. J Virol. 1998;72:7703–7706. doi: 10.1128/jvi.72.9.7703-7706.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma DP, Ramsay AJ, Maguire DJ, Rolph MS, Ramshaw IA. Interleukin-4 mediates down regulation of antiviral cytokine expression and cytotoxic T-lymphocyte responses and exacerbates vaccinia virus infection in vivo. J Virol. 1996;70:7103–7107. doi: 10.1128/jvi.70.10.7103-7107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Den Broek M, Bachmann MF, Kohler G, Barner M, Escher R, Zinkernagel R, Kopf M. IL-4 and IL-10 antagonize IL-12-mediated protection against acute vaccinia virus infection with a limited role of IFN-gamma and nitric oxide synthetase 2. J Immunol. 2000;164:371–378. doi: 10.4049/jimmunol.164.1.371. [DOI] [PubMed] [Google Scholar]
- 24.Scott JE, ElKhal A, Freyschmidt EJ, MacArthur DH, McDonald D, Howell MD, Leung DY, Laouar A, Manjunath N, Bianchi T, Boes M, Oettgen HC, Geha RS. Impaired immune response to vaccinia virus inoculated at the site of cutaneous allergic inflammation. J Allergy Clin Immunol. 2007;120:1382–1388. doi: 10.1016/j.jaci.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Howell MD, Jones JF, Kisich KO, Streib JE, Gallo RL, Leung DY. Selective killing of vaccinia virus by LL-37: implications for eczema vaccinatum. J Immunol. 2004;172:1763–1767. doi: 10.4049/jimmunol.172.3.1763. [DOI] [PubMed] [Google Scholar]
- 26.Kawakami Y, Tomimori Y, Yumoto K, Hasegawa S, Ando T, Tagaya Y, Crotty S, Kawakami T. Inhibition of NK cell activity by IL-17 allows vaccinia virus to induce severe skin lesions in a mouse model of eczema vaccinatum. J Exp Med. 2009;206:1219–1225. doi: 10.1084/jem.20082835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reefer AJ, Satinover SM, Solga MD, Lannigan JA, Nguyen JT, Wilson BB, Woodfolk JA. Analysis of CD25hiCD4+ “regulatory” T-cell subtypes in atopic dermatitis reveals a novel T(H)2-like population. J Allergy Clin Immunol. 2008;121:415–422. e413. doi: 10.1016/j.jaci.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Ou LS, Goleva E, Hall C, Leung DY. T regulatory cells in atopic dermatitis and subversion of their activity by superantigens. J Allergy Clin Immunol. 2004;113:756–763. doi: 10.1016/j.jaci.2004.01.772. [DOI] [PubMed] [Google Scholar]
- 29.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J Exp Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toka FN, Suvas S, Rouse BT. CD4+ CD25+ T cells regulate vaccine-generated primary and memory CD8+ T-cell responses against herpes simplex virus type 1. J Virol. 2004;78:13082–13089. doi: 10.1128/JVI.78.23.13082-13089.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haeryfar SM, DiPaolo RJ, Tscharke DC, Bennink JR, Yewdell JW. Regulatory T cells suppress CD8+ T cell responses induced by direct priming and cross-priming and moderate immunodominance disparities. J Immunol. 2005;174:3344–3351. doi: 10.4049/jimmunol.174.6.3344. [DOI] [PubMed] [Google Scholar]
- 32.Belkaid Y. Role of Foxp3-positive regulatory T cells during infection. Eur J Immunol. 2008;38:918–921. doi: 10.1002/eji.200738120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320:1220–1224. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 35.Belyakov IM, Earl P, Dzutsev A, Kuznetsov VA, Lemon M, Wyatt LS, Snyder JT, Ahlers JD, Franchini G, Moss B, Berzofsky JA. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc Natl Acad Sci U S A. 2003;100:9458–9463. doi: 10.1073/pnas.1233578100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu R, Johnson AJ, Liggitt D, Bevan MJ. Cellular and humoral immunity against vaccinia virus infection of mice. J Immunol. 2004;172:6265–6271. doi: 10.4049/jimmunol.172.10.6265. [DOI] [PubMed] [Google Scholar]
- 37.Liu L, Zhong Q, Tian T, Dubin K, Athale SK, Kupper TS. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat Med. 16:224–227. doi: 10.1038/nm.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aung S, Tang YW, Graham BS. Interleukin-4 diminishes CD8(+) respiratory syncytial virus-specific cytotoxic T-lymphocyte activity in vivo. J Virol. 1999;73:8944–8949. doi: 10.1128/jvi.73.11.8944-8949.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howell MD, Gallo RL, Boguniewicz M, Jones JF, Wong C, Streib JE, Leung DY. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006;24:341–348. doi: 10.1016/j.immuni.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Matsui M, Moriya O, Yoshimoto T, Akatsuka T. T-bet is required for protection against vaccinia virus infection. J Virol. 2005;79:12798–12806. doi: 10.1128/JVI.79.20.12798-12806.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, Borrow P, Tough DF. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009–1015. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen KB, Watford WT, Salomon R, Hofmann SR, Pien GC, Morinobu A, Gadina M, O’Shea JJ, Biron CA. Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science. 2002;297:2063–2066. doi: 10.1126/science.1074900. [DOI] [PubMed] [Google Scholar]
- 43.Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 44.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–530. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 47.Kohyama S, Ohno S, Isoda A, Moriya O, Belladonna ML, Hayashi H, Iwakura Y, Yoshimoto T, Akatsuka T, Matsui M. IL-23 enhances host defense against vaccinia virus infection via a mechanism partly involving IL-17. J Immunol. 2007;179:3917–3925. doi: 10.4049/jimmunol.179.6.3917. [DOI] [PubMed] [Google Scholar]
- 48.Howell MD, Novak N, Bieber T, Pastore S, Girolomoni G, Boguniewicz M, Streib J, Wong C, Gallo RL, Leung DY. Interleukin-10 downregulates anti-microbial peptide expression in atopic dermatitis. J Invest Dermatol. 2005;125:738–745. doi: 10.1111/j.0022-202X.2005.23776.x. [DOI] [PubMed] [Google Scholar]
- 49.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 50.Sher A, Coffman RL. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu Rev Immunol. 1992;10:385–409. doi: 10.1146/annurev.iy.10.040192.002125. [DOI] [PubMed] [Google Scholar]
- 51.Liu L, Xu Z, Fuhlbrigge RC, Pena-Cruz V, Lieberman J, Kupper TS. Vaccinia virus induces strong immunoregulatory cytokine production in healthy human epidermal keratinocytes: a novel strategy for immune evasion. J Virol. 2005;79:7363–7370. doi: 10.1128/JVI.79.12.7363-7370.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, Khoury S, Oukka M, Kuchroo VK. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 54.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 55.Oyoshi MK, Elkhal A, Kumar L, Scott JE, Koduru S, He R, Leung DY, Howell MD, Oettgen HC, Murphy GF, Geha RS. Vaccinia virus inoculation in sites of allergic skin inflammation elicits a vigorous cutaneous IL-17 response. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0904021106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lopez Kostka S, Dinges S, Griewank K, Iwakura Y, Udey MC, von Stebut E. IL-17 promotes progression of cutaneous leishmaniasis in susceptible mice. J Immunol. 2009;182:3039–3046. doi: 10.4049/jimmunol.0713598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.