Abstract
The intestine consists of epithelial cells that secrete digestive enzymes and mucus (gland cells), absorb food particles (enterocytes), and produce hormones (endocrine cells). Intestinal cells are rapidly turned over and need to be replaced. In cnidarians, mitosis of differentiated intestinal cells accounts for much of the replacement; in addition, migratory, multipotent stem cells (interstitial cells) contribute to the production of intestinal cells. In other phyla, intestinal cell replacement is solely the function of stem cells entering the gut from the outside (such as in case of the neoblasts of platyhelmints) or intestinal stem cells located within the midgut epithelium (as in both vertebrates or arthropods). We will attempt in the following to review important aspects of midgut stem cells in different animal groups: where are they located, what types of lineages do they produce, and how do they develop. We will start out with a comparative survey of midgut cell types found across the animal kingdom; then briefly look at the specification of these cells during embryonic development; and finally focus on the stem cells that regenerate midgut cells during adult life. In a number of model systems, including mouse, zebrafish and Drosophila, the molecular pathways controlling ISC proliferation and the specification of intestinal cell types are under intensive investigation. We will highlight findings of the recent literature, focusing on aspects that are shared between the different models and that point at evolutionary ancient mechanisms of intestinal cell formation.
Keywords: intestine, stem cell, cell fate, development, evolution
1. Midgut cell types across the animal kingdom
Enterocytes
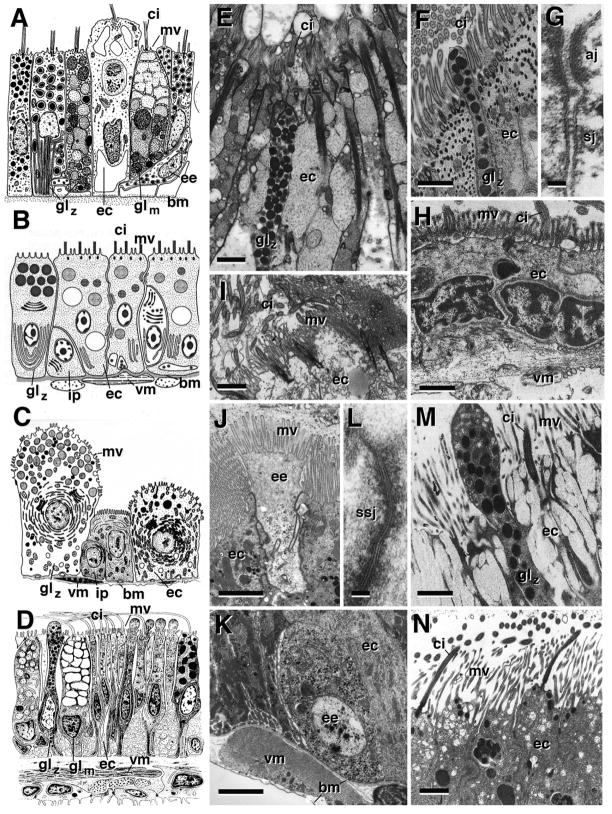
The uptake of food particles by phagocytosis or pinocytosis, followed by intracellular lysosomal degradation, represented the beginning of a digestive system at a stage before a luminal gut with an epithelial lining was in place. Present day single celled animals (protists) and sponges (porifera) metabolize nutrients by intracellular digestion. Ultrastructural specializations for intracellular digestion are a well developed Golgi complex producing lysosomes, and an endocytotic machinery required for phagocytosis. Membrane specializations such as motile cilia/flagellae and microvilli help capturing and immobilizing food particles. The choanocytes lining the inner chambers of sponges, characterized by an apical flagellum surrounded by a microvillar collar, represent prototypical enterocytes (Willenz and van de Vyver, 1982; Leys and Eerkes-Medrano, 2006). Cells similar in structure and (phagocytic) function are found in other invertebrate clades, including cnidaria (Davis, 1975; Chapman, 1978; Goldberg, 2002; Fig. 1A, E), ctenophores (Hernandez-Nicaise, 1991), and platyhelminths (Bowen et al., 1974; 1976; Jones et al., 2000; Fig. 1B). Even though, with the emergence of gland cells secreting enzymes into the gut lumen, digestion switched from intracellular to extracellular in most animal taxa, motile cilia represent a hallmark of enterocytes in many invertebrate taxa, including lower deuterostomes (Fig. 1D, M, N) and lophotrochozoans (Fig. 1F–I); motile cilia appear to be mostly absent from enterocytes of chordates and ecdysozoans (Fig. 1C, J–L). Microvilli, which greatly increase the area of the apical enterocyte membrane facing the gut lumen, become prominent in many animals. Long, densely packed microvilli form the light microscopically visible “brush border” seen in enterocytes of vertebrates, arthropods and other taxa (Fig. 1J, N).
Fig. 1.
Cytology of the intestine. A–D: Schematic renderings of intestinal cell types in cnidarian [A; Tripedalia cystophora (Cubozoa)], lophotorchozoa [B; Microstomum lineare (Platyhelminthes, Macrostomida)]; ecdysozoa [C; Hyalomma asiaticum (Arthropoda, Chelicerata)]; deuterostomia (D; Henricia sp. (Asteroidea)]. E–N: Electron micrographs depicting ultrastructural details of intestinal cells in cnidarian (Mycetophyllia reesi; E), lophotrochozoans [F, nemertine (Nemertopsis bivittata); G, H: sipunculid (Phascolion sp); I: entoproct (Loxosomella elegans)]; ecdysozoans [J, K: arthropod (Drosophila melanogaster); L: nematode (Rhigonema sp)], and deuterostomes [M: echinoderm (Henricia sp.); N: cephalochordate (Branchiostoma virginiae)].
Panels are taken from (with permission): A: Chapman,1978 (p.153); B: Rieger, 1991 (p.70); C: Coons and Alberti, 1999 (p.395); D: Chia and Koss, 1994 (p.221); E: Goldberg, 2002 (p.254); F: Turbeville, 1991 (p.310); G, H: Rice, 1993 (p.272); I: Nielsen and Jespersen, 1997 (p.22); L: Wright, 1991 (p.158); M: Chia and Koss, 1994 (p.225); N: Ruppert, 1997 (p.441).
Abbreviations: aj adherens junction; bm basement membrane; ci cilia; ec enterocyte; ee endocrine cell; gl glandular cell; glm mucus producing glandular cell; glz zymogen glandular cells; ip intestinal progenitor cell; mv microvilli; sj septate junction; ssj smooth septate junction; vm visceral muscle.
Bars: 1μm (E, F, H, I, J, K, M, N); 50nm (G, L)
Glandular cells
In animals with an enclosed gut, absorption of food materials can be greatly improved by enzymes that are secreted into the gut lumen where they break down macromolecules (extracellular digestion). Specialized zymogenic gland cells act to produce large amounts of digestive enzymes that are exocytotically released at the apical membrane. Ultrastructural features of gland cells are an increased endoplasmic reticulum and electron-dense vesicles (“granules”) in which enzymes are transported from the ER to the apical membrane (Fig. 1A, B, E, F). One finds gland cells in all bilaterians, in cnidarians and ctenophores, and even in the ventral epithelium of placozoans, primitive metazoa which have not yet developed an enclosed gut (Grell and Ruthmann, 1991; Schierwater et al., 2009). Gland cells may have apical microvilli and/or cilia in some taxa (e.g., Fig. 1A, E); in others, they are devoid of these apical specializations (Fig. 1D, M).
Aside from zymogenic cells secreting digestive enzymes, mucus producing gland cells are common in most animal taxa. Mucus, made of proteoglycans and glycaminoglycans, protects the luminal surface of the intestinal epithelium, and serves specialized functions in ion and water transport (Cioffi, 1979; Gupta, 1989). Ultrastructurally, mucus cells, such as the goblet cells of the vertebrate gut, differ from zymogenic glands by the low electron density of the secretory vesicles (Fig. 1A, D).
With the emergence of highly corrosive enzyme mixtures secreted into the gut lumen the necessity arose to seal the luminal surface of the intestinal epithelium from its basal surface, thereby preventing leakage of enzymes into the tissue. To that end one finds specialized intercellular junctions, such as tight junctions in chordates, and septate junctions in invertebrates (Lord and di Bona, 1976), which connect the sub-apical membranes of enterocytes and gland cells (Fig. 1G). A special type of septate junction, called smooth septate junction or continuous junction, is characteristic of enterocytes in arthropods and other ecdysozoans (Lane et al., 1984, 1994; Tepass et al., 1994; Fig. 1L).
Endocrine cells
A number of peptides and small molecules are able to modulate the secretion of enzymes, the beating of cilia, or the contraction of muscle fibers, both in the outer body wall, as well as in the intestine. In multicellular animals, the production of such active compounds is restricted to specialized cells: endocrine cells, which release their products as hormones into the surrounding tissue or blood vessels, and neurons, which discharge active molecules as neurotransmitters at specialized intercellular contacts (synapses). In cnidarians and some other invertebrate phyla (e.g., echinoderms) one finds both endocrine cells and sensory neurons as integral part of the gut epithelium (Chapman, 1978; Westfall et al., 1991; Chia and Koss, 1991). These cells receive chemical and/or mechanical stimuli, associated with ingested food, at their apical membrane which contacts the gut lumen. Hormones/neurotransmitter are packaged into vesicles and transported to the basal cell pole, where they are released into the interstitial space/blood vessels, or transported via nerve fibres to synapses. In most animal taxa, sensory neurons are no longer part of the gut epithelium, but endocrine cells, recognizable by their basal cell body containing characteristic dense core vesicles, are ubiquitous (Fig. 1A, J, K).
Stem cells
The different cell types of the midgut have a very limited life span due to the heavy strain put on them by corrosive enzymes and mechanical gut function. How are these cell populations maintained throughout the life span of the animal? In the simplest scenario, seen in cnidarians, differentiated cells divide mitotically (David and Campbell, 1972; Schmidt and David, 1986). However, in most other animal groups, differentiated gut cells have lost the ability to undergo mitosis. Instead, populations of undifferentiated, mitotically active cells form part of the gut. These intestinal stem cells (ISCs), recognizable by their small size, basal location, and relative lack of secretory vesicles or lysosomes (Fig. 1B, C), undergo mitosis in a highly controlled manner, maintaining their own number, as well as giving rise to the differentiated cell types of the gut.
The mechanism by which ISCs divide and reproduce the gut epithelium may vary significantly among different animals. For example, in the mammalian intestine, the progeny of ISCs does not differentiate right away, but proliferates at a high rate (transient amplifying cells) before exiting the cell cycle (Crosnier et al., 2006). By contrast, in insects such as Drosophila, transient amplification does not occur, and (a subset of) the progeny of ISCs differentiates directly into enterocytes or secretory cells (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). Another dimension along which stem cells may vary is their relationship to the different gut cell types. It is possible that several intrinsically different ISC populations, each one responsible for a particular cell type (or subset of cell types), exist. Alternatively, a multipotent ISC could produce all cell types. Or, the ISC gives rise to only one cell type, which then “transdifferentiates” over its life time into other cell types, without intervening mitosis. That the latter possibility applies at least in some cases is suggested by findings in crustaceans where stem cells produce gland cells which secondarily become enterocytes (see below). We will have a closer look at how intestinal stem cells generate the different gut cell types in part 3 of this review. First, however, we will briefly summarize pertinent findings that explain how the midgut with its different cell types emerges during embryonic development.
2. Embryonic development of the midgut
2.1. Specification of the endoderm
The midgut is derived from the endoderm, a population of cells that is transported into the interior of the embryo through the process of gastrulation. Molecular determinants of endoderm are expressed early in development. In many taxa, maternally produced mRNAs (e.g., vegT in amphibians; Zhang et al., 1998; Yasuo and Lemaire, 1999) are localized to the yolk rich (vegetal) pole of the oocyte. When the oocyte cleaves into blastomeres, blastomeres derived from the vegetal pole inherit these messages, and as a result turn on sets of zygotic genes specifying endodermal fate. Aside from the directed localization of maternal determinants, activation of the Wnt signaling pathway plays a crucial role in specifying endodermal fate among blastomeres in many metazoans (Bei et al., 2002; Ettensohn, 2006; Lee et al., 2007; Henry et al., 2008; Darras et al., 2011).
Downstream of maternal factors and Wnt activity, several group of transcription factors seem to be universally involved in endoderm development (Fig. 2A). One group encompasses members of the GATA binding proteins. GATA is the short DNA sequence found in the regulatory domain of many genes that are regulated by GATA binding transcription factors. Several GATA factors function exclusively during blood cell formation (hematopoiesis), whereas others are required in the endoderm (Bossard and Zaret, 1998; Weber et al., 2000) and the cardiac mesoderm. GATA transcription factors as determinants of endoderm have been also identified in the invertebrate models C. elegans and Drosophila (Zhu et al., 1997; Maduro et al., 2005; Murakami et al., 2005; Boeck et al., 2011). Drosophila has three GATA family members of which at least one, encoded by the gene serpent (srp) acts as a determinant for endoderm. The nematode C. elegans also possesses several GATA proteins. One of these, end-1, is expressed in the endodermal E-lineage that gives rise to the midgut. Besides the GATA genes, another group of conserved transcription factors is intimately involved with the endoderm: the HNF/fork head genes. HNF proteins form a family of transcription factors first identified in mammals where they control a large number of liver specific genes. Several members of this family, such as HNF3b, is already expressed in the endoderm of gastrulating mouse embryos (Ang et al., 1993). HNF/forkhead genes play a similar role in Drosophila and C. elegans (Weigel et al., 1989; Azzaria et al., 1996). The role of GATA and/or fork head genes in gut regionalization has also been observed in various lophotrochozoans (polychate annelids: Boyle and Seaver, 2008; 2010; sipunculids: Boyle and Seaver, 2010; planarians: Martín-Durán and Romero, 2011) and lower deuterostomes (echinoderms: Oliveri et al., 2006; ascidians: Olsen and Jeffery, 1997) which supports conservation across the major bilaterian clades (de-Leon, 2011). Research on the cnidarian Nematostella (Martindale et al., 2004) suggests that the role of GATA and fork head in endodermal specification extends even deeper into the animal tree.
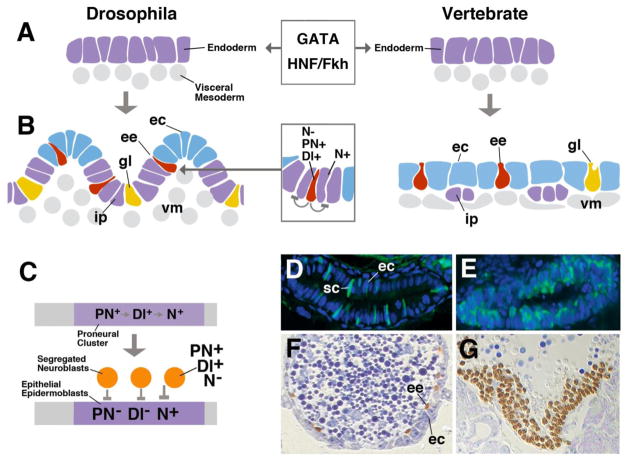
Fig. 2.
Development of the intestine. A: Specification of the endoderm by conserved transcriptional regulators of the GATA and HNF/Fkh family. B: Specification of intestinal cell types, controlled by Notch signaling pathway. C: The proneural-neurogenic gene cassette in the neurectoderm. D: Section of wild type larval zebrafish intestine, depicting enterocytes (blue) and secretory cells (green). E: In the mutant mind bomb (mib; abolishes Dl function), all intestinal cell express secretory fate. F: Section of wild type Drosophila midgut, showing enterocytes (ec; blue) and endocrine cells (brown). Loss of Delta (G) causes transformation of enterocytes into endocrine cells.
Abbreviations: Dl Delta; ec enterocyte; ee endocrine cell; gl gland cell; ip intestinal progenitor; N Notch; PN proneural gene; sc secretory cell (endocrine and glandular); vm visceral mesoderm
2.2. Specification of midgut cell types
In a second phase of gut development the endoderm splits up into different cell types (Fig. 2B). Developmental studies carried out in several model organisms showed that a gene cassette known from a wealth of previous studies on neural development, the proneural-neurogenic gene network (Fig. 2C), also appears to be central to the molecular mechanism controlling the selection of different gut cell types. Proneural genes encode transcriptional regulators that belong to the large family of basic helix-loop-helix (bHLH) proteins, including the Achaete-Scute proteins and their vertebrate homologs (e.g., Mash-1 in mouse), and Atonal and its vertebrate homologs (Math and neurogenins in mouse; reviewed in Campuzano and Modolell, 1992; Kageyama et al., 1995; Guillemot, 1999; Lo et al., 2002; Cachero et al., 2011). Proneural genes are switched on in the embryonic endoderm at the time when different cell types start to become specified. The upstream mechanism responsible to upregulate proneural gene expression in the developing intestine is still largely unknown; global endoderm determinants, such as GATA or HNF/Fkh, are among the factors that activate transcription of the proneural genes (Jacquemin et al., 2000; d’Angelo et al., 2010). The expression of proneural genes in the endoderm promotes the fate of secretory (glandular and endocrine) cells. Alongside with the proneural genes, the Notch signaling pathway (encoded by genes originally identified as “neurogenic genes” in Drosophila; Campos-Ortega, 1995) gets activated and prompts endoderm to adopt the fate of enterocytes. The dynamic balance between proneural and neurogenic gene activity determines the ratio of cell types developing from the endoderm.
The mouse proneural gene Math-1 is expressed in the primordium of the intestine at embryonic stage E16.5, and becomes restricted to emerging secretory cells by E18.5. Loss of Math1 results in the absence of both glandular cells and endocrine cells (Yang et al., 2001). Another proneural group member, neurogenin 3, acts downstream of Math-1 in endocrine cells; loss of neurogenin 3 ablates several endocrine cell populations, in particular glucagon, somatostatin, and gastrin expressing cells (Jenny et al., 2002; Lee et al., 2002). A mutation in the human ngn 3 homolog shows the same pathology and is the cause for a type of congenital malabsorptive diarrhea (Wang et al., 2006). The expression of proneural genes in the developing midgut is inhibited by Notch activity through a lateral inhibition mechanism. The Notch ligand Delta accumulates in secretory cells of zebrafish and mouse gut (Crosnier et al., 2005) and activates the Notch cascade in neighboring cells, which turn transcription factors specifying enterocyte fate (e.g., Hes-1; Jensen et al., 2000). Loss of Notch activity in zebrafish or mouse causes a conversion of gut enterocytes into secretory cells (Crosnier et al., 2005; van Es et al., 2005; Fig. 2D, E).
The proneural-neurogenic gene cassette also controls cell fate in the developing midgut of Drosophila. In Drosophila, enterocytes have both absorptive and zymogenic function; dedicated gland cells are few in number, and are restricted to a narrow segment of the midgut (Filshie et al., 1971; Dubreuil, 2004). However, endocrine cells, producing a variety of different peptides, are scattered throughout the length of the gut (Veenstra et al., 2008). As in vertebrates, proneural genes (e.g., the bHLH transcription factor Lethal of scute) are expressed in the embryo endoderm, and then become restricted to the endocrine cell population (Tepass and Hartenstein, 1995; Hartenstein et al., 2010; Takashima et al., 2011). Notch activity is high in enterocytes, and loss of Notch or Delta results in a complete conversion of endoderm into endocrine cells (Fig. 2F, G). The activation of Notch causes the opposite phenotype: enteroendocrine cells are reduced in number. The conservation of the genetic mechanism underlying the selection of midgut cell types in derived members of the deuterostomes (human, mouse, zebrafish) and the ecdysozoa (Drosophila) indicates that this mechanism is ancient, and most likely shared among all metazoa. As molecular and “instant genetic tools” such as RNAi are now available for members of all animal taxa, we will soon learn whether this prediction is correct.
3. Cell replacement and regeneration in the adult gut
3.1. Replacement of intestine by mitosis and migrating stem cells in the gastrodermis of Cnidarians
Cnidarians are divided into two major clades, the Anthozoa (sea anemones and corals) and the Medusozoa, the later of which includes the Scyphozoa (true jellyfish), Cubozoa (box jellies) and Hydrozoa (hydroids and siphonophores; Collins, 2009). In all groups, the gut represents a sac with a single opening for the intake and expulsion of food. The hydrozoan Hydra, probably the best-studied cnidarian, has one of the simplest guts of any animal. The epithelium lining the Hydra gut (gastrodermis) consists of a column of cells which include, besides the generic enterocytes and glandular cells, sensory neurons and nematocytes (the stinging cells unique to cnidarians)(Davis, 1975; Wood, 1979). Most of these cells can be further divided into several subtypes, although it is not always clear whether these variants represent different cell fates, or changes to a cell’s morphology during its life (Murate et al., 1997). The lineage of the intestinal cells of Hydra is surprisingly complex. Enterocytes divide mitotically at a constant rate and thereby maintain their number without the need for a stem cell precursor (David and Campbell, 1972). Gland cells divide as well, but require additional input from stem cells (“interstitial” or I-cells) to keep their number constant (Schmidt and David, 1986). A predominant role of I-cells in the production of gland cells has also been observed in Hydra attenuata (Bode et al., 1987). Neurons and nematocytes are exclusively produced by I-cells. It appears that I-cells migrate from the ectoderm into the gastrodermis to generate these cell types (David and Gierer, 1974; Smid and Tardent, 1984), although it has also been suggested that nematocytes generated in the ectoderm are swallowed by Hydra and relocated into the gastrodermis.
In other hydrozoans, some aspects of gut stem cell dynamics are similar to Hydra, while others are not. Unlike Hydra, BrdU incorporation and gene expression studies support an endodermal origin for I-cells in Hydractinia and Clytia (Kroiher, Plickert, & Müller, 1990; Leclère et al., 2012; Plickert, Kroiher, & Munck, 1988; Rebscher, Volk, Teo, & Plickert, 2008), and possibly Podocoryne (Seipel, Yanze, & Schmid, 2004). In Hydractinia, endodermal epithelial cell maintenance does not appear to require input from I-cells, which is similar to Hydra. But under certain conditions, Hydractinia I-cells can generate epithelial cells. When I-cells are chemically removed from Hydractinia polyps, and mutant I-cells are introduced, all cells—including enterocytes—eventually take on the mutant phenotype (Müller & Teo, 2004). Additionally, during colony fusion, I-cells from transgenically labeled Hydractinia have been observed moving into the wild-type colony, generating new epithelial cells (Künzel et al., 2010). In Podocoryne, isolated endodermal epithelial cells, when combined with striated muscle cells, will transdifferentiate into the various enterocytes of the endoderm, as well as I-cells and germline cells (Schmid, Wydler, & Alder, 1982). Together, this data suggests that the three non-overlapping cell niches of Hydra do not apply to all hydrozoans, and these animals have evolved a variety of pathways to generate enterocytes.
Non-hydrozoan cnidarians lack an obvious I-cell homologue, and there are currently no cell-tracing experiments clarifying which lineages form the enterocytes. However, it is likely that self-renewing endodermal epithelial cells take on many of the digestive roles of canonical enterocytes (similar to Hydra, e.g. McNeil, 1981), and that more advanced secretory and gland cells are generated from transdifferentiation of the epithelial cell (see Gold and Jacobs, this issue, for a more detailed discussion of this hypothesis). Recent work on transgenic Nematostella suggests that larval endodermal epithelial cells can differentiate into neurons (Nakanishi, Renfer, Technau, & Rentzsch, 2012), so it seems reasonable to hypothesize that they might generate other cell types in the gut as well.
3.2. Replacement of intestine by migrating stem cells (neoblasts) in platyhelminths
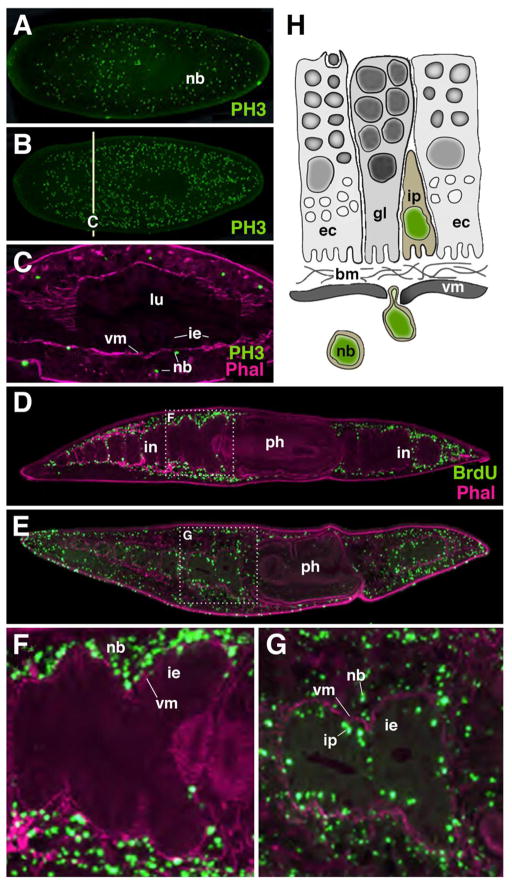
Platyhelminthes (flatworms) represents a large and diverse phylum among the bilaterian super taxon lophotrochozoa. Similar to hydrozoa mentioned above, flatworms possess motile, multipotent stem cells, called neoblasts (Shibata et al., 2010; Baguna, 2012). All differentiated tissues, including cells of the intestine, have lost the capability to divide. Only neoblasts, distributed all over the mesenchyme that fills the clefts between intestine and body wall, divide continuously (Fig. 4A–C). Attracted by as yet unidentified signals, neoblasts invade tissues and differentiate into all cell types. The replacement of intestinal cells, including glandular cells and phagocytic enterocytes, by neoblasts moving through the basement membrane into the gut has been followed in a recent study (Forsthoefel et al., 2011) which applied pulse-chase experiments with Bromo-deoxy uridine (BrdU, a marker for replicating cells. The study demonstrated that incorporation of BrdU-positive neoblasts occurred evenly throughout all regions of the intestine (Fig. 4D–G). Thus, as stated above for the cnidarian gastrodermis, the flatworm intestine does not have spatially restricted growth zones from where newly produced cells spread out to replace old cells; rather, growth and cell replacement occurs ubiquitously all over the gut epithelium.
Fig. 4.
Replacement of intestinal cells in platyhelminth. A–C: proliferating neoblasts (nb), labeled with anti-Phosphohistone 3 (PH3; green). A: starving animal; B: One day after feeding; C: cross section of animal shown in B. Note that neoblasts are located outside the intestinal epithelium (ie). Visceral muscle layer (vm; labeled by phallodin; magenta) intervenes between intestinal epithelium and neoblasts. D–G: Movement of neoblasts (labeled with BrdU; green) into gut epithelium. After chase period of 1 day (D, F) neoblasts are still outside the gut (note visceral muscle layer between neoblasts and epithelium). After chase period of 5 days (E, G) neoblasts have moved through muscle and are incorporated into the gut as intestinal progenitors (ip). H: Schematic representation of neoblast movement into gut.
Abbreviations: bm basement membrane; ec enterocyte; gl gland cell; ie intestinal epithelium; ip intestinal progenitor; nb neoblast; vm visceral mesoderm
Little is known about intestinal cell maintenance in other lophotrochozoan species. Several reports support the idea that mitosis of differentiated cells can occur (e.g., in bryozoan; Mukai et al., 1997). However, details regarding their proliferatory activity and lineage characteristics are not known.
3.3. Replacement of intestine by resident stem cells of the midgut in insects and other arthropods
Insects and other arthropod taxa have clusters of undifferentiated, mitotically active cells scattered all over the midgut (Lehane, 1998; Fig. 5A). These intestinal stem cells are located at the basal surface of the midgut, sandwiched in between the visceral muscle fibers and the epithelium. In Drosophila, ISCs emerge as “left overs” of the intestinal progenitors that form the adult gut (Hartenstein et al., 2010; Takashima et al., 2011). In the fly larva, adult intestinal progenitors form clusters of dividing cells scattered throughout the differentiated larval midgut. With the onset of metamorphosis, most of these cells differentiate into adult midgut; a small number of them remains undifferentiated and give rise to the population of intestinal stem cells found in the adult midgut. ISCs are motile cells that divide frequently; for example, the cells of the adult Drosophila midgut are renewed approximately four times during the eight week life span of the fly (Jiang et al., 2009).
Fig. 5.
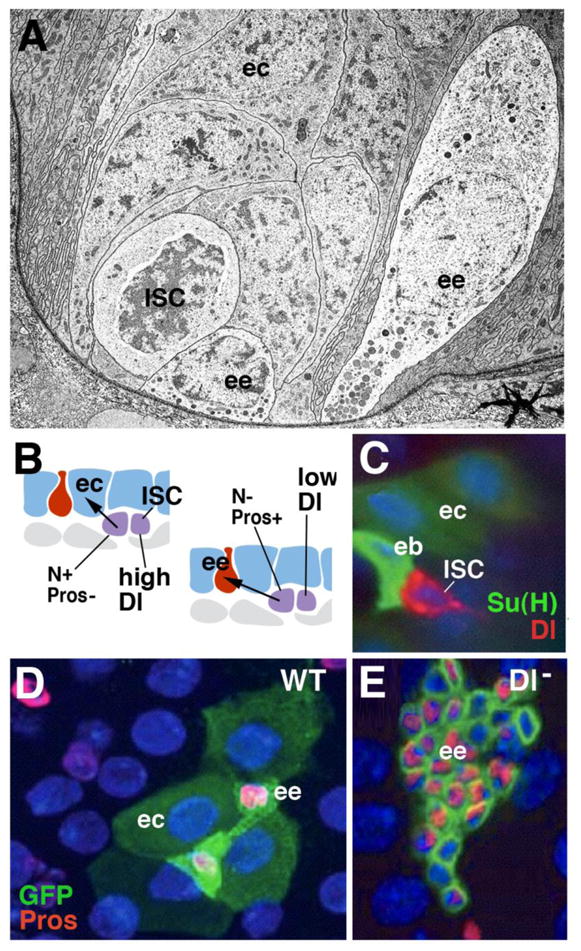
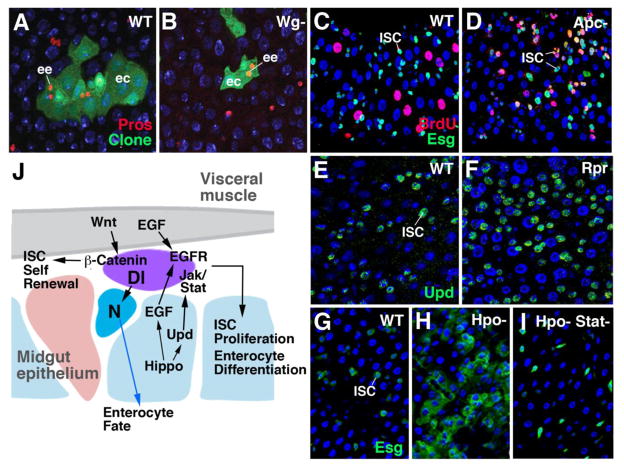
Cell replacement in the adult Drosophila midgut. A: Electron micrograph of basal portion of midgut epithelium, showing enteroendocrine cells (ee) in close spatial association with proliferating stem cell “nests” (ISC; from Lehane, 1998, with permission). B: Schematic representation of adult midgut, showing differentiated enterocytes (blue; ec) and endocrine cells (red; ee), and proliferating intestinal stem cells (ISC; purple). High level of Dl expression in ISC (left) triggers high N activity in one daughter cell (enteroblast; eb), which inhibits proneural gene prospero (pros) and causes differentiation as enterocyte. Low Dl levels (right) allow pros to be expressed, promoting fate of endocrine cell. C: Confocal image showing pair of daughter cells resulting from ISC division; one daughter (ISC) expresses high Dl levels(red); the other one (eb) has turned up N activity, monitored by reporter Su(H) (green). C: Wild-type clone (labeled green), containing four enterocytes and two endocrine cells. Clones derived from stem cells lacking Delta (D) Has increased number of cells which all express endocrine fate. Panels C–E: from Ohlstein and Spradling, 2007 (with permission)
ISCs are multipotent stem cells that produce both enterocytes and endocrine cells, as well as gland cells. Gland cells occur only in a small central segment of the Drosophila midgut (the so called “copper cells”; Dubreuil, 2004; Strand and Micchelli, 2011). The production of digestive enzymes is apparently taken on by the enterocytes, rather than gland cells. In other arthropods, including lobsters (crustacea) and ticks (chelicerates), gland cells and enterocytes (digestive cells) may represent sequential stages in the developmental pathway of the same cells. For example, in the decapod lobster Homarus americanus, stem cells produce gland cells, which subsequently “transdifferentiate” into absorptive enterocytes (reviewed in Icely and Nott, 1992).
Genetic studies in Drosophila demonstrated that, as described for the embryonic endoderm, the specification of cell fate in the ISCs of the adult midgut is also controlled by the balance of proneural gene expression and Notch signaling activity. Dividing, undifferentiated ISCs express the gene escargot (esg; Ohlstein and Spradling, 2006; Micchelli and Perrimon, 2006). Triggered by an as yet unknown mechanism, proneural genes, notably the homeobox gene prospero (pros), but also the bHLH genes asense (ase) are activated, which promote the endocrine fate (Bardin et al., 2010). Notch activity is required to divert the fate decision of ISCs from endocrine to enterocyte. Notch inhibits pros, and activates other, enterocyte-specific transcription factors, such as E(spl) (homolog of vertebrate Hes1; Bardin et al., 2010). Notch activity is promoted by the Notch ligand Delta, which is expressed in a cyclical pattern in ISCs (Ohlstein and Spradling, 2007; Fig. 5B, C). At high levels of Dl expression, signaling between the two daughter cells takes place, with the outcome that Notch is activated in one of the daughters, which then adopts the fate of an enterocyte. If the level of Dl in the ISC is low, it will generate an endocrine cell. If Dl, or N, is removed, no enterocytes are formed; ISCs divide faster, and produce only endocrine cells (Ohlstein and Spradling, 2007; Fig. 5D, E). The mechanism controlling the cyclic expression level of Dl in ISCs is not yet understood. Signals acting from the differentiated intestinal cells, as well as the adjacent visceral musculature, seem to play an important role. These same signals, notably Wingless/Wnt, is also pivotal in maintaining the population of stem cells, and we will have a closer look the way they act in the context of stem cell renewal and its control by “niche derived” signals in section 4 below.
3.4. Replacement of intestine by resident stem cells and transient amplifying cells in vertebrates
The digestive epithelium of the mammalian small intestine has enlarged its surface by producing finger-like processes, termed villi, and invaginations, called crypts (Fig. 6A). The epithelium of villi is composed of enterocytes, glandular cells (goblet cells) and endocrine cells; crypts contain a type of gland cell involved in immune function, called Paneth cell. In addition, crypts are the site of cell renewal (Fig. 6B, C). Two types of ISCs are located in the crypts. One type (“label retaining cell” or LRC) cycles very slowly; the other one (“active stem cell”) cycles faster and is responsible for the rapid turnover of intestinal cells (Li and Clevers, 2010; Fig. 6A). LRCs are located in the lateral walls of the crypts; active stem cells are intermingled with Paneth cells at the crypt bottom, and are therefore also called ‘crypt-base columnar cells (CBCs). Progeny of the intestinal stem cells are pushed towards the upper region of the crypts. These cells keep dividing at a high frequency (transient amplifying cells) and, after a limited number of cell divisions, exit the cell cycle. Postmitotic cells move upward into the villus epithelium where they differentiate into the different types of intestinal cells (Crosnier et al., 2006; Scoville et al., 2008; Fig. 6A).
Fig. 6.
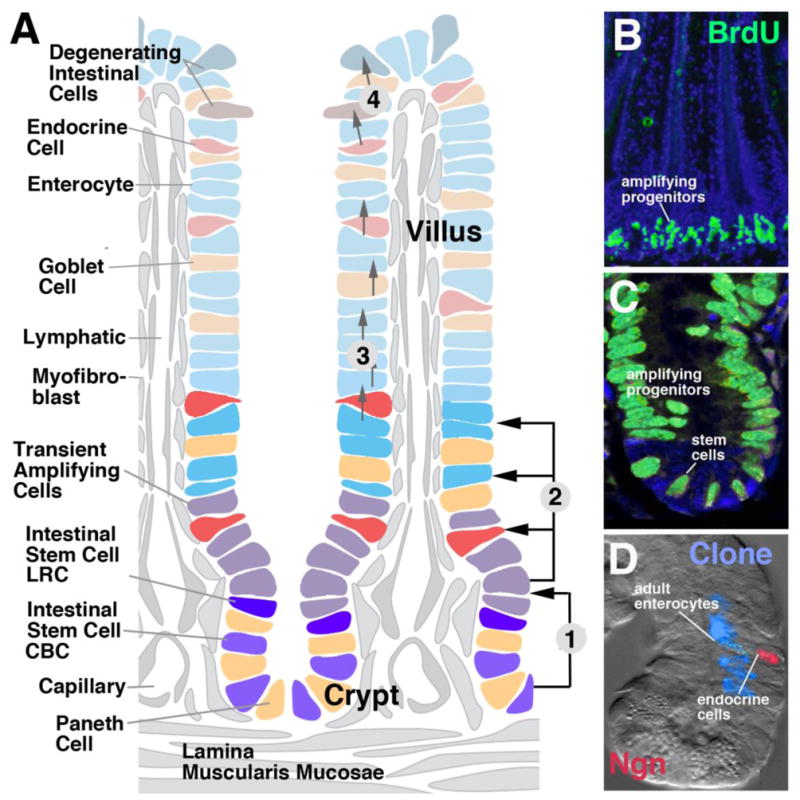
Cell replacement in the mammalian small intestine. A: Schematic depiction of intestinal villus and crypt, showing distribution of stem cells, transient amplifying cells, and differentiated cell types. Numbered arrows indicate cell movements: (1) Stem cells at crypt bottom give rise to transient amplifying cells moving towards crypt neck; (2) Transient amplifying cells produce all cell types of adult intestine; (3) Postmitotic cells stream towards tips of villi; (4) Damaged postmitotic cells at tips are sloughed off into intestinal lumen. B (low magnification) and C (high magnification): BrdU-labeled proliferating cells in crypt of mouse intestinal epithelium (from Aiken and Roth, 1992, with permission). D: Clone derived from labeled progenitor, containing enterocytes and one endocrine cell ([abeled with probe against neurogenin (Ngn; red); from Bjerknes and Cheng, 2006, with permission].
The characteristic cell replacement system of the adult intestine, consisting of crypt-associated ISCs and transient amplifying cells, emerges in the late embryo. During earlier stages, the mammalian endoderm forms a multilayered tube in which all cells are mitotically active (Henning et al., 1994; Crosnier et al., 2005). All endoderm cells undergo symmetric divisions, resulting in an increase in surface area. This eventually leads to the formation of an epithelial monolayer folded into villi and crypts. Cells located in the villi exit the mitotic cycle and differentiate; cells in the crypts remain mitotically active and form the stem cells and transient amplifying cells characteristic of the mature intestine.
Clonal analyses demonstrate that stem cells of the mammalian intestine are pluripotent. Labeled clones, originating from individual stem cells, include enterocytes, as well as secretory and/or endocrine cells (Bjerknes and Cheng, 2006; Fig. 6D). Cell-cell interactions among the proliferating transient amplifying cells and/or postmitotic cells found at the junction between crypt and villus account for cell fate decisions. We find again a prominent role of the proneural-neurogenic gene cassette. The mouse proneural gene Math-1 reaches high levels of expression in the zone of transient amplifying progenitors. Subsequently it becomes restricted to postmitotic secretory (glandular and endocrine) cells. Neurogenin 3 is specifically expressed downstream of Math1 in endocrine cells. As in the embryo, knock-out of Math1 or Ngn3 cause gradual loss of the respective cell types (Yang et al., 2001; Jenny et al., 2002; Lee et al., 2002; reviewed in Schonhoff et al., 2004). Expression of Hes-1, a bHLH factor activated by Notch signaling, occurs in enterocytes surrounding Hes-1 negative secretory cells (Jensen et al., 2000). Reducing N activity in adult gut, as in the embryo, causes an overproduction of secretory cells at the expense of enterocytes (Fre et al., 2011a, b).
4. Maintenance of stem cells: the stem cell niche and niche signaling
Aside from producing the differentiated intestinal cell types, ISCs have the task of maintaining their own number. The mechanism by which stem cells produce both more stem cells, in addition to differentiated cells, is described by one of two models, the predetermined model and the stochastic model (Miller et al., 2005). According to the predetermined model, individual stem cells divide asymmetrically. Molecular determinants expressed prior to mitosis become localized to one cell pole, and, during cytokinesis, end up in only one of the two daughter cells. Neural stem cells (neuroblasts) of the Drosophila nervous system represent a prime example for asymmetric division. These cells express the proneural gene prospero. Prior to each division, Pros protein is trafficked to one pole of the neuroblast; during mitosis, it becomes part of the daughter cell arising at that pole. Pros becomes active in this cell, inhibiting cell division, and activating neural specific genes. The other daughter cell, lacking Pros, continues to divide (Chia et al., 2001; Reichert, 2011).
The second model explaining the production of two cell populations, called stochastic model, postulates that individual stem cells divide symmetrically and produce a pool of progeny that “fill up” a certain space. As long as they reside within this space, operationally defined as the stem cell niche, they receive a signal that maintains their status as undifferentiated dividing stem cells. As proliferation continues, daughter cells stochastically “fall out” of the niche and, losing the niche signal, differentiate or enter a phase of transient amplification. Mammalian hematopoietic stem cells (HSCs) residing in the bone marrow may be maintained in a stochastic mechanism. HSCs are located adjacent to the osteoblast layer that lines the bone marrow cavity. Osteoblasts form (part of) the niche: they emit signals, such as angiopoietin 1 (Ang 1), which maintain the stemness of HSCs. Once daughter cells move away from the osteoblast layer, they are no longer exposed to the niche signals, and enter the phase of transient amplification (Arai et al., 2004; 2005).
We will in the last section of this review explore the maintenance of stem cells in the vertebrate and Drosophila intestine. These studies begin to provide answers to the questions (1) what cells constitute the niche environment of ISCs; (2) what are the signals produced by the niche; (3) how do these signals act on the ISCs; (4) how do ISCs and niches emerge during development.
4.1. Stem cell maintenance in the mammalian intestine
We had seen above that ISCs are located in the crypts of the intestinal epithelium. The crypt epithelium and surrounding tissue provides the niche environment maintaining ISCs. By contrast, tissues of the villi promote differentiation. Adjacent to the epithelium of the small intestine are several types of mesenchymal cells, notably the smooth muscle cells surrounding the lymphatic vessel in the core of the villus, and the myofibroblasts that form a network around capillaries near the intestinal epithelium (Yen and Wright, 2006; Scoville et al., 2008; McLin et al., 2009; Shaker and Rubin, 2010; Powell et al., 2011). Smooth muscle cells and myofibroblasts also contact the epithelium of the crypts. Myofibroblasts are the most likely source of signals acting on ISCs in the crypt. In addition, Paneth cells, which are intermingled with the stem cells at the crypt bottom, also produce signals that maintain stem cell renewal (Sato et al., 2011). Thus, glandular cells of the crypt epithelium, as well as neighboring mesodermal tissues, represent the niche environment for the ISCs of the vertebrate intestine.
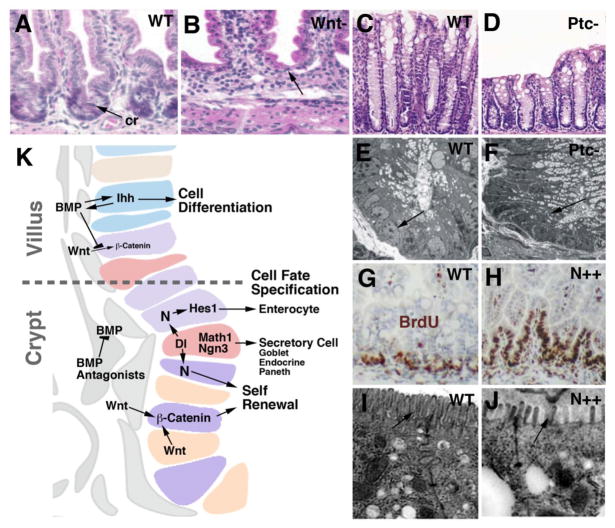
Central to the network of signaling interactions taking place in the ISC niche are components of the Wnt/Wingless pathway (Haegebarth and Clevers, 2009; Yeung et al., 2011; Silva et al., 2011; Fig. 7A, B, K). Wnt proteins are secreted by myofibroblasts, as well as Paneth cells; as discussed below, they also play a pivotal role in the Drosophila midgut. Wnt/wg signals act through nuclear translocation of b-catenin, which, by complexing with other transcription factors, activates target genes. Among these are regulators of the cell cycle, such as c-myc, which promotes the G1-S transition (Fevr et al., 2007). To prevent continued cell division in the villi, Wnt activity is restricted to the crypt by the graded expression of several other signals, notably Wnt antagonists, Bone Morphogenetic Protein (BMP), and Hedgehog (Scoville et al., 2008; Yeung et al., 2011; Fig. 7K). BMP is emitted by myofibroblasts of the villi, where it is maintained by Hedgehog signals (Indian hedgehog, Ihh), derived from the villus epithelium. The removal of BMP signals causes the stem cell population to invade the villi. Conversely, constitutively active Hh signaling results in premature enerocyte differentiation and depletion of stem cells in the crypt (Fig. 7C–F). Myofibroblasts surrounding the crypt epithelium produce the BMP antagonists Noggin and Gremlin, which inhibit the BMP pathway and thereby allow the Wnt signal to be active (Scoville et al., 2008; Yeung et al., 2011).
Fig. 7.
Maintenance of stem cells in the mammalian gut. A, B: Histological section (H&E staining) of wild type mouse intestine (A) and mutant intestine 4 days after inactivation of beta-catenin (Wnt pathway). Note absence of crypt cells. C, D: Section of wild type mouse intestine and Patched (Ptc) mutant intestine. Mutant in Ptc causes constitutively active Hedgehog signaling. Note reduced length of villi. Electron micrographs (E, F) show that immature stem cells and progenitors that populate crypt of wild type (arrow in E) are replaced by cylindrical, differentiated enterocytes in Ptc mutant. G–J: Role of Notch signaling. Constitutively active Notch causes an expansion of proliferating stem cells and progenitors (BrdU labeled; brown) into villi. At the same time, enterocyte differentiation (note long microvilli of wild type brushborder; arrow in I) is inhibited (short and irregularly spaced microvilli indicated by arrow in J). K: Schematic image of intestinal epithelium at villus-crypt boundary, depicting signaling interactions involved in controlling proliferation and differentiation of intestinal cells.
Panels A, B: from Fevr et al., 2007 (with permission); C–F: from van Dop et al., 2009; G–J: Fre et al., 2005
The second signaling pathway required for ISC maintenance is the Notch pathway, that we encountered above in the context of specifying enterocyte fate. It is not yet clear whether these two functions of Notch in (1) maintaining stem cells, and (2) inhibiting secretory/endocrine cells are separable, or whether they represent aspects of one single signaling interaction. The Notch ligand Delta (Dl) is expressed by emerging secretory (glandular and endocrine) cells in the crypt (Stamataki et al., 2011). As in the embryo, these cells also express the proneural genes Math1 and Neurogenin 3. The expression of proneural genes block further division and promote secretory fate; the expression of Dl acts on neighboring cells, i.e., ISCs and transient amplifying cells, and prevents them from adopting a secretory cell fate. In other words, N activity, possibly in conjunction with Wnt activity, promotes the division of stem cells and transient amplifying cells. Overexpression of Notch, using a promoter active in all epithelial cells, results in loss of secretory cells and increased proliferation (Fig. 7G, H). Loss of N or Dl depletes the intestine of stem cells and enterocytes (Fre et al., 2011). The effect of constitutively active Notch on enterocyte differentiation in the adult gut seems puzzling, in particular in light of Notch function on midgut development in Drosophila, discussed below. Thus, activated Notch has a negative impact on enterocyte differentiation: microvilli of enterocytes remained short and more widely spaced, failing to form the conspicuous brush border of fully differentiated enterocytes (Fre et al., 2005; Fig. 7I, J). In addition, the apoptosis of enterocytes is increased. These data show that Notch activation is essential for maintaining ISCs, which then proceed to form enterocytes, but needs to be downregulated to physiological levels for terminal differentiation of enterocytes to occur.
4.2. Signaling pathways controlling ISC maintenance in the Drosophila intestine
ISCs of the Drosophila midgut form evenly distributed clusters of 1–4 cells. It was initially thought that all ISCs undergo stereotypic, asymmetric divisions, resulting in one daughter that continues to cycle, and a second daughter that becomes postmitotic (called enteroblast) and adopts either an enterocyte fate or endocrine fate (Ohlstein and Spradling, 2006; Micchelli and Perrimon, 2006). Recent studies (e.g., de Navascues et al., 2012) indicate that the division pattern of ISCs is more varied, with a good sized number of ISC division resulting in two daughters that continue to divide into ISCs, or two daughters that differentiate. Similar to the vertebrate intestine, Wnt/Wg signaling plays a key role in maintaining Drosophila ISCs (Lin et al., 2008; Lee et al., 2009; Xu et al., 2011; Fig. 8J). Cellular clones derived from ISCs lacking Wnt pathway activity are smaller than wild-type clones (Lin et al., 2008; Fig. 8A, B). Conversely, overactivation of the Wnt pathway, caused by mutations in the adenomatous polyposis coli (apc) gene, increases the number of ISC clusters, and the number of dividing cells per cluster (Lee et al., 2009; Fig. 8C, D). The ligand, Wg, is expressed in a subset of visceral muscle cells (Lin et al., 2008). DWnt-4, another member of the Drosophila Wnt family, is expressed more widely in the entire visceral musculature (ST and VH, unpublished).
Fig. 8.
Maintenance of stem cells in the Drosophila midgut. A, B: Clone (green) derived from single ISC after three weeks; WT (A) and beta-catenin mutant (no Wg signaling). C, D: ISCs are labeled by expression of the escargot (Esg) gene (green). Number of proliferating ISCs (BrdU-positive, yellow) is enhanced in animals lacking the apc gene (D; hyperactivity of Wg signaling), compared to wild type (C). E, F: Number of ISCs expressing the Jak/Stat signal Unpaired (Upd; green) is increased following the activation of Reaper (Rpr; panel F) which induces enterocyte cell death. G, H, I: ISCs are labeled by expression of Esg (green). Loss of causes strong increase in ISC number (H). Concomitant block of Jak/Stat pathway rescues this phenotype (I). J: Schematic image of Drosophila midgut, depicting signaling interactions involved in controlling proliferation and differentiation of intestinal cells.
In addition to Wnt/Wg, three other pathways were shown to act on Drosophila ISCs. They include the EGFR, Jak/Stat and Hippo pathways. Ligands activating EGFR are expressed by visceral muscle cells and by differentiated enterocytes (Jiang et al., 2011; Xu et al., 2011). Ligands for the JAK/STAT pathway (Upd, Upd1, Upd2) are found in enterocytes, as well as in ISCs themselves (Jiang et al., 2009; Liu et al., 2010). Taken together, these findings suggest that the Drosophila midgut does not contain a spatially restricted niche; rather, all cell midgut cell types, including visceral muscle fibers as well as epithelial enterocytes, contribute signals that promote self renewal of ISCs.
The EGFR and JAK/STAT pathways act to maintain gut homeostasis under normal and pathological conditions. Experimental injury or bacterial infection of enterocytes results in an increase of Upd expression (Jiang et al., 2009) which, by activating Jak/Stat, accelerates ISC proliferation (Fig. 8E, F). Recent data show that the Hippo pathway acts upstream of JAK/STAT in the regenerative response to cellular stress (Shaw et al., 2010; Ren et al., 2010). Thus, cellular stress in enterocytes blocks Hippo, which results in an increase in the production of Upd and EGFR ligands (Karpovicz et al., 2010; Fig. 8G–I).
The role of Notch signaling in midgut ISC self renewal is complex. Some aspects of Notch function are clearly conserved between Drosophila and vertebrates. Thus, as described in previous sections, loss of N activity in ISCs will cause these cells to adopt an endocrine fate. Conversely, activation of N causes ISCs to become enterocytes. However, in contrast to the situation in vertebrates, activated Notch, rather than increasing proliferation, blocks proliferation; and reduction in Notch activity, at least transiently, increases proliferation (Ohlstein and Spradling, 2006; 2007; Micchelli and Perrimon, 2006). This opposite effect of N on ISC proliferation does not reflect a general, species-related difference; in many proliferating cells of Drosophila, including larval neuroblasts (Bowman et al., 2008), as well as imaginal discs, Notch activity drives proliferation and inhibits differentiation. Likewise, in vertebrates, loss of N activity is coupled with increased proliferation in a number of scenarios (Surendran et al., 2010). It is possible that the different cytological characteristics of ISCs in vertebrate and Drosophila may, at least in part, explain the opposite response of this cell type to N activity. The dividing ISCs of the Drosophila midgut are mesenchymal cells which do not form part of the gut epithelium. Midgut epithelial cells themselves are strictly postmitotic. This is not the case in vertebrates, where proliferating stem cells or progenitors of the gut, and many other tissues, are integrated into the epithelium. Now let us assume that the effect of N activity in stem cells is primarily to maintain, or to promote, the epithelial configuration of these cells. What that implies in Drosophila ISCs is that these cells, being mesenchymal to begin with, undergo a mesenchymal-epithelial transition and thereby become enterocytes. If one further assumes that the midgut epithelial environment no longer supports the option of mitosis (possibly due to factors involving the junctional complex), it becomes clear that elevated N activity in nascent enterocytes will not be able to promote proliferation. By contrast, in vertebrates, ISCs are epithelial cells, and proliferation is a process that takes place within the epithelial configuration. Elevated N activity will maintain the epithelial cytotype, and proliferation.
We had started out this section by pointing out that stem cell proliferation creates two different pools of cells, more stem cells and differentiated cells, and that two different models, predetermined and stochastic asymmetry, can explain this outcome. Which one applies to the ISCs in the Drosophila and vertebrate midgut? The division pattern of Drosophila ISCs has been called asymmetric, because the two daughter cells express different fates, one cell remaining ISC, the other proceeding towards differentiation. We now know that this does not apply to all ISC divisions (de Navascues et al., 2012). Even in the cases where daughter cells do have different fates, the mitotic events themselves are not asymmetric in the strict cell biological sense, because there are no known cytoplasmic determinants that become distributed to only one daughter cell, and not the other. The asymmetric outcome of these divisions is most likely due to extrinsic signals that reach one daughter and not the other. For example, if one considers that signals derived from the visceral musculature that surrounds the basal surface of the midgut epithelium play a role in stem cell maintenance, it would follow that daughter cells with broader contact to the muscle are more exposed to the signal, and would remain stem cells. In this model, orientation of the mitotic spindle would be important, since it dictates the positioning of the daughter cells emerging from a stem cell division. It has been reported that spindle orientation in Drosophila ISCs is indeed directed at an angle of 29±14deg relative to the horizontal plane, which causes, in mitotic events, that one daughter cell keeps a basal position, adjacent to the visceral musculature, whereas the other daughter cell is pushed away from this location (Ohlstein and Spradling, 2007). The basal daughter cell retains its fate as a stem cell, the more apical daughter goes on and differentiates. Non-random spindle orientations have also recently been reported for ISCs in the mammalian intestine (Quyn et al., 2010). If one takes these reports serious, and incorporates directed spindle orientation as an important factor of the mechanism maintaining stem cells, one arrives at a model that lies between the predetermined and stochastic one. Thus, as assumed in the stochastic model, it is a signal from the (niche) environment, and not asymmetrically distributed intracellular factors, that dictates whether a cell remains stem cell or not. However, given the intrinsically fixed spindle orientation, most mitotic events will lead to an asymmetric outcome, because they deliver daughter cells into different positions relative to the niche signal.
Conclusions
We have discussed in this review that the midgut in all animal taxa comprises digestive enterocytes, as well as secretory glandular and endocrine cells. Ultrastructural (and presumably functional) characteristics of these cells are highly conserved. We assume that these cell types arose as epithelial cells with apical cilia and microvilli; these characterers are still maintained in enterocytes of non-bilaterians, as well as most bilaterian clades. Cilia are absent in most, if not all, ecdysozoans, as well as in chordates. All intestinal cell types may have originally proliferated, thereby compensating for the continuous cell loss by wear and tear. This mechanism still exists in cnidarians. In bilaterians, stem cells that either migrate into the gut (in platyhelminths) or reside in the gut as epithelial cells (as in chordates) or sub-epithelial cells (as in insects) take over the function of intestinal cell replacement. Molecular pathways that maintain stem cells and dictate the fate of the different intestinal cells produced by these stem cells are highly conserved, further supporting the idea that the mechanism of intestinal cell renewal was established early in animal evolution. It is of interest that the same gene cassette responsible to define the fate of glandular cells and endocrine cells also acts in the selection of neuronal progenitors, which indicates that these cell types are developmentally and evolutionarily related. One may speculate that in primitive metazoans prior to the appearance of a nervous system, specialized epithelial cells located in the epidermis and the intestinal lining became sensitive to stimuli, and secreted metabolites that helped in nutrient digestion or uptake (secretory cells), or in evoking adaptive responses in other tissues (endocrine cells). The proneural-neurogenic gene cassette might already at this early stage in evolution have acted to control the balance between regular epithelial cells and specialized secretory/endocrine cells. Endocrine cells might have constituted the preadaptive cell type from which neurons evolved, by adding to the secretory/regulative function of this cell type the structural component of forming long processes and synapses. This hypothetical scenario would explain the highly conserved molecular mechanism central to the specification of neurons and secretory cells.
Fig. 3.
Cell division (in green) using EdU labeling over a two hour period in various cnidarians. Interestingly, in all of these images cell division appears scattered, as opposed to being localized to a stem cell niche (A) Gastrodermis of young medusa of the jellyfish Aurelia; several gastrodermal filaments are highlighted in yellow. (B) Sectioned polyp of Aurelia, with a yellow line demarcating epithelium from gastrodermis. Note the higher level of cell division in the epithelium, which is similar to Hydra. (C) Higher magnification of Aurelia polyp gastrodermis, showing that at least some cell division occurs in endodermal epithelial cells, as in Hydra. (D–E) Mesenteries of the sea anemone Nematostella, again emphasizing the diffuse nature of cell proliferation. (A–C) Tyrosinated tubulin in red, nuclear staining in blue, (D–E) Acetylated tubulin in orange, nuclear staining in blue.
Acknowledgments
This work was supported by Grant NIH/1 R01 GM087373 to Volker Hartenstein. D.A.G. gratefully acknowledges funding from an NIH Training Grant in Genomic Analysis and Interpretation T32HG002536, and the National Aeronautics and Space Administration (NASA) Astrobiology Program.
References
- Aiken KD, Roth KA. Temporal differentiation and migration of substance P, serotonin, and secretin immunoreactive enteroendocrine cells in the mouse proximal small intestine. Dev Dyn. 1992;194:303–310. doi: 10.1002/aja.1001940406. [DOI] [PubMed] [Google Scholar]
- Ang SL, Wierda A, Wong D, Stevens KA, Cascio S, Rossant J, Zaret KS. The formation and maintenance of the definitive endoderm lineage in the mouse: involvement of HNF3/forkhead proteins. Development. 1993;119(4):1301–1315. doi: 10.1242/dev.119.4.1301. [DOI] [PubMed] [Google Scholar]
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118(2):149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Arai F, Hirao A, Suda T. Regulation of hematopoietic stem cells by the niche. Trends Cardiovasc Med. 2005;15(2):75–79. doi: 10.1016/j.tcm.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Azzaria M, Goszczynski B, Chung MA, Kalb JM, McGhee JD. A fork head/HNF-3 homolog expressed in the pharynx and intestine of the Caenorhabditis elegans embryo. Dev Biol. 1996;178(2):289–303. doi: 10.1006/dbio.1996.0219. [DOI] [PubMed] [Google Scholar]
- Baguñà J. The planarian neoblast: the rambling history of its origin and some current black boxes. Int J Dev Biol. 2012;56(1–3):19–37. doi: 10.1387/ijdb.113463jb. [DOI] [PubMed] [Google Scholar]
- Bardin AJ, Perdigoto CN, Southall TD, Brand AH, Schweisguth F. Transcriptional control of stem cell maintenance in the Drosophila intestine. Development. 2010;137(5):705–714. doi: 10.1242/dev.039404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei Y, Hogan J, Berkowitz LA, Soto M, Rocheleau CE, Pang KM, Collins J, Mello CC. SRC-1 and Wnt signaling act together to specify endoderm and to control cleavage orientation in early C. elegans embryos. Dev Cell. 2002;3(1):113–125. doi: 10.1016/s1534-5807(02)00185-5. [DOI] [PubMed] [Google Scholar]
- Bjerknes M, Cheng H. Neurogenin 3 and the enteroendocrine cell lineage in the adult mouse small intestinal epithelium. Dev Biol. 2006;300:722–735. doi: 10.1016/j.ydbio.2006.07.040. [DOI] [PubMed] [Google Scholar]
- Bode HR, Heimfeld S, Chow MA, Huang LW. Gland cells arise by differentiation from interstitial cells in Hydra attenuata. Dev Biol. 1987;122(2):577–85. doi: 10.1016/0012-1606(87)90321-6. [DOI] [PubMed] [Google Scholar]
- Boeck ME, Boyle T, Bao Z, Murray J, Mericle B, Waterston R. Specific roles for the GATA transcription factors end-1 and end-3 during C. elegans E-lineage development. Dev Biol. 2011;358(2):345–355. doi: 10.1016/j.ydbio.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossard P, Zaret KS. GATA transcription factors as potentiators of gut endoderm differentiation. Development. 1998;125(24):4909–4917. doi: 10.1242/dev.125.24.4909. [DOI] [PubMed] [Google Scholar]
- Bowen ED, Ryder TA, Thompson JA. The fine structure of the planarian Polycelis tenuis Iijima. II. The intestine and gastrodermal phagocytosis. Protoplasma. 1974;79:1–17. doi: 10.1007/BF02055779. [DOI] [PubMed] [Google Scholar]
- Bowen ED, Ryder TA, Dark C. The effects of starvation on the planarian worm Polycelis tenuis Iijima. Cell Tissue Res. 1976;169(2):193–209. doi: 10.1007/BF00214208. [DOI] [PubMed] [Google Scholar]
- Bowman SK, Rolland V, Betschinger J, Kinsey KA, Emery G, Knoblich JA. The tumor suppressors Brat and Numb regulate transit-amplifying neuroblast lineages in Drosophila. Dev Cell. 2008;14(4):535–546. doi: 10.1016/j.devcel.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle MJ, Seaver EC. Developmental expression of foxA and gata genes during gut formation in the polychaete annelid, Capitella sp. I. Evol Dev. 2008;10:89–105. doi: 10.1111/j.1525-142X.2007.00216.x. [DOI] [PubMed] [Google Scholar]
- Boyle MJ, Seaver EC. Expression of FoxA and GATA transcription factors correlates with regionalized gut development in two lophotrochozoan marine worms Chaetopterus (Annelida) and Themiste lageniformis (Sipuncula) EvoDevo. 2010;1:2. doi: 10.1186/2041-9139-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachero S, Simpson TI, Zur Lage PI, Ma L, Newton FG, Holohan EE, Armstrong JD, Jarman AP. The gene regulatory cascade linking proneural specification with differentiation in Drosophila sensory neurons. PLoS Biol. 2011;9(1):e1000568. doi: 10.1371/journal.pbio.1000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Ortega JA. Genetic mechanisms of early neurogenesis in Drosophila melanogaster. Mol Neurobiol. 1995;10(2–3):75–89. doi: 10.1007/BF02740668. [DOI] [PubMed] [Google Scholar]
- Campuzano S, Modolell J. Patterning of the Drosophila nervous system: the achaete-scute gene complex. Trends Genet. 1992;8:202–208. doi: 10.1016/0168-9525(92)90234-u. [DOI] [PubMed] [Google Scholar]
- Chapman DM. Microanatomy of the cubopolyp, Tripedalia cystophora (Class Cubozoa) Helgoländer wiss Meeresunters. 1978;31:128–168. [Google Scholar]
- Chia FS, Koss R. Asteroidea. In: Harrison FW, Chia TS, editors. Microscopic Anatomy of Invertebrates. Vol. 14. Wiley Liss; New York: 1994. pp. 169–246. [Google Scholar]
- Chia W, Cai Y, Morin X, Tio M, Udolph G, Yu F, Yang X. The cell cycle machinery and asymmetric cell division of neural progenitors in the Drosophila embryonic central nervous system. Novartis Found Symp. 2001;237:139–15. doi: 10.1002/0470846666.ch11. [DOI] [PubMed] [Google Scholar]
- Cioffi M. The morphology and fine structure of the larval midgut of a moth (Manduca sexta) in relation to active ion transport. Tissue Cell. 1979;11(3):467–479. doi: 10.1016/0040-8166(79)90057-0. [DOI] [PubMed] [Google Scholar]
- Collins AG. Recent insights into cnidarian phylogeny. Smithsonian Contrib Mar Sci. 2009;38:139–149. [Google Scholar]
- Coons LB, Alberti G. Acari: Ticks. In: Harrison FW, Foelix AF, editors. Microscopic Anatomy of Invertebrates. 8B. Wiley Liss; New York: 1999. pp. 267–514. [Google Scholar]
- Crosnier C, Vargesson N, Gschmeissner S, Ariza-McNaughton L, Morrison A, Lewis J. Delta-Notch signalling controls commitment to a secretory fate in the zebrafish intestine. Development. 2005;132:1093–1104. doi: 10.1242/dev.01644. [DOI] [PubMed] [Google Scholar]
- Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- D’Angelo A, Bluteau O, Garcia-Gonzalez MA, Gresh L, Doyen A, Garbay S, Robine S, Pontoglio M. Hepatocyte nuclear factor 1alpha and beta control terminal differentiation and cell fate commitment in the gut epithelium. Development. 2010;137(9):1573–1582. doi: 10.1242/dev.044420. [DOI] [PubMed] [Google Scholar]
- Darras S, Gerhart J, Terasaki M, Kirschner M, Lowe CJ. β-catenin specifies the endomesoderm and defines the posterior organizer of the hemichordate Saccoglossus kowalevskii. Development. 2011;138(5):959–970. doi: 10.1242/dev.059493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David CN, Campbell RD. Cell cycle kinetics and development of Hydra attenuata. I. Epithelial cells. J Cell Sci. 1972;11(2):557–568. doi: 10.1242/jcs.11.2.557. [DOI] [PubMed] [Google Scholar]
- David CN, Gierer A. Cell cycle kinetics and development of Hydra attenuata. III. Nerve and nematocyte differentiation. J Cell Sci. 1974;16(2):359–375. doi: 10.1242/jcs.16.2.359. [DOI] [PubMed] [Google Scholar]
- Davis LE. Histological and ultrastructural studies of the basal disk of Hydra. III. The gastrodermis and the mesoglea. Cell Tissue Res. 1975;162(1):107–118. doi: 10.1007/BF00223266. [DOI] [PubMed] [Google Scholar]
- de-Leon SB. The conserved role and divergent regulation of foxa, a pan-eumetazoan developmental regulatory gene. Dev Biol. 2011;357(1):21–26. doi: 10.1016/j.ydbio.2010.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Navascués J, Perdigoto CN, Bian Y, Schneider MH, Bardin AJ, Martínez-Arias A, Simons BD. Drosophila midgut homeostasis involves neutral competition between symmetrically dividing intestinal stem cells. EMBO J. 2012;31(11):2473–2485. doi: 10.1038/emboj.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil RR. Copper cells and stomach acid secretion in the Drosophila midgut. Int J Biochem Cell Biol. 2004;36(5):745–752. doi: 10.1016/j.biocel.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Ettensohn CA. The emergence of pattern in embryogenesis: regulation of beta-catenin localization during early sea urchin development. Sci STKE. 2006;2006(361):pe48. doi: 10.1126/stke.3612006pe48. [DOI] [PubMed] [Google Scholar]
- Fevr T, Robine S, Louvard D, Huelsken J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol. 2007;27(21):7551–7559. doi: 10.1128/MCB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filshie BK, Poulson DF, Waterhouse DF. Ultrastructure of the copper-accumulating region of the Drosophila larval midgut. Tissue Cell. 1971;3(1):77–102. doi: 10.1016/s0040-8166(71)80033-2. [DOI] [PubMed] [Google Scholar]
- Forsthoefel DJ, Park AE, Newmark PA. Stem cell-based growth, regeneration, and remodeling of the planarian intestine. Dev Biol. 2011;356(2):445–459. doi: 10.1016/j.ydbio.2011.05.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435(7044):964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- Fre S, Hannezo E, Sale S, Huyghe M, Lafkas D, Kissel H, Louvi A, Greve J, Louvard D, Artavanis-Tsakonas S. Notch lineages and activity in intestinal stem cells determined by a new set of knock-in mice. PLoS One. 2011;6(10):e25785. doi: 10.1371/journal.pone.0025785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fre S, Bardin A, Robine S, Louvard D. Notch signaling in intestinal homeostasis across species: the cases of Drosophila, Zebrafish and the mouse. Exp Cell Res. 2011;317(19):2740–2747. doi: 10.1016/j.yexcr.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Goldberg WM. Gastrodermal structure and feeding responses in the scleractinian Mycetophyllia reesi, a coral with novel digestive filaments. Tissue Cell. 2002;34(4):246–261. doi: 10.1016/s0040-8166(02)00008-3. [DOI] [PubMed] [Google Scholar]
- Grell KG, Ruthmann A. Placozoa. In: Harrison FW, Westfall JA, editors. Microscopic Anatomy of Invertebrates. Vol. 2. Wiley Liss; New York: 1991. pp. 13–28. [Google Scholar]
- Guillemot F. Vertebrate bHLH genes and the determination of neuronal fates. Exp Cell Res. 1999;253:357–364. doi: 10.1006/excr.1999.4717. [DOI] [PubMed] [Google Scholar]
- Gupta BL. The relationship of mucoid substances and ion and water transport, with new data on intestinal goblet cells and a model for gastric secretion. 1989;43:81–110. [PubMed] [Google Scholar]
- Haegebarth A, Clevers H. Wnt signaling, lgr5, and stem cells in the intestine and skin. Am J Pathol. 2009;174(3):715–21. doi: 10.2353/ajpath.2009.080758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartenstein V, Takashima S, Adams KL. Conserved genetic pathways controlling the development of the diffuse endocrine system in vertebrates and Drosophila. Gen Comp Endocrinol. 2010;166(3):462–469. doi: 10.1016/j.ygcen.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasebe T, Kajita M, Iwabuchi M, Ohsumi K, Ishizuya-Oka A. Thyroid hormone-regulated expression of nuclear lamins correlates with dedifferentiation of intestinal epithelial cells during Xenopus laevis metamorphosis. Dev Genes Evol. 2001;221:199–208. doi: 10.1007/s00427-011-0371-7. [DOI] [PubMed] [Google Scholar]
- Henning SJ, Rubin DC, Shulman R. Ontogeny of the intestinal mucosa. In: Johnson LR, editor. Physiology of the gastrointestinal tract. New York NY: Raven press; 1994. pp. 571–610. [Google Scholar]
- Henry JQ, Perry KJ, Wever J, Seaver E, Martindale MQ. Beta-catenin is required for the establishment of vegetal embryonic fates in the nemertean, Cerebratulus lacteus. Dev Biol. 2008;317(1):368–379. doi: 10.1016/j.ydbio.2008.02.042. [DOI] [PubMed] [Google Scholar]
- Hernandez-Nicaise ML. Ctenophora. In: Harrison FW, Westfall JA, editors. Microscopic Anatomy of Invertebrates. Vol. 2. Wiley Liss; New York: 1991. pp. 359–418. [Google Scholar]
- Icely JD, Nott JAR. Digestion and absorption: digestive system and associated organs. In: Harrison FW, Humes AG, editors. Microscopic Anatomy of Invertebrates. Vol. 10. Wiley Liss; New York: 1992. pp. 147–201. [Google Scholar]
- Jacquemin P, Durviaux SM, Jensen J, Godfraind C, Gradwohl G, Guillemot F, Madsen OD, Carmeliet P, Dewerchin M, Collen D, Rousseau GG, Lemaigre FP. Transcription factor hepatocyte nuclear factor 6 regulates pancreatic endocrine cell differentiation and controls expression of the proendocrine gene ngn3. Mol Cell Biol. 2000;20(12):4445–4454. doi: 10.1128/mcb.20.12.4445-4454.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny M, Uhl C, Roche C, Duluc I, Guillermin V, Guillemot F, Jensen J, Kedinger M, Gradwohl G. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 2002;21:6338–6347. doi: 10.1093/emboj/cdf649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137(7):1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Grenley MO, Bravo MJ, Blumhagen RZ, Edgar BA. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell. 2011;8(1):84–95. doi: 10.1016/j.stem.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MK, Hughes-Stamm SR, East RM, Cribb TH. Ultrastructure of the digestive tract of Gyliauchen nahaensis (Platyhelminthes, Digenea), an inhabitant of the hindgut of herbivorous fishes. J Morphol. 2000;246(3):198–211. doi: 10.1002/1097-4687(200012)246:3<198::AID-JMOR4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Kageyama R, Sasai Y, Akazawa C, Ishibashi M, Takebayashi K, Shimizu C, Tomita K, Nakanishi S. Regulation of mammalian neural development by helix-loop-helix transcription factors. Crit Rev Neurobiol. 1995;9:177–188. [PubMed] [Google Scholar]
- Karpowicz P, Perez J, Perrimon N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development. 2010;137(24):4135–4145. doi: 10.1242/dev.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroiher M, Plickert G, Müller WA. Pattern of cell proliferation in embryogenesis and planula development of Hydractinia echinata predicts the postmetamorphic body pattern. Roux’s Arch Dev Biol. 1990;199(3):156–163. doi: 10.1007/BF01681488. [DOI] [PubMed] [Google Scholar]
- Künzel T, Heiermann R, Frank U, Müller W, Tilmann W, Bause M, Nonn A, Helling M, Schwarz RS, Plickert G. Migration and differentiation potential of stem cells in the cnidarian Hydractinia analysed in eGFP-transgenic animals and chimeras. Dev Biol. 2010;348(1):120–9. doi: 10.1016/j.ydbio.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Lane NJ, Harrison JB, Lee WM. Intercellular junctions in the hepatopancreas of the lobster Nephrops norvegicus. Biol Cell. 1984;52(3):267–277. doi: 10.1111/j.1768-322x.1985.tb00346.x. [DOI] [PubMed] [Google Scholar]
- Leclère L, Jager M, Barreau C, Chang P, Le Guyader H, Manuel M, Houliston E. Maternally localized germ plasm mRNAs and germ cell/stem cell formation in the cnidarian Clytia. Dev Biol. 2012;364(2):236–48. doi: 10.1016/j.ydbio.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Lee CS, Perreault N, Brestelli JE, Kaestner KH. Neurogenin 3 is essential for the proper specification of gastric enteroendocrine cells and the maintenance of gastric epithelial cell identity. Genes Dev. 2002;16:1488–1497. doi: 10.1101/gad.985002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PN, Kumburegama S, Marlow HQ, Martindale MQ, Wikramanayake AH. Asymmetric developmental potential along the animal-vegetal axis in the anthozoan cnidarian, Nematostella vectensis, is mediated by Dishevelled. Dev Biol. 2007;310(1):169–86. doi: 10.1016/j.ydbio.2007.05.040. [DOI] [PubMed] [Google Scholar]
- Lee WC, Beebe K, Sudmeier L, Micchelli CA. Adenomatous polyposis coli regulates Drosophila intestinal stem cell proliferation. Development. 2009;136(13):2255–2264. doi: 10.1242/dev.035196. [DOI] [PubMed] [Google Scholar]
- Lehane MJ. The Midgut. In: Harrison FW, Locke M, editors. Microscopic Anatomy of Invertebrates. Wiley Liss; New York: 1998. pp. 725–746. [Google Scholar]
- Leys SP, Eerkes-Medrano DI. Feeding in a calcareous sponge: particle uptake by pseudopodia. Biol Bull. 2006;211(2):157–171. doi: 10.2307/4134590. [DOI] [PubMed] [Google Scholar]
- Li HJ, Ray SK, Singh NK, Johnston B, Leiter AB. Basic helix-loop-helix transcription factors and enteroendocrine cell differentiation. Diabetes Obes Metab. 2011;13(Suppl 1):5–12. doi: 10.1111/j.1463-1326.2011.01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327(5965):542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Xu N, Xi R. Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature. 2008;455(7216):1119–1123. doi: 10.1038/nature07329. [DOI] [PubMed] [Google Scholar]
- Lin G, Xi R. Intestinal stem cells, muscular niche and Wingless signaling. Fly. 2010;2(6):310–312. doi: 10.4161/fly.7428. [DOI] [PubMed] [Google Scholar]
- Liu W, Singh SR, Hou SX. JAK-STAT is restrained by Notch to control cell proliferation of the Drosophila intestinal stem cells. J Cell Biochem. 2010;109(5):992–999. doi: 10.1002/jcb.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo L, Dormand E, Greenwood A, Anderson DJ. Comparison of the generic neuronal differentiation and neuron subtype specification functions of mammalian achaete-scute and atonal homologs in cultured neural progenitor cells. Development. 2002;129:1553–1567. doi: 10.1242/dev.129.7.1553. [DOI] [PubMed] [Google Scholar]
- Lord BA, DiBona DR. Role of the septate junction in the regulation of paracellular transepithelial flow. J Cell Biol. 1976;71(3):967–972. doi: 10.1083/jcb.71.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maduro MF, Hill RJ, Heid PJ, Newman-Smith ED, Zhu J, Priess JR, Rothman JH. Genetic redundancy in endoderm specification within the genus Caenorhabditis. Dev Biol. 2005;284(2):509–22. doi: 10.1016/j.ydbio.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Martín-Durán JM, Romero R. Evolutionary implications of morphogenesis and molecular patterning of the blind gut in the planarian Schmidtea polychroa. Dev Biol. 2011;352:164–176. doi: 10.1016/j.ydbio.2011.01.032. [DOI] [PubMed] [Google Scholar]
- Martindale MQ, Pang K, Finnerty JR. Investigating the origins of triploblasty: ‘mesodermal’ gene expression in a diploblastic animal, the sea anemone Nematostella vectensis (phylum, Cnidaria; class, Anthozoa) Development. 2004;131:2463–2474. doi: 10.1242/dev.01119. [DOI] [PubMed] [Google Scholar]
- McLin VA, Henning SJ, Jamrich M. The role of the visceral mesoderm in the development of the gastrointestinal tract. Gastroenterology. 2009;136(7):2074–2091. doi: 10.1053/j.gastro.2009.03.001. [DOI] [PubMed] [Google Scholar]
- McNeil PL. Mechanisms of nutritive endocytosis. I. Phagocytic versatility and cellular recognition in Chlorohydra digestive cells, a scanning electron microscope study. J Cell Sci. 1981;49:311–39. doi: 10.1242/jcs.49.1.311. [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439(7075):475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Miller SJ, Lavker RM, Sun TT. Interpreting epithelial cancer biology in the context of stem cells: tumor properties and therapeutic implications. Biochim Biophys Acta. 2005;1756:25–52. doi: 10.1016/j.bbcan.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Müller WA, Teo R, Frank U. Totipotent migratory stem cells in a hydroid. Dev Biol. 2004;275(1):215–24. doi: 10.1016/j.ydbio.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Mukai H, Terakado K, Reed CG. Bryozoa. In: Harrison FW, Woolacott RM, editors. Microscopic Anatomy of Invertebrates. Vol. 13. Wiley Liss; New York: 1997. pp. 45–206. [Google Scholar]
- Murakami R, Okumura T, Uchiyama H. GATA factors as key regulatory molecules in the development of Drosophila endoderm. Dev Growth Differ. 2005;47(9):581–589. doi: 10.1111/j.1440-169X.2005.00836.x. [DOI] [PubMed] [Google Scholar]
- Murate M, Kishimoto Y, Sugiyama T, Fujisawa T, Takahashi-Iwanaga H, Iwanaga T. Hydra regeneration from recombined ectodermal and endodermal tissue. II. Differential stability in the ectodermal and endodermal epithelial organization. J Cell Sci. 1997;110(Pt 16):1919–1934. doi: 10.1242/jcs.110.16.1919. [DOI] [PubMed] [Google Scholar]
- Nakanishi N, Renfer E, Technau U, Rentzsch F. Nervous systems of the sea anemone Nematostella vectensis are generated by ectoderm and endoderm and shaped by distinct mechanisms. Development. 2012;139(2):347–57. doi: 10.1242/dev.071902. [DOI] [PubMed] [Google Scholar]
- Nielsen C, Jespersen A. Entoprocta. In: Harrison FW, Woollacott RM, editors. Microscopic Anatomy of Invertebrates. Vol. 14. Wiley Liss; New York: 1997. pp. 13–44. [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential Notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- Oliveri P, Walton KD, Davidson EH, McClay DR. Repression of mesodermal fate by foxa, a key endoderm regulator of the sea urchin embryo. Development. 2006;133(21):4173–4181. doi: 10.1242/dev.02577. [DOI] [PubMed] [Google Scholar]
- Olsen CL, Jeffery WR. A forkhead gene related to HNF-3beta is required for gastrulation and axis formation in the ascidian embryo. Development. 1997;124(18):3609–3619. doi: 10.1242/dev.124.18.3609. [DOI] [PubMed] [Google Scholar]
- Plickert G, Kroiher M, Munck A. Cell proliferation and early differentiation during embryonic development and metamorphosis of Hydractinia echinata. Development. 1988;103(4):795–803. doi: 10.1242/dev.103.4.795. [DOI] [PubMed] [Google Scholar]
- Powell DW, Pinchuk IV, Saada JI, Chen X, Mifflin RC. Mesenchymal cells of the intestinal lamina propria. Annu Rev Physiol. 2011;73:213–237. doi: 10.1146/annurev.physiol.70.113006.100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quyin AJ, Appleton PL, Carey FA, Steele RFC, Barker N, Clevers H, Ridgway RA, Sansom OJ, Näthke IS. Spindle orientation bias in gut epithelial stem cell compartments. Cell Stem Cell. 2010;6:175–181. doi: 10.1016/j.stem.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Rebscher N, Volk C, Teo R, Plickert G. The germ plasm component Vasa allows tracing of the interstitial stem cells in the cnidarian Hydractinia echinata. Dev Dyn. 2008;237(6):1736–45. doi: 10.1002/dvdy.21562. [DOI] [PubMed] [Google Scholar]
- Reichert H. Drosophila neural stem cells: cell cycle control of self-renewal, differentiation, and termination in brain development. Results Probl Cell Differ. 2011;53:529–546. doi: 10.1007/978-3-642-19065-0_21. [DOI] [PubMed] [Google Scholar]
- Ren F, Wang B, Yue T, Yun EY, Ip YT, Jiang J. Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proc Natl Acad Sci U S A. 2010;107(49):21064–210649. doi: 10.1073/pnas.1012759107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME. Sipuncula. In: Harrison FW, Rice ME, editors. Microscopic Anatomy of Invertebrates. Vol. 12. Wiley Liss; New York: 1993. pp. 237–326. [Google Scholar]
- Rieger R. Turbellaria. In: Harrison FW, Bogitsh J, editors. Microscopic Anatomy of Invertebrates. Vol. 2. Wiley Liss; New York: 1991. pp. 1–140. [Google Scholar]
- Ruppert EE. Cephalochordata (Acrania) In: Harrison FW, Ruppert EE, editors. Microscopic Anatomy of Invertebrates. Vol. 15. Wiley Liss; New York: 1997. pp. 349–504. [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469(7330):415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierwater B, de Jong D, Desalle R. Placozoa and the evolution of Metazoa and intrasomatic cell differentiation. Int J Biochem Cell Biol. 2009;41(2):370–379. doi: 10.1016/j.biocel.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Schmid V, Wydler M, Alder H. Transdifferentiation and regeneration in vitro. Dev Biol. 1982;92(2):476–88. doi: 10.1016/0012-1606(82)90193-2. [DOI] [PubMed] [Google Scholar]
- Schmidt T, David CN. Gland cells in Hydra: cell cycle kinetics and development. J Cell Sci. 1986;85:197–215. doi: 10.1242/jcs.85.1.197. [DOI] [PubMed] [Google Scholar]
- Schonhoff SE, Giel-Moloney M, Leiter AB. Development and differentiation of gut endocrine cells. Endocrinology. 2004a;145:2639–2644. doi: 10.1210/en.2004-0051. [DOI] [PubMed] [Google Scholar]
- Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology. 2008;134(3):849–864. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- Seipel K, Yanze N, Schmid V. The germ line and somatic stem cell gene Cniwi in the jellyfish Podocoryne carnea. Int J Dev Biol. 2004;48(1):1–7. doi: 10.1387/ijdb.15005568. [DOI] [PubMed] [Google Scholar]
- Shaker A, Rubin DC. Intestinal stem cells and epithelial-mesenchymal interactions in the crypt and stem cell niche. Transl Res. 2010;156(3):180–187. doi: 10.1016/j.trsl.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RL, Kohlmaier A, Polesello C, Veelken C, Edgar BA, Tapon N. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development. 2010;137(24):4147–4158. doi: 10.1242/dev.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata N, Rouhana L, Agata K. Cellular and molecular dissection of pluripotent adult somatic stem cells in planarians. Dev Growth Differ. 2010;52(1):27–41. doi: 10.1111/j.1440-169X.2009.01155.x. [DOI] [PubMed] [Google Scholar]
- Silva AC, Filipe M, Steinbeisser H, Belo JA. Characterization of Cer-1 cis-regulatory region during early Xenopus development. Dev Genes Evol. 2011;221:29–41. doi: 10.1007/s00427-011-0357-5. [DOI] [PubMed] [Google Scholar]
- Smid I, Tardent P. Migration of I-cells from ectoderm to endoderm in Hydra attenuata Pall (Cnidaria, Hydrozoa) and their subsequent differentiation. Dev Biol. 1984;106(2):469–477. doi: 10.1016/0012-1606(84)90246-x. [DOI] [PubMed] [Google Scholar]
- Stamataki D, Holder M, Hodgetts C, Jeffery R, Nye E, Spencer-Dene B, Winton DJ, Lewis J. Delta1 expression, cell cycle exit, and commitment to a specific secretory fate coincide within a few hours in the mouse intestinal stem cell system. PLoS One. 2011;6(9):e24484. doi: 10.1371/journal.pone.0024484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M, Micchelli CA. Quiescent gastric stem cells maintain the adult Drosophila stomach. Proc Natl Acad Sci U S A. 2011;108(43):17696–17701. doi: 10.1073/pnas.1109794108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surendran K, Selassie M, Liapis H, Krigman H, Kopan R. Reduced Notch signaling leads to renal cysts and papillary microadenomas. J Am Soc Nephrol. 2010;21(5):819–832. doi: 10.1681/ASN.2009090925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima S, Adams KL, Ortiz PA, Ying CT, Moridzadeh R, Younossi-Hartenstein A, Hartenstein V. Development of the Drosophila entero-endocrine lineage and its specification by the Notch signaling pathway. Dev Biol. 2011;353(2):161–172. doi: 10.1016/j.ydbio.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U, Hartenstein V. The development of cellular junctions in the Drosophila embryo. Dev Biol. 1994;161(2):563–596. doi: 10.1006/dbio.1994.1054. [DOI] [PubMed] [Google Scholar]
- Tepass U, Hartenstein V. Neurogenic and proneural genes control the specification of non-neuronal cell types in the Drosophila endoderm. Development. 1995;121:393–405. doi: 10.1242/dev.121.2.393. [DOI] [PubMed] [Google Scholar]
- Turbeville JM. Nmertinea. In: Harrison FW, Bogitsh, editors. Microscopic Anatomy of Invertebrates. Vol. 3. Wiley Liss; New York: 1991. pp. 285–328. [Google Scholar]
- Van Dop WA, Uhmann A, Wijgerde M, Sleddens-Likels A, Heijmans J, Offerhaus GJ, van den Beergh Weerman MA, Boeckxstaens GE, Hommes DW, Hardwick JC, Hahn H, van den Brink GR. Depletion of the colonic epithelial precursor cell compartment upon conditional activation of the Hedgehog pathway. Gastroenterology. 2009;136:2195–2203. doi: 10.1053/j.gastro.2009.02.068. [DOI] [PubMed] [Google Scholar]
- van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- Veenstra JA, Agricola HJ, Sellami A. Regulatory peptides in fruit fly midgut. Cell Tissue Res. 2008;334:499–516. doi: 10.1007/s00441-008-0708-3. [DOI] [PubMed] [Google Scholar]
- Wang J, Cortina G, Wu SV, Tran R, Cho JH, Tsai MJ, Bailey TJ, Jamrich M, Ament ME, Treem WR, Hill ID, Vargas JH, Gershman G, Farmer DG, Reyen L, Martín MG. Mutant neurogenin-3 in congenital malabsorptive diarrhea. N Engl J Med. 2006;355(3):270–280. doi: 10.1056/NEJMoa054288. [DOI] [PubMed] [Google Scholar]
- Weber H, Symes CE, Walmsley ME, Rodaway AR, Patient RK. A role for GATA5 in Xenopus endoderm specification. Development. 2000;127(20):4345–4360. doi: 10.1242/dev.127.20.4345. [DOI] [PubMed] [Google Scholar]
- Weigel D, Jürgens G, Küttner F, Seifert E, Jäckle H. The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell. 1989;57(4):645–658. doi: 10.1016/0092-8674(89)90133-5. [DOI] [PubMed] [Google Scholar]
- Westfall JA, Wilson JD, Rogers RA, Kinnamon JC. Multifunctional features of a gastrodermal sensory cell in Hydra: three-dimensional study. J Neurocytol. 1991;20(4):251–261. doi: 10.1007/BF01235543. [DOI] [PubMed] [Google Scholar]
- Willenz P, Van de Vyver G. Endocytosis of latex beads by the exopinacoderm in the fresh water sponge Ephydatia fluviatilis: an in vitro and in situ study in SEM and TEM. J Ultrastruct Res. 1982;79(3):294–306. doi: 10.1016/s0022-5320(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Wood RL. The fine structure of the hypostome and mouth of HydraII. Transmission electron microscopy. Cell Tissue Res. 1979;199(2):319–338. doi: 10.1007/BF00236142. [DOI] [PubMed] [Google Scholar]
- Wright KA. Nematoda. In: Harrison FW, Ruppert EE, editors. Microscopic Anatomy of Invertebrates. Vol. 4. Wiley Liss; New York: 1991. pp. 111–195. [Google Scholar]
- Xu N, Wang SQ, Tan D, Gao Y, Lin G, Xi R. EGFR, Wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Dev Biol. 2011;354(1):31–43. doi: 10.1016/j.ydbio.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- Yasuo H, Lemaire P. A two-step model for the fate determination of presumptive endodermal blastomeres in Xenopus embryos. Curr Biol. 1999;9(16):869–879. doi: 10.1016/s0960-9822(99)80391-1. [DOI] [PubMed] [Google Scholar]
- Yen TH, Wright NA. The gastrointestinal tract stem cell niche. Stem Cell Rev. 2006;2(3):203–212. doi: 10.1007/s12015-006-0048-1. [DOI] [PubMed] [Google Scholar]
- Yeung TM, Chia LA, Kosinski CM, Kuo CJ. Regulation of self-renewal and differentiation by the intestinal stem cell niche. Cell Mol Life Sci. 2011:2513–2525. doi: 10.1007/s00018-011-0687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret K. Developmental competence of the gut endoderm: genetic potentiation by GATA and HNF3/fork head proteins. Dev Biol. 1999;209(1):1–10. doi: 10.1006/dbio.1999.9228. [DOI] [PubMed] [Google Scholar]
- Zhang J, Houston DW, King ML, Payne C, Wylie C, Heasman J. The role of maternal VegT in establishing the primary germ layers in Xenopus embryos. Cell. 1998;94(4):515–524. doi: 10.1016/s0092-8674(00)81592-5. [DOI] [PubMed] [Google Scholar]
- Zhu J, Hill RJ, Heid PJ, Fukuyama M, Sugimoto A, Priess JR, Rothman JH. end-1 encodes an apparent GATA factor that specifies the endoderm precursor in Caenorhabditis elegans embryos. Genes Dev. 1997;11(21):2883–2896. doi: 10.1101/gad.11.21.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]