Abstract
Background
Subjective cognitive impairment (SCI) in older persons without manifest symptomatology is a common condition with a largely unclear prognosis. We hypothesized that (1) examining outcome for a sufficient period by using conversion to mild cognitive impairment (MCI) or dementia would clarify SCI prognosis, and (2) with the aforementioned procedures, the prognosis of SCI subjects would differ significantly from that of demographically matched healthy subjects, free of SCI, termed no cognitive impairment (NCI) subjects.
Methods
A consecutive series of healthy subjects, aged ≥40 years, presenting with NCI or SCI to a brain aging and dementia research center during a 14-year interval, were studied and followed up during an 18-year observation window. The study population (60 NCI, 200 SCI, 60% female) had a mean age of 67.2 ± 9.1 years, was well-educated (mean, 15.5 ± 2.7 years), and cognitively normal (Mini-Mental State Examination, 29.1 ± 1.2).
Results
A total of 213 subjects (81.9% of the study population) were followed up. Follow-up occurred during a mean period of 6.8 ± 3.4 years, and subjects had a mean of 2.9 ± 1.6 follow-up visits. Seven NCI (14.9%) and 90 SCI (54.2%) subjects declined (P < .0001). Of NCI decliners, five declined to MCI and two to probable Alzheimer’s disease. Of SCI decliners, 71 declined to MCI and 19 to dementia diagnoses. Controlling for baseline demographic variables and follow-up time, Weibull proportional hazards model revealed increased decline in SCI subjects (hazard ratio, 4.5; 95% confidence interval, 1.9–10.3), whereas the accelerated failure time model analysis with an underlying Weibull survival function showed that SCI subjects declined more rapidly, at 60% of the rate of NCI subjects (95% confidence interval, 0.45–0.80). Furthermore, mean time to decline was 3.5 years longer for NCI than for SCI subjects (P = .0003).
Conclusions
These results indicate that SCI in subjects with normal cognition is a harbinger of further decline in most subjects during a 7-year mean follow-up interval. Relevance for community populations should be investigated, and prevention studies in this at-risk population should be explored.
Keywords: Subjective cognitive impairment, Subjective cognitive complaints, Brain aging, Cognition, Mild cognitive impairment, Outcome studies, Longitudinal studies, Dementia, Risk factors for dementia, Neuropsychological testing
1. Introduction
Subjective cognitive impairment (SCI) is a common condition in older persons. A review found three studies of prevalence in community-residing persons ≥ 65 years [1–4]. SCI prevalence ranged from 25% to 56%. During the past three decades, approximately one third of persons coming to our outpatient brain aging and dementia, university hospital–based, clinical research center have presented with these subjective deficits in the absence of subtle or overtly manifest cognitive decline.
The prognosis of SCI is a personal concern for many older persons and a public health concern for the medical community. The prognostic import of these symptoms might also have scientific relevance in the evolution of cognitive impairment in aging.
SCI can occur in apparently healthy persons in the absence of objective evidence of cognitive impairment or psychopathology. Subjective cognitive complaints can also occur in association with mild cognitive impairment (MCI) [5–7], dementia [5], depression [8–10], anxiety [11], numerous medical illnesses, and various medications. Hence, there is a need for studies of the prognosis of persons with SCI who are otherwise healthy.
Results from present studies of SCI prognosis, which have generally used dementia as an outcome criterion, are varied. One recent study [12] queried subjects at baseline with the question, “Do you have memory complaints?” Subjects aged ≥55 years at baseline were followed up for a mean of 9 years. A three times greater risk of Alzheimer’s disease (AD) was observed in the highly educated subject group without cognitive impairment at baseline who had memory complaints, in comparison with subjects who denied complaints. The increase in risk of AD associated with subjective memory complaints was much lower in the subject group with low education (specifically, limited to primary education). In these less educated persons, the presence of subjective complaints of memory problems was associated with 1.5 times greater risk of subsequent AD than in persons who did not report complaints. However, a limitation of this study is that there was no baseline assessment of the presence of depression or of affective symptomatology. This is a potentially serious limitation because as noted earlier, depression is associated with subjective complaints of memory and cognitive impairment and an increased risk of subsequent AD.
Another study queried cognitively normal, community-residing persons aged ≥65 years with the question whether they had “memory loss in the past year” [13]. A “yes” or “no” answer was required. Subjects who responded affirmatively were more than twice as likely to develop dementia during the subsequent 5 years. The association between subjective memory loss (SML) and dementia was maintained after adjusting for depressive symptoms. However, the association with subsequent dementia was not significant after adjustment for a baseline cognitive score. The authors noted that only 15% of those seniors who stated they had memory loss during the past year developed dementia during the subsequent 5 years, and they concluded that “as a clinical predictor, the presence of SML [subjective memory loss] is very insensitive and non-specific” and that SML alone “is unlikely to be a useful predictor of dementia.”
A third study examined community residents aged ≥65 years during a 3-year period [14]. Subjects were asked, “Do you have trouble with your memory?” The subjects who complained of memory trouble scored significantly lower on the cognitive screening measure and higher on the depression assessment. However, subjective memory trouble “did not predict faster cognitive decline or dementia over 3 years.”
The variability observed in current studies of SCI might be due in part to the need for an adequate follow-up interval and partly because of the absence of an MCI outcome criterion in current published systematic outcome studies. In accord with a long-standing published estimate, initially forwarded in 1986, that the MCI stage that eventuates in AD lasts a mean of approximately 7 years and that the preceding SCI stage that eventuates in AD lasts a mean of approximately 15 years [15], we herein investigate the hypothesis that (1) a clearer view of the import of SCI might emerge from a study that examines prognosis with MCI as an outcome criterion, in addition to dementia, during an adequate follow-up interval and in otherwise healthy subjects, and (2) the prognosis of SCI subjects will be worse than that of demographically matched, similarly healthy subjects who are free of SCI, termed no cognitive impairment (NCI) subjects.
MCI is a condition in which subtle objective cognitive impairment is present that is not of sufficient magnitude for a diagnosis of dementia [6,7,16]. In otherwise healthy older populations, MCI is a frequent precursor of dementia. However, progression to dementia in MCI subjects occurs over many years. A review of 19 longitudinal studies comprising clinic attendees or community-residing volunteers found an overall conversion rate in MCI subjects to dementia of 10% per year [17]. In research populations including our own study population [18], approximately a 16% to 18% annual conversion rate of MCI to AD has been observed [18,19]. Hence, MCI, a condition lasting many years before the advent of the dementia of AD, is likely to yield a much higher conversion rate for SCI prognostic studies than studies that use progression to dementia exclusively. Therefore, we conducted a study of SCI prognosis in otherwise healthy older persons by using decline to MCI, as well as dementia, as a primary outcome criterion.
2. Methods
2.1. Subjects and study background
Subjects were community-dwelling persons, >40 years of age, recruited by referral or public announcement to participate in a longitudinal study on cognitive aging. The title of the primary project grant that supported the present study, funded by the U.S. National Institute on Aging (NIA) (AG03051) of the U.S. National Institutes of Health from 1982 until 2003 was “Aging and Dementia: Longitudinal Course of Subgroups.” An explicit hypothesis of this grant from inception was that the prognosis of subjects with SCI would differ from that of other identified subgroups (stages of aging and dementia). After being enrolled in this longitudinal grant, subjects continued to be followed up at specified intervals until demise. Adequate and optimally long follow-up intervals would provide a useful and important view of the nature and origins of the successive stages of brain aging, and AD is explicit in the hypotheses that resulted in the funding of this grant in 1982 and the renewal of funding in 1987, 1992, and 1997.
For the selection of the study population, medical, neurologic, psychiatric, neuropsychological, neuroradiologic, and clinical laboratory evaluations were conducted to exclude subjects with conditions that might interfere with or confound cognitive functioning apart from SCI [20,21].
Criteria for exclusion in the selection of the study population included the following: (1) presence of MCI [7,16] or dementia; (2) history of significant head trauma, seizures, mental retardation, or neurologic disorder; (3) any focal signs of significant brain pathology from the medical or neurologic evaluations; (4) diagnosis of cerebrovascular disease on the basis of either a history of clinically significant cerebral infarction or a modified Hachinski Ischemic Score ≥4 [20] or evidence of infarction from the brain neuroimaging evaluation (generally magnetic resonance imaging scans or, in a small minority of cases, computed tomography scans); (5) significant history of drug or alcohol abuse; (6) history of schizophrenia or major affective disorder, including any subjects with Hamilton Depression Scale (Ham-D) [21] scores of ≥16; (7) cardiac, pulmonary, vascular, metabolic, or hematologic conditions of sufficient severity to adversely affect cognition or functioning; (8) other physical impairment of sufficient severity to adversely affect cognition or functioning; (9) significant abnormality in laboratory evaluations that included comprehensive metabolic values, complete blood counts, urinalysis, serum B12 and folate, thyroid function tests (specifically triiodothyronine [T3], thyroxine [T4], and thyroid-stimulating hormone levels), and screening for syphilis; and (10) active usage of any medications that might significantly affect cognitive functioning.
The New York University Institutional Review Board approved all aspects of this study, and written informed consent was obtained from all subjects.
2.2. Study design
2.2.1. Staging for magnitude of cognitive functioning
Subjects were assessed for presence or absence of SCI and/or objective cognitive impairment at baseline by using the Global Deterioration Scale (GDS) for age-associated cognitive decline and dementia [22]. Validity and reliability of this widely used staging procedure have been reviewed [23,24]. GDS stage 1 subjects are normatively functioning and free of subjective complaints or objective evidence of cognitive impairment (NCI), whereas GDS stage 2 subjects are normatively functioning and have subjective complaints in the absence of objectively manifest deficits (SCI). NCI or SCI subjects were potential study participants. The precise criteria used for differentiating these stages for this investigation were as follows (from Reisberg et al [22]):
GDS stage 1: “No subjective complaints of memory deficit. No memory deficits evident on clinical interview” [22].
The clinical interview referred to in this study included the Brief Cognitive Rating Scale (BCRS), which specifically assesses the presence of subjective impairments in various areas including concentration and calculation, recent memory, remote memory, orientation, and functioning abilities [25]. Importantly, the BCRS also assesses the presence of objective deficits in each of these domains.
GDS stage 2: “Subjective complaints of memory deficit, most frequently in the following areas: (a) forgetting where one has placed familiar objects; (b) forgetting names one formerly knew well. No objective evidence of memory deficit on clinical interview. No objective deficit in employment or social situations. Appropriate concern with respect to symptomatology” [22].
Once again the clinical interview referred to in assessing the GDS in this study includes the BCRS, which assesses the presence of both subjective and objective deficits in considerable detail.
Subjects at GDS stage 3 have mildly manifest deficits, consistent with a diagnosis of MCI [7,16]. The precise criteria for assessing GDS stage 3 are described in the following paragraphs. It should be noted in this regard that the GDS stage is the most appropriate stage for a subject on the basis of the descriptors.
GDS stage 3: “Earliest clear-cut deficits. Manifestations in more than one of the following areas: (a) the subject may have gotten lost when travelling to an unfamiliar location; (b) co-workers become aware of the subject’s relatively poor performance; (c) word and name finding deficit may become evident to intimates; (d) the subject may read a passage or book and retain relatively little material; (e) the subject may demonstrate decreased facility remembering names upon introduction to new people; (f) the subject may have lost or misplaced an object of value; (g) concentration deficit may be evident on clinical testing. Objective evidence of memory deficit is obtained only with an intensive interview. Decreased performance in demanding employment and social settings. Denial begins to become manifest in the subject. Mild to moderate anxiety frequently accompanies the symptoms” [22].
As with the other GDS stages, the intensive interview in this as well as in our other studies includes the BCRS, which assesses subjects in considerable detail on the parameters described earlier, as well as on related symptomatology.
Subjects with GDS stage ≥4 fulfill Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria for dementia of progressively increasing severity (maximum, stage 7) [26].
2.2.2. Population selection and procedures
During the enrollment period (January 1, 1984 to December 31, 1997), there was prescreening as well as continued screening eligibility assessment until subject entry at baseline. Specifically, initial subject contact with the research center was typically by telephone, where the subject would speak with a center coordinator who would make an initial assessment of the suitability of the subject for our longitudinal research study (prescreening). Subjects with, for example, major depression requiring treatment or uncontrolled acute medical illness would be referred elsewhere for appropriate treatment. Also, subjects with obvious exclusionary conditions, such as a history of stroke, psychiatric hospitalization for schizophreniform illness or major depression, or seizure disorder, would be informed of their ineligibility for our general longitudinally studied, research population. If the coordinator assessed the subject as potentially eligible for this research study population, then the subject would be sent a questionnaire regarding their medical history and medications taken. From the information provided in the questionnaire, eligibility for the general research population would be further assessed. Subjects who appeared to be eligible, ie, who appeared to fulfill the inclusion and exclusion criteria, would be scheduled for the evaluations. These evaluations included medical, neurologic, psychiatric, neuropsychological, and clinical laboratory evaluations. If evident exclusionary factors or other conditions requiring treatment were uncovered during these evaluations, these subjects might be appropriately referred for treatment elsewhere and excluded from the general research, longitudinally studied population during the course of these evaluations. Subjects who successfully completed all of the evaluation procedures were entered into the research database. These subjects were further evaluated for eligibility for the general, longitudinally studied research population in rounds attended by the study physicians and other study personnel. Eligible subjects were then recommended for entry into the longitudinally studied research population. The principal investigator (B. R.) made final decisions, when necessary, regarding eligibility of the subjects for entry into the general, grant supported, longitudinally studied research population. Once entered, every effort was made to maintain and follow up study subjects at the specified intervals. Follow-ups were conducted and data were collected as completely as was possible, irrespective of the medical or other conditions that occurred in the subject until and including demise, at which point efforts were made for neuropathologic evaluations from brain donations.
Although subjects with cognitive complaints contacted the research center for participation quite frequently, persons without complaints were considerably more difficult to recruit for the study population. Therefore, efforts were made to reach out to spouses of the general aging and dementia research population and other volunteers to recruit subjects who were free of complaints or evidence of cognitive impairment who were eligible for this longitudinally studied, grant-supported research population.
A total of 340 subjects with NCI or SCI completed the initial, pre-rounds screening and were entered into the database during the period from January 1, 1984 until December 31, 1997. This resulted in 260 (60 NCI and 200 SCI) cognitively normal, apparently healthy subjects who fulfilled the inclusion and exclusion criteria and who were therefore entered into the baseline study population and scheduled for follow-ups. Subjects were followed up at approximately 2-year intervals from January 1, 1984 until December 31, 2001 with more comprehensive evaluations (eg, by using an additional brain magnetic resonance imaging or computed tomography scan evaluation), repeated every fourth year. All follow-up evaluations were performed without reference to prior evaluation results. Once entered, subjects were followed up for as long a period as possible until demise, although the results were censored at the cutoff date of December 31, 2001 for the purposes of this particular reported study.
2.2.3. Follow-up and outcome groups
The primary outcome variable was defined dichotomously as cognitive stability or decline. Outcome was stable if, at the time of the final follow-up evaluation, the subject had been observed to have remained NCI or SCI at every follow-up period. Subjects were categorized as having declined if a diagnosis of MCI or dementia was noted at a periodic follow-up observation. The first follow-up period with decline was used for the analyses. Time to decline was (1) time to progression to MCI or dementia or, if no progression, (2) time to the last follow-up before 2002.
2.2.4. Cognitive-behavioral and neuropsychological assessments
Mental status assessment. Mini-Mental State Examination (MMSE) scores were obtained [27].
-
Clinical cognitive and cognitively based functioning examination was also performed by using the Brief Cognitive Rating Scale (BCRS) [25]. Axis 1 to 5 scores, reported herein, respectively examine the following: (1) concentration and calculation; (2) recent memory; (3) remote memory; (4) orientation; and (5) daily functioning, including executive tasks. Each BCRS axis is, per design, enumerated on a 7-point scale that is optimally concordant with the other BCRS axes and with the GDS stages in brain aging, MCI, and AD. This optimally concordant weighting of BCRS axes 1 to 5 has been previously investigated in a series of studies that have verified these properties [23]. For example, the BCRS clinical interview scores the presence of subjective complaints on each respective axis as a “2” and the absence of subjective complaints as a “1.” Therefore, by design and definition, the BCRS axes distinguish NCI from SCI subjects on the respective parameters measured. Specifically, to cite some examples, in BCRS axis 2, Recent Memory, a score of 1 indicates “no objective or subjective evidence of deficit in recent memory,” and a score of 2 indicates “subjective impairment only (e.g., forgetting names more than formerly).” For BCRS axis 5, Functioning, a score of 1 indicates “no difficulty, either subjectively or objectively,” and a score of 2 indicates “complaints of forgetting location of objects; subjective work difficulties.” A study that included 75 subjects at GDS stage 2 found that the mean score on the BCRS Recent Memory axis was also 2, and that the mean score on the BCRS Functioning axis was also 2. Hence, these measures are optimally weighted for assessment of subjective memory at the GDS stage 2 level. The same relationships between these BCRS axes and the GDS stages are found to proceed with the evolution of impairment in cross-sectional studies of MCI subjects and of subjects in the mild, moderate, moderately severe, and severe stages of probable AD. These studies were conducted in subjects “free of [other] medical, psychiatric, neurologic, or neuroradiologic conditions that might interfere with cognition” [23].
To cite another example, in BCRS axis 2, Recent Memory, a score of 3 indicates “deficit in recall of specific events evident upon detailed questioning, no deficit in the recall of major recent events.” For BCRS axis 5, Functioning, a score of 3 indicates “decreased job functioning evident to co-workers; difficulty in travelling to new locations.” In the study cited earlier that included 48 subjects at GDS stage 3 corresponding to MCI, the mean score on the BCRS Recent Memory axis was also 3, as was the mean score on the Functioning axis. Hence, per design, the BCRS axes are optimally concordant with the cross-sectionally assessed progression of brain aging and dementia pathology in MCI and probable AD. As would be expected for a measure constructed with the properties of the BCRS axes, the axes are also strongly intercorrelated with the progression of brain aging, MCI, and probable AD pathology. In a study of 50 subjects with a severity range from GDS stage 1 (NCI) to GDS stage 6 (moderately severe AD), Pearson intercorrelations of BCRS axes 1 to 5 ranged from 0.83 to 0.94 (all P ≤ .001) [25]. Pearson correlations of each of the 5 BCRS axes and the GDS scores were 0.9 (all P ≤ .001) [25].
Affective status was assessed with the Hamilton Depression Scale (HAM-D) [21]. Comprehensive behavioral changes were assessed by using the Behavioral Pathology in Alzheimer’s Disease (BEHAVE-AD) rating scale [28] (introduced in 1987). The BEHAVE-AD assesses 25 behavioral symptoms that occur in AD patients, each of which is rated in accordance with severity on a 4-point rating scale from not present (zero) to severe (3 points). Each severity rating is anchored to specific symptomatic descriptors. The symptoms are grouped into seven categories of behavioral disturbance: (A) paranoid and delusional ideation; (B) hallucinations; (C) activity disturbances; (D) aggressiveness; (E) diurnal rhythm disturbances; (F) affective disturbances; and (G) anxieties and phobias. The maximum possible disturbance score is 75.
Neuropsychometric evaluation. Memory: Assessed by using three subtests of the Guild Memory Scale [29], specifically (1) paragraph recall, initial and delayed; (2) paired associate recall of pairs of familiar words, initial and delayed; and (3) design recall of abstract shapes. Working memory: Evaluated with digit span subtests of the Wechsler Intelligence Scale Revised (WAIS-R), forward and backward [30]. Perceptual motor skill: Assessed with the WAIS-R digit symbol substitution subtest (DSST). Language function: Assessed with the WAIS-R vocabulary subtest.
Combined psychometric score: The Psychometric Deterioration Score (PDS) is derived from an equal weighting of the nine tests included in the test battery above [31]. The PDS is designed so that a higher score indicates greater impairment.
2.2.5. Statistical analyses
2.2.5.1. Comparison between NCI and SCI subject groups for decline
Differences in outcome between NCI and SCI groups were assessed with the Fisher exact test to compare proportions of subjects who declined. The Savage two-sample test for event time [32,33] was used to compare the mean decline time.
2.2.5.2. Prediction of degree of decline using survival analysis
Likelihood test indicated that the event time distribution is consistent with the Weibull distribution. Weibull proportional hazards model was used to determine the hazard rate of progressing by baseline group, NCI or SCI, controlling for demographic variables (specifically age, gender, and years of education) and follow-up time.
The following model was fitted to the data set: Λ(T) = Λ0 (T) exp(α1 *Group + α2 * Age + α3 *Gender + α4 *Education + α5 *Follow-up), where Λ(T) is the hazard function of progressing and Λ0(T) is the baseline hazard function. Furthermore, the additional contribution of other variables to decline hazard rate was examined.
2.2.5.3. Prediction of time of decline using the accelerated failure time model
Survival functions of the baseline groups, NCI and SCI, were compared by using the Kaplan-Meier method. A survival event was defined as an event in a subject who did not decline to MCI or dementia.
The accelerated failure time model was used to determine the time to decline by baseline group, controlling for demographic variables and follow-up time. The following accelerated failure time model was fitted to the data set: logT = β0 + β1 *Group + β2 * Age + β3 *Gender + β4 *Education + β5 *FollowUp + σε, where T is the event time (time to decline for decliners and time to follow-up for non-decliners who are thus censored) following the Weibull distribution, and ε is a random error.
The additional contribution of other variables to the decline time was also examined.
3. Results
The database consisted of 340 subjects aged ≥40 years with normative cognitive functioning (NCI or SCI) seen during the enrollment period. A total of 80 subjects were excluded because of comorbidities considered to be of possible relevance for cognitive functioning. A post hoc analysis did not reveal significant differences between the excluded subjects (n = 80) and the eligible subjects (n = 260) in terms of diagnostic grouping (NCI versus SCI), age, gender, education level, or MMSE scores.
Of 260 subjects comprising the study population at baseline, follow-up was completed in 213 subjects (81.9%; 47 NCI and 166 SCI) (Table 1). Subject groups followed and not followed did not differ in baseline categorization (NCI versus SCI), age, or MMSE scores. There were differences in gender (more men lost to follow-up, marginally significant, P = .05) and education (mean, 15.7 and 14.7 years for subjects followed up and not followed up, respectively; P = .02).
Table 1.
The study population: subjects entered, followed, and lost to follow-up*
| Total entered (n = 260) | Followed (n = 213) | Not followed (n = 47)† | P value (followed vs not followed) | |
|---|---|---|---|---|
| Baseline category | ||||
| NCI (%) | 60 (23.1) | 47 (22.1) | 13 (27.7) | .45‡ |
| SCI (%) | 200 (76.9) | 166 (77.9) | 34 (72.3) | |
| Age, mean (SD), y | 67.2 (9.1) | 66.8 (9.0) | 69.2 (9.7) | .22 |
| Gender | ||||
| Female (%) | 156 (60) | 134 (63) | 22 (47) | .05§ |
| Male (%) | 104 (40) | 79 (37) | 25 (53) | |
| Education, mean (SD), y | 15.5 (2.7) | 15.7 (2.6) | 14.7 (3.1) | .02¶ |
| Income, mean (SD)** | 36.4 (23.7), n = 207 | 35.3 (23.2), n = 175 | 42.7 (25.6), n = 32 | .11 |
| MMSE score, mean (SD) | 29.1 (1.2) | 29.1 (1.1) | 28.9 (1.3) | .57 |
Abbreviations: NCI, no cognitive impairment; SCI, subjective cognitive impairment; SD, standard deviation; y, years.
Values are expressed as means and SDs or as numbers (percentages).
The 47 subjects seen at baseline but lost to follow-up consisted of subjects who (1) refused further participation (n = 19); (2) moved from the area (n = 1); (3) could not be located (n = 10); (4) were dropped for other reasons (n = 8) (eg, uncooperative or noncomplaint with the research procedures [n = 6], inability to be properly evaluated because of loss of vision [n = 1]); or died (n = 9).
Value for proportion of NCI versus SCI subjects followed in comparison with those not followed.
Value for proportion of females versus males in subjects followed in comparison with those not followed.
P < .05.
In thousands of U.S. dollars at the time of the evaluation. Income was defined as the subject’s total combined family income (including wages, retirement income, and interest income) in the prior 12-month period. The number of subjects is somewhat lower than for other characteristics because many subjects preferred not to disclose this information.
3.1. Baseline characteristics of subjects followed up
There were significant baseline differences between NCI and SCI groups in age (mean, 64.1 and 67.5 years, respectively; P = .02) and MMSE scores (mean, 29.6 and 29.0, respectively; P < .001) for subjects who were followed up (Table 2). There were no significant differences in gender or education. The NCI from SCI subjects are distinguished as anticipated from the design of the BCRS axes, although it is worthy of note that the magnitude of subjective complaints of orientation deficits in SCI subjects is relatively low. Several behavioral variables showed significantly, or for two variables marginally significantly, higher (worse) scores in SCI subjects in comparison with the NCI subjects at baseline (Table 2). These included the total score on the HAM-D (marginally significant, P = .05) and individual scores for HAM-D item 5 (disturbed sleep) and HAM-D item 7 (feelings of incapacity, fatigue or weakness, or loss of interest in activities). The only BEHAVE-AD item that showed a significant difference (P = .009) in the SCI subjects in comparison with the NCI subject group at baseline was item 23 (anxieties), with SCI subjects having higher scores.
Table 2.
Baseline characteristics of followed subjects*
| Baseline category | NCI (n = 47) | SCI (n = 166) | P value |
|---|---|---|---|
| Age, mean (SD), y | 64.1 (8.9) | 67.5 (8.9) | .020† |
| Gender | |||
| Females, no. (%) | 26 (55) | 108 (65) | .230 |
| Males, no. (%) | 21 (45) | 58 (35) | |
| Education, y | 16.1 (2.4) | 15.6 (2.6) (n = 164) | .295 |
| MMSE | 29.6 (0.8) | 29.0 (1.2) | <.001‡ |
| BCRS, total score | 5.74 (0.9) | 8.97 (1.6) (n = 164) | <.001‡ |
| BCRS axis 1: concentration and calculation | 1.28 (0.6) | 2.23 (0.8) (n = 164) | <.001‡ |
| BCRS axis 2: recent memory | 1.23 (0.4) | 2.09 (0.4) (n = 164) | <.001‡ |
| BCRS axis 3: remote memory | 1.13 (0.3) | 1.65 (0.7) (n = 164) | <.001‡ |
| BCRS axis 4: orientation | 1.00 (0.0) | 1.23 (0.4) (n = 164) | <.001‡ |
| BCRS axis 5: daily functioning | 1.11 (0.3) | 1.77 (0.5) (n = 164) | <.001‡ |
| HAM-D total score | 3.13 (3.2) | 4.43 (4.2) | .050 |
| HAM-D item 5: disturbed nighttime sleep | 0.11 (0.3) | 0.36 (0.6) | .009§ |
| HAM-D item 7: fatigue, weakness, disinterest | 0.19 (0.5) | 0.52 (0.8) | .009§ |
| HAM-D item 8: slowness, decreased concentration | 0.26 (0.6) | 0.41 (0.6) | .133 |
| HAM-D item 11: anxiety symptoms | 0.11 (0.3) | 0.24 (0.6) | .143 |
| BEHAVE-AD total score | 0.69 (1.4) (n = 36) | 1.29 (1.7) (n = 130) | .059 |
| BEHAVE-AD item 23: miscellaneous anxieties | 0.24 (0.4) (n = 37) | 0.50 (0.5) (n = 131) | .009§ |
| PDS | 1.86 (0.9) (n = 42) | 2.18 (1.2) (n = 156) | .100 |
| Paragraphs initial recall | 8.07 (2.6) (n = 46) | 7.28 (3.0) | .109 |
| Paragraphs delayed recall | 9.96 (3.1) (n = 46) | 9.12 (3.6) | .152 |
| Paired associates initial recall | 5.04 (2.1) (n = 46) | 4.65 (2.3) | .300 |
| Paired associates delayed recall | 5.59 (2.5) (n = 46) | 5.01 (2.7) | .196 |
| Memory for designs | 6.20 (2.3) (n = 45) | 5.50 (2.2) | .061 |
| Digit span forwards | 6.93 (1.4) (n = 44) | 6.89 (1.2) (n = 159) | .858 |
| Digit span reverse | 5.59 (1.5) (n = 44) | 5.46 (1.4) (n = 159) | .591 |
| Digit symbol substitution test | 55.43 (11.0) (n = 46) | 51.43 (12.7) (n = 165) | .053 |
| WAIS vocabulary | 67.98 (9.8) (n = 46) | 66.38 (12.6) | .428 |
NCI, no cognitive impairment; SCI, subjective cognitive impairment; y, years; MMSE, Mini-Mental State Examination; BRCS, Brief Cognitive Rating Scale; HAM-D, Hamilton Depression Scale; BEHAVE-AD, Behavioral Pathology in Alzheimer’s Disease rating scale; PDS, Psychometric Deterioration Score; WAIS, Wechsler Adult Intelligence Scale.
Values are expressed as mean (standard deviation) unless otherwise indicated. For the HAM-D Scale and for the BEHAVE-AD, only the subscale items for which significant between-group differences were found at baseline, or in the case of HAM-D items 8 and 11 in contributing to the odds of subsequent decline (Table 4), are shown in the table. These subscale items assess the following mood and behavior-related variables: HAM-D item 5, disturbed sleep during the night; HAM-D item 7, thoughts and feelings of incapacity, fatigue or weakness relating to activities, or loss of interest in or less time spent in activities; HAM-D item 8, slowness of thought and speech, and/or impaired ability to concentrate, and/or decreased motor activity; HAM-D item 11, physiologic concomitants of anxiety such as gastrointestinal symptoms, dry mouth, cardiac palpitations, sweating, and others; and BEHAVE-AD item 23, miscellaneous anxieties, such as regarding money, the future, health, and memory.
P < .05
P < .001
P < .01, P values for the differences between NCI and SCI groups.
Psychometric performance was lower on all tasks in the SCI group at baseline; however, only the DSST difference was marginally significant (P = .05).
3.2. Follow-up findings
Follow-up time from baseline to the final evaluation did not differ significantly between NCI (6.7 ± 3.1 years) and SCI subjects (6.8 ± 3.4 years) (Table 3). Both groups had a mean of 3 follow-up visits (2.9 ± 1.6 for the total subject population followed up) after baseline. Decline was observed in 97 of the 213 subjects followed up (45.5%). More SCI subjects (90 of 166, 54.2%) declined as compared with NCI subjects (7 of 47, 14.9%) (P < .0001, Fisher exact test). Mean time to observation of decline for NCI subjects (8.8 years) was longer than for SCI subjects (5.3 years) (Savage two-sample test for event time, P = .0003).
Table 3.
Follow-up characteristics of subjects*
| Baseline category | Total followed up (n = 213) | NCI (n = 47) | SCI (n = 166) | P value | |||
|---|---|---|---|---|---|---|---|
| Total time of follow-up, y | 6.8 ± 3.4 | 6.7 ± 3.1 | 6.8 ± 3.4 | .80† | |||
| Average no. of follow-up visits | 2.92 ± 1.6 | 2.81 ± 1.5 | 2.95 ± 1.6 | .60† | |||
| Stable (n = 116) | Decline (n = 97) | Stable (n = 40) | Decline (n = 7) | Stable (n = 76) | Decline (n = 90) | ||
| Time to decline, y | NA | 5.5 ± 3.0 | NA | 8.8 ± 4.5 | NA | 5.3 ± 2.7 | .0003‡§ |
| Total time of follow-up, y | 5.8 ± 2.9 | 7.9 ± 3.6 | 6.0 ± 2.4 | 10.7 ± 3.6 | 5.8 ± 3.1 | 7.7 ± 3.5 | .72, .03¶** |
Abbreviations: NCI, no cognitive impairment; SCI, subjective cognitive impairment; y, years; NA, not applicable.
Values are means ± standard deviations.
P values for the differences between NCI and SCI groups.
Savage two-sample test for event time.
P < .001.
P values for the differences between NCI and SCI groups in total time of follow-up for stable subjects and decliners, respectively.
P < .05.
Of the seven NCI subjects who declined, five progressed to MCI at follow-up (71.4%), and two progressed to probable AD [34] (in one case, mild AD [GDS stage 4] and in the other case, moderately severe AD [GDS stage 6]). There were no significant medical comorbidities in any of these NCI subjects who progressed to MCI or AD.
Of the 90 SCI subjects who declined, 71 progressed to MCI (78.9%) and 19 to dementia. Of the 71 SCI subjects at baseline who progressed to MCI, there were no significant medical comorbidities in 65 of the subjects (91.5%). Of the remaining six subjects, one had developed hyperthyroidism, one developed notable depression symptomatology, another developed depression that was successfully treated with sertraline (25 mg daily), another had suffered a head injury that had resulted in loss of consciousness, one had coexisting notable vitamin B12 deficiency, and the sixth subject had a 1-cm aneurysm in the circle of Willis that was found on a magnetic resonance imaging scan of the head. Of the 19 SCI subjects at baseline who progressed to dementia, 12 progressed to mild dementia (GDS stage 4), four to moderate dementia (GDS stage 5), and three to moderately severe dementia (GDS stage 6). Of these 19 baseline SCI subjects who progressed to dementia, 12 progressed to probable AD (63.2%) that was mild in six (GDS stage 4), moderate in four (GDS stage 5), and moderately severe in two cases (GDS stage 6). Of the remaining seven SCI subjects who progressed to dementia diagnoses other than probable AD, dementia was associated with a cerebrovascular dementia diagnosis (mild, GDS stage 4) in two cases, with depression in one (mild, GDS stage 4), with hydrocephalus (mild, GDS stage 4) in one, with thyroid disease (mild, GDS stage 4) in one case, with endocrine disease more generally in one case, which included both adult-onset (type 2) diabetes mellitus and a partial thyroidectomy (mild, GDS stage 4). One subject had possible AD at follow-up. This subject with moderately severe dementia (GDS stage 6 at follow-up) had suffered head trauma, resulting in a skull fracture at the time of the final follow-up evaluation.
In survival analysis with the Weibull proportional hazards model that controlled for baseline demographic variables and follow-up time and examined the hazard rates of progressing, it was found that the hazard ratio of SCI subjects to NCI subjects was 4.5 (95% confidence limits, 1.9 to 10.3).
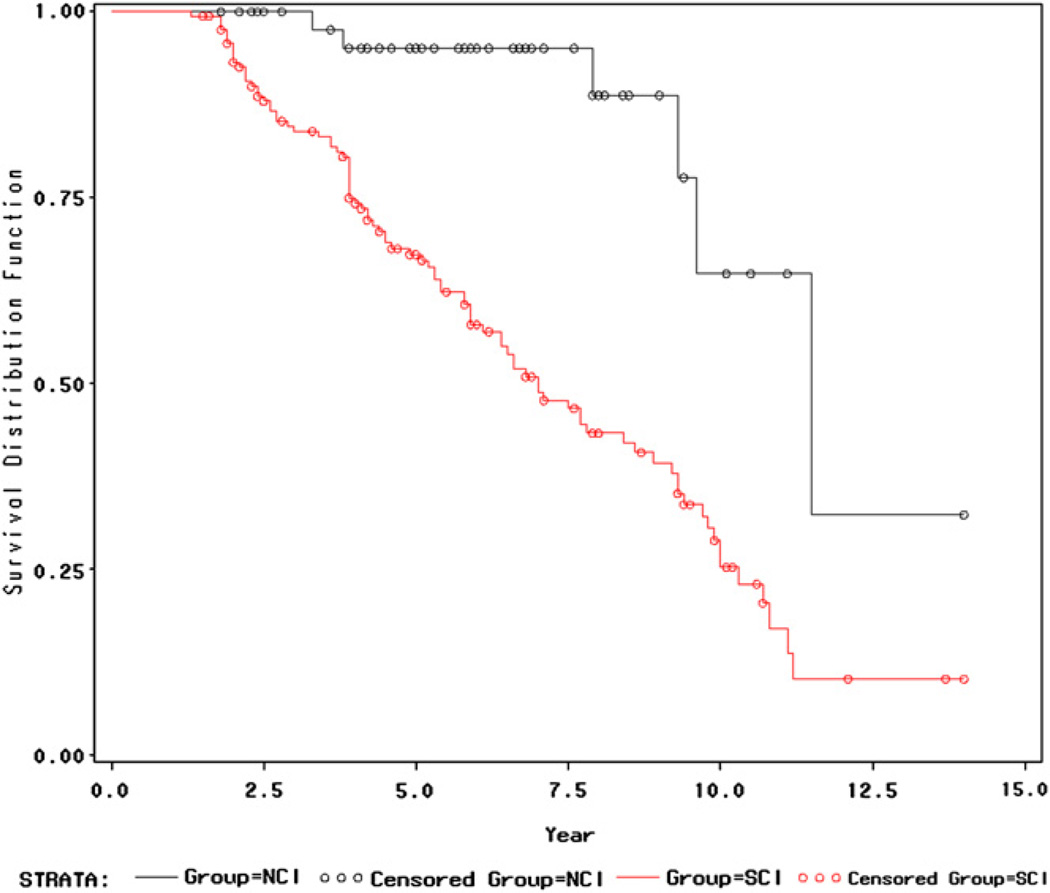
Survival analysis with the Kaplan-Meier method indicated a significant difference between baseline NCI and SCI groups in the absence over time of decline to MCI or dementia (P < .0001 for both the Wilcoxon test and the Log-Rank test) (Fig. 1).
Fig. 1.
Plot of survival distribution functions for the NCI and SCI baseline groups. The y-axis is the probability of not declining to MCI or dementia. The x-axis is the time (in years) to decline. This survival curve plot extends until observation year 14. There were no SCI subject observations beyond year 14. One NCI subject was followed up beyond year 14. This subject was observed to decline at year 16. There was a significant difference between the NCI and the SCI baseline groups in the survivor function of absence of decline to MCI or dementia in favor of the NCI baseline group (P < .0001, Wilcoxon test and Log-Rank test).
Survival analysis was also performed with an accelerated failure time model that controlled for demographic variables and follow-up time and examined the time to decline on the basis of baseline group. SCI subjects took on average a 60% shorter time to decline than NCI subjects (estimated ratio of the expected decline time, 0.60; 95% confidence limits, 0.45 to 0.80).
Accelerated failure time model analyses, with an underlying Weibull survival function, which controlled for the effect of baseline group as well as baseline demographic variables and follow-up time, found significant additional effects on the time to decline were contributed by virtually the same baseline variables contributing significantly to the hazards of decline (Table 4). Such resemblance is anticipated because of the equivalence between the Weibull proportional hazards model and the accelerated failure time model analyses with an underlying Weibull survival function [35]. The slight discrepancies between some P values are due to numeric methods and the fact that these model parameters are in general non-linear functions of each other. These variables contributing to time to decline are (1) the MMSE score (marginally significant, P = .05); (2) total score on the BCRS (P = .006); (3) BCRS Axis 1 (concentration and calculation; P = .004); (4) BCRS Axis 5 (functioning and self-care; P = .03); (5) two individual HAM-D items, item 8 (slowness of thought and speech and/or impaired concentration and/or decreased motor activity; P = .03) and item 11 (somatic anxiety; P = .007); (6) the composite psychometric variable, the PDS (P < .0001); and (7) seven of the nine individual psychometric variables.
Table 4.
Variables contributing to the hazard ratio and time to decline after controlling for baseline group membership (NCI vs SCI), demographic variables,* and follow-up time
| Hazard ratio for additional variable† | P value† | Percent change in decline time‡ | P value‡ | |
|---|---|---|---|---|
| MMSE | 0.9 (0.7 to 1.0) | .058 | 5.1 (−0.1 to 10.5) | .054 |
| BCRS total score | 1.2 (1.0 to 1.3) | .011§ | −5.8 (−10.4 to −2.0) | .006¶ |
| BCRS axis 1: concentration and calculation | 15 (1.1 to 2.0) | .004¶ | −13.1 (−21.3 to −4.9) | .004¶ |
| BCRS axis 2: recent memory | 1.2 (0.8 to 1.9) | .453 | −7.7 (−22.1 to 8.3) | .335 |
| BCRS axis 3: remote memory | 1.1 (0.8 to 1.5) | .611 | −3.0 (−13.9 to 9.4) | .603 |
| BCRS axis 4: orientation | 1.3 (0.8 to 2.2) | .258 | −11.3 (−25.9 to 6.2) | .181 |
| BCRS axis 5: daily functioning | 1.5 (1.0 to 2.4) | .065 | −17.3 (−29.5 to −2.0) | .030§ |
| HAM-D total score** | 1.0 (1.0 to 1.1) | .263 | −1.0 (−3.0 to 1.0) | .266 |
| HAM-D item 8: slowness, decreased concentration | 1.4 (1.0 to 2.0) | .047§ | −12.2 (−22.1 to −9.5) | .031§ |
| HAM-D item 11: anxiety symptoms | 1.6 (1.2 to 2.2) | .004¶ | −14.8 (−23.7 to −3.9) | .007¶ |
| BEHAVE-AD total score††> | 1.0 (0.9 to 1.2) | .747 | −1.0 (−5.8 to 4.1) | .666 |
| BEHAVE-AD item 23: miscellaneous anxieties | 1.3 (0.8 to 2.0) | .230 | −10.4 (−22.9 to 5.1) | .178 |
| PDS‡‡ | 1.7(1.3 to 2.1) | .000§§ | −17.3 (−23.7 to −10.4) | .000§§ |
| Paragraphs delayed recall | 0.9 (0.9 to 1.0) | .028§ | 3.0 (0.2 to 5.1) | .035§ |
| Paired associates initial recall | 0.9 (0.8 to 1.0) | .006¶ | 5.1 (1.0 to 8.3) | .005¶ |
| Paired associates delayed recall | 0.9 (0.9 to 1.0) | .005¶ | 5.1 (1.0 to 8.3) | .004¶ |
| Memory for designs | 0.8 (0.8 to 0.9) | .001¶ | 6.2 (3.0 to 10.5) | .001¶ |
| Digit span reverse | 0.8 (0.7 to 1.0) | .031§ | 7.3 (2.0 to 12.7) | .012§ |
| Digit symbol substitution test | 0.99 (0.97 to 1.00) | .080 | 0.6 (0.0 to 1.1) | .047§ |
| WAIS vocabulary | 0.97 (0.96 to 0.99) | .0003§§ | 0.9 (0.4 to 1.3) | .001¶ |
NOTE. See Table 2 for variable mean scores and standard deviations.
Abbreviations: NCI, no cognitive impairment; SCI, subjective cognitive impairment; MMSE, Mini-Mental State Examination; BRCS; Brief Cognitive Rating Scale; HAM-D, Hamilton Depression Scale; BEHAVE-AD, Behavioral Pathology in Alzheimer’s Disease rating scale; PDS, Psychometric Deterioration Score; WAIS, Wechsler Adult Intelligence Scale.
Age, gender, and educational level.
Estimated hazard ratios for decline and the corresponding 95% confidence intervals and P values for each variable are obtained through survival analyses controlling for baseline group, demographic variables, and follow-up time.
Percent change in and the corresponding 95% confidence interval for the expected survival time for each unit increase in the variable. Thus for the MMSE (marginally significant), each additional point is associated with a 5.1% increase in expected time to decline. For BCRS total scores, for example, each additional point is associated with a 5.8% decrease in expected time to decline.
P < .05.
P < .01.
Two constituent HAM-D item scores are significant, items 8 and 11. For definitions of these items, see the footnote in Table 2. Other constituent HAM-D items are not shown.
One individual item score on behavioral pathology in BEHAVE-AD is significant, item 23 (see Table 2 footnote for definition). Other constituent BEHAVE-AD items are not shown.
Individual tests from the PDS that are not shown (paragraphs initial recall and digit span forward) are not found to be significant. Note that the PDS and its individual tests are of the opposite signs, and thus their contributions to the percent change in decline time are in the opposite directions as well.
P < .001.
3.3. Variables associated with decline within the diagnostic categories
Few NCI subjects (n = 7) manifested decline at follow-up. Nevertheless, it is instructive to examine the variables associated with decline in the NCI and SCI subject groups separately (Table 5). For both diagnostic categorizations, age was significantly associated with decline (P < .01). Fewer years of education were associated with decline in the SCI subjects (P < .01). The only neuropsychometric measure associated with decline in the NCI subjects was the DSST (P = .02). In contrast, poorer performance in baseline MMSE scores, BCRS total scores, BCRS Axis 1 and 5 scores, PDS scores, and six of nine psychometric test scores in the SCI subjects were associated strongly (P < .01) with subsequent decline. One mood variable, HAM-D item 12, gastrointestinal symptoms (eg, appetite loss), was associated with subsequent decline in the NCI subjects (P = .02). Also, one mood symptom, HAM-D item 10, psychological anxiety, was associated with subsequent decline in the SCI subjects (P = .02).
Table 5.
Baseline characteristics of stable versus decline outcome groups within the NCI and SCI baseline categories
| Baseline category |
NCI (n = 47) |
SCI (n = 166) |
||||
|---|---|---|---|---|---|---|
| Outcome | Stable (n = 40) | Decline (n = 7) | P value* | Stable (n = 76) | Decline (n = 90) | P value† |
| Age, mean, y | 62.54 (8.39) | 73.04 (5.94) | .007‡ | 65.28 (8.93) | 69.43 (8.39) | .002‡ |
| Education, y | 16.00 (2.36) | 16.57 (2.51) | .561 | 16.21 (1.96) | 15.08 (2.99) | .005‡ |
| MMSE | 29.63 (0.87) | 29.71 (0.49) | .793 | 29.29 (1.09) | 28.71 (1.20) | .002‡ |
| BCRS, total score§ | 5.68 (0.89) | 6.14 (0.90) | .204 | 8.54 (1.70) | 9.32 (1.50) | .002‡ |
| BCRS axis 1: concentration and calculation | 1.27 (0.60) | 1.29 (0.76) | .967 | 2.01 (0.82) | 2.41 (0.72) | .001‡ |
| BCRS axis 5: daily functioning | 1.08 (0.27) | 1.29 (0.49) | .099 | 1.65 (0.48) | 1.88 (0.47) | .002‡ |
| HAM-D, total score§ | 3.35 (3.36) | 1.86 (2.12) | .264 | 4.07 (4.28) | 4.74 (4.14) | .302 |
| HAM-D item 10: tension, worry | 0.53 (0.78) | 0.00 | .086 | 0.43 (0.74) | 0.76 (0.93) | .016¶ |
| HAM-D item 12: decreased appetite | 0.00 | 0.14 (0.38) | .015¶ | 0.01 (0.12) | 0.02 (0.15) | .665 |
| BEHAVE-AD total score§ | 0.76 (1.48) (n = 33) | 0.00 (n = 3) | .338 | 1.16 (1.72) (n = 58) | 1.39 (1.68) (n = 74) | .438 |
| PDS§ | 1.80 (1.00) (n = 35) | 2.18 (0.54) | .331 | 1.75 (0.96) (n = 72) | 2.56 (1.20) (n = 84) | .000** |
| Paired associates initial recall | 5.21 (2.20) (n = 39) | 4.14 (1.35) | .226 | 5.22 (2.31) | 4.17 (2.22) | .003‡ |
| Paired associates delayed recall | 5.85 (2.56) (n = 39) | 4.14 (1.46) | .096 | 5.66 (2.47) | 4.47 (2.78) | .004‡ |
| Digit span forwards | 6.86 (1.57) (n = 37) | 7.29 (0.95) | .484 | 7.26 (1.14) (n = 73) | 6.58 (1.21) (n = 86) | .000** |
| Digit span reverse | 5.54 (1.57) (n = 37) | 5.86 (1.10) | .614 | 5.86 (1.31) (n = 73) | 5.12 (1.43) (n = 86) | .001‡ |
| Digit symbol substitution test | 57.00 (11.0) (n = 39) | 46.71 (7.0) | .021¶ | 54.55 (13.6) | 48.76 (11.2) | .003‡ |
| WAIS vocabulary | 67.39 (10.0) (n = 39) | 71.29 (7.93) | .335 | 70.49 (6.71) | 62.91 (15.2) | .000** |
NOTE. Values are expressed as means and standard deviations (SDs).
Abbreviations: NCI, no cognitive impairment; SCI, subjective cognitive impairment; y, years; MMSE, Mini-Mental State Examination; BCRS, Brief Cognitive Rating Scale; HAM-D, Hamilton Depression Scale; BEHAVE-AD, Behavioral Pathology in Alzheimer’s Disease rating scale; PDS, Psychometric Deterioration Score; WAIS, Wechsler Adult Intelligence Scale.
For the NCI baseline group, P values for the outcome group differences were obtained with the Wilcoxon Rank Sum Test.
For the SCI baseline group, P values for the outcome group differences were obtained from independent samples t tests.
P < .01.
For the BCRS, HAM-D, the BEHAVE-AD scale, and the individual constituent tests of the PDS, only individual items or tests that are found to be significant in either the NCI or the SCI subject groups are shown. HAM-D item 10 assesses psychic anxiety including subjective tension and irritability and worrying about minor matters. HAM-D item 12 assesses loss of appetite.
P < .05.
P < .001.
4. Discussion
These results indicate that otherwise healthy community-residing older persons presenting to an outpatient cognitive-dementia research center with SCI are much more likely to manifest subsequent decline than similarly aged persons presenting without these complaints. With a mean 7-year observation window, we found that SCI subjects had 4.5 times the risk of progressing to an MCI or dementia diagnosis than persons free of these symptoms, after controlling for age and other relevant factors. It was also observed that a majority, 54% in this sample, of persons with SCI manifested decline to MCI or dementia during the 7-year mean observation window. This decline in the SCI subjects was in marked contrast to a 15% occurrence of decline in NCI subjects, who were also 3.4 years younger at baseline but took an average 3.5 years longer to decline when decline occurred.
Persons with SCI come to clinicians with the belief that they have a problem. They fear the development of AD. Does SCI inexorably progress to MCI and ultimately AD, or is it inherently less stable, varying with as yet undetermined factors (microvascular disease and/or mood, for example)? Data from the present study and other studies such as those that have been referenced herein are becoming available that address these important questions.
Persons presenting with SCI symptoms had a mean MMSE score of 29.0, an eminently normal score. Nevertheless, this was significantly lower than the NCI cohort, with a mean MMSE of 29.6. We hypothesize that this difference is not only significant but also “real” in that the SCI persons are experiencing cognitive losses that, while evident in aggregate, are well within the normal range for individuals. This normal cognition functioning in SCI persons is exemplified by the normal MMSE scores.
Support for this hypothesis from the perspective of a dementia outcome comes from the study of St John and Montgomery [13]. This study found an association between subjective memory loss (SML) and subsequent dementia. However, after adjustment for the baseline cognitive status score, the association between SML and subsequent dementia was not significant. The authors of this study concluded that “the most likely explanation of these findings is that those with SML [subjective memory loss] are experiencing early pre-clinical cognitive loss, which is also apparent as a lower score.” These authors continue with the statement that “in essence, those with early pre-clinical cognitive loss are accurately reporting these early changes, which only becomes overt dementia at a later time” [13].
Observations of future decliners within the SCI subject group from the present study are also consistent with the hypothesis that lower cognitive test scores, even if these scores are within the normal range, are predictive of future decline. Future SCI decliners within the SCI subject group were found to have significantly lower scores than stable SCI subjects at baseline, a mean of 5 years before the observation of decline, on the MMSE and on six of the nine psychometric measures studied, as well as on the composite psychometric score. Consonant with these data is our finding that SCI persons with specific complaints or evidence of concentration and calculation deficit and functional deficit (BCRS Axes 1 and 5) are significantly more likely to manifest subsequent decline (Table 5).
Neuropathologic data have been collected from a series of 2,369 staged brains from nonselected autopsy cases. The subjects were aged 25 to 95 years, and the series included both nondemented and demented cases. The data from this study indicate that a majority of cases coming for autopsy aged between 60 and 65 years manifest Alzheimer’s type neurofibrillary changes, and 20% or more show these changes in the fourth decade of life [36]. The origins of eventually clinically manifest AD might be at very early periods of human development [37].
Hence, current observations and theories of AD origins fit well with the hypothesis that AD might become manifest many years before overt symptoms of dementia or even MCI are observed. In addition, studies in the present research population have indicated physiologic changes in SCI versus age-matched NCI subjects in terms of increased urinary cortisol levels and decreased brain metabolism [38,39]. The latter changes seem to be on a continuum with those of AD.
Most decliners in the present study declined to an MCI diagnosis. A previous study of MCI subject outcomes in our population of subjects fulfilling the rigorous inclusion and exclusion criteria described herein indicated that during a 4-year period, two thirds of MCI cases declined to a dementia diagnosis [18]. In >75% of these baseline MCI subjects, decline occurred to probable AD [18,34]. In terms of other or associated conditions in this prior study, of 74 subjects who declined to MCI, 18 subjects (24.3%) declined to dementia mixed with notable other conditions, specifically significant affective symptomatology (n = 2), dementia with evidence of stroke (n = 7), hydrocephalus (n = 1), thyroid dysfunction (n = 2), B12 or folate deficiency (n = 1), or other or undetermined types of dementia at follow-up (n = 5) [18]. Even in these 18 subjects who developed dementia associated with additional conditions, it is likely that AD remained the major pathogenic event in most subjects. Therefore, there is evidence that decline to MCI in the present study might be associated with decline to the eventual dementia of AD.
As noted in a recent consensus statement, “the term mild cognitive impairment was first used in association with stage 3 of the GDS” [7]. The original GDS publication in 1982 referred to GDS stage 3 as a stage of “mild cognitive decline” [22]. Subsequently, in 1988 in association with the publication of cross-sectional data showing significant decrements in numerous psychometric tests, as well as the MMSE, and other standard “dementia” tests, in GDS stage 3 subjects, in comparison with GDS stage 2 subjects, we first referred to GDS stage 3 as mild cognitive impairment (MCI) [31]. In association with our first publication of longitudinal data demonstrating decline to dementia in significant proportions of MCI subjects when followed up over years, we reinforced the terminology “mild cognitive impairment” and its associated implications for the medical and scientific community in the title and content of our 1991 publication [16]. In publications from 1989 until the present, MCI has been extensively characterized using the GDS stage 3 definition [40]. For example, between 1989 and 1999, the nature of behavioral disturbances, neurologic reflex and release sign changes, motor changes, equilibrium and coordination changes, changes in hippocampal atrophy, and electrophysiologic characteristics of MCI subjects in comparison with ostensibly cognitively normal (GDS stage 1 or 2) and/or mild dementia (GDS stage 4) subjects were all extensively described in the published literature (see reference [40] for citations).
In 1999, the nascent publications on MCI of Petersen et al [41] appeared, and in 2001, Petersen et al [6] proposed new criteria for the definition of MCI. These new criteria were the following: (1) memory complaint, preferably corroborated by an informant; (2) objective memory impairment; (3) normal general cognitive function; (4) intact activities of daily living; and (5) not demented. It was and is clear that the clinical entities described by the GDS stage 3 definition of MCI and by the definition of MCI by Petersen et al in 2001 [6] are essentially the same. For example, outcome studies that use the GDS 3 MCI definition and subsequently, the MCI definition by Petersen et al [6], have found nearly identical annual conversion rates to dementia, with very similar research clinical populations [18,19].
However, as previously noted by us and others, we believe there are important differences between the GDS stage 3 MCI definition and the MCI definition by Petersen et al [6] (2001) [7,40]. For example, the GDS stage 3 definition notes executive level functional deficits in association with MCI, whereas the 2001 Petersen et al definition does not allude to such deficits, emphasizing only “intact activities of daily living.” Also, the GDS stage 3 MCI definition emphasizes generalized cognitive decrements, whereas the 2001 MCI criteria of Petersen et al emphasize “objective memory impairment.” Most importantly, in terms of the present investigation and associated findings, the GDS MCI definition does not require the presence of subjective memory complaints, whereas the MCI definition by Petersen et al (2001) does require “memory complaint.” As discussed in a recent consensus publication, “Many individuals with mild cognitive impairment deny they have the disorder and do not report symptoms, although they nevertheless show signs of cognitive impairment consistent with the disorder that are evident to clinicians and informants. The GDS 3 definition of mild cognitive impairment - unlike the preceding GDS 2 stage of subjective cognitive impairment - does not require memory complaints; only signs of the disorder are required for GDS stage 3 assignment” [7].
Our research, published a quarter century ago in 1985, indicates the problems with the subjective complaint criterion for MCI [5]. Subjective cognitive impairment is the necessary pathognomonic criterion for GDS 2 (SCI). When we query subjects and ask them “what kind of problems do you have with memory?,” subjects in GDS stage 2, on average, complain of a mild to moderate level of memory problems [5]. In MCI (GDS stage 3), in aggregate, subjects complain of a moderately severe level of memory problems, although some subjects deny these problems. Subsequently, in mild dementia (GDS stage 4) subject complaints with respect to the severity of their memory problems decreases slightly, and subsequently with the progression of the severity of dementia, the magnitude of subject-reported complaints continues to decrease, returning to the GDS stage 2 level in moderately severe (GDS stage 6) dementia patients. We have concluded from these and related observations that subjective complaints are probably not a desirable criterion for MCI, where manifest, albeit subtle, clinical symptomatology occurs.
In 2004 the International Working Group (IWG) on Mild Cognitive Impairment issued a statement with recommended criteria for MCI that appears to have resolved many of the ostensible disparities between the GDS stage 3 MCI definition and the MCI definition by Petersen et al (2001) [6] by moving much closer to the GDS stage 3 definition in all areas of disparity [42]. Specifically, the IWG criteria do not require self-report of decline for MCI, and hence they circumvent the issue of denial. Rather, the IWG criteria suggest that MCI subjects have “self and/or informant report and impairment on objective cognitive tasks; and/or evidence of decline over time on objective cognitive tasks.” Also, the IWG criteria recommend a clinical rather than a psychometric definition of MCI, analogous to the GDS/BCRS interview approach. Also, the IWG criteria suggest the following functional definition of MCI, “preserved basic activities of daily living/minimal impairment in complex instrumental functions,” which appears to be quite concordant with the executive level functional deficits emphasized in the GDS definition of MCI. Finally, like the GDS definition of MCI and in contrast with the 2001 Petersen et al criterion for MCI, the IWG report notes that “A wide range of cognitive functions appear to decline in persons who will be later diagnosed with AD...including memory, attention, language, visuospatial skill, perceptual speed and executive functioning.” This latter statement is consonant with the GDS 3 MCI definition, the BCRS interview used in association with this definition, and our prior research findings [23].
The requirement of subjective complaints for a diagnosis of SCI as embodied in the GDS stage 2 definition, and the absence of a requirement of subjective complaints for the definition of MCI as embodied in the GDS stage 3 definition of MCI, are of great importance and merit further discussion. Our research has shown that subjective complaints frequently increase in magnitude in subjects from GDS stage 2 (SCI) to GDS stage 3 (MCI) [5]. However, these subjective complaints do not increase from GDS stage 3 (MCI) to GDS stage 4 (mild AD), and the magnitude of subjective complaints actually decreases as the dementia of AD progresses. These observations explicate current research findings that subjective cognitive complaints in MCI subjects have no value in the prediction of subsequent impairment [43]. Clearly, these MCI results with respect to subjective complaints are in marked contrast to the SCI, GDS stage 2, results being reported in this article and predicted by our prior work and usage [5].
Future investigations should address generalizability of the present findings and other questions raised by this study. For example, this was a relatively well-educated subject population, typical of medical center cognitive research centers but not typical of the general population. Also, this study sample was selected for the absence of significant comorbidity of relevance for cognition. While recognizing these distinctive features of the subjects in the present investigation, it is also worth noting that subjective cognitive impairment associated with aging is presently being demonstrated to occur in substantial proportions of very diverse human populations. For example, in a rural, Chinese-speaking islet near mainland China, nearly half of residents aged ≥65 years in two surveyed townships were found to state that they had “trouble with [their] memory” [14]. In marked contrast to the present study population, the majority of these Chinese residents were illiterate. In another study conducted in remote river communities in the Amazon rainforest, 70% of residents surveyed aged ≥50 years (mean age, 62.3 years; 67% illiterate) were found to have subjective cognitive impairment [44].
Hence, complaints of cognitive impairment associated with aging do not appear to occur characteristically, exclusively, or even predominantly in more educated or industrial or “modern” societies or settings. Indeed, the highest prevalence of SCI yet to be reported appears to come from the study of Brucki and Nitrini [44] conducted in the remote, predominantly illiterate Amazon rainforest population. Perhaps these observations with respect to SCI are best understood in the context of the well-known education effect in dementia studies, an effect that indicates an increased risk for dementia in general and AD more particularly, in less educated persons [45–47]. This education effect has also been observed in longitudinal studies of the likelihood of subsequent decline to dementia in our own population. For example, in a 4-year prospective longitudinal study of decline to dementia in general or decline to AD, more particularly, conducted in baseline NCI, SCI, and MCI subjects (GDS stages 1, 2, or 3) from the present NIA grant-supported general research population, subjects who declined to dementia more generally, or AD more specifically, had mean lower education levels of 2.0 years and 2.2 years, respectively, than persons who did not decline (P < .001) [18]. In the present investigation, this education effect was also observed in the SCI subject group. Subjects with SCI who subsequently declined had significantly lower mean educational levels, a mean of >1 year less education, than stable subjects (Table 5).
We conclude that in parallel with continuing investigations clarifying the nature and prognosis of SCI, the present findings raise the exciting possibility of conducting preventative studies of MCI and eventual AD at a much earlier point than previously, approximately one to two decades before the appearance of overt dementia. It is possible that some medications might be most efficacious in the SCI stage of the evolution of the illness process.
For example, of the four currently approved and used AD medications in the United States, two medications, rivastigmine and galantamine, have been approved only for mild to moderate dementia in AD, and one medication, memantine, has been approved only for moderate to severe dementia in AD. The remaining medication, donepezil, is the only medication that has been demonstrated to be efficacious across the spectrum of AD dementia from mild to severe [48]. As we learn more about the origins of AD, it is possible that agents that reduce environmental exposure to toxins [49], antioxidants, beta or gamma secretase inhibitors, or other treatment approaches might prove to be most useful in slowing the progression of AD in the SCI stage. Information is accruing that will facilitate such studies. As Khachaturian et.al [50] have recently noted in their consensus report entitled “A roadmap for the prevention of dementia,” “additional targets that need to be explored particularly for the prodromal stages of AD, include … disrupted synaptic function … Other targets that should be explored for the relevance to AD … include those related to cerebrovascular disease, plasticity, and neurogenesis. Investigations of these and other targets could lead to more clarity in the question of how and when intervention should or could begin”. The value of forestalling the onset of eventual AD by any increment of time would be of incalculable benefit both in diminishing financial burden of societies throughout the world and, much more importantly, in relieving the human suffering that accompanies this prevalent condition of later life.
5. Glossary
5.1. The general grant-supported, research population; alternatively referred to as the “general research population” or the “general research, longitudinally studied population”
This comprised all subjects entered into the longitudinal follow-up study, irrespective of age range, stage, diagnosis at baseline (eg, no cognitive impairment [NCI], subjective cognitive impairment [SCI], mild cognitive impairment [MCI], or probable Alzheimer’s disease [AD]), or temporal range of entry into the study (the first subjects entered into the study had baseline evaluations in February 1978).
5.2. The study population
These are subjects fulfilling the selection criteria for the study reported in this publication. These selection criteria included (1) enrollment in the general research population from January 1, 1984 until December 31, 1997; (2) a diagnosis of no cognitive impairment (NCI) (synonymous with global deterioration scale [GDS] stage 1) or subjective cognitive impairment (SCI) (synonymous with GDS stage 2); (3) age at enrollment ≥40 years; and (4) fulfillment of all of the specific inclusion criteria and exclusion criteria, enumerated in the methods section (2.1) of the publication. It should be noted that many of the exclusion criteria also apply to the general research population.
5.3. Excluded subjects
Subjects screened for eligibility within the enrollment period (from January 1, 1984 until December 31, 1997), aged 40 or above, with normative cognitive functioning (NCI, GDS stage 1 or SCI, GDS stage 2), who were entered into the research database but found to be ineligible for the longitudinal grant and the present study because of the presence of medical conditions that might interfere with cognition or progress or recur as to interfere with cognition, apart from age and SCI.
5.4. Subjects not followed
These were subjects enrolled in the study population and who were part of the baseline study population, having fulfilled all eligibility criteria for the study, who had agreed and consented to longitudinal study follow-up, but who could not be followed. Reasons for loss to follow-up included the following: (1) refusal to return for follow-up; (2) the subject could not be located; (3) the subject died before they could be followed; (4) the subject was dropped from the study for non-compliance, the development of blindness, or other reasons; or (5) the subject had moved out of the follow-up area.
5.5. Subjects followed up
These are subjects in the study population who were successfully followed on at least one occasion within the study window, ie, before December 31, 2001. Most of the analyses reported in this publication were conducted on these successfully followed subjects.
Acknowledgments
Supported in part by United States Department of Health and Human Services (DHHS) grants P30 AG08051, AG03051, AG09127, and AG11505 from the National Institute on Aging and by grant MH43486 from the National Institute of Mental Health of the US National Institutes of Health, by grants 90AZ2791, 90AM2552, and 90AR2160 from the United States DHHS Administration on Aging, by a senior Clinical Research Fellowship provided by Forest Laboratories, Inc, by grant M01 RR00096 from the General Clinical Research Center Program of the National Center for Research Resources of the US National Institutes of Health, by the Fisher Center for Alzheimer’s Disease Research Foundation, and by grants from Mr Zachary Fisher and Mrs Elizabeth M. Fisher, Mr William Silberstein, and Mr Leonard Litwin. Additional support is acknowledged from Mr Michael Stern, the Estate of Rosalind Cherry, the Hagedorn Fund, the Harry and Jennie Slayton Foundation, the Sonya Samberg Family Trust, the Leonard Litwin Fund for Alzheimer’s Disease Research, and the Woodbourne Foundation. We acknowledge important contributions of Isabel Monteiro, MD, and Istvan Boksay, MD, PhD, to the collection of the behavioral data in this study. We also acknowledge Nauman Ashraf, MD, Iryna Lobach, PhD, and Imran A. Jamil, MD, for assistance with the preparation of the manuscript.
References
- 1.Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? a review of clinical and population-based studies. Int J Geriatr Psychiatry. 2000;15:983–991. doi: 10.1002/1099-1166(200011)15:11<983::aid-gps238>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Gagnon M, Dartigues JF, Mazaux JM, Dequae L, Letenneur L, Giroire JM, et al. Self-reported memory complaints and memory performance in elderly French community residents: results of the PA-QUID Research Program. Neuroepidemiology. 1994;13:145–154. doi: 10.1159/000110373. [DOI] [PubMed] [Google Scholar]
- 3.Tobiansky R, Blizard R, Livingston G, Mann A. The Gospel Oak Study stage IV: the clinical relevance of subjective memory impairment in older people. Psychol Med. 1995;25:779–786. doi: 10.1017/s0033291700035029. [DOI] [PubMed] [Google Scholar]
- 4.Blazer DG, Hays JC, Fillenbaum GG, Gold DT. Memory complaint as a predictor of cognitive decline: a comparison of African American and White elders. J Aging Health. 1997;9:171–184. doi: 10.1177/089826439700900202. [DOI] [PubMed] [Google Scholar]
- 5.Reisberg B, Gordon B, McCarthy M, Ferris SH, de Leon MJ. Insight and denial accompanying progressive cognitive decline in normal aging and Alzheimer’s disease. In: Stanley B, editor. Geriatric psychiatry: ethical and legal issues. Washington, DC: American Psychiatric Press; 1985. pp. 37–79. [Google Scholar]
- 6.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia—mild cognitive impairment (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 7.Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, et al. Mild cognitive impairment. Lancet. 2006;367:1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 8.Bolla KI, Lindgren KN, Bonaccorsy C, Bleecker ML. Memory complaints in older adults. Fact or fiction? Arch Neurol. 1991;48:61–64. doi: 10.1001/archneur.1991.00530130069022. [DOI] [PubMed] [Google Scholar]
- 9.O’Connor DW, Pollitt PA, Roth M, Brook PB, Reiss BB. Memory complaints and impairment in normal, depressed, and demented elderly persons identified in a community survey. Arch Gen Psychiatry. 1990;47:224–227. doi: 10.1001/archpsyc.1990.01810150024005. [DOI] [PubMed] [Google Scholar]
- 10.Feehan M, Knight RG, Partridge FM. Cognitive complaint and test performance in elderly patients suffering depression or dementia. Int J Geriatr Psychiatry. 1991;6:287–293. [Google Scholar]
- 11.Jorm AF, Christensen H, Korten AE, Jacomb PA, Henderson AS. Memory complaints as a precursor of memory impairment in older people: a longitudinal analysis over 7–8 years. Psychol Med. 2001;31:441–449. [PubMed] [Google Scholar]
- 12.van Oijen M, de Jong FJ, Hofman A, Koudstaal PJ, Breteler MMB. Subjective memory complaints, education, and risk of Alzheimer’s disease. Alzheimer Dement. 2007;3:92–97. doi: 10.1016/j.jalz.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 13.St John P, Montgomery P. Are cognitively intact seniors with subjective memory loss more likely to develop dementia? Int J Geriatr Psychiatry. 2002;17:814–820. doi: 10.1002/gps.559. [DOI] [PubMed] [Google Scholar]
- 14.Wang P-N, Wang S-J, Fuh J-L, Teng E-L, Liu C-Y, Lin C-H, et al. Subjective memory complaint in relation to cognitive performance and depression: a longitudinal study of a rural Chinese population. J Am Geriatr Soc. 2000;48:295–299. doi: 10.1111/j.1532-5415.2000.tb02649.x. [DOI] [PubMed] [Google Scholar]
- 15.Reisberg B. Dementia: a systematic approach to identifying reversible causes. Geriatrics. 1986;41(4):30–46. [PubMed] [Google Scholar]
- 16.Flicker C, Ferris SH, Reisberg B. Mild cognitive impairment in the elderly: predictors of dementia. Neurology. 1991;41:1006–1009. doi: 10.1212/wnl.41.7.1006. [DOI] [PubMed] [Google Scholar]
- 17.Bruscoli M, Lovestone S. Is MCI really just early dementia? a systematic review of conversion studies. Int Psychogeriatr. 2004;16:129–140. doi: 10.1017/s1041610204000092. [DOI] [PubMed] [Google Scholar]
- 18.Kluger A, Ferris SH, Golomb J, Mittelman MS, Reisberg B. Neuropsychological prediction of decline to dementia in nondemented elderly. J Geriatr Psychiatry Neurol. 1999;12:168–179. doi: 10.1177/089198879901200402. [DOI] [PubMed] [Google Scholar]
- 19.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 20.Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol. 1980;7:486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reisberg B, Ferris SH, de Leon MJ, Crook T. The global deterioration scale for assessment of primary degenerative dementia. Am J Psych. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 23.Reisberg B, Franssen E, Bobinski M, Auer S, Monteiro I, Boksay I, et al. Overview of methodologic issues for pharmacologic trials in mild, moderate, and severe Alzheimer’s disease. Int Psychogeriatr. 1996;8:159–193. doi: 10.1017/s1041610296002566. [DOI] [PubMed] [Google Scholar]
- 24.Reisberg B. Global measures: utility in defining and measuring treatment response in dementia. Int Psychogeriatr. 2007;19:421–456. doi: 10.1017/S1041610207005261. [DOI] [PubMed] [Google Scholar]
- 25.Reisberg B, Ferris SH. The Brief Cognitive Rating Scale (BCRS) Psychopharmacol Bull. 1988;24:629–636. [PubMed] [Google Scholar]
- 26.Reisberg B. Diagnostic criteria in dementia: a comparison of current criteria, research challenges and implications for DSM-V. J Geriatr Psychiatry Neurol. 2006;19:137–146. doi: 10.1177/0891988706291083. [DOI] [PubMed] [Google Scholar]
- 27.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 28.Reisberg B, Borenstein J, Salob SP, Ferris SH, Franssen E, Georgotas A. Behavioral symptoms in Alzheimer’s disease: phenomenology and treatment. J Clin Psychiatry. 1987;48(5 suppl):9–15. [PubMed] [Google Scholar]
- 29.Gilbert JG, Levee RF. Patterns of declining memory. J Gerontol. 1971;26:70–75. doi: 10.1093/geronj/26.1.70. [DOI] [PubMed] [Google Scholar]
- 30.Wechsler D. The measurement and appraisal of adult intelligence. 4th ed. Baltimore, MD: Williams & Wilkins; 1958. [Google Scholar]
- 31.Reisberg B, Ferris SH, de Leon MJ, Sinaiko E, Franssen E, Kluger A, et al. Stage-specific behavioral, cognitive, and in vivo changes in community residing subjects with age-associated memory impairment and primary degenerative dementia of the Alzheimer type. Drug Dev Res. 1988;15:101–114. [Google Scholar]
- 32.Savage IR. Contributions to the theory of rank order statistics - the two-sample case. Ann Math Stat. 1956;27:590–616. [Google Scholar]
- 33.Hsieh HK. Encyclopedia of statistical sciences. Vol 8. New York, NY: Wiley; 1988. Savage test; pp. 267–270. [Google Scholar]
- 34.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 35.Cox DR, Oakes D. Analysis of survival data. London, UK: Chapman and Hall; 1984. [Google Scholar]
- 36.Braak H, Braak E. Aspects of cortical destruction in Alzheimer’s disease. In: Hyman BT, Duyckaerts C, Christen Y, editors. Connections, cognition and Alzheimer’s disease. (Research and perspectives in Alzheimer’s disease) Berlin, Heidelberg: Springer-Verlag; 1997. pp. 1–16. [Google Scholar]
- 37.Bloss CS, Delis DC, Salmon DP, Bondi MW. Decreased cognition in children with risk factors for Alzheimer’s disease. Biol Psychiatry. 2008;64:904–906. doi: 10.1016/j.biopsych.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolf OT, Dziobek I, McHugh P, Sweat V, de Leon MJ, Javier E, et al. Subjective memory complaints in aging are associated with elevated cortisol levels. Neurobiol Aging. 2005;26:1357–1363. doi: 10.1016/j.neurobiolaging.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Mosconi L, De Santi S, Brys M, Tsui WH, Pirraglia E, Glodzik-Sobanska L, et al. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol Psychiatry. 2008;63:609–618. doi: 10.1016/j.biopsych.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reisberg B, Ferris SH, Kluger A, Franssen E, Weigel J, de Leon MJ. Mild cognitive impairment (MCI): a historical perspective. Int Psychogeriatr. 2008;20:18–31. doi: 10.1017/S1041610207006394. [DOI] [PubMed] [Google Scholar]
- 41.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 42.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment: beyond controversies, towards a consensus—report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 43.Purser JL, Fillenbaum GG, Wallace RB. Memory complaint is not necessary for diagnosis of mild cognitive impairment and does not predict 10-year trajectories of functional disability, word recall, or short portable mental status questionnaire limitations. J Am Geriatr Soc. 2006;54:335–338. doi: 10.1111/j.1532-5415.2005.00589.x. [DOI] [PubMed] [Google Scholar]
- 44.Brucki SMD, Nitrini R. Subjective memory impairment in a rural population with low education in the Amazon rainforest: an exploratory study. Int Psychogeriatr. 2009;21:164–171. doi: 10.1017/S1041610208008065. [DOI] [PubMed] [Google Scholar]
- 45.Katzman R. Education and the prevalence of dementia and Alzheimer’s disease. Neurology. 1993;43:13–20. doi: 10.1212/wnl.43.1_part_1.13. [DOI] [PubMed] [Google Scholar]
- 46.Mortimer JA, Graves AB. Education and other socioeconomic determinants of dementia and Alzheimer’s disease. Neurology. 1993;43:S39–S44. [Google Scholar]
- 47.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on incidence of Alzheimer’s disease. JAMA. 1994;271:1004–1010. [PubMed] [Google Scholar]
- 48.Thomson Healthcare Inc. Physicians’ desk reference. 62nd ed. Montvale, NJ: Thomson Healthcare Inc; 2008. pp. 2214–2218.pp. 2373–2378.pp. 1181–1185.pp. 1075–1079. [Google Scholar]
- 49.Wu J, Basha MR, Brock B, Cox DP, Cardozo-Pelaez F, McPherson CA, et al. Alzheimer’s disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb):evidence for a developmental origin and environmental link for AD. J Neurosci. 2008;28:3–9. doi: 10.1523/JNEUROSCI.4405-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khachaturian ZS, Petersen RC, Gauthier S, Buckholtz N, Corey-Bloom JP, Fillit H, et al. A roadmap for the prevention of dementia: the inaugural Leon Thal Symposium. Alzheimer Dement. 2008;4:156–163. doi: 10.1016/j.jalz.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]