Abstract
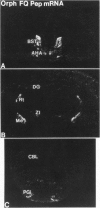
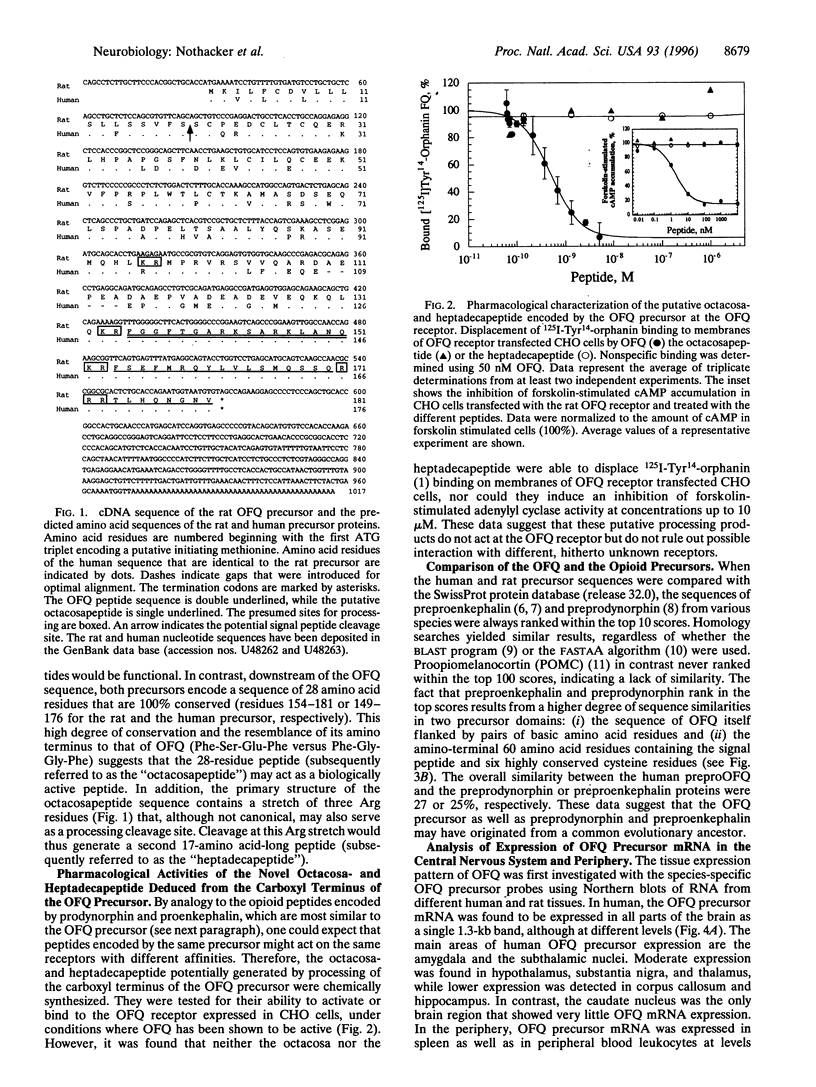
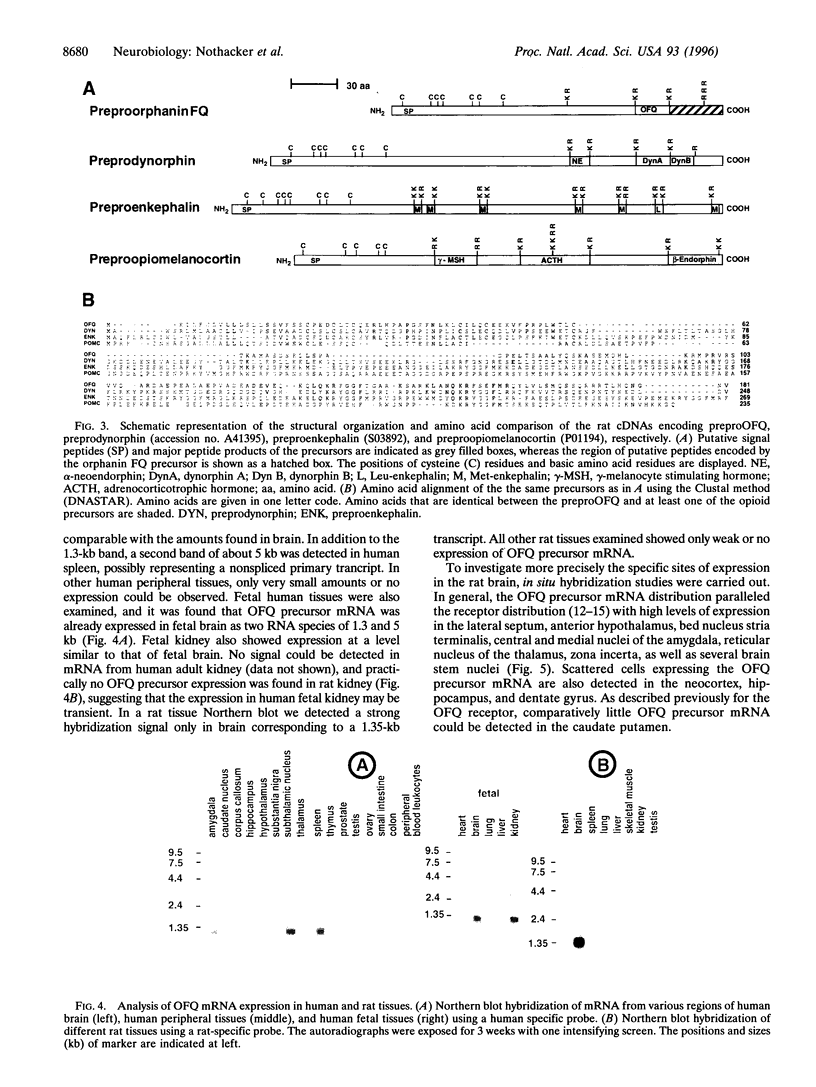
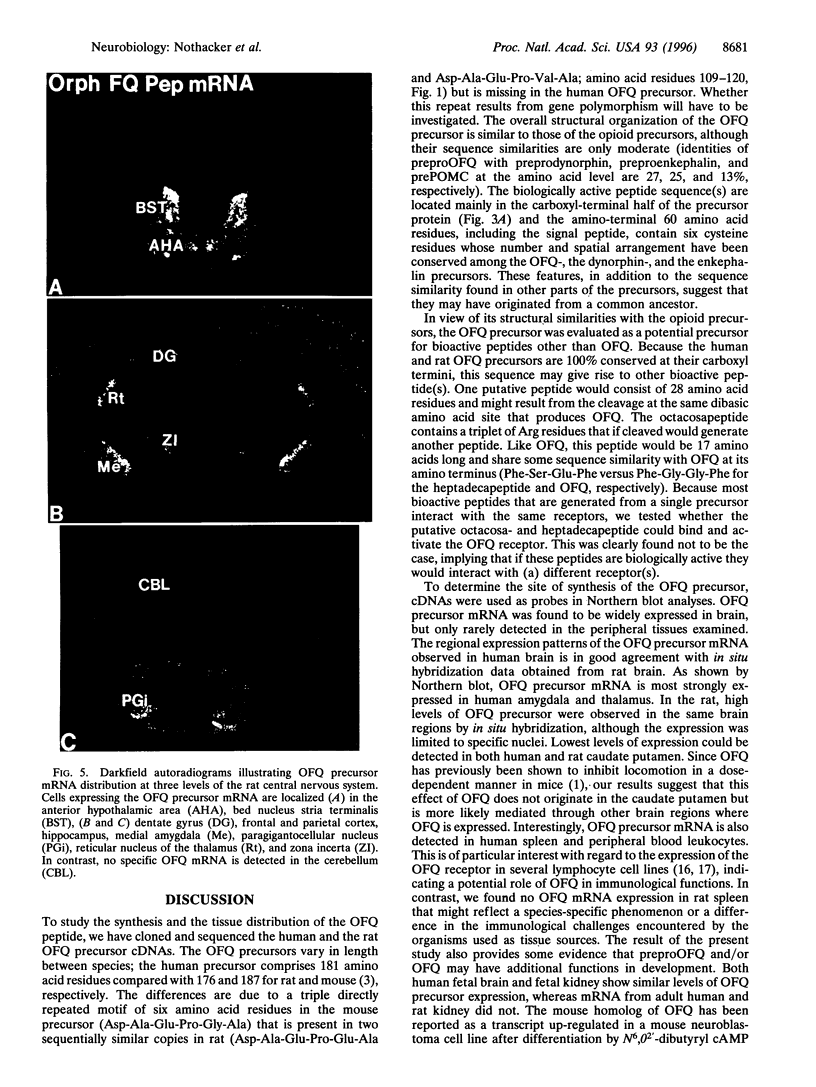
The heptadecapeptide orphanin FQ (OFQ) is a recently discovered neuropeptide that exhibits structural features reminiscent of the opioid peptides and that is an endogenous ligand to a G protein-coupled receptor sequentially related to the opioid receptors. We have cloned both the human and rat cDNAs encoding the OFQ precursor proteins, to investigate whether the sequence relationships existing between the opioid and OFQ systems are also found at the polypeptide precursor level, in particular whether the OFQ precursor would encode several bioactive peptides as do the opioid precursors, and to study the regional distribution of OFQ sites of synthesis. The entire precursor protein displays structural homology to the opioid peptide precursors, especially preprodynorphin and preproenkephalin. The predicted amino acid sequence of the OFQ precursor contains a putative signal peptide and one copy of the OFQ sequence flanked by pairs of basic amino acid residues. Carboxyl-terminal to the OFQ sequence, the human and rat precursors contain a stretch of 28 amino acids that is 100% conserved and thus may encode novel bioactive peptides. Two peptides derived from this stretch were synthesized but were found to be unable to activate the OFQ receptor, suggesting that if they are produced in vivo, these peptides would likely recognize receptors different from the OFQ receptor. To begin analyzing the sites of OFQ mRNA synthesis, Northern analysis of human and rat tissues were carried out and showed that the OFQ precursor mRNA is mainly expressed in the brain. In situ hybridization of rat brain slices demonstrated a regional distribution pattern of the OFQ precursor mRNA, which is distinct from that of the opioid peptide precursors. These data confirm that the OFQ system differs from the opioid system at the molecular level, although the OFQ and opioid precursors may have arisen from a common ancestral gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bunzow J. R., Saez C., Mortrud M., Bouvier C., Williams J. T., Low M., Grandy D. K. Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a mu, delta or kappa opioid receptor type. FEBS Lett. 1994 Jun 27;347(2-3):284–288. doi: 10.1016/0014-5793(94)00561-3. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Seeburg P., Hoffman B. J., Gage L. P., Udenfriend S. Molecular cloning establishes proenkephalin as precursor of enkephalin-containing peptides. Nature. 1982 Jan 21;295(5846):206–208. doi: 10.1038/295206a0. [DOI] [PubMed] [Google Scholar]
- Halford W. P., Gebhardt B. M., Carr D. J. Functional role and sequence analysis of a lymphocyte orphan opioid receptor. J Neuroimmunol. 1995 Jun;59(1-2):91–101. doi: 10.1016/0165-5728(95)00030-6. [DOI] [PubMed] [Google Scholar]
- Kakidani H., Furutani Y., Takahashi H., Noda M., Morimoto Y., Hirose T., Asai M., Inayama S., Nakanishi S., Numa S. Cloning and sequence analysis of cDNA for porcine beta-neo-endorphin/dynorphin precursor. Nature. 1982 Jul 15;298(5871):245–249. doi: 10.1038/298245a0. [DOI] [PubMed] [Google Scholar]
- Lachowicz J. E., Shen Y., Monsma F. J., Jr, Sibley D. R. Molecular cloning of a novel G protein-coupled receptor related to the opiate receptor family. J Neurochem. 1995 Jan;64(1):34–40. doi: 10.1046/j.1471-4159.1995.64010034.x. [DOI] [PubMed] [Google Scholar]
- Meunier J. C., Mollereau C., Toll L., Suaudeau C., Moisand C., Alvinerie P., Butour J. L., Guillemot J. C., Ferrara P., Monsarrat B. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995 Oct 12;377(6549):532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Mollereau C., Parmentier M., Mailleux P., Butour J. L., Moisand C., Chalon P., Caput D., Vassart G., Meunier J. C. ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett. 1994 Mar 14;341(1):33–38. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Inoue A., Kita T., Nakamura M., Chang A. C., Cohen S. N., Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979 Mar 29;278(5703):423–427. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Furutani Y., Takahashi H., Toyosato M., Hirose T., Inayama S., Nakanishi S., Numa S. Cloning and sequence analysis of cDNA for bovine adrenal preproenkephalin. Nature. 1982 Jan 21;295(5846):202–206. doi: 10.1038/295202a0. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinscheid R. K., Nothacker H. P., Bourson A., Ardati A., Henningsen R. A., Bunzow J. R., Grandy D. K., Langen H., Monsma F. J., Jr, Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995 Nov 3;270(5237):792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Saito Y., Maruyama K., Saido T. C., Kawashima S. N23K, a gene transiently up-regulated during neural differentiation, encodes a precursor protein for a newly identified neuropeptide nociceptin. Biochem Biophys Res Commun. 1995 Dec 14;217(2):539–545. doi: 10.1006/bbrc.1995.2809. [DOI] [PubMed] [Google Scholar]
- Wick M. J., Minnerath S. R., Lin X., Elde R., Law P. Y., Loh H. H. Isolation of a novel cDNA encoding a putative membrane receptor with high homology to the cloned mu, delta, and kappa opioid receptors. Brain Res Mol Brain Res. 1994 Nov;27(1):37–44. doi: 10.1016/0169-328x(94)90181-3. [DOI] [PubMed] [Google Scholar]
- Wick M. J., Minnerath S. R., Roy S., Ramakrishnan S., Loh H. H. Expression of alternate forms of brain opioid 'orphan' receptor mRNA in activated human peripheral blood lymphocytes and lymphocytic cell lines. Brain Res Mol Brain Res. 1995 Sep;32(2):342–347. doi: 10.1016/0169-328x(95)00096-b. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]