Abstract
Titanium dioxide (TiO2) nanoparticles (NPs) are manufactured worldwide in large quantities for use in a wide range of applications including pigment and cosmetic manufacturing. Although TiO2 is chemically inert, TiO2 NPs can cause negative health effects, like respiratory tract cancer in rats. However, the mechanisms involved in TiO2-induced genotoxicity and carcinogenicity have not been clearly defined and are poorly studied in vivo. The present study investigates TiO2 NP-induced genotoxicity, oxidative DNA damage and inflammation in a mice model. We treated wild type mice with TiO2 NPs in drinking water and determined the extent of DNA damage using the comet assay, the micronuclei assay, the γ-H2AX immuno-staining assay and by measuring 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels and, as genetic instability end point, DNA deletions. We also determined mRNA levels of inflammatory cytokines in the peripheral blood. Our results show that TiO2 NPs induced 8-OHdG, γ-H2AX foci, micronuclei and DNA deletions. The formation of γ-H2AX foci, indicative of DNA double strand breaks, was the most sensitive parameter. Inflammation was also present as characterized by a moderate inflammatory response. Together these results describe the first comprehensive study of TiO2 NP induced genotoxicity in vivo in mice, possibly caused by a secondary genotoxic mechanism associated with inflammation and/or oxidative stress. Given the growing use of TiO2 NPs, these findings raise concern about potential health hazards associated with TiO2 NP exposure.
Keywords: TiO2, nanoparticles, mice, genetic instability, DNA damage, inflammation
INTRODUCTION
Titanium dioxide (TiO2) accounts for 70% of the total production volume of pigments worldwide (1). It is widely used to provide whiteness and opacity to products such as paints, plastics, papers, inks, food colorants, and toothpastes. TiO2 is also used in cosmetic and skin care products, particularly in sun-blocks, where it helps to protect the skin from ultraviolet light, especially in the case of nano-sized particles (< 100 nm). Nevertheless, TiO2 has recently been re-classified by the International Agency for Research on Cancer (IARC) as Group 2B carcinogen: “possibly carcinogenic to humans” (2). The reason for this new classification stems from the fact that high concentrations of pigment-grade (< 2.5 μm) and ultrafine (< 100 nm) TiO2 dust can cause respiratory tract cancer in exposed rats (3, 4). However, it should be noted that epidemiologic studies of workers exposed to pigment-grade TiO2 conducted so far have not been able to detect an association between occupational exposure to TiO2 and an increased risk for lung cancer (5, 6). Genotoxicity studies, which measure different types of DNA damage (e.g. gene mutations, chromosomal damage, DNA strand break formation) are an important part of cancer research and risk assessment of potential carcinogens. These studies help to understand possible mechanisms causing tumor induction. As such, in vivo mechanisms underlying TiO2 NP tumor induction are still unclear.
Since NPs diameter does not exceed a hundred nanometers at maximum, they are able to penetrate cells (7) and interfere with several sub-cellular mechanisms. Indeed, some studies show that some NPs can penetrate into cell nuclei and hence may directly interfere with the structure and function of genomic DNA (8). Additionally, after oral administration in mice, TiO2 particles were shown to translocate to systemic organs such as liver and spleen, as well as lung and peritoneal tissues (9). Genotoxicity studies have been performed to understand the carcinogenic potential of TiO2 NPs, using assays that measure mutations in genes (e.g. Ames/Salmonella and Hypoxanthineguanine PhosphoRibosyl Transferase (HPRT) assays) (10-12), chromosomal damage representing possible clastogenic activity of the particles (e.g. micronuclei) (11, 13-16) and DNA strand breakage (e.g. alkaline comet assay) (11, 14). Except for one, these studies were conducted in vitro in cultured cells, but conflict in their results. Half of the studies show that TiO2 NPs are genotoxic in cell lines (11, 13, 14, 16) while the other half show that TiO2 NPs are not (10, 12, 15). The rationale for these conflicting results is not clear since different cell types, doses and NP sizes have been used. Some studies suggest possible mechanisms for TiO2 NP genotoxicity. TiO2 NPs might damage DNA directly or indirectly via oxidative stress and/or inflammatory responses. Two recent studies show a direct chemical interaction between TiO2 NPs and DNA, through the DNA phosphate group, but a link to mutagenesis has not been proven (17, 18). On the other hand, other studies show that TiO2 NPs can cause DNA damage indirectly through inflammation (19-22) and generation of reactive oxygen species (ROS) (13, 14, 23, 24).
So far, most NPs genotoxicity studies have focused on cell culture systems but confirmation from animal experiments, more relevant to human exposure, is required. To further understand TiO2 NP toxicity in vivo, we studied effect of TiO2 NP exposure on genotoxicity, DNA damage and inflammation in mice. To evaluate inflammation in mice, we determined mRNA expression of both pro- and anti-inflammatory cytokines. To assess DNA damage, we used the γ-H2AX and the comet assays to evaluate DNA strand breaks, the micronuclei (MN) assay to estimate chromosomal damage and the measure of 8-OHdG levels using HPLC to determine oxidative DNA damage. We also used an in vivo DNA deletion assay, which allows visual detection of DNA deletion events within the pink-eyed unstable (pun) locus in developing mouse embryos (25), which can detect environmental as well as genetic cancer predisposing factors (25). Our results show that TiO2 P25 NP can induce 8-OHdG, γ-H2AX foci, MN, DNA deletions and inflammation markers in a mice model. Therefore, this study suggests that TiO2 NPs are genotoxic in vivo.
MATERIALS & METHODS
Mouse care and breeding
C57Bl/6Jpun/pun mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). The C57Bl/6Jpun/pun background is essentially identical to C57Bl/6J with the exception of a naturally occurring 70 kb internal duplication in the pink-eyed dilution (p) gene, termed the pink-eyed unstable (pun) allele. Mice were housed and cared for under standard specific pathogen- free conditions and according to ARC and IACUC regulations. Mice were given a standard, autoclaved diet from Harlan Teklad (Harlan Teklad No 8656), and sterilized water ad libitum. Mice were housed in a 12-hours light/ dark cycle. Pregnancy was timed by checking for vaginal plugs, with noon of the day of discovery counted as 0.5 days post coitum (dpc). Four to five months old mice were used for all experiments.
Titanium Dioxide nanoparticles preparation and exposure
“Aeroxide” P25 TiO2 (Degussa, now Evonik, Germany,) NPs were chosen for this study. The crystal structure is a mixture of 75 % anatase and 25% rutile TiO2, purity was at least 99.5% TiO2, and primary particle size was 21 nm with a specific surface area of 50 ± 15 m2.g−1. These NPs have been used in many of the previous mammalian studies (14, 15, 23, 26-29). Using Dynamic Light Scattering in water revealed that the size of TiO2 NPs agglomerates ranged from 21 nm to 1446 nm and the mean size was 160 +/− 5 nm. About 70% of particles have a size of 160 nm. Solutions of dispersed TiO2 NPs were prepared by ultra-sonication (Solid state/Ultrasonic FS-14, Fisher Scientific) for 15 min in drinking water at 60, 120, 300 and 600 μg/ml concentrations just before use. We measured TiO2 NP supplemented water intake at the end of experiments in each cage, which housed 2-3 mice, and calculated an average daily water intake per mouse. Daily TiO2 NP-water intake ranged from 3 to 7 ml per mouse, consistent with normal daily water intake. Doses were calculated using a 30g average weight per mouse, and an average of 5 ml of water intake per day. The exposure was 5 days in adult males. For in utero exposure, pregnant dams were given NPs supplemented drinking water for 10 days from 8.5 to 18.5 dpc at a concentration of 300μg/ml. Water was used as negative control.
In vivo DNA deletion assay
To evaluate genotoxicity of TiO2 NPs we employed an intra-chromosomal duplication of 70 kb fragment spanning exons 6–18 of the pink-eyed dilution (p) gene in mice (termed pun mutation). When a DNA deletion event occurs between these duplications, the pun allele reverts to the wild type p gene. Reconstitution of the wild type p gene can be seen as a single pigmented cell or a clone of pigmented cells on the un-pigmented RPE in the transgenic mice and represents a DNA deletion as a permanent genotoxic event (30). Pregnant mice were treated with TiO2 NPs, and then offspring were sacrificed at 20 days of age. Their eyes were extracted and dissected to display the RPE for the deletion/eye-spot assay as described previously (25). One RPE corresponds to one eye.
Alkaline Comet assay
Peripheral blood was collected by sub-mandibular vein puncture (before treatment and after treatment) in an EDTA coated tube. The comet assay was performed as previously described (31). On average, from 3 slides 150 to 200 randomly captured comets per sample were analyzed. Results were expressed as averaged tail moment values ± SEM.
Micronuclei assay (MN)
The micronuclei assay was performed as described elsewhere (32). Three μl aliquots of the peripheral blood were collected as described above and smeared on slides and stained into Giemsa stain for 1.5 min. Approximately 2000 erythrocytes were scored per animal to estimate the frequency of micronucleated erythrocytes.
Bone marrow preparation
Animals were sacrificed with an overdose of isoflurane after 5 days treatment with TiO2 NPs in drinking water. Both femora were dissected, and marrow cells were flushed out with 1 ml PBS and pipetted several times. The cell suspension was centrifuged at 1000 rpm for 5 minutes, the supernatant withdrawn, and the cell pellet resuspended and placed on a clean glass slide.
γ-H2AX assay, RNA Isolation and Quantitative Real-Time PCR
The γ-H2AX assay was performed with bone marrow cells, and the RNA isolation for quantitative real time PCR was carried out on peripheral blood. These assays were performed as described elsewhere (32).
Determination of oxidative DNA damage by measuring 8-OHdG
Mouse livers were isolated just after 5 days treatment with NPs and immediately frozen in liquid nitrogen and homogenized under liquid nitrogen. 8-Hydroxy-2′-deoxyguanosine also referred to as 8-OHdG level was measured using HPLC with electron capture detection system as previously described in (33).
Statistical analysis
For the deletion assay, the comet assay, the MN assay and 8-OHdG and mRNA levels of cytokines, we used the student t-test to compare untreated mice with treated mice. For the γ-H2AX experiment, the percentage of positive cells for control groups vs. treated groups was compared via χ2 test. In addition to the t-test, for comet assay data, the Wilcoxon test for matched paired data was also used to compare the effect of TiO2 NPs on the Tail moment before and after NPs treatment. The difference was considered significant at the 95% confidence level (p < 0.05) and highly significant at the 99% confidence level (p ≤ 0.01).
RESULTS
TiO2 NPs increased the frequency of DNA deletions
We used the DNA deletion assay to evaluate in vivo genotoxicity of TiO2 NPs. We quantified the number of eye-spots per RPE as a measure of DNA deletions in in utero exposed mice. TiO2 NPs treated mice had an average of 8.13 ± 1.70 eye-spots per RPE versus 6.42 ± 1.47 eye-spots per RPE in non-treated mice (Fig. 1). TiO2 NP exposed mice displayed a significant increase in eye-spots (27%) compared to unexposed mice (p = 0.019), suggesting that after maternal oral exposure, TiO2 NPs increased DNA deletion frequency in fetuses.
Fig. 1.
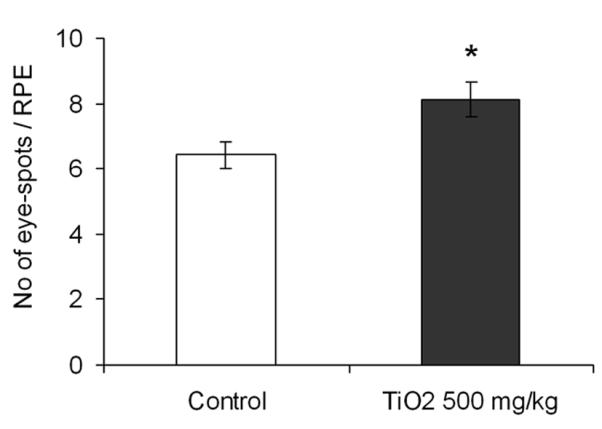
Frequency of DNA deletions in control and TiO2 NP treated mice. One RPE corresponds to one eye. Mice were treated with NPs during embryonic development at a total dose of 500 mg/kg. The results shown are the mean numbers of eye-spots per RPE ± error of the mean (SEM), with n = 42 eyes for control and n = 53 eyes for TiO2 NP treated mice, * p < 0.05.
TiO2 NPs induced γ-H2AX foci
Phosphorylation of histone H2AX on serine 139 occurs at sites flanking DNA DSBs providing a measure of the number of DSBs within a cell (34). We used this assay to compare DSB formation in bone marrow of mice with and without TiO2 NP treatment.
The γ-H2AX foci formation increased by about 10, 20, 25 and 30% following treatment with 50, 100, 250 and 500 mg/kg TiO2 NPs, respectively, in comparison to untreated mice (Fig. 2). Percentage of γ-H2AX positive cells increased with TiO2 NP concentration in a clear dose-dependent manner (p < 0.001). These data provided evidence that after oral administration, TiO2 NPs induce DSBs in bone marrow cells.
Fig. 2.
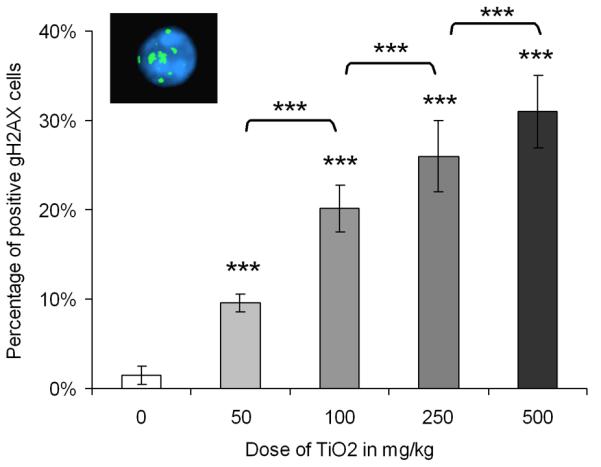
Percentage of positive γ-H2AX cells in bone marrow in untreated and TiO2 NP treated mice, and a picture of a positive γ-H2AX cell with more than 4 foci. Each bar represents the mean of 5 mice ± SEM, ***, p < 0.001, TiO2 NP treated versus control.
TiO2 NPs increased DNA strand breaks
DNA stand breaks (double-strand breaks, single-strand breaks, and/or strand breaks induced by alkali-labile sites) were measured by the alkaline comet assay in mice peripheral blood before and after treatment. Tail moment significantly increased after TiO2 NP treatment (Fig. 3). The average tail moment was 0.0102 ± 0.001 before treatment and 0.0137 ± 0.0011 after TiO2 NP treatment. TiO2 NPs increased DNA strand breaks in white blood cells from peripheral blood by 34 % (p = 0.001 with t-test and p = 0.04 with the Wilcoxon test).
Fig. 3.
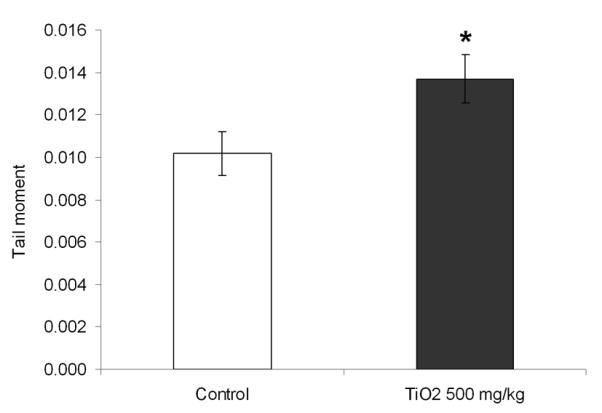
Frequency of DNA strand breaks in mice before and after treatment with 500 mg/kg TiO2 NPs. DNA damage is represented by the Tail Moment. Mean ± SEM, n= 5 mice/group is shown, *, p < 0.05, compared with untreated mice.
TiO2 NPs induced micronuclei
The MN assay was used to detect chromosomal damage in erythrocytes from peripheral blood. The incidence of MN serves as an index of clastogenicity. MN frequency increased significantly only at the highest (500 mg/kg) dose of TiO2 NPs used (p = 0.009) (Fig. 4). At this dose, the average MN frequencies for untreated mice were 4.3 ± 0.93 versus 9.2 ± 1.07 per 2000 RBC for TiO2 NP treated mice, which resulted in a 2.1- fold increase in MN formation. This result showed at high dose TiO2 NPs induced detectable clastogenicity in mice peripheral blood.
Fig. 4.
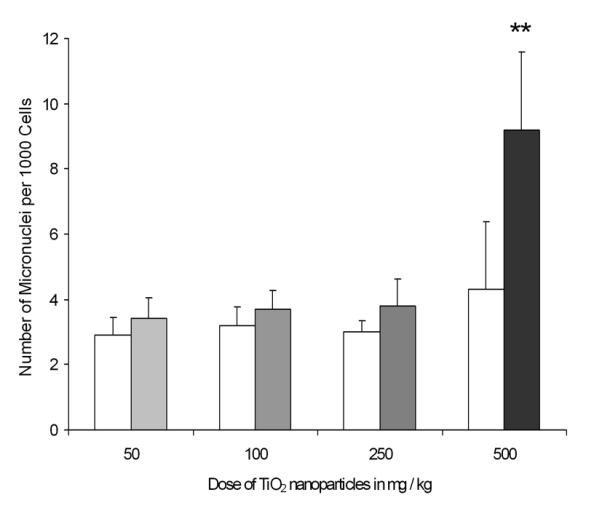
Frequency of MN in mice before and after TiO2 NPs treatment in peripheral blood erythrocytes. Open bars represent untreated controls and grey bars represent TiO2 NPs treated mice. Each bar represents the mean of 5 mice ± SEM., *, p < 0.01 compared with untreated mice.
TiO2 NPs induced oxidative DNA damage
We examined the degree of oxidative DNA damage by measuring the level of 8-OHdG in DNA isolated from TiO2 NP-treated and -untreated mouse livers. The level of 8-OHdG was significantly higher in TiO2 NP treated than untreated mice (p = 0.04, Fig. 5). The average number of 8-OHdG per 106dG was 4.25 ± 0.66 for untreated mice and 6.43 ± 0.58 for TiO2 NP treated mice resulting in a 1.5 fold increase at 500 mg/kg TiO2 NP. This suggested that TiO2 NPs induced oxidative DNA damage in liver.
Fig. 5.
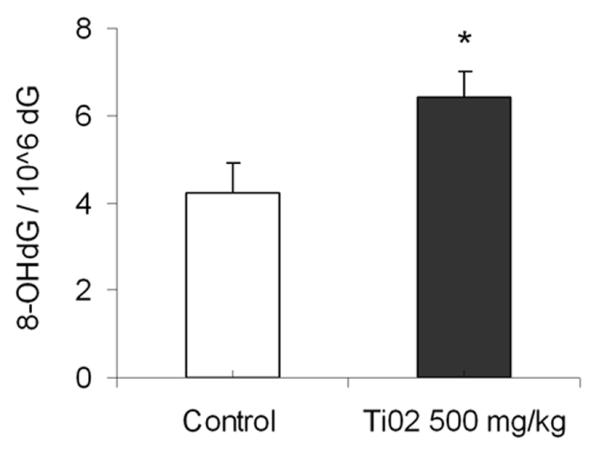
The level of 8-OHdG in untreated and 500 mg/kg TiO2 NP treated mouse livers. Mean ± SEM, n = 5 mice/group, *, p < 0.05, compared with untreated mice.
TiO2 NPs induced a pro-inflammatory response
We quantified mRNA transcripts of three Th1/pro-inflammatory cytokines (T-helper cell type 1) and three Th2/ anti-inflammatory (T-helper cell type 2) cytokines in the peripheral blood. After treatment, the pro-inflammatory cytokines Tumor necrosis factor- α (Tnf-α), Interferon-γ (Ifn-γ) and the mouse ortholog of Interleukin-8 (KC) were significantly up-regulated (p = 0.01, p = 0.02, p = 0.05 respectively) (Fig. 6A). A general up-regulation of these cytokines may be due to the effects of circulating TiO2 NPs directly in the peripheral blood, suggesting systemic distribution, and direct activation of a pro-inflammatory response. To the contrary, anti-inflammatory cytokines with generally opposing function were not up-regulated, including Transforming Growth Factor- β (TGF-β), Interleukin-10 (IL-10), and IL-4 (Fig. 6B). TiO2 NPs did not induce an anti-inflammatory response, which mean they did not inhibit the production and release of pro-inflammatory mediators.
Fig. 6.
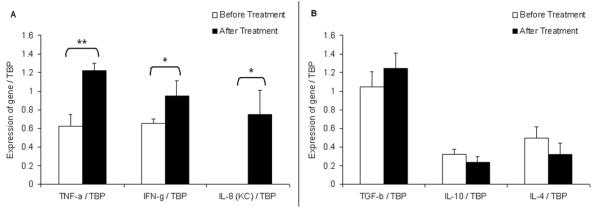
TiO2 NPs at 500 mg/kg induce pro-inflammatory cytokines but not anti-inflammatory ones. Open bars represent untreated controls and black bars represent TiO2 NPs treated mice. A. Expression of pro-inflammatory cytokine panel relative to TBP, the internal control gene. *:p < 0.05, **: p <0.01 by Student’s t-test for treatment comparisons. B. Expression of anti-inflammatory cytokine panel relative to TBP. Student’s t-test revealed no significant differences. For each graph, n = 5 mice/group.
DISCUSSION
Here we report for the first time that TiO2 NPs are genotoxic and clastogenic in vivo in mice. We showed that TiO2 NPs (500 mg/kg) induce not only DNA single and double strand breaks but also chromosomal damage. The formation of γ-H2AX foci, which show DSBs formation, was the most sensitive parameter and demonstrated a consistent dose-dependent response. Concerning health relevance, DSBs are much more damaging in terms of genetic instability than SSBs and oxidative DNA damage which are transient. Our results extend previous in vitro findings with the MN and comet assays in several human cells and Syrian hamster embryo cells (11, 13, 14, 16), although they have not been detected in some studies (12, 15). Differences in response between studies may be due to how TiO2 NPs differ in terms of TiO2 production, particle size, degree of aggregation, preparation method (sonication), incubation conditions, dose and susceptibility between cell types (35, 36), implying that more studies are needed to determine the conditions in which TiO2 NP genotoxicity occurs.
To date very few in vivo genotoxicity studies have been carried out with NPs. A chronic exposure to TiO2 NPs at concentrations that produce chronic pulmonary inflammation was associated with an increased incidence of tumors in rat lungs (4). So far only two in vivo genotoxicity studies have been reported, which showed that in vivo TiO2 NPs increased Hprt mutation frequency in alveolar epithelial cells (10) but did not induce DNA adduct formation in rat lungs (27). Our study showed for the first time that in vivo after oral exposure, TiO2 NPs induce DNA strand breaks and chromosomal damage in bone marrow and/or peripheral blood, which may help to further understand potential mechanisms of TiO2 NPs carcinogenicity. We also found that maternal exposure to 500 mg/kg TiO2 NPs during gestation results in significantly elevated frequencies of DNA deletions in offspring. This result is a major finding since it demonstrates for the first time that in utero exposure of fetuses via the mother causes an increase in large deletions in offspring. Taken together our findings show that TiO2 NPs orally administrated, induce genotoxicity systemically in organs, such as blood, bone marrow and even the embryo.
Surprisingly, human studies have not been able to detect any relation between TiO2 occupational exposure and cancer risk (5, 6, 37), but these studies have methodological and epidemiological limitations as reviewed by the National Institute of Occupational Safety and Health (NIOSH) (38). In addition, these studies were not designed to investigate the relationship between TiO2 particle size and lung cancer risk, an important question for assessing the potential occupational carcinogenicity of TiO2. Indeed Dankovic et al. comparing several tumor studies, pointed out TiO2 NPs produced lung tumors in rats at a much lower dose than fine particles (< 250 nm) (10 and 250 mg/m3 for NPs and fine particles respectively) (4). Although TiO2 NPs are prone to forming agglomerates of more than 100 nm in solution, these agglomerates are apparently not stable and appear to dissociate in bodily fluids and tissues, which could be an explanation for TiO2 NPs higher toxicity. It has also been reported that TiO2 NP surface interactions are weak (21). In addition, an inhalation study showed that TiO2 NP agglomerates of ~700 nm dissociate into smaller units after deposition in the lung (22). Even if NPs aggregate into bigger size agglomerate, their primary particle sizes remain a significant trait which impacts their toxicity. Further human studies would be necessary in order to understand NP health effects. For instance, one could use blood-based assays similar to those performed in this study in a future molecular epidemiology study in occupational settings. Furthermore, TiO2 is also used in food colorants and toothpaste. This supports the notion that NP ingestion constitutes a relevant route of exposure in humans and underscores the significance of the findings of our study. In addition, given the capability of NPs to enter the systemic blood circulation, NPs may pose hazard to a variety of other organs, as we have shown here.
As previously suggested, a possible mechanism for NP caused genotoxicity might be via oxidative stress (39). Indeed, previous studies showed that TiO2 NPs have hydroxyl radical activity (40-42), and can also trigger ROS production in different cell lines (14, 43) upon interaction with the cell membrane or even in cell free environment (24, 44). We confirmed these results in our in vivo experiment by showing oxidative DNA damage (8-OHdG) increase in mouse livers after TiO2 NP treatment. Also TiO2 NP-increased DNA deletions during fetal development might be a result of oxidative genome damage. As previously discussed (45), oxidative stress is particularly hazardous in replicating cells. For instance, oxidative DNA lesions (e.g. 8-OHdG, SSBs or stalled replication forks) can lead to DSBs after replication, which can result in recombination, thus producing permanent genome rearrangements. As shown in yeast, oxidative mutagens might be powerful inducers of DNA deletions (46). Because embryonic cells are generally characterized by a high replication index, they might be particularly susceptible to oxidative genome damage.
We have also observed an inflammatory reaction as shown by changes in cytokine expression in peripheral blood, in which TiO2 NPs could be exerting direct inflammatory effects on circulating innate cells and Th1 effector cells. The inflammatory response involving recruitment and activation of phagocytes is capable of causing oxidative bursts that may serve as a possible explanation for the observed genotoxicity to peripheral leukocytes, MN formation, oxidative DNA damage in liver cells and DNA deletion induction in fetal RPE. Since we showed that in mice TiO2 NPs induce an inflammatory reaction and oxidative DNA damage, it is tempting to speculate that the mechanism underlying TiO2 NP genotoxicity might be a secondary (indirect) genotoxicity pathway as suggested by Dankovic and coworkers (4). Secondary genotoxicity is considered to be the key aspect of some particle toxicity, e.g. quartz, silica, because of their ability to elicit persistent inflammation in vivo (10, 47). This implies that particles have pro-oxidant and pro-inflammatory activity, leading to genotoxicity.
In summary, our study showed for the first time that TiO2 NPs induce clastogenicity, genotoxicity, oxidative DNA damage and inflammation in vivo in mice. These results have been observed after only 5 days of treatment via drinking water, and in multiple organs suggesting a systemic effect. We also showed that in utero exposure to TiO2 NPs results in an increased frequency in DNA deletions in the fetus. These results represent the first comprehensive in vivo genotoxicity study of TiO2 NPs. These data suggest that we should be concerned about a potential risk of cancer or genetic disorders especially for people occupationally exposed to high concentrations of TiO2 NPs and that it might be prudent to limit ingestion of TiO2 NPs through nonessential drug additives, food colors etc.
ACKNOWLEDGEMENTS
The authors thank Susanne Henning at the UCLA Center for Human Nutrition for performing HPLC. We are thankful to Dr Gopal Iyer at the UCLA Department of Chemistry and Biochemistry for Dynamic Light Scattering measurements. We also wish to thank Dr. Fabien Nogué, Dr Norman Arnheim and especially Dr. Jean-Louis Plouhinec for their very interesting comments on the manuscript. This research was supported by NIH, RO1 grant No. ES09519 to RHS and NIH 1RO3 CA133928-01 to RR.
REFERENCES
- 1.Baan R, Straif K, Grosse Y, et al. Carcinogenicity of carbon black, titanium dioxide, and talc. The Lancet Oncology. 2006;7:295–6. doi: 10.1016/s1470-2045(06)70651-9. [DOI] [PubMed] [Google Scholar]
- 2.IARC . Carbon black, titanium dioxide and Non-Asbestiform Talc. Volume 93. International Agency for Research on Cancer (IARC); Lyon, France: Monographs on the evaluation of carcinogenic risks to humans. in preparation. [Google Scholar]
- 3.Borm PJ, Schins RP, Albrecht C. Inhaled particles and lung cancer, part B: paradigms and risk assessment. Int J Cancer. 2004;110:3–14. doi: 10.1002/ijc.20064. [DOI] [PubMed] [Google Scholar]
- 4.Dankovic D, Kuempel E, Wheeler M. An approach to risk assessment for TiO2. Inhal Toxicol. 2007;19(Suppl 1):205–12. doi: 10.1080/08958370701497754. [DOI] [PubMed] [Google Scholar]
- 5.Boffetta P, Soutar A, Cherrie JW, et al. Mortality among workers employed in the titanium dioxide production industry in Europe. Cancer Causes Control. 2004;15:697–706. doi: 10.1023/B:CACO.0000036188.23970.22. [DOI] [PubMed] [Google Scholar]
- 6.Fryzek JP, Chadda B, Marano D, et al. A Cohort Mortality Study among Titanium Dioxide Manufacturing Workers in the United States. Journal of Occupational and Environmental Medicine. 2003;45:400–9. doi: 10.1097/01.jom.0000058338.05741.45. [DOI] [PubMed] [Google Scholar]
- 7.Park S, Lee YK, Jung M, et al. Cellular Toxicity of Various Inhalable Metal Nanoparticles on Human Alveolar Epithelial Cells. Inhalation Toxicology. 2007;19:59–65. doi: 10.1080/08958370701493282. [DOI] [PubMed] [Google Scholar]
- 8.Chen M, von Mikecz A. Formation of nucleoplasmic protein aggregates impairs nuclear function in response to SiO2 nanoparticles. Experimental Cell Research. 2005;305:51–62. doi: 10.1016/j.yexcr.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Jani PU, McCarthy DE, Florence AT. Titanium dioxide (rutile) particle uptake from the rat GI tract and translocation to systemic organs after oral administration. International Journal of Pharmaceutics. 1994;105:157–68. [Google Scholar]
- 10.Driscoll KE, Deyo LC, Carter JM, et al. Effects of particle exposure and particle-elicited inflammatory cells on mutation in rat alveolar epithelial cells. Carcinogenesis. 1997;18:423–30. doi: 10.1093/carcin/18.2.423. [DOI] [PubMed] [Google Scholar]
- 11.Wang JJ, Sanderson BJ, Wang H. Cyto- and genotoxicity of ultrafine TiO2 particles in cultured human lymphoblastoid cells. Mutat Res. 2007;628:99–106. doi: 10.1016/j.mrgentox.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Warheit DB, Hoke RA, Finlay C, et al. Development of a base set of toxicity tests using ultrafine TiO2 particles as a component of nanoparticle risk management. Toxicol Lett. 2007;171:99–110. doi: 10.1016/j.toxlet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Gurr JR, Wang AS, Chen CH, Jan KY. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology. 2005;213:66–73. doi: 10.1016/j.tox.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Kang SJ, Kim BM, Lee YJ, Chung HW. Titanium dioxide nanoparticles trigger p53-mediated damage response in peripheral blood lymphocytes. Environ Mol Mutagen. 2008;49:399–405. doi: 10.1002/em.20399. [DOI] [PubMed] [Google Scholar]
- 15.Linnainmaa K, Kivipensas P, Vainio H. Toxicity and cytogenetic studies of ultrafine titanium dioxide in cultured rat liver epithelial cells. Toxicology in Vitro. 1997;11:329–35. doi: 10.1016/s0887-2333(97)00000-3. [DOI] [PubMed] [Google Scholar]
- 16.Rahman Q, Lohani M, Dopp E, et al. Evidence that ultrafine titanium dioxide induces micronuclei and apoptosis in Syrian hamster embryo fibroblasts. Environ Health Perspect. 2002;110:797–800. doi: 10.1289/ehp.02110797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li S, Zhu H, Zhu R, et al. Impact and mechanism of TiO2 nanoparticles on DNA synthesis in vitro. Science in China Series B - Chemistry. 2008;51:367–72. [Google Scholar]
- 18.Zhu R-R, Wang S-L, Zhang R, Sun X-Y, Yao S-D. A Novel Toxicological Evaluation of TiO2 Nanoparticles on DNA Structure. Chinese Journal of Chemistry. 2007;25:958–61. [Google Scholar]
- 19.Chen HW, Su SF, Chien CT, et al. Titanium dioxide nanoparticles induce emphysema-like lung injury in mice. Faseb J. 2006;20:2393–5. doi: 10.1096/fj.06-6485fje. [DOI] [PubMed] [Google Scholar]
- 20.de Haar C, Hassing I, Bol M, Bleumink R, Pieters R. Ultrafine but not fine particulate matter causes airway inflammation and allergic airway sensitization to co-administered antigen in mice. Clin Exp Allergy. 2006;36:1469–79. doi: 10.1111/j.1365-2222.2006.02586.x. [DOI] [PubMed] [Google Scholar]
- 21.Grassian VH, O’Shaughnessy PT, Adamcakova-Dodd A, Pettibone JM, Thorne PS. Inhalation exposure study of titanium dioxide nanoparticles with a primary particle size of 2 to 5 nm. Environ Health Perspect. 2007;115:397–402. doi: 10.1289/ehp.9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oberdorster G, Ferin J, Lehnert BE. Correlation between particle size, in vivo particle persistence, and lung injury. Environ Health Perspect. 1994;102(Suppl 5):173–9. doi: 10.1289/ehp.102-1567252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Federici G, Shaw BJ, Handy RD. Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): Gill injury, oxidative stress, and other physiological effects. Aquatic Toxicology. 2007;84:415–30. doi: 10.1016/j.aquatox.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Veranth JM, Kaser EG, Veranth MM, Koch M, Yost GS. Cytokine responses of human lung cells (BEAS-2B) treated with micron-sized and nanoparticles of metal oxides compared to soil dusts. Part Fibre Toxicol. 2007;4:2. doi: 10.1186/1743-8977-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reliene R, Bishop AJ, Aubrecht J, Schiestl RH. In vivo DNA deletion assay to detect environmental and genetic predisposition to cancer. Methods Mol Biol. 2004;262:125–39. doi: 10.1385/1-59259-761-0:125. [DOI] [PubMed] [Google Scholar]
- 26.Dick CA, Brown DM, Donaldson K, Stone V. The role of free radicals in the toxic and inflammatory effects of four different ultrafine particle types. Inhal Toxicol. 2003;15:39–52. doi: 10.1080/08958370304454. [DOI] [PubMed] [Google Scholar]
- 27.Gallagher J, Heinrich U, George M, et al. Formation of DNA adducts in rat lung following chronic inhalation of diesel emissions, carbon black and titanium dioxide particles. Carcinogenesis. 1994;15:1291–9. doi: 10.1093/carcin/15.7.1291. [DOI] [PubMed] [Google Scholar]
- 28.Rehn B, Seiler F, Rehn S, Bruch J, Maier M. Investigations on the inflammatory and genotoxic lung effects of two types of titanium dioxide: untreated and surface treated. Toxicology and Applied Pharmacology. 2003;189:84–95. doi: 10.1016/s0041-008x(03)00092-9. [DOI] [PubMed] [Google Scholar]
- 29.Xia T, Kovochich M, Brant J, et al. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006;6:1794–807. doi: 10.1021/nl061025k. [DOI] [PubMed] [Google Scholar]
- 30.Reliene R, Schiestl RH. Mouse models for induced genetic instability at endogenous loci. Oncogene. 2003;22:7000–10. doi: 10.1038/sj.onc.1206904. [DOI] [PubMed] [Google Scholar]
- 31.Reliene R, Pollarda J, Sobol Z, et al. N-acetyl cysteine protects against ionizing radiation-induced DNA damage but not against cell killing in yeast and mammals. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2009;665:37–43. doi: 10.1016/j.mrfmmm.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 32.Westbrook AM, Wei B, Braun JB, Schiestl RH. Intestinal mucosal inflammation leads to systemic genotoxicity in mice. Cancer Research. 2009;69:4827–34. doi: 10.1158/0008-5472.CAN-08-4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reliene R, Fischer E, Schiestl RH. Effect of N-acetyl cysteine on oxidative DNA damage and the frequency of DNA deletions in atm-deficient mice. Cancer Res. 2004;64:5148–53. doi: 10.1158/0008-5472.CAN-04-0442. [DOI] [PubMed] [Google Scholar]
- 34.Ismail IH, Wadhra TI, Hammarsten O. An optimized method for detecting gamma-H2AX in blood cells reveals a significant interindividual variation in the gamma-H2AX response among humans. Nucleic Acids Res. 2007;35:e36. doi: 10.1093/nar/gkl1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tao F, Kobzik L. Lung macrophage-epithelial cell interactions amplify particle-mediated cytokine release. Am J Respir Cell Mol Biol. 2002;26:499–505. doi: 10.1165/ajrcmb.26.4.4749. [DOI] [PubMed] [Google Scholar]
- 36.Thevenot P, Cho J, Wavhal D, Timmons RB, Tang L. Surface chemistry influences cancer killing effect of TiO2 nanoparticles. Nanomedicine: Nanotechnology, Biology and Medicine. 2008;4:226–36. doi: 10.1016/j.nano.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garabrant DH, Fine LJ, Oliver C, Bernstein L, Peters JM. Abnormalities of pulmonary function and pleural disease among titanium metal production workers. Scand J Work Environ Health. 1987;13:47–51. doi: 10.5271/sjweh.2087. [DOI] [PubMed] [Google Scholar]
- 38.NIOSH . NIOSH Current Intelligence Bulletin. National Institute for Occupational Safety and Health, DHHS (NIOSH) publication; Cincinnati: 2005. Evaluation of Health Hazard and Recommendations for Occupational Exposure to Titanium Dioxide. Volume. [Google Scholar]
- 39.Schins RP, Knaapen AM. Genotoxicity of poorly soluble particles. Inhal Toxicol. 2007;19(Suppl 1):189–98. doi: 10.1080/08958370701496202. [DOI] [PubMed] [Google Scholar]
- 40.Donaldson K, Beswick PH, Gilmour PS. Free radical activity associated with the surface of particles: a unifying factor in determining biological activity? Toxicol Lett. 1996;88:293–8. doi: 10.1016/0378-4274(96)03752-6. [DOI] [PubMed] [Google Scholar]
- 41.Gilmour P, Brown DM, Beswick PH, et al. Surface free radical activity of PM10 and ultrafine titanium dioxide: A unifying factor in their toxicity? The Annals of Occupational Hygiene. 1997;41:32–8. [Google Scholar]
- 42.Zhang Q, Kusaka Y, Sato K, et al. Differences in the extent of inflammation caused by intratracheal exposure to three ultrafine metals: Role of free radicals. Journal of Toxicology and Environmental Health, Part A. 1998;53:423–38. doi: 10.1080/009841098159169. [DOI] [PubMed] [Google Scholar]
- 43.Helfenstein M, Miragoli M, Rohr S, et al. Effects of combustion-derived ultrafine particles and manufactured nanoparticles on heart cells in vitro. Toxicology. 2008;253:70–8. doi: 10.1016/j.tox.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 44.Sayes CM, Wahi R, Kurian PA, et al. Correlating nanoscale titania structure with toxicity: a cytotoxicity and inflammatory response study with human dermal fibroblasts and human lung epithelial cells. Toxicol Sci. 2006;92:174–85. doi: 10.1093/toxsci/kfj197. [DOI] [PubMed] [Google Scholar]
- 45.Reliene R, Schiestl RH. Antioxidant N-acetyl cysteine reduces incidence and multiplicity of lymphoma in Atm deficient mice. DNA Repair (Amst) 2006;5:852–9. doi: 10.1016/j.dnarep.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Brennan RJ, Swoboda BE, Schiestl RH. Oxidative mutagens induce intrachromosomal recombination in yeast. Mutat Res. 1994;308:159–67. doi: 10.1016/0027-5107(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 47.Schins RPF. Mechanisms of genotoxicity of particles and fibers. Inhalation Toxicology. 2002;14:57–78. doi: 10.1080/089583701753338631. [DOI] [PubMed] [Google Scholar]


