Abstract
The yeast DEL assay is an effective method for measuring intrachromosomal recombination events resulting in DNA deletions that when occurring in mammalian cells are often associated with genomic instability and carcinogenesis. Here we used the DEL assay to measure γ-ray-induced DNA deletions throughout different phases of yeast culture growth. Whereas yeast survival differed by only up to twofold throughout the yeast growth phase, proliferating cells in lag and early exponential growth phases were tenfold more sensitive to ionizing radiation-induced DNA deletions than cells in stationary phase. Radiation-induced DNA deletion potential was found to correlate directly with the fraction of cells in S/G2 phase. The ability of the antioxidants l-ascorbic acid and DMSO to protect against radiation-induced DNA deletions was also measured within the different phases of yeast culture growth. Yeast cells in lag and early exponential growth phases were uniquely protected by antioxidant treatment, whereas nondividing cells in stationary phase could not be protected against the induction of DNA deletions. These results are compared with those from mammalian cell studies, and the implications for radiation-induced carcinogenesis and radioprotection are discussed.
INTRODUCTION
Ionizing radiation exposure produces a variety of DNA damages in cells, which includes strand crosslinks, base damages, single-strand breaks (SSBs) and double-strand breaks (DSBs) (1). Cells respond to this damage through complex molecular signaling pathways that can activate cellular responses such as DNA repair, gene expression, growth arrest and apoptosis (2). Cells sustaining radiation-induced damage may exhibit delayed effects such as genomic instability and may ultimately become carcinogenic (3). Both acute DNA damage induced by radiation and the subsequent cellular responses are influenced by a variety of factors, including radiation quality, dose rate, dose fractionation, cell/tissue type, cell cycle phase and cell environment physiology [for a comprehensive review, see ref. (4)].
Throughout the 1920s to 1940s, early studies aimed at determining sensitivity to radiation throughout the cell cycle were performed in a variety of organisms. The results of these pioneering studies lacked agreement as to which cell cycle stage is the most radiosensitive and as a whole were inconclusive (5). In 1961, in studies made possible by the development of the in vitro clonogenic survival assay of Puck and Marcus 5 years previously (6), Terasima and Tolmach definitively measured the clonogenic radiosensitivity of HeLa cells synchronized by mitotic harvest throughout the cell cycle (7). HeLa cells in M phase were the most sensitive to X-ray cell killing, G1 and G2 were the most radioresistant, and S-phase cells were intermediately sensitive. These results have been reproduced in other mammalian cell lines generally yielding the same variation in cell cycle radiosensitivity (8). In 1961 and 1962, Dewey and Humphrey reported measurements of the sensitivity of mouse fibroblasts to γ-ray-induced chromosomal aberrations (9, 10); similar to the earlier observation (7), cells irradiated in S and G2 phases were up to twofold more sensitive to chromosomal aberrations than G1 cells. These results were later reproduced in numerous follow-up studies using multiple cell types and collectively established G2 to be the most sensitive to radiation-induced aberrations (11-14).
Likewise, the genotoxic effects of radiation also vary with cell cycle position. Multiple attempts were made in the 1970s to determine a relationship between radiation-induced mutation sensitivity and cell cycle phase (15-17), but no firm conclusions were established until 1980, when H. J. Burki published his study using synchronized CHO cells. Here G1 was demonstrated to be the most sensitive cell cycle phase for X-ray-induced Hprt mutations for each dose between 1 and 8 Gy, early S phase to be slightly less radiosensitive, and late S phase to be relatively resistant (18). These results were upheld by later studies reporting G1 to be the most sensitive phase for mutation induction by radiation (19-22).
The results from experiments aimed at quantifying cell cycle phase sensitivity in yeast have thus far offered mixed correlations with results from in vitro mammalian cell studies. Budding yeast cells in S and M phase are more resistant to radiation cell killing than nonbudding cells in G1 (23, 24), in opposition to that observed in mammalian cell cultures (7, 8). Proliferating yeast cells (predominant in S/G2) exhibit greater X-ray-induced chromosomal loss (monosomy) than stationary (G1/G0)-phase yeast cells (25). Yeast cells are most sensitive to radiation-induced mutations in the G1 phase, less sensitive in early S, and least sensitive in late S/G2 (26), well modeling that observed in mammalian cell studies (18-22).
Here we use the yeast DEL assay to measure the sensitivity of radiation-induced DNA deletions with respect to cell cycle phase. The DEL assay is an efficient system for measuring intrachromosomal recombination events characterized by deletion of 6 kb of genomic DNA (27). The RS112 yeast strain carries an internal disruption cassette at the genomic his3 locus; deletion here restores wild-type HIS3 and phenotypic histidine prototrophy. The DEL assay was established previously as a marker for DNA deletions, a subset of genome rearrangements that, when occurring in mammalian cells may be involved in carcinogenesis (28, 29). In validating studies, the DEL assay detected 47 of 50 EPA-listed carcinogens, and for 60 compounds of known carcinogenic activity, it correlated 92% positively with animal carcinogenicity data (30). Ionizing radiation is a potent inducer of DNA deletion events using the DEL assay (28, 31, 32). Here we observed that DNA deletions are significantly more strongly induced in yeast by radiation when cells are in the S/G2 phase compared to G1/G0, which may be important for the best mode of use of the assay. Furthermore, we observed that antioxidant treatment uniquely protected proliferating yeast cells but not stationary cells from radiation-induced DNA deletions.
MATERIALS AND METHODS
Yeast Strain, Media and Reagents
The diploid S. cerevisiae strain RS112 (MATa/MATα ura3-52/ura3-52 leu2-3,112/leu2-Δ98 trp5-27/TRP5 arg4-3/ARG4 ade2-40/ade2-101 ilv1-92/ILV1 HIS3∷pRS6/his3Δ200 LYS2/lys2-801) was used to measure DNA intrachromosomal recombination events at the his3 locus. Synthetic complete (SC or +13) medium was prepared as yeast nitrogen base 0.67%, glucose 2%, agar 2% plus the following amino acids and bases per 900 ml of distilled water: 60 mg each of adenine sulfate, l-isoleucine, l-leucine, l-lysine–HCl, l-tyrosine, 45 mg of l-arginine–HCl, l-histidine–HCl, l-methionine, uracil, 90 mg of l-tryptophan. SC medium lacking histidine (–his) was made as above but without addition of histidine. SC medium lacking leucine (–leu) was made as above but without leucine, and the following was added per 900 ml of distilled water after autoclaving: 18 mg uracil, 36 mg adenine sulfate, and 18 mg l-histidine. For liquid medium preparation, agar was not added.
l-Ascorbic acid (CAS No. A-0278) and DMSO (CAS No. D2650) were purchased from Sigma.
γ Irradiation
Yeast cells were irradiated with 1000 Gy in a Mark I irradiator (J. L. Shepherd and Associates, Glendale, CA) with a 137C γ-ray source at dose rate of ~16.1 Gy/min.
Yeast Proliferation and DEL Assay
The yeast DEL assay was used to score radiation-induced cell killing, DNA deletions and radioprotection using agar plates. Individual clones of RS112 were grown on –leu plates for 56 h at 30°C and then up to 4 weeks at 4°C. A single clone of RS112 was inoculated in ~7 ml –leu medium at 30°C and at subsequent times in 2- to 6-h intervals for 30 h and aliquots were taken and resuspended in distilled water to measure cell density, cell cycle composition, and radiation-induced deletion events. Yeast cells were sonicated for ~8 min prior to scoring cell density and cell cycle distribution. Cell density was measured with a hemocytometer. Cell cycle was assessed by scoring budding yeast (S and G2) and nonbudding yeast (G1 and G0); an average of 266 cells was scored for each individual measurement at each time.
Radiation-induced deletion events were measured as follows: Cells were exposed to 1000 Gy γ rays. Then irradiated cells were plated at 200,000 per –his and 1,000 per +13 plate and unirradiated control cells were plated 100,000 per –his and 100 per +13 plate, each in duplicate. Plates were then incubated at 30°C for 48 h, after which colonies were counted. Survival was calculated by dividing the number of colonies counted on +13 plates by the number of cells plated and the plating efficiency obtained from unirradiated control yeast measurements. The number of colonies scored on –his plates was used to calculate the number of deletion events per 10,000 surviving yeast. The measurements at 0 and 30 min were generated by suspending individual clones directly in either 1 ml water or 1 ml –leu liquid medium, respectively. Both were irradiated ~30 min after inoculation, but the former measurement was considered as time 0 since yeast cells do not proliferate while in water. Experiments without radioprotector measurement were performed in quadruplicate at each time.
For experiments done with l-ascorbic acid (1 mM) and DMSO (1% v/v), two aliquots were taken at each time for irradiation, and compounds were added to a single aliquot ~20 min prior to γ-ray treatment; both antioxidant-treated and nontreated samples were exposed simultaneously to γ rays, and deletion recombination and survival were measured as above. Samples were taken 4–30 h after inoculation. Experiments with radioprotectors were performed in triplicate with control experiments performed in parallel, also in triplicate. Statistical significance was calculated using the two-tailed Student’s t test.
RESULTS
Yeast Proliferation and Radiation-Induced Cytotoxicity and Genotoxicity
Yeast proliferation was measured in liquid cultures for 30 h after inoculation. Single clones comprising of ~106 cells were inoculated in 7 ml –leu medium, and yeast density was measured every 4 h for 24 h and again at 30 h. This time window included the lag, exponential and stationary phases of yeast growth (Fig. 1A, Fig. 2).
FIG. 1.
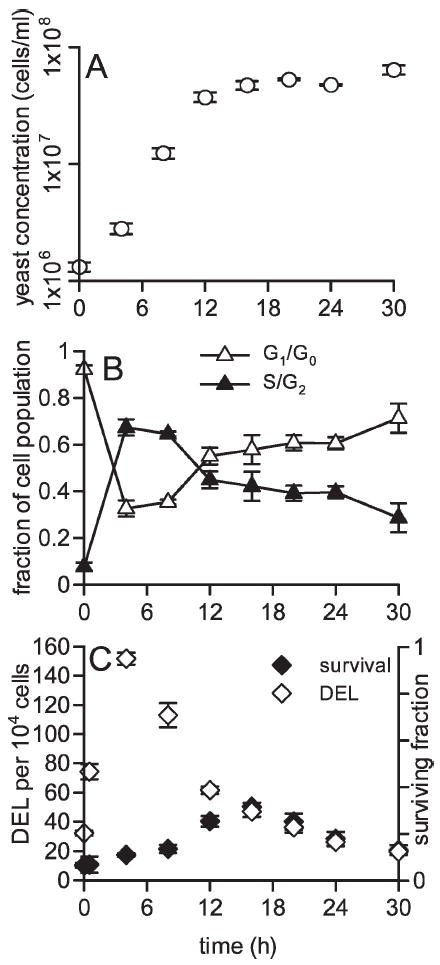
Panel A: Yeast cell density measured in cell cultures as a function of time after inoculation. Panel B: The fractions of cells in G1/G0 and S/G2 in the same cultures. G1/G0 cells were measured by scoring nonbudding yeast and S/G2 cells were measured by scoring budding yeast cells. Panel C: Radiation-induced cell killing and homologous DNA deletion (DEL) events per 104 surviving cells in cell cultures irradiated with 1000 Gy at different times after inoculation. For all panels, each point is the mean of four independent experiments ± SEM.
FIG. 2.
Representative images of budding and non-budding yeast from 0-, 2-, 8- and 30-h cultures are presented in panels A, B, C and D, respectively.
Cell cycle composition was also measured at the same time for 30-h yeast culture growth. Nonbudding yeast observed via microscopy were classified as G1 or G0 phase and budding yeast as S or G2 phase. Initial cultures at time zero were comprised predominantly (>90%) of G1/G0 cells, after 4 h of growth, yeast cultures were comprised predominantly (~75%) of S and G2 cells (Fig. 1B). With time, the fraction of cells in S and G2 diminished as cells entered stationary phase characterized by being primarily in G0 and/or G1 phases.
At each time, aliquots of yeast cultures were exposed to 1000 Gy γ rays and radiation cell killing and DNA deletion events (DEL) were measured. Both end points demonstrated measurable changes in magnitude throughout the three phases of yeast growth. Prior to inoculation, 1000 Gy γ rays induced 32.3 deletion events per 104 surviving cells (Fig. 1C). Four-hour cultures, in which the greatest fraction of cells were in S/G2, were the most sensitive to deletion events induced by 1000 Gy, with 152 induced events per 104 surviving cells. Between 4 and 30 h, as yeast populations began to reaccumulate in G1 and G0 phases, the sensitivity of radiation-induced deletion events decreased correspondingly. After 30 h growth, yeast cultures were well into stationary phase (Fig. 1A) were comprised mostly of G0 cells (Fig. 1B), and only 19.5 deletion events per 104 cells were induced by radiation.
The magnitude of radiation-induced cytotoxicity was also measured throughout the first three phases of cell growth. Yeast cultures just entering stationary phase were the most resistant to γ-ray-induced cell killing (Fig. 1C). Yeast in lag phase and in the end of stationary phase were most sensitive to radiation cytotoxicity. The difference between the maximum and minimum sensitivity of yeast to radiation cytotoxicity throughout 30 h of culture growth was approximately twofold.
Radioprotection and Yeast Culture Growth
The ability of antioxidants DMSO and l-ascorbic acid to protect against cell killing and induction of DNA deletions by radiation was determined throughout yeast growth. DMSO (1%) offered some protection against 1000 Gy γ-ray induced DNA deletions in 4-, 8- and 12-h cultures, with P = 0.061, P = 0.047 and P = 0.16, respectively. At these times, cells are in the exponential growth phase. DMSO did not protect at other times when cells were in stationary phase (Fig. 3A). DMSO also uniquely protected against 1000-Gy-induced cyto-toxicity after 4 and 8 h of growth (P < 0.01 at both times), but protection against cytotoxicity was not observed at later times.
FIG. 3.
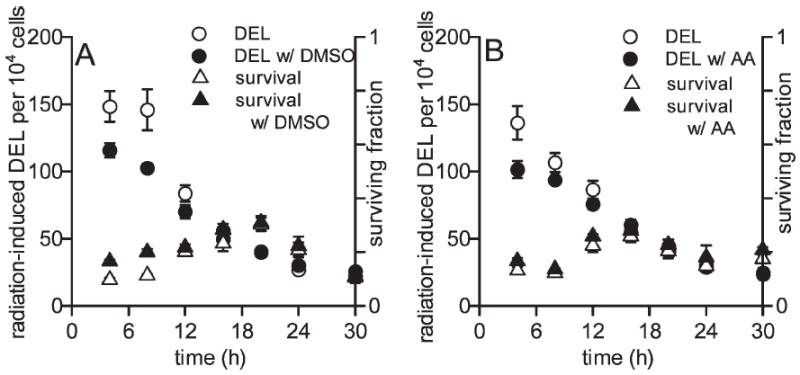
Protection against 1000 Gy γ-ray-induced cell killing and DNA deletions in yeast culture grown for 4 to 30 h by DMSO (panel A) and ascorbic acid (panel B). Protection against deletion events was generally present only during stages of yeast exponential growth and not during stationary phase. Each measurement is the mean of three independent experiments ± SEM.
Yeast cells incubated with 1 mM ascorbic acid during radiation exposure exhibit a response that is similar to that observed with DMSO but reduced in magnitude. Ascorbic acid reduced γ-ray-induced DNA deletions early in exponential yeast growth at 4 and 8 h, but this was not significant (P < 0.07 and P = 0.16, respectively). No protective trend was observed as cells entered stationary phase (Fig. 3B). Protection against cell killing by ascorbic acid was similarly strongest after 4 h and 8 h of growth (P = 0.10 and P = 0.037, respectively), but significant protection was not observed at other times when yeast cells were in stationary phase.
DISCUSSION
Radiation cytotoxicity and genotoxicity were measured throughout yeast culture growth. Radiation cell killing varied by as much as twofold throughout the first three phases of yeast growth (Fig. 1C). Cells were most resistant to radiation cell killing when cultures were entering stationary phase; such resistance did not correlate with cell cycle. Induction of DNA deletion by radiation varied approximately eightfold throughout the phases of yeast growth, and cells were most sensitive to radiation-induced deletions when cultures were in exponential growth, when most cells are in S/G2 phase. For measurements taken throughout the first 30 h of cell growth, the magnitude of radiation-induced DNA deletions correlated positively with the fraction of cells in S/G2 (Fig. 4). The correlation of sensitivity to DNA deletion after irradiation with the fraction of cells in S/G2 is highly significant (P < 0.0001) using a Pearson test (r = 0.775 with 68 degrees of freedom).
FIG. 4.
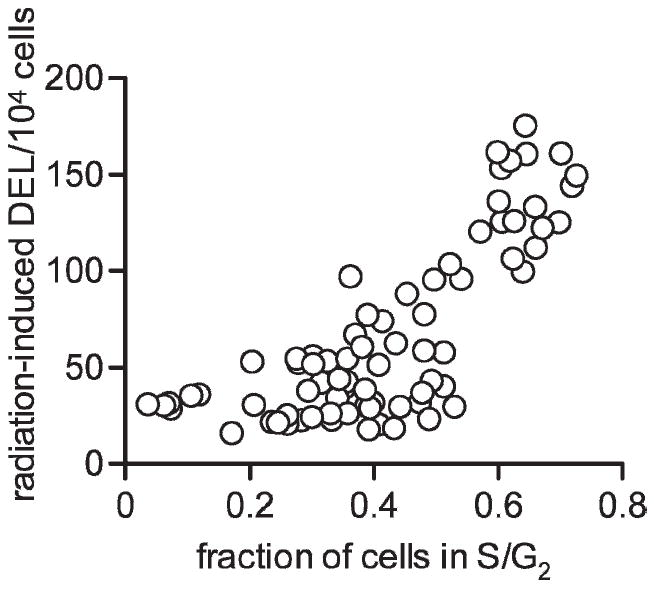
Radiation-induced DNA deletions (DEL) per 104 surviving cells as a function of the fraction of cells in S/G2 for 70 independent measurements. Cultures with more cells in S/G2 were more susceptible to radiation-induced DNA deletions. This correlation was highly significant (P < 0.0001) as calculated by Pearson test.
The variation of the radiation sensitivity of yeast with growth phase observed here complements that reported in previous studies. In multiple studies, budding yeast cells have been observed to be more resistant to radiation inactivation than nonbudding yeast (23, 24, 33). Generally, exponentially growing yeast cells are more resistant to radiation cell killing than cells in stationary phase or G1 (34). Similarly, medium-starved cells (presumed to be in G0 or G1) are more sensitive to radiation inactivation than nonstarved yeast (33, 35). Here yeast cells in late exponential growth are most resistant to radiation cell killing, supporting the observations of Tippins and Parry (34). Furthermore, cells in late stationary phase were most sensitive to radiation cell killing, paralleling the heightened radio-sensitivity of medium-starved yeast observed by Raju et al. (33) and Laser (35).
Previous reports related the relationship between radiation-induced recombination to cell cycle stage. In synchronized yeast cells, the magnitude of both X-ray-induced intra- and intergenic recombination was greatest in G1 cells, lower in S and least in G2 (36). Using arrested cells, X-ray-induced homologous recombination (HR) is greater in G1 than G2, but sister chromatid recombination induction is higher in G2 than G1 (37). Cells arrested in S and G2 phases are more sensitive to γ-ray-induced deletion events than cells arrested in the G1 phase (32) in agreement with our present data. Here, using dividing cells, cultures were comprised predominantly of S/G2-phase cells were as much as eightfold more sensitive to γ-ray-induced deletions than cultures comprised of G0 and G1 cells (Fig. 1).
The severe susceptibility of dividing cells to radiation-induced intrachromosomal recombination compared to nondividing cells observed here is noteworthy. First, the effects of radiation-induced damage fixation in different periods of the cell cycle or different phases of cell growth is biologically relevant. Throughout the body, many cells are nondividing (G0) while others are constantly dividing; this is well modeled by cell cultures in log growth. Thus studies of the effects of radiation on cells in vitro in exponentially dividing cells may not always be relevant to the effects of radiation on tissue in vivo. Second, the cellular response and repair of DNA damage, specifically DSBs, is regulated by cyclin-dependent kinases (CDKs) and thus is dependent on cell cycle stage (38). Cells in G1 repair DSBs using the non-homologous end-joining (NHEJ) pathway, whereas cells in S and G2 repair DSBs predominantly by HR (39). These repair pathways are highly conserved and remain dependent on the cell cycle in both yeast (40) and mammalian cells (41, 42). In agreement with this are our data on the yeast sensitivity to radiation-induced deletion events that correlate with the S/G2 cell cycle stage (Figs. 1 and 4) since deletion events are formed primarily through homologous intrachromosomal (deletion) recombination (28).
The capacity of antioxidants to protect against radiation-induced damages exclusively in dividing cell cultures may have implications for radioprotection in humans. Whereas cell cycle-specific sensitivity to radiation-induced toxicities has been well investigated, studies of cell cycle-dependent radioprotection capacity have been sparse thus far. Here dividing cells were protected with antioxidant treatment against radiation-induced DNA deletions, yet nondividing cells were not protected under the same conditions (Fig. 3). Antioxidants protect against radiation primarily by reducing indirect oxidative base damage caused by radiolytic ROS (43). Base damage lesions have been shown previously to induce deletion events when cells are allowed to undergo DNA replication (44). Thus the observation that proliferating cell cultures are better protected by antioxidant treatment against DNA deletion events is fitting and may have implications for radioprotection against carcinogenesis. The yeast DEL assay is a model for studying radiation-induced homologous DNA deletions (28, 31, 32). Genome rearrangements, specifically including DNA deletions between repetitive elements, are present in many if not most human cancers [for review, see ref. (45)]. Using the DEL assay to qualify antioxidant protection against DNA deletions offers a system for measuring radioprotection against particular genomic rearrangement events involved in carcinogenesis. That observed here (Fig. 3) suggests that rapidly dividing cells are more sensitive to radiation carcinogenesis but are also uniquely protectable with antioxidant treatment against the same. This suggestion is complemented in part by the atomic bomb epidemiological studies in which human tissues comprised of rapidly proliferating cells were observed to be more susceptible to radiation carcinogenesis than slowly dividing tissues (46, 47).
Currently, we are using the yeast DEL assay in a high-throughput format (48) to screen for novel radioprotectors. The data reported here are important for determining the optimal window of sensitivity for screening with the DEL assay. Furthermore, the traditional DEL assay protocol using agar plates use 17 h of incubation with the chemicals to be tested, which encompasses several generations of cell division (49), including the most sensitive phase as determined here. If rapidly dividing yeast cells are hypersensitive to chemically induced deletion recombination like they are to ionizing radiation-induced deletion recombination, this may be one of the reasons why the DEL assay detects carcinogens with greater sensitivity than other genotoxicity assays (30).
Finally, a comparison can be made between cell cycle sensitivity to radiation damages measured in mammalian and yeast in vitro systems. Specific cell cycle stage sensitivity to radiation-induced cell killing, chromosomal aberrations, mutation induction and homologous DNA deletions is tabulated with experimental data from yeast and mammalian cells in Table 1. Conflicting results exist for cell cycle stage dependence on radiation cell killing, yet analogous results exist for mutation induction. Radiation cell killing likely has little relevance to cancer, but mutation induction and intrachromosomal homologous deletion events are both associated with carcinogenesis. Both yeast and mammalian in vitro cultures are most susceptible to radiation mutations early in the cell cycle, but yeast in later stages of the cell cycle are most sensitive to homologous DNA deletion formation. Cell cycle sensitivity to radiation-induced homologous DNA deletions in mammalian cells (50) is thus far undetermined, but studies of this are warranted.
TABLE 1.
Comparison of Yeast and Mammalian Cells for Cell Cycle Dependence of Sensitivity to Ionizing Radiation for Cell Killing and Genotoxicity
| Yeast | Mammalian cells | |
|---|---|---|
| Cell killing | G1 > S and G2 (23, 24) | M > S > G1 and G2 (7, 8) |
| Chromosomal aberrations | No experimental data | G2 > S > G1 (9-14) |
| Mutation induction | G1 > early S > late S and G2 (26) | G1 > early S > late S (18-22) |
| Homologous DNA deletions | S and G2 > G1 and G0 (this study) | No experimental data |
Notes. Cell cycle phase sensitivity to ionizing radiation was tabulated for measurements performed in yeast and mammalian cell systems. The cell cycle phases are listed in order of most sensitive to least sensitive to radiation exposure.
Acknowledgments
KH was funded in part by an NIH National Institute of Biomedical Imaging and Bioengineering training grant and a NASA Graduate Student Researchers Program (GSRP) research fellowship. This work was supported by project 1 to RHS of NIH grant 1 U19 AI 67769-01 to William McBride.
References
- 1.Ward JF. DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Prog Nucleic Acid Res Mol Biol. 1988;35:95–125. doi: 10.1016/s0079-6603(08)60611-x. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt-Ullrich RK, Dent P, Grant S, Mikkelsen RB, Valerie K. Signal transduction and cellular radiation responses. Radiat Res. 2000;153:245–257. doi: 10.1667/0033-7587(2000)153[0245:stacrr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki K, Ojima M, Kodama S, Watanabe M. Radiation-induced DNA damage and delayed induced genomic instability. Oncogene. 2003;22:6988–6993. doi: 10.1038/sj.onc.1206881. [DOI] [PubMed] [Google Scholar]
- 4.Pouget JP, Mather SJ. General aspects of the cellular response to low- and high-LET radiation. Eur J Nucl Med. 2001;28:541–561. doi: 10.1007/s002590100484. [DOI] [PubMed] [Google Scholar]
- 5.Sparrow AH. X-ray sensitivity changes in meiotic chromosomes and the nucleic acid cycle. Proc Natl Acad Sci USA. 1944;30:147–155. doi: 10.1073/pnas.30.7.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puck TT, Marcus PI. Action of X-rays on mammalian cells. J Exp Med. 1956;103:653–666. doi: 10.1084/jem.103.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terasima T, Tolmach LJ. Changes in X-ray sensitivity of HeLa cells during the division cycle. Nature. 1961;190:1210–1211. doi: 10.1038/1901210a0. [DOI] [PubMed] [Google Scholar]
- 8.Hall EJ. Radiobiology for the Radiologist. 3. Lippincott; Philadelphia: 1988. [Google Scholar]
- 9.Dewey WC, Humphrey RM. Relative radiosensitivity of different phases in the life cycle of L-P59 mouse fibroblasts and ascites tumor cells. Radiat Res. 1962;16:503–530. [PubMed] [Google Scholar]
- 10.Dewey WC, Humphrey RM. Relative radiosensitivity of different phases in the life cycle of L-P59 mouse fibroblasts. Radiat Res. 1961;14:461. [PubMed] [Google Scholar]
- 11.Chu EHY, Giles NH, Passano K. Types and frequencies of human chromosome aberrations induced by X-rays. Proc Natl Acad Sci USA. 1961;47:830–839. doi: 10.1073/pnas.47.6.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu TC, Dewey WC, Humphrey RM. Radiosensitivity of cells of Chinese hamster in vitro in relation to the cell cycle. Exp Cell Res. 1962;27:441–452. doi: 10.1016/0014-4827(62)90010-1. [DOI] [PubMed] [Google Scholar]
- 13.Dewey WC, Humphrey RM. Restitution of radiation-induced chromosomal damage in Chinese hamster cells related to the cell’s life cycle. Exp Cell Res. 1964;35:262–276. doi: 10.1016/0014-4827(64)90094-1. [DOI] [PubMed] [Google Scholar]
- 14.Dewey WC, Humphrey RM, Sedita BA. Cell cycle kinetics and radiation-induced chromosomal aberrations studies with C14 and H3 labels. Biophys J. 1966;6:247–260. doi: 10.1016/S0006-3495(66)86654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arlett CF, Potter J. Mutation to 8-azaguanine resistance induced by gamma-radiation in Chinese hamster cell line. Mutat Res. 1971;13:59–65. doi: 10.1016/0027-5107(71)90125-4. [DOI] [PubMed] [Google Scholar]
- 16.Carlson JH, Dewey WC, Hopwood LE. X-ray-induced mutants resistant to 8-azaguanine. II. Cell cycle dose response. Mutat Res. 1976;34:465–480. doi: 10.1016/0027-5107(76)90223-2. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe M, Horikawa M. Analyses of differential sensitivities of synchronized HeLa S3 cells to radiations and chemical carcinogens during the cell cycle. Mutat Res. 1977;44:413–426. doi: 10.1016/0027-5107(77)90099-9. [DOI] [PubMed] [Google Scholar]
- 18.Burki HJ. Ionizing radiation-induced 6-thioguanine-resistant clones in synchronous CHO cells. Radiat Res. 1980;81:76–84. [PubMed] [Google Scholar]
- 19.O’Neill JP, Flint KB. X-ray induction of 6-thioguanine-resistant mutants in division arrested, G0/G1 phase Chinese hamster ovary cells. Mutat Res. 1985;150:443–450. doi: 10.1016/0027-5107(85)90141-1. [DOI] [PubMed] [Google Scholar]
- 20.Grdina DJ, Sigdestad CP. Chemical protection and cell-cycle effects on radiation-induced mutagenesis. Cell Prolif. 1992;25:23–29. doi: 10.1111/j.1365-2184.1992.tb01434.x. [DOI] [PubMed] [Google Scholar]
- 21.Tauchi H, Nakamura N, Sawada S. Cell cycle dependence for the induction of 6-thioguanine-resistant mutations: G2/M stage is distinctively sensitive to 252Cf neutrons but not to 60Co gamma-rays. Int J Radiat Biol. 1993;63:465–481. doi: 10.1080/09553009314550631. [DOI] [PubMed] [Google Scholar]
- 22.Chernikova SB, Lindquisk KL, Elkind MM. Cell cycle-dependent effects of wortmannin on radiation survival and mutation. Radiat Res. 2001;155:826–831. doi: 10.1667/0033-7587(2001)155[0826:ccdeow]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Beam CA, Mortimer RK, Wolfe RG, Tobias CA. The relation of radioresistance to budding in Saccharomyces cerevisiae. Arch Biochem Biophys. 1954;49:110–122. doi: 10.1016/0003-9861(54)90172-1. [DOI] [PubMed] [Google Scholar]
- 24.Langguth EN, Beam CA. Repair mechanisms and cell cycle dependent variations in X-ray sensitivity of diploid yeast. Radiat Res. 1973;53:226–234. [PubMed] [Google Scholar]
- 25.Parry JM, Sharp D, Tippins RS, Parry EM. Radiation-induced mitotic and meiotic aneuploidy in the yeast. Mutat Res. 1979;61:37–55. doi: 10.1016/0027-5107(79)90005-8. [DOI] [PubMed] [Google Scholar]
- 26.Kelly SL, Merrill C, Parry JM. Cyclic variations in sensitivity to X-irradiation during meiosis in Saccharomyces cerevisiae. Mol Gen Genet. 1983;191:312–318. doi: 10.1007/BF00334832. [DOI] [PubMed] [Google Scholar]
- 27.Schiestl RH. Nonmutagenic carcinogens induce intrachromosomal recombination in yeast. Nature. 1989;337:285–288. doi: 10.1038/337285a0. [DOI] [PubMed] [Google Scholar]
- 28.Brennan RJ, Schiestl RH. Persistent genomic instability in the yeast Saccharomyces cerevisiae induced by ionizing radiation and DNA-damaging agents. Radiat Res. 2001;155:768–777. doi: 10.1667/0033-7587(2001)155[0768:pgiity]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 29.Bishop AJ, Schiestl RH. Homologous recombination and its role in carcinogenesis. J Biomed Biotechnol. 2002;2:75–85. doi: 10.1155/S1110724302204052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ku WW, Aubrecht J, Mauthe RJ, Schiestl RH, Fornace AJ., Jr Genetic toxicity assessment: employing the best science for human safety evaluation Part VII: Why not start with a single test: a transformational alternative to genotoxicity hazard and risk assessment. Toxicol Sci. 2007;99:20–25. doi: 10.1093/toxsci/kfm147. [DOI] [PubMed] [Google Scholar]
- 31.Schiestl RH, Gietz RD, Mehta RD, Hastings PJ. Carcinogens induce intrachromosomal recombination in yeast. Carcinogenesis. 1989;10:1445–1455. doi: 10.1093/carcin/10.8.1445. [DOI] [PubMed] [Google Scholar]
- 32.Galli A, Schiestl RH. On the mechanism of UV and gamma-ray-induced intrachromosomal recombination in yeast cells synchronized in different stages of the cell cycle. Mol Gen Genet. 1995;248:301–310. doi: 10.1007/BF02191597. [DOI] [PubMed] [Google Scholar]
- 33.Raju MR, Gnanapurani M, Stackler B, Madhvanath U, Howard J, Lyman JT, Manney TR, Tobias CA. Influence of linear energy transfer on the radioresistance of budding haploid yeast cells. Radiat Res. 1972;51:310–317. [PubMed] [Google Scholar]
- 34.Tippins RS, Parry JM. A comparison of the radiosensitivity of stationary, exponential, and G1 phase wild type and repair deficient yeast cultures: supporting evidence for stationary phase yeast cells being in G0. Int J Radiat Biol. 1982;41:215–220. doi: 10.1080/09553008214550231. [DOI] [PubMed] [Google Scholar]
- 35.Laser H. Some observations on irradiation effects in yeast. Radiat Res. 1962;16:471–482. [PubMed] [Google Scholar]
- 36.Esposito RE. Genetic recombination in synchronized cultures of Saccharomyces cerevisiae. Genetics. 1967;59:191–210. doi: 10.1093/genetics/59.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kadyk LC, Hartwell LH. Replication-dependent sister chromatid recombination in rad1 mutants of Saccharomyces cerevisiae. Genetics. 1993;133:469–487. doi: 10.1093/genetics/133.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ira G, Paellicioli A, Balijja A, Wang X, Fiorani S, Carotenuto W, Liberi G, Bressan D, Wan L, Foiani M. DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature. 2004;431:1011–1017. doi: 10.1038/nature02964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aylon Y, Liefshitz B, Kupiec M. The CDK regulates repair of double-strand breaks by homologous recombination during the cell cycle. EMBO J. 2004;23:4868–4875. doi: 10.1038/sj.emboj.7600469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zierhut C, Diffley JF. Break dosage, cell cycle stage and DNA replication influence DNA double strand break response. EMBO J. 2008;27:1875–1885. doi: 10.1038/emboj.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delacote F, Lopez BS. Importance of the cell cycle phase for the choice of the appropriate DSB repair pathway, for genome stability maintenance: the trans-S double-strand break repair model. Cell Cycle. 2008;7:33–38. doi: 10.4161/cc.7.1.5149. [DOI] [PubMed] [Google Scholar]
- 42.Shrivastav M, De Haro LP, Nickoloff JA. Regulation of DNA double-strand break repair pathway choice. Cell Res. 2008;18:134–147. doi: 10.1038/cr.2007.111. [DOI] [PubMed] [Google Scholar]
- 43.Blakely WF, Fuciarelli AF, Wegher BJ, Dizdaroglu M. Hydrogen peroxide-induced base damage in deoxyribonucleic acid. Radiat Res. 1990;121:338–343. [PubMed] [Google Scholar]
- 44.Galli A, Schiestl RH. Cell division transforms mutagenic lesions into deletion-recombinagenic lesions in yeast cells. Mutat Res. 1999;429:13–26. doi: 10.1016/s0027-5107(99)00097-4. [DOI] [PubMed] [Google Scholar]
- 45.Varshavsky A. Targeting the absence: Homozygous DNA deletions as immutable signposts for cancer therapy. Proc Natl Acad Sci USA. 2007;104:14935–14940. doi: 10.1073/pnas.0706546104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Preston DL, Kusumi S, Tomonaga M, Izumi S, Ron E, Kuramoto A, Kamada N, Dohy H, Matsui T, Mabuchi K. Cancer incidence in atomic bomb survivors. Part III: Leukemia, lymphoma and multiple myeloma, 1950–1987. Radiat Res. 1994;137(Suppl):S68–S97. [PubMed] [Google Scholar]
- 47.Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, Mabuchi K, Kodama S. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res. 2007;168:1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 48.Hontzeas N, Hafer K, Schiestl RH. Development of a microtiter plate version of the yeast DEL assay amenable to high-throughput toxicity screening of chemical libraries. Mutat Res. 2007;634:228–234. doi: 10.1016/j.mrgentox.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Brennan RJ, Schiestl RH. Detecting carcinogens with the yeast DEL assay. Methods Mol Biol. 2004;262:111–124. doi: 10.1385/1-59259-761-0:111. [DOI] [PubMed] [Google Scholar]
- 50.Aubrecht J, Rugo RE, Schiestl RH. Carcinogens induce intrachromosomal recombination in human cells. Carcinogenesis. 1995;16:2841–2846. doi: 10.1093/carcin/16.11.2841. [DOI] [PubMed] [Google Scholar]


