Abstract
Background
A total number of 14 valid species of Diphyllobothrium tapeworms have been described in literature to be capable of causing diphyllobothriosis, with D. latum being the major causative agent of all human infections. However, recent data indicate that some of these infections, especially when diagnosed solely on the basis of morphology, have been identified with this causative agent incorrectly, confusing other Diphyllobothrium species with D. latum. Another widely distributed species, D. dendriticum, has never been considered as a frequent parasite of man, even though it is found commonly throughout arctic and subarctic regions parasitizing piscivorous birds and mammals. Recent cases of Europeans infected with this cestode called into question the actual geographic distribution of this tapeworm, largely ignored by medical parasitologists.
Methodology and Results
On the basis of revision of more than 900 available references and a description and revision of recent European human cases using morphological and molecular (cox1) data supplemented by newly characterized D. dendriticum sequences, we updated the current knowledge of the life-cycle, geographic distribution, epidemiological status, and molecular diagnostics of this emerging causal agent of zoonotic disease of man.
Conclusions
The tapeworm D. dendriticum represents an example of a previously neglected, probably underdiagnosed parasite of man with a potential to spread globally. Recent cases of diphyllobothriosis caused by D. dendriticum in Europe (Netherlands, Switzerland and Czech Republic), where the parasite has not been reported previously, point out that causative agents of diphyllobothriosis and other zoonoses can be imported throughout the world. Molecular tools should be used for specific and reliable parasite diagnostics, and also rare or non-native species should be considered. This will considerably help improve our knowledge of the distribution and epidemiology of these human parasites.
Introduction
Diphyllobothriosis is a human disease caused by fish tapeworms (or broad tapeworms) of the genus Diphyllobothrium Cobbold, 1858 (Cestoda: Diphyllobothriidea). It represents the most important fish-borne zoonosis caused by tapeworms [1]. Most of the cases are asymptomatic, but in about one out of five cases, diarrhoea, abdominal pain, or discomfort occurs [1]. Humans get infected by eating raw, insufficiently cooked, or marinated freshwater and marine fish. Increasing popularity of dishes based on raw fish meat, such as sushi, sashimi, carpaccio, or ceviche, significantly increases the risk of acquiring the parasite, even in the most developed countries. As many as 14 species of Diphyllobothrium have been described as capable of causing diphyllobothriosis, with D. latum (Linnaeus, 1758) being the dominant species in human infections. Together with D. nihonkaiense Yamane, Kamo, Bylund et Wikgren, 1986, D. latum is also considered to be the most pathogenic for man [1].
Routine diagnostics of human infections are currently based on morphological observations of relatively small eggs with an operculum and/or segments (proglottides) with median genital pores. Such cases are mostly identified as D. latum or as unspecified Diphyllobothrium infections. However, recent data indicate that some of these infections, especially when diagnosed solely on the basis of morphology, have been misidentified. It is thus highly probable that prevalence of other human-infecting Diphyllobothrium species is currently underestimated. A molecular diagnostic based on genetic markers such as cox1 gene has helped identify new cases of diphyllobothriosis in non-endemic regions, which would indicate import of these parasites to new geographical areas. This would be the case in recent D. latum re-emergence in the Alpine region of Central Europe (France, Italy and Switzerland) [1]–[4].
Diphyllobothrium latum has circumboreal distribution, with most cases reported in northern Europe, Russia (Karelia, Siberia, and Far East), and North America (Canada and Alaska), but has been recently found also in South America (Chile) [1]. Another human-infecting species, D. nihonkaiense, seems to dominate the northern Pacific region, whereas D. pacificum (Nybelin, 1931) is endemic to the southern Pacific (coast of South America) [1]. Another widely distributed species, D. dendriticum (Nitzsch, 1824), has never been considered as an important or frequent parasite of man [5], [6], even though it is rather common in arctic regions [7]. This cestode typically parasitizes arctic and subarctic piscivorous birds (such as gulls) and mammals (such as foxes or bears); human infections have been generally considered accidental [1].
The literature was searched in Rosenberg (1977) [8] and in online databases Web of Knowledge and Pubmed with the key words “Diphyllobothrium” and “Diphyllobothrium dendriticum” (July 2013).
Brief Historical Overview
Diphyllobothrium dendriticum was originally described as Bothriocephalus dendriticus from Larus tridactylus (now Ricca tridactyla) by Nitzsch (1824) in northern Germany [9]. The first documented case of human infection with this tapeworm was reported by Cholodkovsky (1916) as Dibothriocephalus minor from a man living on the banks of Lake Baikal, Russia [10]. Another species described from man in Russia was Diphyllobothrium strictum by Talysin (1932) on Olkhon Island of Lake Baikal and Diphyllobothrium nenzi Petrov, 1938 from the lower Pechora River [11], [12]. However, all these species are considered to be synonyms of D. dendriticum because they are morphologically indistinguishable [13]–[16].
Thus, most diphyllobothriosis cases caused by D. dendriticum were reported from the region of Siberia, especially from the surroundings of Lake Baikal, along with a few from north Canada and Alaska [5], [14]. In contrast, there have been no reliable records of autochthonous human infection in Europe, and all recent reports from Europe most probably represent imported infections (see below) [1], [7].
The actual proportion of diphyllobothriosis caused by D. dendriticum is difficult to estimate from the literature because voucher material has rarely been deposited in museum collections for later scrutiny and is seldom suitable for molecular analyses (samples fixed in formalin or AFA, i.e., a mixture of alcohol, formalin, and acetic acid). In addition, several species of Diphyllobothrium may occur sympatrically, such as D. latum and D. dendriticum in Siberia, and D. alascense Rausch et Williamson, 1985, D. dalliae Rausch, 1956, D. dendriticum, D. latum, D. ursi Rausch, 1954, and recently recognised D. nihonkaiense in North America, which further complicates correct diagnosis [1], [17].
Recent Human Cases
Two clinical cases of D. dendriticum infection from Switzerland were confirmed recently by molecular methods [18], [19]. One case was reported from a 59-year-old woman who had visited Norway, Canada, and Alaska one and six years before the infection was detected, respectively. This woman also consumed fish frequently. The other case was a four-year-old boy who had visited tropical Asia and ate smoked or poorly cooked fish there. He had also consumed imported Atlantic salmon (Salmo salar) from Norway and perch (Perca fluviatilis) from Switzerland.
The third case, falsely assigned as a D. latum infection, was reported from the Netherlands. Subsequent molecular phylogenetic analysis proved that the 31-year-old man was infected with D. dendriticum (present data, Figure 1). This man had visited Brazil five months prior to the infection was detected and successfully treated, but anamnestic data do not allow to make a safe conclusion on the origin of the infection [20].
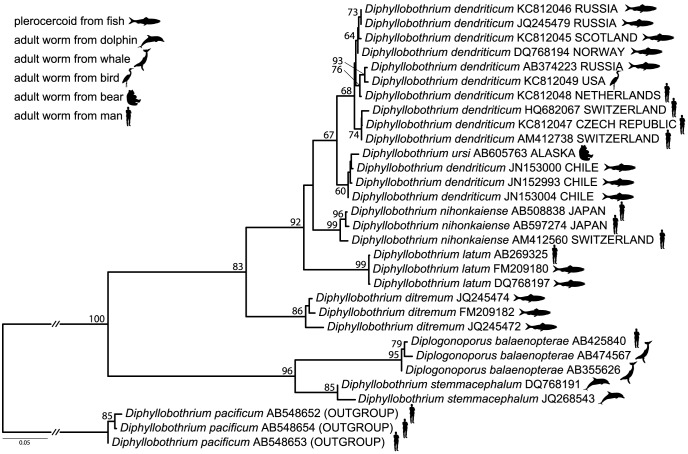
Figure 1. Maximum likelihood (ML) estimate of Diphyllobothrium dendriticum phylogenetic relationships based on currently available cox1 sequences of human-infecting Diphyllobothrium species and their close relatives computed in Garli 2.0.
Nucleotide data matrix was 1563; codon positions were analyzed separately according to the partition scheme and models (TrN+I) (F81) (TrN+I+G) chosen according to the BIC in PartitionFinder 1.0.1. Nodal support values depict bootstrap support proportions >50 based on 1,000 repetitions estimated in Garli. Note that the D. pacificum branch was shortened by a factor of two. Newly obtained sequences are shown in bold type; country of origin is listed for D. dendriticum infections.
The most recent case was recorded in the Czech Republic. A 28-year-old woman that had spent summer 2010 as a seasonal worker in a fish processing plant located in Klawock, southeast Alaska, found a large piece of a tapeworm strobila (∼30 cm) on Jan 5, 2011 (Figure 2). She was treated by a single dose of praziquantel (Cesol), 750 mg, on that day, and the three subsequent stool laboratory checks on January 10–13 returned negative. She acknowledged eating barbecued fish (especially sockeye and pink salmon, O. nerka and O. gorbuscha) and occasionally other wild salmonids like Arctic cisco (Coregonus autumnalis) during her summer job. Molecular phylogenetic analyses based on sequences of the complete cytochrome c oxidase subunit 1 (cox1) and partial large subunit nuclear ribosomal DNA (lsrDNA) genes unequivocally determined the cestode as D. dendriticum (Figure 1).
Figure 2. Morphology of Diphyllobothrium dendriticum.
(A–E, G, H) Human case from the Czech Republic. (A) Whole worm. (B, C) Whole mount of gravid proglottids and their detail. (D) Sagittal section of gravid proglottids. (E, I) Egg in light microscope and scanning electron micrograph (SEM). (F) Scolex (SEM). (G) Lateral extremities of gravid segments (SEM). (H) Whole worm. (F, H) SEM of the specimen from experimentally infected hamster.
Morphology and Differential Diagnosis
Diphyllobothrium dendriticum is a large tapeworm, with body (strobila) length reaching up to 1 m and width up to 1 cm, respectively (Figure 2). The strobila consists of several hundred segments (proglottids), each containing one set of male and female genital organs (Figure 2). Depending on the host and physiological state, the body can vary considerably in size and shape [5]. The head (scolex) is usually spatulate in shape, but its shape varies according to the state of contraction. Neck (proliferation zone between the scolex and strobila) is present in relaxed specimens. Gravid segments, i.e., segments containing fully formed eggs in the uterus, are usually wider (0.82–10.0 mm) than long (0.13–2.1 mm) and have concave lateral margins, with more or less pointed projections formed bilaterally at each segmental junction (Figure 2) [5].
Testes are spherical, numerous, in the medulla (i.e., internal to the inner longitudinal musculature), and confluent between segments and across the anterior margin of the segment. The cirrus-sac (terminal part of the male reproductive organ) is round in dorsoventral view, but oblique in sagittal section (Figure 2). The external seminal vesicle is small, less than half-size of the cirrus-sac, muscular, dorsal to the cirrus-sac, and not visible from the ventral side (Figure 2). The common genital pore (joint opening of the cirrus-sac and vagina) is median, at about anterior third (21–37%) of the length of the segment, situated on an elevation densely covered with papillae (nipples), often elliptical in shape (Figure 2).
The ovary is bilobed, near the posterior margin of the segment, and variable in shape. Vitelline follicles are numerous, small, spherical, situated in the cortex (i.e. external to the inner longitudinal musculature), and confluent between segments and across the anterior margin of the segment. The uterus is tubular, forms six to eight loops reaching up to the cirrus-sac, opening on the ventral side of the segment at its midline, immediately posterior to the genital pore. The eggs are thick-shelled, operculate, 50–70 µm long, and 30–52 µm wide; their surface is almost smooth with a few shallow pits (Figure 2) [14], [18], [19, present study].
Routine diagnostics of diphyllobothriosis is mainly based on the finding of the eggs in stool samples. The size and shape of the operculate eggs, with their minute terminal knobs, are characteristic for the genus, but their species identification is usually impossible because of high intraspecific variation [17], [21], [22]. The species of Diphyllobothrium are most readily distinguished by the shape and size of the scolex, neck, and male genital organs (visible in sagittal sections), i.e., morphological features that are not possible to evaluate in most clinical cases (the scolex and neck are rarely found and proglottides are deformed or decomposed as a result of treatment with anthelminthics or inappropriate sample processing).
Diphyllobothrium dendriticum differs from the remaining human-infecting species (D. latum, D. nihonkaiense, and D. pacificum) by featuring more concave lateral margins of gravid segments, a more dorsal external seminal vesicle in relation to the cirrus-sac (not visible from the ventral side) and some segments with vitelline follicles confluent at its anterior part [5]. Recently, a molecular test based on multiplex PCR of mitochondrial cox1 gene has been developed, allowing for a quick differential diagnosis between the common human-infecting Diphyllobothrium species [23].
Molecular Diagnosis and Systematics
Molecular diagnostic methods based on analyses of the cox1 gene sequences proved capable of reliably distinguishing among the species of human-infecting broad fish tapeworms. The cox1 gene represents a widely used molecular barcoding marker for species determination of various groups of animals, whose elevated rate of sequence evolution allowed for accumulation of a sufficient number of nucleotide substitutions that are capable of distinguishing one tapeworm species from another. Even a partial sequence of this gene is able to determine the correct species. However, universal primers that amplify the entire cox1 gene of any Diphyllobothrium species exist and should preferably be used, as they provide significantly more data. Species-specific primers that anneal at distinct positions of the cox1 gene then form the basis of a molecular diagnostic method based on multiplex PCR that allows for rapid differential diagnosis of the human-infecting Diphyllobothrium species [23].
Phylogenetic analyses have continuously indicated a close relationship of D. dendriticum, D. latum, and D. nihonkaiense. The last common human parasite, D. pacificum, is apparently a more distantly related taxon, most probably forming a basal lineage of the genus Diphyllobothrium [3], [23]–[27]. Out of the molecular markers used, cox1 performs best in both phylogenetic reconstructions and comparative diagnostic analyses. Just recently, the phylogenetic status of D. ursi, another human-infecting species described by Rausch (1954) from bear (Ursus arctos) in Alaska, was assessed [28], [29]. In these analyses inferred from cox1 gene sequences, validity of this North American species was supported [29].
A phylogenetic tree based on currently available cox1 sequences supplemented by new data on several isolates from intermediate and definitive hosts (5 new sequences; see Table 1) suggests a sister-group relationship of D. dendriticum and D. nihonkaiense, D. latum being the sister of the two (Figure 1). However, this branching pattern never gets statistically supported and tends to change according to the method of analysis. The present analysis also questioned the correct determination of Diphyllobothrium dendriticum samples from Oncorhynchus mykiss from Chile that formed a distinct lineage with D. ursi apart from the remaining D. dendriticum representatives [30]. Based on the current data, however, it is not possible to test if D. dendriticum from Chile was in fact misdiagnosed D. ursi or if D. ursi represents a large form of D. dendriticum from bears (Figure 1).
Table 1. Sequences of Diphyllobothrium dendriticum used in phylogenetic analysis (Figure 1).
| Access. No. | Stage | Host | Locality (possible origin in parentheses) | Authority |
| KC812046 | plerocercoid | Coregonus autumnalis | Lake Baikal, Russia | new sequence |
| JQ245479 | plerocercoid | Coregonus autumnalis | Lake Baikal, Russia | Suleymanov et al. (unpublished article) |
| KC812045 | plerocercoid | Coregonus lavaretus | Loch Lomond, Scotland, UK | new sequence |
| DQ768194 | plerocercoid | Salvelinus alpinus | Fjellfrosvatn Lake, Norway | Yera et al. 2008 [3] |
| AB374223 | plerocercoid | Salvelinus leucomaenis | Lake Azabachye, Kamchatka, Russia | Arizono et al. 2009 [53] |
| KC812049 | adult | Larus hyperboreus | Kansas, USA | new sequence |
| KC812048 | adult | Homo sapiens | Netherlands (Brazil) | new sequence** |
| HQ682067 | adult | Homo sapiens | Switzerland | de Marval et al. 2013 [19] |
| KC812047 | adult | Homo sapiens | Czech Republic (Alaska) | new sequence |
| AM412738 | adult | Homo sapiens | Bern, Switzerland | Wicht et al. 2008 [18] |
| AB605763* | adult | Ursus arctos middendorffi | Kodiak Island, Alaska, USA | Yamasaki et al. 2012 [29] |
| JN152993* | plerocercoid | Oncorhynchus mykiss | Tarahuin Lake, Chile | Rozas et al. 2012 [30] |
| JN153000* | plerocercoid | Oncorhynchus mykiss | Tarahuin Lake, Chile | Rozas et al. 2012 [30] |
| JN153004* | plerocercoid | Oncorhynchus mykiss | Natri Lake, Chile | Rozas et al. 2012 [30] |
sequences of Diphyllobothrium ursi;
see van Doorn et al. 2005 [20].
The origin of D. dendriticum in South America is not known. It has been probably imported by migratory birds such as Sterna hirundo, S. paradisea, and Larus pipixcan on their visits to South America [31]. Completing of the life-cycle was most likely possible due to the introduction of the second intermediate host—rainbow trout O. mykiss—at the beginning of the 20th century. However, native fish, such as Galaxias maculatus, G. platei, Diplomystes composensis, Percichthys trucha and several others are also infected with Diphyllobothrium plerocercoids in Chile [32].
Life Cycle and Epidemiology
The life cycle of D. dendriticum is similar to life cycles of other Diphyllobothrium species, all of which include three hosts [1]. Planktonic copepods serve as the first intermediate hosts in which the larval stage, called procercoid, develops. The second larval stage or metacestode, called plerocercoid, develops in freshwater and anadromous fishes, especially salmonids [5]. However, D. dendriticum plerocercoids were found in more than 50 species of 12 families of freshwater fish (Abyssocottidae, Atherinopsidae, Balitoridae, Comephoridae, Cottidae, Cottocomephoridae, Gadidae, Galaxiidae, Gasterosteidae, Osmeridae, Percichthyidae, and Salmonidae) [33]. Despite this extraordinarily wide spectrum of fish hosts, D. dendriticum has never been reported from naturally or experimentally infected perch (Perca fluviatilis) or pike (Esox lucius), which are the principal second intermediate hosts of D. latum in the Palaearctic region [5], [34].
Plerocercoids of D. dendriticum are usually encysted within the visceral organs or body cavity, whereas records of free larvae in the muscles of Pacific salmons and trouts such as Oncorhynchus nerca and O. clarki should be considered doubtful unless verified using molecular markers [5], [35]. Humans can become infected either by consuming raw or undercooked visceral organs, e.g., liver and ovaries, or flesh of fish that were gutted, but where plerocercoids remained on the ventral abdominal flap attached to the fillet or migrated to the musculature [36], [37].
Adults of D. dendriticum have been found in birds of nine families (Accipitridae, Alcidae, Corvidae, Gaviidae, Laridae, Pandionidae, Pelecanidae, Podicipedidae, and Sternidae), with the majority of the records coming from gulls (Laridae) [38], [39], [33]. The prevalence of infection in these principal definitive hosts is usually low (1–25%) [40]–[43], except for gulls in the endemic area of Baikal, where up to 76% Larus argentatus were infected [13], [39], [44]. Common definitive hosts are also mammals, such as the arctic fox (Alopex lagopus) with prevalence of 4–15% reported from Greenland and Iceland [45]–[47].
The prepatent period of D. dendriticum is short, less than two weeks in man, with maximum egg shedding in late summer and fall [6], [7], [36]. The parasite longevity in the definitive host is assumed to last only four to six months [7], but Wicht et al. (2008) reported a patient infected with D. dendriticum with two years of chronically relapsing diarrhoea [18].
Geographical Distribution and Endemic Areas
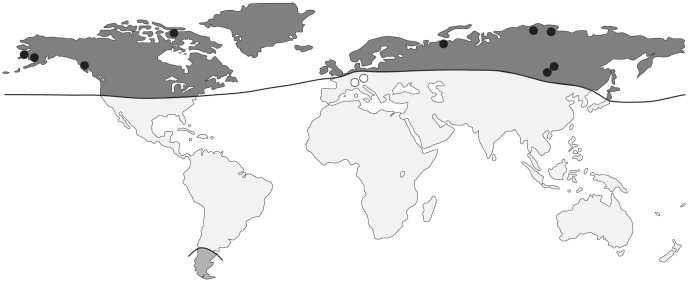
The original distribution of D. dendriticum is circumboreal, but the parasite was allegedly found also in trout introduced to Argentina and Chile (see Molecular Diagnosis and Systematics) (Figure 3) [30], [31], [48]. The geographical distribution of D. dendriticum and D. latum overlaps; however, D. dendriticum tends to predominate in arctic regions, where it infects salmonids and coregonids, whereas D. latum infections are characteristic for more subarctic and temperate areas (Figure 3) [7].
Figure 3. Geographical distribution and human cases of Diphyllobothrium dendriticum.
Black dots represent autochthonous human cases; white dots represent imported human cases. Black line delimits the area of D. dendriticum distribution (grey colour).
In the Lake Baikal region, the prevalence of cases with D. dendriticum decreased markedly from almost 30% in 1929 to less than 0.01% in 2005–2007 [13], [49], however, the prevalence in other regions of Russia (Buryatia, Krasnoyarsk district, Taymyr Peninsula, and Yakutia) remains rather high (up to 14%) [49]–[51] (2012 email from I. Kutyrev, Ulan-Ude to the senior author; unreferenced).
Another endemic area of D. dendriticum is arctic North America, but the number of cases is much lower compared to Siberia [5], [17]. At least ten human records have been confirmed from Alaska, Nunavut and British Columbia, mostly from native Inuit populations, but the real numbers are unknown [5, present study].
Medical Importance and Control
Diphyllobothriosis is not a life-threatening disease and most human cases are mild or even asymptomatic [1]. Since the causative agents of numerous clinical cases have not been reliably identified, it is not possible to distinguish differences in the pathogenicity of individual species, including D. dendriticum. However, we assume that D. dendriticum does not represent a serious human pathogen that would cause disease with severe clinical signs. Nevertheless, some infections can result in chronically relapsing diarrhoea and thus require the attention of medical doctors and adequate treatment [18].
Rolf Vik infected himself with D. dendriticum while in the United States working on Oncorhynchus clarkii in order to take the adult tapeworms back to Norway to make comparisons with Norwegian species. He did not observe any health problems [5].
Treatment of diphyllobothriosis is effective, with praziquantel being the drug of choice. Prophylaxis is also straightforward—the key measure is to avoid consumption of raw or undercooked fish. However, consumption of raw or lightly pickled fish is considered a traditional delicacy by numerous nationalities and this habit has gained ground quickly around the world.
Prevention of water contamination through waste water purification in sewage plants represents another way of controlling the disease. However, its impact is limited in the case of D. dendriticum because a variety of definitive hosts might serve as reservoirs of the disease in a given area. The elevated mobility of these reservoir hosts (especially piscivorous birds) enables the parasite to disseminate eggs over large areas, thus representing a serious obstacle in the control of diphyllobothriosis caused by D. dendriticum.
Climate change will result in faunal shift (global warming) and will influence parasites, especially those that undergo temperature-dependent development [52]. This also concerns broad fish tapeworms (Diphyllobothrium spp.), including D. dendriticum, the life cycle of which is realized in a freshwater environment (first intermediate hosts are planktonic copepods and second intermediate hosts are teleost fishes). Climate change and global warming certainly represent new challenges to assess their impact on ecosystems, including aquatic ones, with corresponding impact on parasite distribution [52].
Conclusions
Parasitic infections caused by tapeworms (Cestoda) do not generally represent a serious public health concern in developed countries, with relatively very few exceptions such as echinococcosis, sparganosis, and cysticercosis,. Nevertheless, several food-borne diseases and/or zoonoses have emerged during the last decades as a result of global trade (transport of fresh fish “on the ice”), increased mobility of people, and their changing eating habits, i.e., increased popularity of raw or undercooked food. The tapeworm Diphyllobothrium dendriticum represents an example of a previously neglected, probably underdiagnosed parasite of man with potential to spread globally.
Recent cases of diphyllobothriosis caused by D. dendriticum in Europe (Netherlands, Switzerland and Czech Republic), where the parasite has not been reported previously, represent evidence that causative agents of zoonoses can be imported throughout the world. It is thus necessary to pay attention also to previously rare or non-native parasites. Molecular tools should be used for specific and reliable diagnostics, which may considerably help improve our knowledge of the distribution and epidemiology of these human parasites.
Key Learning Points
On the basis of revision of more than 900 available references and a description and revision of recent European human cases using morphological data, we updated the current knowledge of the life cycle, geographic distribution, and epidemiological status of this emerging causal agent of zoonotic disease of man.
The molecular (cox1) data supplemented by five newly characterized D. dendriticum sequences and molecular diagnostics of this emerging disease were added and discussed.
The tapeworm Diphyllobothrium dendriticum represents an example of a previously neglected, probably underdiagnosed parasite of man with a potential to spread globally.
Five Key Papers in the Field
Scholz T, Garcia HH, Kuchta R, Wicht B (2009) Update on the human broad tapeworm (genus Diphyllobothrium), including clinical relevance. Clin Microbiol Rev 22: 146–160.
de Marval F, Gottstein B, Weber M, Wicht B (2013) Imported diphyllobothriasis in Switzerland: molecular methods to define a clinical case of Diphyllobothrium infection as Diphyllobothrium dendriticum, August 2010. Euro Surveill 18: 31–36.
Wicht B, Ruggeri-Bernardi N, Yanagida T, Nakao M, Peduzzi R, et al. (2010) Inter- and intra-specific characterization of tapeworms of the genus Diphyllobothrium (Cestoda: Diphyllobothriidea) from Switzerland, using nuclear and mitochondrial DNA targets. Parasitol Int 59: 35–39.
Dupouy-Camet J, Peduzzi R (2004) Current situation of human diphyllobothriasis in Europe. Eurosurveillance 9: 31–34.
Curtis MA, Bylund G (1991) Diphyllobothriasis: fish tapeworm disease in the circumpolar north. Arctic Med Res 50: 18–25.
Acknowledgments
The authors are indebted to numerous persons who kindly provided valuable information, as well as those who sent clinical samples of tapeworms for molecular evaluation. Special thanks are due to Karin Andersen (Norway), Naoki Arizono (Japan), Oleg Ditrich (Czech Republic), Jean Dupouy-Camet (France), Anindo Choudhury (United States), Ivan Kutyrev (Russia), Kateřina Leštinová (Czech Republic), Barbara Wicht (Switzerland), and Helène Yera (France).
Funding Statement
Grant Agency of the Czech Republic (project No. P506/12/1632). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Scholz T, García HH, Kuchta R, Wicht B (2009) Update on the human broad tapeworm (genus Diphyllobothrium), including clinical relevance. Clin Microbiol Rev 22: 146–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wicht B, Ruggeri-Bernardi N, Yanagida T, Nakao M, Peduzzi R, et al. (2010) Inter- and intra-specific characterization of tapeworms of the genus Diphyllobothrium (Cestoda: Diphyllobothriidea) from Switzerland, using nuclear and mitochondrial DNA targets. Parasitol Int 59: 35–39. [DOI] [PubMed] [Google Scholar]
- 3. Yera H, Nicoulaud J, Dupouy-Camet J (2008) Use of nuclear and mitochondrial DNA PCR and sequencing for molecular identification of Diphyllobothrium isolates potentially infective for humans. Parasite 15: 402–407. [DOI] [PubMed] [Google Scholar]
- 4. Dupouy-Camet J, Peduzzi R (2004) Current situation of human diphyllobothriasis in Europe. Eurosurveillance 9: 31–34. [PubMed] [Google Scholar]
- 5. Andersen KI, Ching HL, Vik R (1987) A review of freshwater species of Diphyllobothrium with redescriptions and the distribution of D. dendriticum (Nitzsch, 1824) and D. ditremum (Creplin, 1825) from North America. Can J Zool 65: 2216–2228. [Google Scholar]
- 6. Curtis MA, Rau ME, Tanner CE, Prichard RK, Faubert GM, et al. (1988) Parasitic zoonoses in relation to fish and wildlife harvesting by Inuit communities in northern Quebec, Canada. Arctic Med Res 47: 693–696. [PubMed] [Google Scholar]
- 7. Curtis MA, Bylund G (1991) Diphyllobothriasis: fish tapeworm disease in the circumpolar north. Arctic Med Res 50: 18–25. [PubMed] [Google Scholar]
- 8.Rosenberg AI (1977) Diphyllobothriides and Diphyllobothrioses of Medical Veterinary Significance: Index of Native and Foreign Literature 1558–1972. Petrozavodsk: Karelia. (In Russian.) [Google Scholar]
- 9.Nitzsch CL (1824) Bothriocephalus. In: Ersch JS, Gruber JG. Allgemeine Encyclopaedie der Wissenschaften und Künste. Leipzig: Gleditsch. pp. 94–97. [Google Scholar]
- 10. Cholodkovsky (1916) Sur un nouveau parasite de l'homme. Zool Zh 1: 231–237. [Google Scholar]
- 11. Petrov MI (1938) New diphyllobothriid of man. Med Parazitol 7: 406–414 (In Russian.). [Google Scholar]
- 12. Talysin T (1932) Dibothriocephalus strictus n. sp. Menschenparasit des Baikalgestades. Z Parasitenkd 4: 722–729. [Google Scholar]
- 13. Chizhova TP, Gofman-Kadoshnikov PB (1960) The natural focus of diphyllobothriosis in the Baikal lake and its pattern. Med Parazitol 29: 168–176. [PubMed] [Google Scholar]
- 14. Serdyukov AM (1972) Diphyllobothrium dendriticum (Nitzsch, 1824) – parasite of man in Tumensky district. Parazitologiya 6: 419–425 (In Russian.). [Google Scholar]
- 15.Delyamure SL, Skryabin AS, Serdiukov AM (1985) Diphyllobothriata – flatworm parasites of man, mammals and birds. Ponova TI, Sonc MD, editors. Moscow, Russia: Nauka. 200 p. (In Russian.) [Google Scholar]
- 16.Kamo H (1999) Guide to identification of diphyllobothriid cestodes. Tokyo, Japan: Gendai Kikaku. 146 p. (In Japanese.) [Google Scholar]
- 17. Rausch RL, Hilliard DK (1970) Studies on the helminth fauna of Alaska. XLIX. The occurrence of Diphyllobothrium latum (Linnaeus, 1758) (Cestoda: Diphyllobothriidae) in Alaska, with notes on other species. Can J Zool 48: 1201–1212. [DOI] [PubMed] [Google Scholar]
- 18. Wicht B, de Marval F, Gottstein B, Peduzzi R (2008) Imported diphyllobothriasis in Switzerland: molecular evidence of Diphyllobothrium dendriticum (Nitzsch, 1824). Parasitol Res 102: 201–204. [DOI] [PubMed] [Google Scholar]
- 19. de Marval F, Gottstein B, Weber M, Wicht B (2013) Imported diphyllobothriasis in Switzerland: molecular methods to define a clinical case of Diphyllobothrium infection as Diphyllobothrium dendriticum, August 2010. Eurosurveillance 18: 31–36. [PubMed] [Google Scholar]
- 20. van Doorn HR, van Vugt M, Wetsteyn JC, van Gool T (2005) Infestation with the tapeworm Diphyllobothrium latum after eating raw fish. Ned Tijdschr Genees 149: 2470–2472. [PubMed] [Google Scholar]
- 21. Hilliard DK (1960) Studies on the helminth fauna of Alaska. XXXVIII. The taxonomic significance of eggs and coracidia of some diphyllobothriid cestodes. J Parasitol 46: 703–715. [PubMed] [Google Scholar]
- 22. Andersen KI, Halvorsen O (1978) Egg size and form as taxonomic criteria in Diphyllobothrium . Parasitology 76: 229–240. [DOI] [PubMed] [Google Scholar]
- 23. Wicht B, Yanagida T, Scholz T, Ito A, Jiménez JA, et al. (2010) Multiplex PCR for differential identification of broad tapeworms (Cestoda: Diphyllobothrium) infecting humans. J Clin Microbiol 48: 3111–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wicht B, de Marval F, Peduzzi R (2007) Diphyllobothrium nihonkaiense (Yamane et al., 1986) in Switzerland: First molecular evidence and case reports. Parasitol Int 56: 195–199. [DOI] [PubMed] [Google Scholar]
- 25. Abe N (2009) Identification of the larval cestode plerocercoid found in the body cavities of the japanese smelt Hypomesus transpacificus nipponensis, and review of the literature. J Urban Living Health Assoc 53: 110–116. [Google Scholar]
- 26. Yamasaki H, Kuramochi T (2009) A case of Diphyllobothrium nihonkaiense infection possibly linked to salmon consumption in New Zealand. Parasitol Res 105: 583–586. [DOI] [PubMed] [Google Scholar]
- 27. Mercado R, Yamasaki H, Kato M, Munoz V, Sagua H, et al. (2010) Molecular identification of the Diphyllobothrium species causing diphyllobothriasis in Chilean patients. Parasitol Res 106: 995–1000. [DOI] [PubMed] [Google Scholar]
- 28. Rausch RL (1954) Studies on the helminth fauna of Alaska. XXI. Taxonomy, morphological variation, and ecology of Diphyllobothrium ursi n. sp. provis. on Kodiak island. J Parasitol 40: 540–563. [PubMed] [Google Scholar]
- 29. Yamasaki H, Muto M, Yamada M, Arizono N, Rausch RL (2012) Validity of the bear tapeworm Diphyllobothrium ursi (Cestoda: Diphyllobothriidae) based on morphological and molecular markers. J Parasitol 98: 1243–1247. [DOI] [PubMed] [Google Scholar]
- 30. Rozas M, Bohle H, Sandoval A, Ildefonso R, Navarrete A, et al. (2012) First molecular identification of Diphyllobothrium dendriticum plerocercoids from feral rainbow trout (Oncorhynchus mykiss) in Chile. J Parasitol 98: 1220–1226. [DOI] [PubMed] [Google Scholar]
- 31. Torres P, Franjola R, Figueroa L, Schlatter R, González H, et al. (1981) Researches on Pseudophyllidea (Carus, 1813) in the south of Chile. IV Occurrence of Diphyllobothrium dendriticum (Nitzch). J Helminthol 55: 173–187. [PubMed] [Google Scholar]
- 32. Torres P, Cuevas C, Tang M, Barra M, Franjola R, et al. (2004) Introduced and native fishes as infection foci of Diphyllobothrium spp. in humans and dogs from two localities at Lake Panguipulli in Southern Chile. Comp Parasitol 71: 111–117. [Google Scholar]
- 33.Gibson DI, Bray RA, Harris EA (2005) Host-Parasite Database of the Natural History Museum, London. Avaiable: http://www.nhm.ac.uk/research-curation/scientific-resources/taxonomy-systematics/host-parasites/index.html. Accessed 1 July 2013.
- 34. Kuhlow F (1953) Über die Entwicklung und Anatomie von Diphyllobothrium dendriticum Nitzsch 1824. Z Parasitenkd 16: 1–35. [DOI] [PubMed] [Google Scholar]
- 35. Ching HL (1988) The distribution of plerocercoids of Diphyllobothrium dendriticum (Nitzsch) in sockeye salmon (Oncorhynchus nerka) smolts from Great Central Lake, British Columbia. Can J Zool 66: 850–852. [Google Scholar]
- 36.Freeman RS, Jamieson J. (1972) Parasites of Eskimos at Igloolik and Hall Beach, Northwest Territories. In: Shaphard RJ, Ioth S, editors. Proceedings of the 3rd International Congress on Circumpolar Health. Toronto: University of Toronto Press. pp. 306–315. [Google Scholar]
- 37. Ross P, Olpinski S, Curtis M (1989) Relationships between dietary practice and parasite zoonoses in Northern Québec Inuit communities. Etudes Inuit 13: 33–47. [Google Scholar]
- 38. Markowski S (1949) On the species of Diphyllobothrium occurring in birds and their relation to man and other hosts. J Helminthol 23: 107–126. [DOI] [PubMed] [Google Scholar]
- 39. Nekrasov AV, Pronin NM, Sanzheva SD, Timoshenko TM (1988) Composition of definite hosts of Diphyllobothrium dendriticum (Nitzsch, 1824) and distribution of its imago hemipopulation in the Baikal water basin. Med Parazitol 1988: 69–71 (In Russian.). [PubMed] [Google Scholar]
- 40. Figueroa F, Torres P, Franjola R, Schlatter R (1980) Investigaciones sobre Pseudophyllidea (Carus, 1813) en el sur de Chile. VI. Infección por Diphyllobothrium (Cobbold) en Larus maculipennis Lichtenstein en el lago Calafquén. Bol Chil Parasitol 35: 71–73. [PubMed] [Google Scholar]
- 41. Locke SA, Levy MS, Marcogliese DJ, Ackerman S, McLaughlin JD (2012) The decay of parasite community similarity in ring-billed gulls Larus delawarensis and other hosts. Ecography 35: 530–538. [Google Scholar]
- 42. Vermeer K (1969) Comparison of the helminth fauna of California gulls, Larus californicus, and ring-billed gulls, Larus delawarensis, at Beaverhill and Miquelon Lakes, Alberta. Can J Zool 47: 267–270. [Google Scholar]
- 43. Figueroa F, Torres P, Schlatter R, Asenjo F, Franjola R, et al. (1979) Investigaciones sobre Pseudophyllidea (Carus, 1813) en el sur de Chile. III: Estudio sobre Diphyllobothrium sp. en aves del Lago Calafquén (39°32′S, 72°09′W). Bol Chil Parasitol 34: 13–20. [PubMed] [Google Scholar]
- 44. Nekrasov AV, Pronin NM, Sanzhieva SD, Timoshenko TM (1999) Diversity of helminth fauna in the herring gull (Larus argentatus) from the Baikal Lake: peculiarities of spatial distribution and invasion. Parazitologiya 33: 426–436 (In Russian.). [Google Scholar]
- 45. Rausch RL, Fay FH, Williamson FSL (1983) Helminths of the arctic fox, Alopex lagopus (L.), in Greenland. Can J Zool 61: 1847–1851. [Google Scholar]
- 46. Skirnisson K, Eydal M, Gunnarsson E, Hersteinsson P (1993) Parasites of the arctic fox (Alopex lagopus) in Iceland. J Wildlife Dis 29: 440–446. [DOI] [PubMed] [Google Scholar]
- 47. Kapel CMO, Nansen P (1996) Gastrointestinal helminths of arctic foxes (Alopex lagopus) from different bioclimatological regions in Greenland. J Parasitol 82: 17–24. [PubMed] [Google Scholar]
- 48. Revenga J, Acuic T, Semenas L (1991) Difilobotriasis en salmónidos introducidos en el Parque y Reserva Nacional Nahuel Huapi Argentina: morfológia de plerocercoides. Arch Med Vet 23: 157–163. [Google Scholar]
- 49. Savchenkov MF, Chumachenko IG, Turchinova DA (2008) Diphyllobothriasis in Baikalsky region (epidemiologic observation). Siberian Med J 78: 88–90 (In Russian.). [Google Scholar]
- 50. Artamoshin AS, Frolova AA (1990) Helminthozoonoses in the area of the USSR extreme North. Med Parazitol 1990: 50–54 (In Russian.). [Google Scholar]
- 51. Plyusheva GL, Darchenkova NN, Akimova PF (1997) Spread of medically significant Diphyllobothrium species in Russia. Med Parazitol 1997: 55–60 (In Russian.). [Google Scholar]
- 52. Jenkins EJ, Castrodale LJ, de Rosemond SJC, Dixon BR, Elmore SA, et al. (2013) Tradition and transition: parasitic zoonoses of people and animals in Alaska, northern Canada, and Greenland. Adv Parasitol 82: 33–204. [DOI] [PubMed] [Google Scholar]
- 53. Arizono N, Shedko M, Yamada M, Uchikawa R, Tegoshi T, et al. (2009) Mitochondrial DNA divergence in populations of the tapeworm Diphyllobothrium nihonkaiense and its phylogenetic relationship with Diphyllobothrium klebanovskii . Parasitol Int 58: 22–28. [DOI] [PubMed] [Google Scholar]