Abstract
This review examines neuroimaging and neurocognitive findings on alcohol-related toxicity in adolescents. Teens who meet criteria for alcohol use disorders, as well as those who engage in subdiagnostic binge drinking behaviors, often show poorer neurocognitive performance, alterations in gray and white matter brain structure, and discrepant functional brain activation patterns when compared to nonusing and demographically matched controls. Abnormalities are also observed in teens with a family history of alcoholism, and such differences in neuromaturation may leave youths at increased risk for the development of an alcohol use disorder or increased substance use severity. More prospective investigations are needed, and future work should focus on disentangling preexisting differences from dose-dependent effects of alcohol on neurodevelopment. Intervention strategies that utilize neuroimag-ing findings (e.g., identified weaknesses in particular neural substrates and behavioral correlates) may be helpful in both prevention and intervention campaigns for teens both pre- and postinitiation of alcohol use.
Keywords: adolescence, alcohol, development, imaging
INTRODUCTION
Adolescent Brain Development
The brain is going through great structural and functional transition from childhood into adolescence, and well into young adulthood. Gray matter volume is decreasing as weak synaptic connections are being eliminated. White matter fiber tracts are continuing to develop as myelination is allowing for more efficient and timely communication between brain regions (Giedd 2004, Lebel et al. 2012). There is a temporal specificity pattern as brain regions important for higher-order cognitive functioning (e.g., frontal-subcortical brain regions) reach peak maturity last compared to lower-order sensorimotor regions (Stiles & Jernigan 2010). Healthy brain development throughout childhood and adolescence is important for optimal neurocognitive performance. In fact, even subtle changes in neurodevelopmental trajectories (e.g., changes in brain structural volume, cortical thickness, demyelination) may have neurobehavioral consequences, along with emotional and social implications (e.g., psychopathology, risk-taking behaviors) (Casey et al. 2008, Nagy et al. 2004). Altered brain development due to exposure to drugs of abuse during adolescence, particularly alcohol, can set the stage for cognitive problems into adulthood, which can translate into functional consequences throughout life.
Prevalence of Adolescent Alcohol Use and Drinking Patterns
The Monitoring the Future survey, conducted in the United States, finds alcohol to be the most commonly used intoxicant among eighth-, tenth-, and twelfth-graders. Although lifetime and binge drinking rates decreased in 2011 compared to previous years, 25% of adolescents in all three grades continue to report using alcohol within the past month. Thirteen percent have been drunk or engaged in heavy episodic drinking behaviors (Johnston et al. 2012). Given the important neural development occurring during this time period, along with findings on neurotoxic effects of alcohol in many adult studies (Pfefferbaum et al. 2009, Sullivan et al. 2000), further exploration into the impact of alcohol use on brain maturational changes is critical. The concept of binge drinking, or heavy episodic drinking, is receiving more attention given the prevalence in US society, as 13% of teens have had five or more drinks in a row in the past two weeks. Such trends in substance use patterns in our youth underscore the importance of research on associations between binge drinking, alcohol use disorders, and brain functioning. Links to preexisting differences such as family history are also important for early identification and prevention. Studies on the relationships between binge drinking, alcohol use disorders, genetic vulnerabilities, and cognition are presented in this review. The investigations discussed focus on cognitive assessment and neuroimaging methods, studies best designed to help us understand the neurotoxic effects of alcohol use in adolescence.
BRAIN STRUCTURAL CHANGES IN ADOLESCENT ALCOHOL USE
Gray Matter Volume
Research focused on the neurotoxic effects of alcohol use has identified the cerebral cortex (e.g., prefrontal cortex), limbic system (e.g., hippocampus), and cerebellum as structures vulnerable to the effects of alcohol use (Crews et al. 2005). Notably, these brain regions, particularly cortico-limbic regions, are still undergoing development throughout adolescence and early adulthood. In order to understand how brain structural changes may be associated with alcohol use during adolescence, several cross-sectional magnetic resonance imaging (MRI) investigations have worked to identify differences in gray matter macrostructure volume between adolescent alcohol users compared to teens with minimal alcohol exposure.
In 2000, De Bellis and colleagues examined hippocampal volumes in subjects (approximately ages 13 to 21) with alcohol use disorders compared to demographically matched controls. Between-group differences revealed smaller bilateral hippocampal volumes in teens diagnosed with an alcohol use disorder; total hippocampal volume was positively correlated with age at onset (younger age of initiation, smaller volume) and negatively correlated with duration of an alcohol use disorder (shorter duration, larger volume) (De Bellis et al. 2000). The same research team found smaller prefrontal cortex volumes in teens with an alcohol use disorder compared to controls, along with a sex-by-group interaction, as males with an alcohol use disorder had smaller cerebellar volumes as compared to their same-sex counterparts. Negative correlations were observed between prefrontal cortex gray matter volume and alcohol consumption variables, for example average drinks and maximum drinks per episode (De Bellis et al. 2005).
Investigations from our laboratory have also evaluated prefrontal cortex volumes and temporal lobe structures such as the hippocampus. Nagel and colleagues examined 14 adolescents (ages 15 to 17 years) with an alcohol use disorder and 17 healthy comparison teens. Teens with an alcohol use disorder had significantly smaller left hippocampal volumes than healthy teens (Nagel et al. 2005). Alcohol consumption variables were not related to hippocampal volumes. Similarly, Medina and colleagues (2008) found a group-by-gender interaction with prefrontal cortex volume. Female teens with an alcohol use disorder demonstrated smaller prefrontal cortex volumes, whereas males had larger prefrontal cortex volumes compared to their same-gender controls (Medina et al. 2008). Although these studies are not prospective in nature, they suggest alcohol-related alterations in brain structure development, particularly decreased macrostructure. Interestingly, several studies from our laboratory have found more profound differences in brain structure volume in adolescent alcohol users, and more subtle differences in adolescent marijuana users (Medina et al. 2007, 2009).
The underlying pathological mechanism of alcohol-related changes in brain development remains unclear and speculative, and preexisting differences must be better understood. Additional longitudinal research is needed in this area, and future studies will likely work to address this issue. Family history of an alcohol use disorder is consistently found to be associated with increased likelihood of development of a substance use disorder (Hill et al. 2008, 2011a). Several studies from our laboratory have examined differences in youth with a family history of alcohol use disorder to better elucidate preexisting morphological differences that may have genetic bases. Hanson and colleagues (2010) examined teens with minimal substance use (ages 12 to 14), with and without a family history of alcoholism, and found a group-by-gender interaction. Males with a family history had larger left hippocampi compared to males without a family history. For all adolescents, larger right hippocampal volumes predicted poorer delayed visual memory (Hanson et al. 2010). This work is somewhat consistent with previous findings suggesting that offspring of families positive for alcoholism show differences in limbic brain structures, although research has found smaller amygdala volumes in youth with a high-density family history (Hill et al. 2001).
Two recent studies by Hill and colleagues have looked at brain structure, family history, and genes that may play a role in central nervous system growth, and the etiology and susceptibility of alcohol dependence [e.g., VA/MET alleles of the brain-derived neurotrophic factor (BDNF), GABRA2, the gene that encodes the gamma-aminobutyric acid subunit 2, and short allele of 5-HTT]. Findings include decreased right hemisphere orbitofrontal cortex volume in youth and young adults with a positive family history of alcohol dependence (multiplex), linked with 5-HTT and BDNF genes (Hill et al. 2009). In regard to cerebellum volume, youth and young adults (age range 9 to 29) with a positive family history were found to have greater cerebellum volume; findings were linked to an interaction between BDNF and GABRA2 genes (Hill et al. 2007b, 2011b). Overall, findings suggest that genetic variations support structural brain differences, which may influence the development of alcohol misuse in certain vulnerable individuals.
Cortical Thickness
Cortical thinning is a hallmark of adolescent brain development, as the literature has consistently found decreases in cortical thickness likely related to elimination of weaker synaptic connections (Giedd 2004, Stiles & Jernigan 2010). Yet, limited work has examined how alcohol misuse (e.g., teens with an alcohol use disorder or teens engaging in subdiagnostic episodic drinking) impacts trajectories of cortical refinement. A recent investigation lead by Squeglia and colleagues (2011b) examined how heavy episodic drinking in adolescents ages 16 to 19 related to indices of cortical thickness. Binge-by-gender interactions were seen for cortical thickness in four left frontal brain regions; female bingers had thicker cortices than female controls, whereas male bingers had thinner cortices than male controls. Thicker left frontal cortices corresponded with poorer visuospatial, inhibition, and attention performances for female bingers and worse attention for male bingers. More work is needed to understand the temporal sequence of gray matter cortical thickness discrepancies and to disentangle what is driving these differences (e.g., pruning and/or gray matter loss, myelination).
White Matter Integrity
White matter allows cortical regions to communicate quickly and allows for efficient information processing. White matter fiber tracts are of particular interest because, unlike gray matter structure, development is more protracted throughout late adolescence and early adulthood (Lebel & Beaulieu 2011). Conventional MRI studies have generally found smaller overall white matter volumes in adolescents with alcohol use disorders compared to matched controls, particularly prefrontal cortex white matter (De Bellis et al. 2005), although there is some evidence that the pattern varies by gender (Medina et al. 2008).
Diffusion tensor imaging (DTI) has provided a way to look at more subtle changes in white matter fiber structure, which may be more appropriate for this developmental time period. Two common indices of the integrity of a fiber tract, fractional anisotropy (FA) and mean diffusivity (MD), provide an inference of white matter coherence, compactness, and directional dependence of fiber structure (Le Bihan 2003). Typically, increases in FA and decreases in MD are seen with healthy white matter development (Lebel et al. 2012). Given the more subtle changes (e.g., myelination, intra-/extracellular fluid changes) that may be occurring during this window of development, microstructural changes may not be observable with traditional MRI, and therefore studies have focused on FA and MD.
To better understand the effects of binge drinking on white matter integrity, we looked at heavy episodic drinkers compared to matched controls within our laboratory. We found that binge drinkers, approximately 18 years of age, showed poorer white matter integrity in several white matter regions, including cortical and subcortical projection fibers. Lower FA in six of these regions was linked to severity of use variables, specifically greater lifetime hangover symptoms and higher estimated peak blood alcohol concentrations (McQueeny et al. 2009). In several investigations we have evaluated white matter integrity in adolescent marijuana users (ages 16 to 19) with co-occurring alcohol use. We have generally found poorer white matter integrity in widespread fiber tracts across the brain, including cortical and subcortical tracts, along with corpus callosum (Bava et al. 2009, 2010). In an effort to disentangle the relationships between binge drinking and marijuana use, we looked at differences in 14 binge drinkers, 14 marijuana users with report of co-occurring binge drinking, and 14 matched controls with minimal substance use. Both substance-using groups showed poorer white matter health compared to nonusing controls; however, in several regions (cortical and subcortical), binge drinkers demonstrated poorer white matter health compared to marijuana users and controls (Jacobus et al. 2009).
We have recently identified, in a prospective investigation (Bava et al. 2013), that days of alcohol use from ages 18 to 20 predicted poorer white matter integrity at follow-up 1.5 years later, suggesting that regular heavy alcohol use over development likely has deleterious effects on brain tissue despite possible baseline differences that may relate to genetics. For example, Herting and colleagues (2010) found that youth (ages 11 to 15) with a positive family history had poorer white matter integrity in cortical and subcortical areas, along with associated poorer performance on a task of delay discounting and reaction time prior to initiation of drinking (Herting et al. 2010). We have also observed significant prospective relationships between white matter integrity (i.e., FA) and future risky behaviors measured over 1.5 years (including substance use). For instance, poor white matter integrity (fractional anisotropy) measured at baseline was related to more self-reported substance use and delinquency/aggression 18 months later, highlighting the importance (and potential clinical utility) of the observed cross-sectional differences discussed above (Jacobus et al. 2012).
PRECLINICAL STUDIES
A large body of research exists on the neurochemical, neurobiological, and behavioral changes in adolescent animals exposed to alcohol. An in-depth analysis is beyond the scope of this review; nevertheless, some degree of translation of this work to the clinical field is important to gain perspective on our progress in understanding mechanisms of alcohol-related neurotoxicity in adolescence. Ongoing neurogenesis during adolescence is an important process in development; animal research has suggested that alcohol may interfere with neural stem cell proliferation in areas such as the hippocampus (Morris et al. 2010), driving neurodegeneration and changes in brain structure and function. Similar studies have found differences in the generation and survival of cortical cells in alcohol-exposed adolescent animals compared with controls (Crews et al. 2006, Hansson et al. 2010, Helfer et al. 2009, Koss et al. 2012, Nixon & Crews 2002). Changes observed in human macro- and microstructural MRI studies may reflect tissue loss or remodeling related to inhibition of cell generation and survival. Preclinical studies also suggest that microglia activation, oxidative stress, and proinflammatory changes may be a possible mechanism by which alcohol intoxication, particularly heavy episodic use or binge drinking, leads to neurotoxic degeneration and/or prevents genesis of neurons and glia (Alfonso-Loeches et al. 2012, Crews & Nixon 2009).
It is likely that there is differential neurochemical transmission sensitivity to alcohol during adolescence that contributes to ongoing use and therefore deleterious structural and functional effects. Remodeling of neurochemical processes combined with repeated alcohol exposure during this developmental window, particularly dopamine, GABA, and glutamate processes, may leave teens vulnerable to the rewarding physiological effects of use. For instance, studies have found increases in dopamine release with repeated alcohol administration in adolescent animals in areas such as the nucleus accumbens (Pascual et al. 2009, Philpot et al. 2009), and altered sensitivity to GABA transmission in animals with similar postnatal exposure levels (Fleming et al. 2012, Li et al. 2003). Alcohol-related alterations of neurochemical processes that are undergoing neuro-maturation and refinement may result in more permanent reorganization, resulting in enhanced vulnerability to increased self-administration and addictive-like behaviors into young adulthood.
BRAIN FUNCTION CHANGES IN ADOLESCENT ALCOHOL USERS
In addition to volumetric imaging studies, there has been an increase in the number of functional magnetic resonance imaging studies (fMRI) in the literature focusing on adolescent alcohol users to help determine the impact on neural signaling, which could underlie cognitive differences. fMRI allows us to make inferences about neural activity, or changes in response activation, by measuring the blood oxygen level–dependent (BOLD) signal. In one of the earliest studies from our laboratory, Tapert and colleagues (2001) found that among young women (ages 18 to 25), those with alcohol dependence showed decreased BOLD response during a spatial working memory task in parietal and frontal cortices (Tapert et al. 2001). In 2004, we found that younger adolescents (ages 15 to 17) who drank heavily for approximately two years had increased activation in the parietal lobe and decreased activation in precentral gyrus, precuneus, occipital, and cerebellar regions compared to light drinkers in response to a spatial working memory task (Tapert et al. 2004c). There was a dose-dependent effect, as adolescents who reported more hangover symptoms and alcohol use exhibited greater abnormalities. Although the discrepancy in findings remains unclear, one explanation is that more cumulative lifetime use may lead to a reduced capacity in neural functioning (as described by the former study), as differences observed in younger individuals with a history of alcohol use disorders may reflect both reduced capacity and compensatory mechanisms (latter study). The neural mechanisms are still being elucidated, and it is likely that the deleterious effects of alcohol change throughout development.
Gender and comorbid substance use are important considerations as well. Recently, Squeglia and colleagues (2011a) found binge drinking–by-gender interactions in BOLD activation to a spatial working memory task, with female binge drinkers showing less activation than female controls, and male binge drinkers exhibiting greater response than male controls. For female binge drinkers, less activation was associated with poorer neurocognitive performance; however, the opposite was found true for male binge drinkers (Squeglia et al. 2011a). These findings are consistent with previous work that suggests differences in brain activation between males and females, and increased vulnerability of females to alcohol-related brain changes (Caldwell et al. 2005). Notably, in teens with both an alcohol and marijuana use disorder, we found less inferior frontal and temporal activation and increased medial frontal activation compared to controls in response to the spatial working memory task, suggesting altered neural processing in teens with co-occurring use of these commonly consumed intoxicants (Schweinsburg et al. 2005).
Differences in response to a verbal encoding task (i.e., learning of novel word pairs) have been observed in our laboratory. In a study evaluating 12 adolescent binge drinkers compared to 12 nondrinking controls (ages 16 to 18), we found that bingers showed more response in right superior frontal and bilateral posterior parietal cortices, but less response in occipital cortex during encoding. In addition, controls showed significant activation in the left hippocampus during novel encoding, whereas binge drinkers did not; findings may indicate disadvantaged processing of novel verbal information (Schweinsburg et al. 2010). A follow-up to this investigation in 2011 (also ages 16 to 18) similarly found an increased dorsal frontal and parietal BOLD response among binge drinkers, with decreased inferior frontal response (Schweinsburg et al. 2011). Park and colleagues (2011) evaluated 21 young adult male college students (age 23); half met criteria for an alcohol use disorder. The group of heavy drinkers showed reduced activation in several brain regions, including frontal, temporal, parietal, and cerebellar cortex, compared to controls during a verbal working memory task (Park et al. 2011).
Xiao and colleagues (2012) looked at more complex cognitive processing with an affective decision-making task (the Iowa Gambling Task) in binge drinkers ages 16 to 18. Binge drinkers (compared to matched controls) showed increased BOLD activity in the limbic brain regions (amygdala, insula) compared to nondrinkers. Increased drinking severity was positively related to BOLD activity in the insula and negatively related to BOLD activity in the orbitofrontal cortex (Xiao et al. 2012).
Prospective investigations remain limited; however, we looked at youth ages 12 to 14 prior to initiation of alcohol use and found that teens classified as transitioning to heavy alcohol use by age 18 had less BOLD activation (at baseline) in frontal, temporal, and parietal cortices in response to a go/no-go task of response inhibition at baseline (Norman et al. 2011). Findings support preexisting differences in these teens, which likely evolve to both increases and decreases in BOLD signal; such changes may be reflective of compensatory strategies earlier in development and declines in capacity revealed over time with heavier use. An interesting study by Nees and colleagues (2012) found that reward-related brain activation (e.g., ventral striatum) aided in the prediction of early-onset drinking in adolescents, further supporting the notion that preexisting brain differences, along with personality and behavioral traits, may leave certain youth vulnerable to addictive behaviors (Nees et al. 2012).
Genetics, Vulnerability, and Brain Function
An abundance of work has examined the relationship between brain integrity (structure, function) and genetic familial risk for alcohol use disorders in an effort to understand altered developmental trajectories prior to the development of substance misuse. These studies evaluate healthy teens prior to initiation of any significant alcohol use. In 2004, Schweinsburg and colleagues identified differences in BOLD activation in response to a task of inhibition. Teens (ages 12 to 14) with a positive family history of alcoholism showed decreased inhibitory response in frontal brain regions (Schweinsburg et al. 2004). A similar pattern of findings was observed in 2007 by Hill and colleagues, who found that young adults (average age 23) with a family history of alcoholism showed less BOLD response in frontal and temporal brain regions to an emotion recognition/learning paradigm compared to matched controls (Hill et al. 2007a). In contrast, a study looking at younger adolescents (average age 13) on a Stroop color-word task found increased BOLD activation in frontal regions, cingulate, and insula in participants with a positive family history compared to controls. Discrepant findings likely reflect different stages of development and/or differences in task demands (Silveri et al. 2011).
Heitzeg and colleagues (2008, 2010) have reported differences in both brain activation and affective circuitry in youth with a positive family history as well as more vulnerable children of alcoholics (reporting some degree of alcohol involvement). For example, they report a lack of deactivation in the ventral caudate and prefrontal region in youth with a positive family history that is observed in youth with a negative family history in response to an inhibition paradigm. The authors also report differences in orbitofrontal brain regions and subcortical brain regions in response to emotional stimuli, suggesting that more resilient youth with a family history of alcoholism show greater emotional regulation. In general, these investigations suggest discrepancies in brain activation patterns in youth with a positive family history engaging in higher-risk behaviors (heavier drinking), leaving them particularly vulnerable (Heitzeg et al. 2008, 2010). In 2008, our lab found that youth with a denser family history of an alcohol use disorder had less BOLD activation in response to a simple vigilance condition in frontal and cingulate regions. More activity during the resting condition was also reported in these youths (Spadoni et al. 2008). This study supported preliminary evidence that adolescents with a family history may also have differences in regulating their default mode network.
Several more studies have looked at the BOLD response to more complex reward-anticipation and decision-making tasks in order to understand whether altered reward-related circuitry in mesolimbic brain regions contributes to alcohol-use disorder vulnerabilities. Bjork and colleagues (2008) did not find differences in response to a monetary incentive task between children of alcoholics and matched controls, ages 12 to 16. In a similar study by Yau and colleagues (2012), the authors looked at children (ages 18 to 22) of alcoholics also using a monetary incentive delay task. The authors found underlying differences in the mesolimbic substrates in response to this task in children of alcoholics. Two main findings reported in this investigation are that (a) there is a decreased nucleus accumbens response to reward anticipation in children of alcoholics with a positive family history and low alcohol use, and (b) positive relationships are supported among externalizing, nucleus accumbens activity, and current and lifetime alcohol consumption in children of alcoholics. Discrepancies in these two studies are likely related to developmental trajectories of the reward system, whereas brain response to reward may play a role in risk taking, but this likely varies by stage of neurodevelopment (e.g., age) and interacts with environment (Bjork et al. 2008, Yau et al. 2012).
Paradigms focused more on brain response to complex attention, decision making, or more executive aspects of cognitive control have yielded interesting findings. Cservenka and colleagues (2012) found that, among teenagers ages 13 to 15, youth with a positive family history showed less BOLD activation in the right dorsolateral prefrontal cortex and cerebellum to a decision-making task (Wheel of Fortune) compared to youth with a negative family history; teens with a family history of alcoholism also showed different brain activation patterns in parietal brain regions in response to risky choices compared to chance choices, suggesting aberrant cortical-cerebellar circuitry related to decision making (Cservenka & Nagel 2012). The same authors found youth (approximately age 14) with a positive family history had less BOLD activation during a verbal working memory 2-back paradigm, along with slower reaction times, compared to controls in multiple areas of the prefrontal cortex (Cservenka et al. 2012).
In a prospective investigation, Weiland and colleagues (2012) found that teens higher in resiliency (flexible and adaptive behavioral responses) in early adolescence (ages 12 to 15) had later onset of drinking (ages 18 to 22). Negative relationships were identified between resiliency and BOLD activity in basal ganglia and thalamus during a working memory task. The authors suggest that resiliency may be related to preexisting neuroprotective factors because resiliency was predictive of brain activation differences (Weiland et al. 2012).
New advances in methodology, such as functional connectivity MRI, allow us to look at correlations between brain region activity and make inferences about the strength of brain circuitry. In 2011, Herting and colleagues looked at participants ages 11 to 15 with and without a family history of alcoholism. The authors report poorer connectivity between cerebellar regions and anterior prefrontal cortices in youth with a positive family history. Interestingly, poorer white matter integrity was also found in corresponding fronto-cerebellar white matter tracts (Herting et al. 2011). Our lab conducted a similar investigation examining connectivity between frontoparietal areas in teens ages 12 to 14, and we also found poor connectivity between posterior parietal and dorsolateral prefrontal brain regions compared to controls without a family history of alcoholism; however, differences in corresponding white matter tracts were not observed (Wetherill et al. 2012).
Cue Reactivity, Level of Response, and Brain Function
Cue reactivity is a classically conditioned response to drug stimuli. There have been few studies of cue reactivity in the literature with adolescents, despite reports of craving in the presence of alcohol and other drugs. Understanding brain regions subserving reactivity and craving is important because it may elucidate brain functioning that is preexisting and genetically linked (e.g., family history status) or result in shifted attention to alcohol cues in the environment after heavy use and compromised coping skills in the presence of alcohol, causing increased substance use in some teens after initiation. Pulido and colleagues (2009b) looked at relationships between drinking models (family, friends), family history of an alcohol use disorder, and personal alcohol use on valence to alcohol cues. The sample included young adults ages 18 to 23 (most drinking less than once per week) and found that positive response to cues was dependent on more personal experience (greater current drinking) compared to family history or environment (Pulido et al. 2009b). In a follow-up study by the same author, family history was not associated with brain activation to positive or negative alcohol expectancies per se in nondrinking youth; however, differences in brain response (less neural differentiation to task conditions) were related to more positive alcohol use expectancies (Pulido et al. 2009a).
In two of the earlier cue reactivity studies conducted by Tapert and colleagues (2004a), alcohol-dependent young women (ages 18 to 24) showed an increased BOLD response (compared to nonabusers) during alcohol word presentation trials in prefrontal, insular, subcallosal, and anterior cingulate regions. Increased craving was related to increased BOLD response among the alcohol-dependent women (Tapert et al. 2004a). Another investigation looked at differences in activation in response to alcohol pictures. Teens with alcohol use disorders (ages 14 to 17) showed increased activation to alcoholic beverage pictures in anterior cingulate and prefrontal and limbic brain regions compared to demographically matched controls. Increased brain response was related to more drinks per month (Tapert et al. 2003), highlighting differences in brain activation in heavy users in response to cues, which then likely enhances the vulnerability of teens for future use. More work prior to transition into alcohol abuse/dependence is needed in this area.
Level of response to alcohol is linked to preexisting genetic characteristics. Research has shown that individuals requiring higher doses of alcohol to feel desired effects are more likely to develop an alcohol use disorder (Schuckit & Smith 1996), and there are likely underlying neural substrates that impact cognitive and affective processes related to level of response. We found that in a sample of adolescents ages 15 to 17 with varying degrees of alcohol use (over half with a family history of alcohol use disorder), BOLD activation to a visual working memory task predicted level of response to alcohol. In several cases (e.g., frontal gyrus, temporal gyrus, insula), increased BOLD activation was related to needing more drinks to achieve an effect similar to that of early drinking episodes (Tapert et al. 2004b).
ELECTROPHYSIOLOGICAL FINDINGS
Electroencephalographic (EEG) research can measure resting states or event-related potentials (ERPs; e.g., P300) by electrode recordings placed on the scalp to indirectly measure neural activity, or electrical activity, in the brain linked to resting or stimulus presentation. This imaging technique increases temporal resolution and is more efficient at capturing neural activity changes compared to BOLD imaging; however, spatial resolution is lost. The literature has shown expected changes in ERP throughout development, both childhood and adolescence, as neural remodeling is occurring (Polich et al. 1990). For example, P3 (or P300) is identified as changing with adolescent development. Studies show P3 amplitude increasing with age and P3 latency decreasing with age. Studying ERPs such as P3 provides an opportunity to identify neural substrates underlying cognitive processing.
Several EEG studies have looked at adolescent and young adult binge drinkers. In 2007, Ehlers et al. (2007) found that young adults who engaged in binge drinking during adolescence displayed decreased latency of ERP subcomponent P3 (perhaps related to more rapid maturation, or pruning) and lower P450 amplitude to an emotion recognition task in comparison with controls (Ehlers et al. 2007). A prospective test/retest study in 2009 found latency differences in response to an auditory paradigm (significantly delayed latency) in P1, N2, and P3b after initiation of binge drinking (no between-group differences were observed prior to drinking), suggesting cerebral dysfunction, or slowed brain activity, related to heavy episodic drinking (Maurage et al. 2009). In 2012, the same research group described a cohesive pattern of impairments as evidenced by delayed latency in several ERP components related to cognitive functioning (a face detection task) among university student binge drinkers. The authors describe the findings in light of slowed communication, slowed information processing, and/or reduced neuronal firing intensity (Maurage et al. 2012). In similar binge drinking investigations with university students, Crego and colleagues (2009, 2010) found ERP differences between binge drinkers and controls in response to a visual working memory and attention task. For example, these authors identified alterations in N2, P3, and late positive components compared to controls in frontal and parietal brain regions, suggesting electrophysiological abnormalities for processing information (Crego et al. 2009, 2010).
In a resting state EEG investigation in young adult college-age binge drinkers (approximately age 20) (Courtney & Polich 2010), the authors found increased spectral power in delta and fast-beta bands of binge drinkers. In research exploring the more acute effects of alcohol, Euser and colleagues (2011) found that after a moderate dose of alcohol, males between the ages of 18 and 25 had reduced P300 amplitude to negative feedback in a risky decision-making paradigm. The authors suggest that alcohol use in this population may inhibit sufficient integration of feedback outcomes, or attention allocation, necessary for optimal decision-making performance (Euser et al. 2011).
Research has explored self-report of a family history of alcoholism as a link to possible preexisting electrophysiological differences that may exist and contribute to development of substance use disorders. Research in this area may furnish clues as to whether EEG phenotypes provide useful information about predisposition toward alcohol misuse. Hill and colleagues (1990) conducted one of the earliest studies looking at EEG differences in children (ages 8 to 14) of alcoholics. In high-risk children, they observed greater frontal negativity (N50) and decreased P300 amplitude in response to an event probability task; the authors suggest maturational differences in high-risk children (Hill et al. 1990). In a follow-up prospective study, Hill and colleagues (2000) found that along with family history, reduced visual and auditory P300 amplitude predicted earlier onset of drinking. In support of these findings, McGue and colleagues (2001) found that reduced P300 amplitude was associated with age of first drink in a large sample of 17-year-old adolescents. Personality and behavioral traits (e.g., disinhibition, antisocial behavior, and poor school performance) also were related to age of first drink (McGue et al. 2001).
Twin studies provide the ability to disentangle environment from genetic contributions. In a community-based twin sample, Perlman and colleagues (2009) found evidence for P300 amplitude reduction as an alcoholism endophenotype in adolescents; the authors argue that changes in P300 simply represent the deleterious effects of alcohol use during adolescence (Perlman et al. 2009). Koskinen and colleagues (2011) looked at twins concordant/discordant for alcohol problems and found, in this longitudinal investigation, that P300 amplitude at mean age of 25 (for novel sound) was negatively related to reported alcohol use over adolescence. Evidence for genetic factors influencing the relationship between electrophysiological response and alcohol use is also reported (Koskinen et al. 2011).
Additional work needs to be done in this area to better understand long-term neurophysiolog-ical changes that may occur due to adolescent alcohol use, as well as genetic differences that may predate the onset of use disorders. The present literature appears to suggest that both genetic and behavioral factors contribute to functional brain activity.
NEUROCOGNITIVE PERFORMANCE IN ADOLESCENT ALCOHOL USERS
Several studies have evaluated neurocognitive changes related to alcohol use in adolescence. Earlier cross-sectional investigations have identified poorer performance in many neurocognitive domains, including attention and information processing (Tarter et al. 1995), memory (Brown et al. 2000), visuospatial functioning (Sher et al. 1997), language abilities (Moss et al. 1994), and executive functioning (Moss et al. 1994), when compared with nondrinking controls. Several more recent investigations have supported these earlier findings. For example, Thoma and colleagues (2011) found that consuming more drinks per drinking day was related to poorer performance on measures of attention and executive functioning in adolescents ages 12 to 18. In an investigation of English-and Afrikaans-speaking adolescents (ages 13 to 15), individuals with alcohol dependence performed worse on measures of structured verbal memory and psychomotor speed. In 2012, Parada and colleagues examined undergraduate students ages 18 to 20. The authors found that in comparison with controls, these late adolescents/young adults who engaged in binge drinking performed worse on measures of executive functioning such as working memory. Taken together, these find-ings continue to suggest widespread deleterious effects of alcohol use on neurocognitive abilities spanning childhood to late adolescence (Ferrett et al. 2010, Parada et al. 2012, Thoma et al. 2011).
We have conducted several longitudinal investigations in our laboratory. In 1999, Tapert and colleagues looked at adolescents ages 13 to 19 recruited from inpatient treatment programs. Over four years of follow-up neurocognitive assessments, teens with protracted alcohol and drug use following treatment (along with withdrawal symptoms) had poorer neurocognitive functioning, particularly on measures of attention and visuospatial functioning (Tapert & Brown 1999). A 2002 investigation yielded similar findings: Neuropsychological testing spanned the course of eight years (approximately ages 16 to 24), and alcohol and drug use over the eight-year window predicted attention, learning, and visuospatial functioning (Tapert et al. 2002).
Squeglia and colleagues (2009) compared matched controls (ages 12 to 14) with adolescents who transitioned to heavy or moderate drinking over a three-year follow-up period. The authors found that for females, more drinking days over the follow-up interval predicted worse visuospatial performance at follow-up; however, for boys, more hangover symptoms were associated with worse performance on a test of sustained attention at follow-up (Squeglia et al. 2009). Hanson and colleagues (2011) looked at long-term (10-year) patterns of alcohol and other drug use in teenagers ages 13 to 18. They found that whereas heavier substance use patterns in general were related to poorer learning, memory, attention, and visuospatial processing, heavier use of alcohol in particular (with little other substance use) was related to poorer short-term verbal memory. Interestingly, the authors also found that withdrawal symptoms at each follow-up visit were associated with poorer learning and memory performance, as compared to substance-dependence symptoms (Hanson et al. 2011).
The idea that postdrinking symptoms (hangover/withdrawal) play a deleterious role in brain functioning is increasingly being explored in the literature, as these symptoms may be more detrimental than the quantity of alcohol consumed. Evidence from our own studies show that both self-report of quantity consumed and postdrinking symptoms play a role. In addition to findings by Squeglia et al. (2009) that show negative dose-dependent relationships between drinking and neurocognitive performance, we have also seen negative relationships between alcohol withdrawal symptoms and learning and memory performance. For instance, Mahmood and colleagues found that among adolescent alcohol users ages 15 to 19, greater hangover symptoms were associated with poorer performance on measures of immediate and delayed recall (Mahmood et al. 2010).
Studies are also beginning to explore higher-order, more complex cognitive functioning and alcohol misuse; in particular, risky decision-making paradigms have found differences between adolescent drinkers and matched controls. Johnson and colleagues (2008) looked at tenth-grade adolescents in China and found that binge drinkers showed poorer performance on the Iowa Gambling Task, particularly in their hypersensitivity to reward. Youth with a family history of alcoholism may be genetically linked to preexisting differences in cognitive development (Johnson et al. 2008), mirroring findings from the neuroimaging literature. Nigg and colleagues (2004) found weakness in executive functioning abilities in youth between the ages of 3 and 14 with a family history of alcoholism, suggesting that preexisting differences contribute to risk for alcoholism along with personality traits (Nigg et al. 2004). Despite preexisting differences, studies have clearly shown a link between substance use and declines in neurocognitive functioning with varying degrees of substance misuse (e.g., binge drinking, dependence).
SUMMARY AND CONCLUSIONS
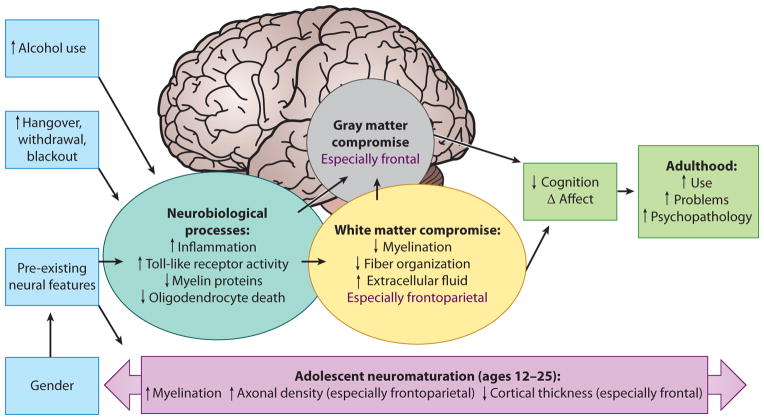
Thirteen percent of eighth-, tenth-, and twelfth-graders are engaging in binge drinking, and less than half (40%) of twelfth-graders report any alcohol use in the past 30 days (Johnston et al. 2012). The degree to which this impacts healthy development is important to society, given that many of our youth are engaging in this behavior while neuromaturation and cellular remodeling are still ongoing and the brain attempts to meet the demands for optimal cognitive processing. Teens who meet criteria for alcohol use disorders, as well as those who engage in subdiagnostic binge drinking behaviors, often show poorer neurocognitive performance, alterations in gray and white matter brain structure, and discrepant functional brain activation patterns when compared with nonusing and demographically matched controls (see Figure 1 for an overall model of how heavy drinking may affect different aspects of adolescent brain development).
Figure 1.
A model of the influence of alcohol on adolescent neuromaturation and neurobehavior.
The studies reviewed above suggest that alterations in gray matter structures, both cortical and subcortical, occur in teens who misuse alcohol. This includes alterations in the overall volume of prefrontal cortex, hippocampus, and amygdala; alcohol-related discrepancies are also observed in cortical thickness measurements. Family history has been shown to play a role in structural brain differences, and accumulating evidence indicates that certain genes may not only predispose someone to develop an alcohol use disorder, but may also be associated with differences in brain morphometry, further increasing vulnerability to addiction. Studies also tend to show consistent differences in white matter integrity in adolescent alcohol users. Notably, poorer white matter integrity is observed in teens with heavy alcohol use histories, as well as in teens engaging in subdiagnostic binge drinking behaviors with co-occurring substance use. In our laboratory we have seen poorer white matter integrity in teens with both marijuana and alcohol use, although alcohol has been shown to have unique and fairly large dose-dependent effects on white matter, which underscores the deleterious effects of this substance by itself on brain integrity. Family history has also been shown to contribute to white matter differences prior to initiation of drinking.
The functional neuroimaging and electrophysiological literature suggests alterations in brain activation with alcohol use during adolescence. Findings suggest that teens who report significant alcohol use may display both increases and decreases in BOLD activation compared to teens with minimal use histories; these findings may reflect compensatory strategies along with early signs of decreased capacity. In younger adults, or in older adolescents with alcohol use disorders, brain activation changes may begin to reflect a decreased capacity of neural signaling as lifetime use substantially increases. Differences in the latency and amplitude of EEG markers can also be seen in youth with alcohol use histories. Gender is also an important variable to consider in both structural and functional neuroimaging because girls and boys peak in brain development at different ages. Subtle research evidence indicates that girls may be slightly more vulnerable to the effects of alcohol than are boys. Preexisting differences, along with personality and environmental factors, may explain altered patterns of brain activation in both boys and girls.
Neurocognitive research focused on alcohol-related problems has found widespread differences in domains including attention, learning and memory, visuospatial processing, and executive functioning. Cross-sectional and prospective work has suggested that cumulative use as well as severity of hangover/withdrawal symptoms are predictive of cognitive functioning regardless of family history, which is likely to account for some differences prior to initiation.
We have found evidence that poor brain integrity in adolescence predicts future substance use (i.e., increased substance use and risky behaviors) over 1.5 years (Jacobus et al. 2012). This means that not only are neural and cognitive changes a result of alcohol consumption and postdrinking symptoms, but also that these changes can perpetuate a negative cycle leading to addiction and increased substance use severity. Cognitive remediation programs provide a potential to decrease the likelihood of future engagement in substance use behaviors. An intervention that would enhance such skills as executive functioning and decision making that could be utilized in treatment programs or mental health clinics may benefit teens who exhibit symptoms of misuse. Although these programs are being utilized with adults (Rupp et al. 2012) and are beginning to be explored prior to initiation of problematic alcohol use (Hollen 1998), limited work has been done with adolescents with alcohol use histories. Prospective neuroimaging investigations to help disentangle preexisting differences from toxicity continue to be important for future investigations. The investigation of how cognitive intervention may prevent future substance use involvement by targeting neural substrates underlying alcohol-related neurotoxicity is an important next step in integrating imaging and cognitive findings with clinical application.
SUMMARY POINTS.
Teens who engage in heavier alcohol use show alterations in gray and white matter brain structure and discrepant functional brain activation patterns.
Teens who report both binge drinking and/or alcohol use disorders also show poorer neurocognitive functioning in most cognitive domains (e.g., learning, memory).
Family history of an alcohol use disorder may leave certain youth more vulnerable to alcohol-related neurotoxicity.
Cumulative alcohol use as well as severity of use likely contribute to changes in neurode-velopmental trajectories.
FUTURE ISSUES.
A better understanding is needed of the preexisting brain differences in youth prior to alcohol use initiation.
Imaging and other biomarkers need to be utilized as predictors of future problem alcohol use and psychopathology.
Interactions must be elucidated between alcohol and other substance use (e.g., marijuana) and brain changes.
Remediation programs must be developed to help enhance cognition and decrease the likelihood of engagement in future substance use behaviors.
Acknowledgments
This review was supported in part by NIAAA R01 AA013419 and NIDA F32 DA032188.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Alfonso-Loeches S, Pascual M, Gomez-Pinedo U, Pascual-Lucas M, Renau-Piqueras J, Guerri C. Toll-like receptor 4 participates in the myelin disruptions associated with chronic alcohol abuse. Glia. 2012;60(6):948–64. doi: 10.1002/glia.22327. [DOI] [PubMed] [Google Scholar]
- Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance users. Psychiatry Res. 2009;173(3):228–37. doi: 10.1016/j.pscychresns.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Jacobus J, Mahmood O, Yang TT, Tapert SF. Neurocognitive correlates of white matter quality in adolescent substance users. Brain Cogn. 2010;72(3):347–54. doi: 10.1016/j.bandc.2009.10.012. Highlights poorer white matter integrity in adolescent alcohol users and associated neurocognitive correlates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Jacobus J, Thayer R, Tapert SF. Longitudinal changes in white matter integrity among adolescent substance users. Alcohol Clin Exp Res. 2013;37(Suppl 1):E181–89. doi: 10.1111/j.1530-0277.2012.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Hommer DW. Incentive-elicited striatal activation in adolescent children of alcoholics. Addiction. 2008;103(8):1308–19. doi: 10.1111/j.1360-0443.2008.02250.x. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcohol Clin Exp Res. 2000;24(2):164–71. [PubMed] [Google Scholar]
- Caldwell LC, Schweinsburg AD, Nagel BJ, Barlett VC, Brown SA, Tapert SF. Gender and adolescent alcohol use disorders on BOLD (blood oxygen level dependent) response to spatial working memory. Alcohol Alcoholism. 2005;40(3):194–200. doi: 10.1093/alcalc/agh134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;1124:111–26. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Polich J. Binge drinking effects on EEG in young adult humans. Int J Environ Res Public Health. 2010;7(5):2325–36. doi: 10.3390/ijerph7052325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crego A, Holguin SR, Parada M, Mota N, Corral M, Cadaveira F. Binge drinking affects attentional and visual working memory processing in young university students. Alcohol Clin Exp Res. 2009;33(11):1870–79. doi: 10.1111/j.1530-0277.2009.01025.x. [DOI] [PubMed] [Google Scholar]
- Crego A, Rodriguez-Holguin S, Parada M, Mota N, Corral M, Cadaveira F. Reduced anterior prefrontal cortex activation in young binge drinkers during a visual working memory task. Drug Alcohol Depend. 2010;109(1–3):45–56. doi: 10.1016/j.drugalcdep.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Crews FT, Buckley T, Dodd PR, Ende G, Foley N, Harper C, Sullivan EV. Alcoholic neurobiology: changes in dependence and recovery. Alcohol Clin Exp Res. 2005;29(8):1504–13. doi: 10.1097/01.alc.0000175013.50644.61. [DOI] [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006;137(2):437–45. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcoholism. 2009;44(2):115–27. doi: 10.1093/alcalc/agn079. Detailed overview of preclinical studies focused on biological mechanisms of alcohol-related neurotoxicity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Herting MM, Nagel BJ. Atypical frontal lobe activity during verbal working memory in youth with a family history of alcoholism. Drug Alcohol Depend. 2012;123(1–3):98–104. doi: 10.1016/j.drugalcdep.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Nagel BJ. Risky decision-making: an FMRI study of youth at high risk for alcoholism. Alcohol Clin Exp Res. 2012;36(4):604–15. doi: 10.1111/j.1530-0277.2011.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, et al. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry. 2000;157(5):737–44. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res. 2005;29(9):1590–600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E, Finnerman G, Gilder D, Lau P, Criado J. P3 components and adolescent binge drinking in Southwest California Indians. Neurotoxicol Teratol. 2007;29(1):153–63. doi: 10.1016/j.ntt.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Euser AS, van Meel CS, Snelleman M, Franken IH. Acute effects of alcohol on feedback processing and outcome evaluation during risky decision-making: an ERP study. Psychopharmacology (Berl) 2011;217(1):111–25. doi: 10.1007/s00213-011-2264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrett HL, Carey PD, Thomas KG, Tapert SF, Fein G. Neuropsychological performance of South African treatment-naive adolescents with alcohol dependence. Drug Alcohol Depend. 2010;110(1–2):8–14. doi: 10.1016/j.drugalcdep.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming RL, Acheson SK, Moore SD, Wilson WA, Swartzwelder HS. In the rat, chronic intermittent ethanol exposure during adolescence alters the ethanol sensitivity of tonic inhibition in adulthood. Alcohol Clin Exp Res. 2012;36(2):279–85. doi: 10.1111/j.1530-0277.2011.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Cummins K, Tapert SF, Brown SA. Changes in neuropsychological functioning over 10 years following adolescent substance abuse treatment. Psychol Addict Behav. 2011;25(1):127–42. doi: 10.1037/a0022350. Longitudinal investigation spanning 10 years focused on the impact of alcohol and other substance use disorders on cognition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Medina KL, Nagel BJ, Spadoni AD, Gorlick A, Tapert SF. Hippocampal volumes in adolescents with and without a family history of alcoholism. Am J Drug Alcohol Abuse. 2010;36(3):161–67. doi: 10.3109/00952991003736397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Nixon K, Rimondini R, Damadzic R, Sommer WH, et al. Long-term suppression of forebrain neurogenesis and loss of neuronal progenitor cells following prolonged alcohol dependence in rats. Int J Neuropsychopharmacol. 2010;13(5):583–93. doi: 10.1017/S1461145710000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg MM, Nigg JT, Yau WY, Zubieta JK, Zucker RA. Affective circuitry and risk for alcoholism in late adolescence: differences in frontostriatal responses between vulnerable and resilient children of alcoholic parents. Alcohol Clin Exp Res. 2008;32(3):414–26. doi: 10.1111/j.1530-0277.2007.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg MM, Nigg JT, Yau WY, Zucker RA, Zubieta JK. Striatal dysfunction marks preexisting risk and medial prefrontal dysfunction is related to problem drinking in children of alcoholics. Biol Psychiatry. 2010;68(3):287–95. doi: 10.1016/j.biopsych.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer JL, Calizo LH, Dong WK, Goodlett CR, Greenough WT, Klintsova AY. Binge-like postnatal alcohol exposure triggers cortical gliogenesis in adolescent rats. J Comp Neurol. 2009;514(3):259–71. doi: 10.1002/cne.22018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Fair D, Nagel BJ. Altered fronto-cerebellar connectivity in alcohol-naive youth with a family history of alcoholism. Neuroimage. 2011;54(4):2582–89. doi: 10.1016/j.neuroimage.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Schwartz D, Mitchell SH, Nagel BJ. Delay discounting behavior and white matter microstructure abnormalities in youth with a family history of alcoholism. Alcohol Clin Exp Res. 2010;34(9):1590–602. doi: 10.1111/j.1530-0277.2010.01244.x. Family history of an alcohol use disorder is related to poorer white matter integrity, which could leave certain youth more vulnerable to development of alcohol-related problems. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, De Bellis MD, Keshavan MS, Lowers L, Shen S, et al. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biol Psychiatry. 2001;49(11):894–905. doi: 10.1016/s0006-3223(01)01088-5. [DOI] [PubMed] [Google Scholar]
- Hill SY, Kostelnik B, Holmes B, Goradia D, McDermott M, et al. fMRI BOLD response to the eyes task in offspring from multiplex alcohol dependence families. Alcohol Clin Exp Res. 2007a;31(12):2028–35. doi: 10.1111/j.1530-0277.2007.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Muddasani S, Prasad K, Nutche J, Steinhauer SR, et al. Cerebellar volume in offspring from multiplex alcohol dependence families. Biol Psychiatry. 2007b;61(1):41–47. doi: 10.1016/j.biopsych.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Shen S, Lowers L, Locke J. Factors predicting the onset of adolescent drinking in families at high risk for developing alcoholism. Biol Psychiatry. 2000;48(4):265–75. doi: 10.1016/s0006-3223(00)00841-6. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Lowers L, Locke-Wellman J, Matthews AG, McDermott M. Psychopathology in offspring from multiplex alcohol dependence families with and without parental alcohol dependence: a prospective study during childhood and adolescence. Psychiatry Res. 2008;160(2):155–66. doi: 10.1016/j.psychres.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Steinhauer S, Park J, Zubin J. Event-related potential characteristics in children of alcoholics from high density families. Alcohol Clin Exp Res. 1990;14(1):6–16. doi: 10.1111/j.1530-0277.1990.tb00438.x. [DOI] [PubMed] [Google Scholar]
- Hill SY, Tessner KD, McDermott MD. Psychopathology in offspring from families of alcohol dependent female probands: a prospective study. J Psychiatr Res. 2011a;45(3):285–94. doi: 10.1016/j.jpsychires.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Wang S, Carter H, Tessner K, Holmes B, et al. Cerebellum volume in high-risk offspring from multiplex alcohol dependence families: association with allelic variation in GABRA2 and BDNF. Psychiatry Res. 2011b;194(3):304–13. doi: 10.1016/j.pscychresns.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Wang S, Kostelnik B, Carter H, Holmes B, et al. Disruption of orbitofrontal cortex laterality in offspring from multiplex alcohol dependence families. Biol Psychiatry. 2009;65(2):129–36. doi: 10.1016/j.biopsych.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollen PJ. Intervention booster: adding a decision-making module to risk reduction and other health care programs for adolescents. J Pediatr Health Care. 1998;12(5):247–55. doi: 10.1016/s0891-5245(98)90205-x. [DOI] [PubMed] [Google Scholar]
- Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR, et al. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicol Teratol. 2009;31(6):349–55. doi: 10.1016/j.ntt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Thayer RE, Trim RS, Bava S, Frank LR, Tapert SF. White matter integrity, substance use, and risk taking in adolescence. Psychol Addict Behav. 2012 doi: 10.1037/a0028235. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CA, Xiao L, Palmer P, Sun P, Wang Q, et al. Affective decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in 10th grade Chinese adolescent binge drinkers. Neuropsychologia. 2008;46(2):714–26. doi: 10.1016/j.neuropsychologia.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings, 2011. Ann. Arbor: Inst. Soc. Res., Univ. Mich; 2012. [Google Scholar]
- Koskinen SM, Ahveninen J, Kujala T, Kaprio J, O’Donnell BF, et al. A longitudinal twin study of effects of adolescent alcohol abuse on the neurophysiology of attention and orienting. Alcohol Clin Exp Res. 2011;35(7):1339–50. doi: 10.1111/j.1530-0277.2011.01470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss WA, Sadowski RN, Sherrill LK, Gulley JM, Juraska JM. Effects of ethanol during adolescence on the number of neurons and glia in the medial prefrontal cortex and basolateral amygdala of adult male and female rats. Brain Res. 2012;1466:24–32. doi: 10.1016/j.brainres.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci. 2011;31(30):10937–47. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60(1):340–52. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4(6):469–80. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Li Q, Wilson WA, Swartzwelder HS. Developmental differences in the sensitivity of hippocampal GABAA receptor-mediated IPSCS to ethanol. Alcohol Clin Exp Res. 2003;27(12):2017–22. doi: 10.1097/01.ALC.0000108390.62394.71. [DOI] [PubMed] [Google Scholar]
- Mahmood OM, Jacobus J, Bava S, Scarlett A, Tapert SF. Learning and memory performances in adolescent users of alcohol and marijuana: interactive effects. J Stud Alcohol Drugs. 2010;71(6):885–94. doi: 10.15288/jsad.2010.71.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurage P, Joassin F, Speth A, Modave J, Philippot P, Campanella S. Cerebral effects of binge drinking: respective influences of global alcohol intake and consumption pattern. Clin Neurophysiol. 2012;123(5):892–901. doi: 10.1016/j.clinph.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Maurage P, Pesenti M, Philippot P, Joassin F, Campanella S. Latent deleterious effects of binge drinking over a short period of time revealed only by electrophysiological measures. J Psychiatry Neurosci. 2009;34(2):111–18. [PMC free article] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink. I Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcohol Clin Exp Res. 2001;25(8):1156–65. [PubMed] [Google Scholar]
- McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, et al. Altered white matter integrity in adolescent binge drinkers. Alcohol Clin Exp Res. 2009;33(7):1278–85. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol Clin Exp Res. 2008;32(3):386–94. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Yang TT, Tapert SF. Prefrontal cortex morphometry in abstinent adolescent marijuana users: subtle gender effects. Addict Biol. 2009;14(4):457–68. doi: 10.1111/j.1369-1600.2009.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol Teratol. 2007;29(1):141–52. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SA, Eaves DW, Smith AR, Nixon K. Alcohol inhibition of neurogenesis: a mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus. 2010;20(5):596–607. doi: 10.1002/hipo.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss HB, Kirisci L, Gordon HW, Tarter RE. A neuropsychologic profile of adolescent alcoholics. Alcohol Clin Exp Res. 1994;18(1):159–63. doi: 10.1111/j.1530-0277.1994.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005;139(3):181–90. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci. 2004;16(7):1227–33. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Nees F, Tzschoppe J, Patrick CJ, Vollstadt-Klein S, Steiner S, et al. Determinants of early alcohol use in healthy adolescents: the differential contribution of neuroimaging and psychological factors. Neuropsy-chopharmacology. 2012;37(4):986–95. doi: 10.1038/npp.2011.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Glass JM, Wong MM, Poon E, Jester JM, et al. Neuropsychological executive functioning in children at elevated risk for alcoholism: findings in early adolescence. J Abnorm Psychol. 2004;113(2):302–14. doi: 10.1037/0021-843X.113.2.302. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83(5):1087–93. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug Alcohol Depend. 2011;119(3):216–23. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada M, Corral M, Mota N, Crego A, Rodriguez Holguin S, Cadaveira F. Executive functioning and alcohol binge drinking in university students. Addict Behav. 2012;37(2):167–72. doi: 10.1016/j.addbeh.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Park MS, Sohn S, Park JE, Kim SH, Yu IK, Sohn JH. Brain functions associated with verbal working memory tasks among young males with alcohol use disorders. Scand J Psychol. 2011;52(1):1–7. doi: 10.1111/j.1467-9450.2010.00848.x. [DOI] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108(4):920–31. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Perlman G, Johnson W, Iacono WG. The heritability of P300 amplitude in 18-year-olds is robust to adolescent alcohol use. Psychophysiology. 2009;46(5):962–69. doi: 10.1111/j.1469-8986.2009.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Rohlfing T, Sullivan EV. Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol Psychiatry. 2009;65(8):680–90. doi: 10.1016/j.biopsych.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot RM, Wecker L, Kirstein CL. Repeated ethanol exposure during adolescence alters the developmental trajectory of dopaminergic output from the nucleus accumbens septi. Int J Dev Neurosci. 2009;27(8):805–15. doi: 10.1016/j.ijdevneu.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Polich J, Ladish C, Burns T. Normal variation of P300 in children: age, memory span, and head size. Int J Psychophysiol. 1990;9(3):237–48. doi: 10.1016/0167-8760(90)90056-j. [DOI] [PubMed] [Google Scholar]
- Pulido C, Anderson KG, Armstead AG, Brown SA, Tapert SF. Family history of alcohol-use disorders and spatial working memory: effects on adolescent alcohol expectancies. J Stud Alcohol Drugs. 2009a;70(1):87–91. doi: 10.15288/jsad.2009.70.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido C, Mok A, Brown SA, Tapert SF. Heavy drinking relates to positive valence ratings of alcohol cues. Addict Biol. 2009b;14(1):65–72. doi: 10.1111/j.1369-1600.2008.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp CI, Kemmler G, Kurz M, Hinterhuber H, Fleischhacker WW. Cognitive remediation therapy during treatment for alcohol dependence. J Stud Alcohol Drugs. 2012;73(4):625–34. doi: 10.15288/jsad.2012.73.625. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry. 1996;53(3):202–10. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, McQueeny T, Nage BJ, Eyler LT, Tapert SF. A preliminary study of functional magnetic resonance imaging response during verbal encoding among adolescent binge drinkers. Alcohol. 2010;44(1):111–17. doi: 10.1016/j.alcohol.2009.09.032. Subdiagnostic binge drinking is associated with less efficient processing of verbal information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, et al. An fMRI study of response inhibition in youths with a family history of alcoholism. Ann N Y Acad Sci. 2004;1021:391–94. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug Alcohol Depend. 2005;79(2):201–10. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Nagel BJ, Eyler LT, Tapert SF. Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction. 2011;106(3):564–73. doi: 10.1111/j.1360-0443.2010.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, Martin ED, Wood PK, Rutledge PC. Alcohol use disorders and neuropsychological functioning in first-year undergraduates. Exp Clin Psychopharmacol. 1997;5(3):304–15. doi: 10.1037//1064-1297.5.3.304. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Rogowska J, McCaffrey A, Yurgelun-Todd DA. Adolescents at risk for alcohol abuse demonstrate altered frontal lobe activation during Stroop performance. Alcohol Clin Exp Res. 2011;35(2):218–28. doi: 10.1111/j.1530-0277.2010.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadoni AD, Norman AL, Schweinsburg AD, Tapert SF. Effects of family history of alcohol use disorders on spatial working memory BOLD response in adolescents. Alcohol Clin Exp Res. 2008;32(7):1135–45. doi: 10.1111/j.1530-0277.2008.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Schweinsburg AD, Pulido C, Tapert SF. Adolescent binge drinking linked to abnormal spatial working memory brain activation: differential gender effects. Alcohol Clin Exp Res. 2011a;35(10):1831–41. doi: 10.1111/j.1530-0277.2011.01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Sorg SF, Schweinsburg AD, Wetherill RR, Pulido C, Tapert SF. Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology. 2011b;220(3):529–39. doi: 10.1007/s00213-011-2500-4. Subdiagnostic binge drinking impacts the cerebral cortex differently in boys and girls. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol Addict Behav. 2009;23(4):715–22. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20(4):327–48. doi: 10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res. 2000;24(5):611–21. [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Baratta MV, Brown SA. fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addict Behav. 2004a;29(1):33–50. doi: 10.1016/j.addbeh.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcohol Clin Exp Res. 2001;25(2):236–45. [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Neuropsychological correlates of adolescent substance abuse: four-year outcomes. J Int Neuropsychol Soc. 1999;5(6):481–93. doi: 10.1017/s1355617799566010. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, et al. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Arch Gen Psychiatry. 2003;60(7):727–35. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: neuropsychological functioning over 8 years in youth. J Int Neuropsychol Soc. 2002;8(7):873–83. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Pulido C, Paulus MP, Schuckit MA, Burke C. Level of response to alcohol and brain response during visual working memory. J Stud Alcohol. 2004b;65(6):692–700. doi: 10.15288/jsa.2004.65.692. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, et al. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcohol Clin Exp Res. 2004c;28(10):1577–86. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Mezzich AC, Hsieh YC, Parks SM. Cognitive capacity in female adolescent substance abusers. Drug Alcohol Depend. 1995;39(1):15–21. doi: 10.1016/0376-8716(95)01129-m. [DOI] [PubMed] [Google Scholar]
- Thoma RJ, Monnig MA, Lysne PA, Ruhl DA, Pommy JA, et al. Adolescent substance abuse: the effects of alcohol and marijuana on neuropsychological performance. Alcohol Clin Exp Res. 2011;35(1):39–46. doi: 10.1111/j.1530-0277.2010.01320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland BJ, Nigg JT, Welsh RC, Yau WY, Zubieta JK, et al. Resiliency in adolescents at high risk for substance abuse: flexible adaptation via subthalamic nucleus and linkage to drinking and drug use in early adulthood. Alcohol Clin Exp Res. 2012;36:1355–64. doi: 10.1111/j.1530-0277.2012.01741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Bava S, Thompson WK, Boucquey V, Pulido C, et al. Frontoparietal connectivity in substance-naive youth with and without a family history of alcoholism. Brain Res. 2012;1432:66–73. doi: 10.1016/j.brainres.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Bechara A, Gong Q, Huang X, Li X, et al. Abnormal affective decision making revealed in adolescent binge drinkers using a functional magnetic resonance imaging study. Psychol Addict Behav. 2012 doi: 10.1037/a0027892. In press. [DOI] [PubMed] [Google Scholar]
- Yau WY, Zubieta JK, Weiland BJ, Samudra PG, Zucker RA, Heitzeg MM. Nucleus accumbens response to incentive stimuli anticipation in children of alcoholics: relationships with precursive behavioral risk and lifetime alcohol use. J Neurosci. 2012;32(7):2544–51. doi: 10.1523/JNEUROSCI.1390-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]