Abstract
Objective
The prefrontal cortex (PFC) receives multiple cortical and subcortical afferents that regulate higher order cognitive functions, many of which emerge late in adolescence. However, it remains unclear how these afferents influence PFC processing, especially in light of the protracted, late adolescent maturation of prefrontal GABAergic function. Here we investigated the role of PFC GABAergic transmission in regulating plasticity elicited from the ventral hippocampus and basolateral amygdala, and how such modulation undergoes functional changes during adolescence in rats.
Methods
In vivo local field potential recordings, combined with prefrontal microinfusion of the GABA-A receptor antagonist picrotoxin, were employed to study the impact of ventral hippocampal and basolateral amygdala high-frequency stimulation on PFC plasticity.
Results
Ventral hippocampal-induced PFC plasticity begins to appear only by postnatal days (P) 45-55 with a transient suppression of the evoked response. A switch from transient to long-lasting depression (LTD) of the PFC response emerges after P55 and throughout adulthood (P65-120). Recordings conducted in the presence of picrotoxin revealed that PFC GABAergic transmission is critical for the expression of LTD. In contrast, basolateral amygdala stimulation resulted in PFC long-term potentiation, a form of plasticity that is already enabled by P30 and is insensitive to picrotoxin.
Conclusions
The development of ventral hippocampal-dependent PFC LTD is contingent upon the recruitment of local prefrontal GABAergic transmission during adolescence whereas plasticity elicited from the basolateral amygdala is not. Thus, different mechanisms contribute to the refinement of prefrontal plasticity during adolescence as inputs from these two regions are critical for shaping PFC functions.
Keywords: adolescence, prefrontal cortex, GABA, local field potentials
Introduction
The prefrontal cortex (PFC) continues its maturation during adolescence, a developmental period during which acquisition of cognitive abilities associated with adult behavior take place (Best and Miller 2010; Casey et al. 2000). The expression and maturation of these prefrontal actions are thought to require proper development of inputs from the ventral hippocampus and amygdala (Maroun and Richter-Levin 2003; Tseng et al. 2009), both of which converge in the prelimbic and infralimbic regions of the PFC (Carmichael and Price 1995; Hoover and Vertes 2007; Ishikawa and Nakamura 2003). Behavioral studies indicate that the ventral hippocampal-PFC pathway is implicated in working memory processes (Floresco et al. 1997; Seamans et al. 1995; Wang and Cai 2006) and inhibitory control (Abela et al. 2012; Chudasama et al. 2012), whereas the amygdala is critical for providing emotional control of prefrontal responses to salient stimuli (Davis and Whalen 2001; Garcia et al. 1999; Gilmartin and Helmstetter 2010; Milad and Quirk 2012; Morgan and LeDoux 1995). Notably, these particular cognitive abilities appear to be impaired in major psychiatric disorders, especially those which arise during adolescence such as schizophrenia, drug abuse, and affective disorders (Andersen and Teicher 2008; Chambers et al. 2001; Paus et al. 2008; Schumann et al. 2011; Spear 2009). Thus, it is likely that prefrontal processing of ventral hippocampal and amygdala transmission undergoes functional remodeling during adolescence to enable better integration of contextual and emotional cues.
It has long been recognized that glutamatergic inputs from the ventral hippocampus play an important role in enabling prefrontal plasticity in adult animals as indicated by the expression of a form of NMDA-dependent LTP following hippocampal tetanic stimulation (Laroche et al. 2000). Although less studied, theta burst stimulation into the amygdala has been shown to elicit a similar form of LTP in the adult PFC in vivo (Maroun and Richter-Levin 2003). In addition to plasticity at glutamatergic synapses, GABAergic interneurons in cortical circuits have been recently recognized as important neural substrates for the development of cognitive functions due to the their ability to inhibit and synchronize neuronal populations (O'Donnell 2011; Uhlhaas and Singer 2011). In this regard, we recently found that prefrontal GABAergic transmission becomes functionally enabled during adolescence to control the adult pattern of PFC network response to ventral hippocampal stimulation (Thomases et al. 2013). Notably, several aspects of hippocampal and amygdala connections to the PFC continue to undergo anatomical changes throughout adolescence (Benes 1989; Cressman et al. 2010; Cunningham et al. 2002). However, it remains unclear how these developing afferents influence prefrontal processing during the adolescent transition to adulthood, especially in light of the protracted maturation of GABAergic function in the PFC (Caballero et al. 2013).
In the present study, we conducted local field potential (LFP) recordings in the medial PFC and determined the role of local GABAergic circuits in modulating prefrontal processing of ventral hippocampal and basolateral amygdala transmission during the transition to adulthood in rats. For this purpose, a high-frequency stimulation protocol was delivered into these regions to induce plasticity in the PFC, and the contribution of prefrontal GABAergic transmission to the response was determined by local infusion of the GABA-A receptor antagonist picrotoxin. This protocol was chosen because it is suitable for assessing developmental changes in medial PFC network function to afferent stimulation (Cass et al. 2013).
Materials and Methods
All electrophysiological recordings were conducted according to the USPHS Guide for Care and Use of Laboratory Animals, and were approved by the Rosalind Franklin University Institutional Animal Care and Use Committee. In the present study, four age groups of male Sprague-Dawley rats (Harlan, Indianapolis, IN) were included (P30-40, P45-55, P65-85, P95-120) based on our recent developmental study showing that maturation of GABAergic function in the PFC occurs late in adolescence (Caballero et al. 2013). They were group housed (2-3 rats/cage) under conditions of constant temperature (21-23°C) and humidity in a 12:12 hour light/dark cycle with food and water available ad libitum. All chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Ventral hippocampal- vs. basolateral amygdala-induced plasticity in the medial PFC by means of LFP recordings in vivo
All recordings were conducted following a previously reported protocol (Cass et al. 2013; Thomases et al. 2013). Briefly, rats were deeply anesthetized with 8% chloral hydrate (400 mg/kg i.p.), placed in a stereotaxic apparatus (ASI instruments, MI), and maintained at 37-38°C (TCAT-2LV controller, Physitemp Instruments, Clifton, NJ). Throughout the recording session, a steady supplementary level of anesthesia (300-400 μL of 8% chloral hydrate per hour) was delivered using a syringe minipump (BASi Baby Bee Syringe Drives, CA) attached to an i.p. cannula (26-gauge butterfly needle). The coordinates for the medial PFC recordings were: 3.2 to 2.7 mm anterior from bregma, 0.8 mm lateral from the midline, 4.2 mm below the brain surface. Prefrontal LFP recordings were conducted using a concentric bipolar electrode (SNE-100 × 50 mm; Rhodes Medical Instruments Inc., Summerland, CA), amplified (Cygnus Technology Inc., Delaware Water Gap, PA), filtered (bandwidth 1-100 Hz) and digitized (Digidata 1440A, Molecular Devices, Sunnyvale, CA) at a sampling rate of 10 kHz. A second concentric bipolar electrode (NE-100 × 50 mm) was used to stimulate the ventral hippocampus (5.8 mm posterior from bregma, 5.2 mm lateral from the midline, 7.3 mm below the brain surface). Single square pulses of 300 μs duration were delivered every 15 s through a computer-controlled pulse generator Master 8 Stimulator (AMPI, Jerusalem, Israel), typically at 0.75 mA stimulating intensity. In the present study, the peak amplitude and slope of the evoked LFP elicited with this intensity falls within the 75-80% range of the input-output response curve (tested from 0.25 mA to 1.0 mA). After 20 min of stable baseline recordings (evoked LFP responses with <20% variability in amplitude and slope), a protocol of high-frequency stimulation at 0.75 mA (50 pulses at 100 Hz/15 s × 4) was delivered into the ventral hippocampus or the basolateral amygdala, and changes in the slope of the evoked response were measured (from onset to peak amplitude of the response) to determine whether input-specific plasticity in the PFC is age-dependent. All time-course plots summarizing the effects of the high-frequency stimulation shown in the figures were created using a bin size window of 2 min (i.e., mean slope value from 8 field responses per data point).
In another set of PFC recordings, high-frequency stimulation-induced plasticity was assessed following local administration of artificial cerebrospinal fluid (aCSF)-containing the GABA-A receptor antagonist picrotoxin. As previously described (Cass et al. 2013; Thomases et al. 2013), a 28-gauge cannula (Plastics One Inc., Roanoke, VA) filled with aCSF-containing picrotoxin (50μM) or aCSF alone was secured to the recording electrode. Typically, the high-frequency stimulation protocol is delivered into the ventral hippocampus or the basolateral amygdala around 30 min after the single infusion of aCSF or aCSF-picrotoxin (1μl total volume at 0.1μl/min). This time window between the infusion and high-frequency stimulation was chosen because the amplitude of the evoked LFP begins to increase during the last 2 min of picrotoxin infusion, an effect that continues until reaching to a steady state, typically by the end of the 10 min post-infusion period. Thus, the 30 min window is needed to enable 20 min of stable baseline recordings before the delivery of the high-frequency stimulation protocol. At the end of the experiments, rats were deeply anesthetized and the brains were quickly removed, fixed in 4% paraformaldehyde overnight, sectioned and stained (cresyl violet), and examined on a microscope to assess the exact location of the electrodes.
Statistical analysis
All the results are expressed as mean ± SEM, and differences among experimental groups were considered statistically significant at p<0.05. Paired t-test was used to assess the within-subject effect of high-frequency stimulation-induced plasticity in the PFC. A two-way ANOVA design was used to determine changes in prefrontal local field potential responses across age groups and treatment conditions (age x treatment).
Results
We begin investigating how ventral hippocampal inputs impact medial PFC plasticity during postnatal development by means of local field potential (LFP) recordings in vivo (Fig 1a). For this purpose, a high-frequency stimulation (HFS) protocol (50 pulses at 100 Hz/15 s x 4) was delivered into the ventral hippocampus, and long-term changes in prefrontal LFP responses to single hippocampal stimulation were determined. Overall analyses revealed an age group effect (F(3,24)=4.7, p=0.01, one-way ANOVA). Medial PFC recordings from the P30-40 age group (n=7) revealed that ventral hippocampal HFS failed to elicit any apparent changes of the evoked LFP response (Fig 1b). In the late adolescent (P45-55, n=7) group, a significant but transient suppression of the prefrontal LFP response was observed following ventral hippocampal HFS (Fig 1c). Relative to baseline, an 18.9 ± 3.2% attenuation of the evoked LFP was observed only during the first 10 min post-HFS period (p<0.006 vs. baseline, paired t-test). Although the magnitude of this initial LFP depression is equivalent to that obtained in the adult PFC (-19.5 ± 2.8% from baseline), only recordings from the latter group revealed a typical pattern of long-term depression (LTD) (Fig 1d-e). In fact, both P65-85 (n=7) and P95-120 (n=7) age groups exhibited similar patterns of prefrontal LTD following ventral hippocampal HFS (Fig 1f). These results indicate that plasticity of ventral hippocampal inputs to the PFC undergo functional remodeling during late adolescence to reach maturity after P55.
Figure 1.
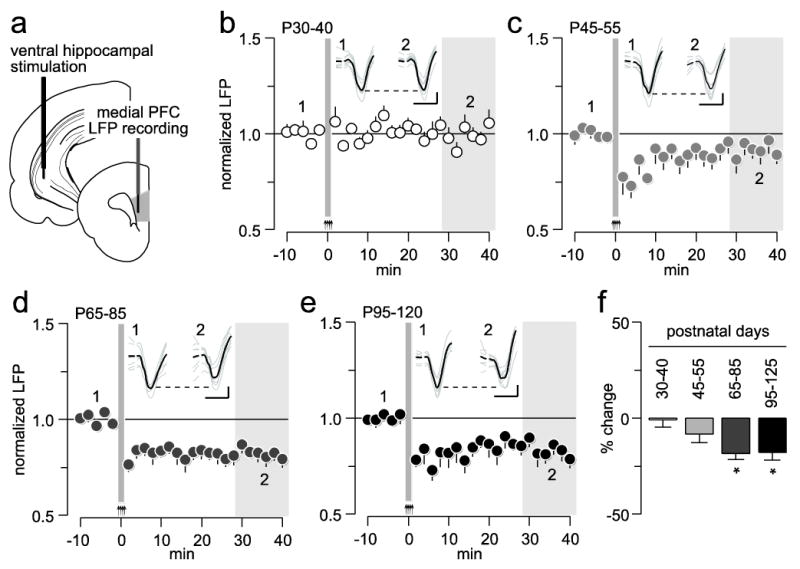
Age-dependent effects of ventral hippocampal high-frequency stimulation (HFS)-induced plasticity in the medial prefrontal cortex (PFC). (a) Diagram depicting the recording arrangement used to study hippocampal-induced plasticity in the medial PFC by means of local field potential (LFP) recordings. (b) Effects of ventral hippocampal high-frequency stimulation (HFS) on medial PFC LFP in postnatal days (P) 30-40 rats (n=7). Arrows and area marked in gray at 0 minute (min) indicate the HFS period. (c) Effects of ventral hippocampal HFS on medial PFC LFP in P45-55 rats (n=7). (d) Effects of ventral hippocampal HFS on medial PFC LFP in P65-85 rats (n=7). (e) Effects of ventral hippocampal HFS on medial PFC LFP in P95-120 rats (n=7). (f) Bar graph summarizing the mean LFP response from the 30-40 min post-HFS (area marked in gray shown in b, c, d, and e; *p<0.02 vs. P30-40, Tukey post-hoc test). Insets: example traces of ventral hippocampal-evoked LFP before (1) and after (2) HFS (calibration bars: 3 mV, 50 ms).
We next asked whether local prefrontal GABAergic transmission contributes to sustaining LTD in the adult PFC. To this end, the GABA-A receptor antagonist picrotoxin (50 μM in aCSF, n=7, see Materials and Methods for details) was delivered into the medial PFC 20 min prior to ventral hippocampal HFS whereas control recordings received aCSF alone (n=6; Fig 2). Results from the P30-40 age group indicate that picrotoxin failed to alter the normal pattern of prefrontal LFP response to ventral hippocampal HFS (Fig 2a). Both aCSF- and picrotoxin-treated PFC from this age group showed similar post-HFS response patterns (Fig 2a). In contrast, when picrotoxin was delivered into the P65-85 PFC (n=7), ventral hippocampal HFS no longer resulted in prefrontal LTD as seen in aCSF controls (n=7; Fig 2b). Instead, a long-term potentiation (LTP) of the evoked LFP response was unveiled in the medial PFC (p<0.0005, baseline vs. 35-40 min post-HFS period, paired t-test; Fig 2c). Two-way ANOVA revealed a significant age x treatment interaction (F(1,23)=60.21, p<0.0001), mainly due to the effects observed in the P65-85 age group. Together, the data demonstrate that the expression of prefrontal LTD requires local GABAergic transmission, a form of plasticity that becomes fully enabled after P55 (Fig 1f). Interestingly, the age-dependent LTP observed when local prefrontal GABAergic transmission is blocked (Fig 2c) indicates that PFC plasticity at glutamatergic synapses also undergoes developmental regulation during adolescence.
Figure 2.
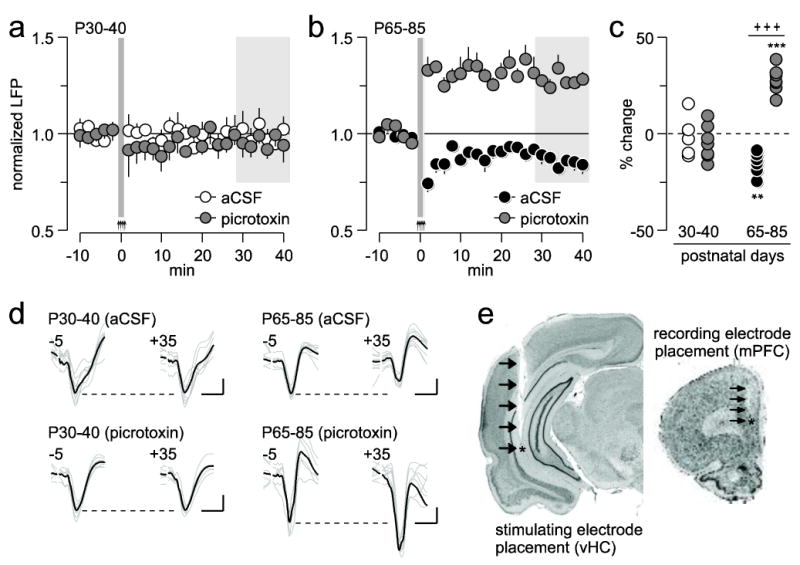
Role of local prefrontal GABAergic transmission in the regulation of ventral hippocampal-induced plasticity in the medial PFC. (a) Effects of local prefrontal microinfusion of aCSF (n=6) or picrotoxin (n=7) on ventral hippocampal HFS-induced PFC plasticity in the P30-40 age group. (b) Effects of local prefrontal microinfusion of aCSF (n=7) or picrotoxin (n=7) on ventral hippocampal HFS-induced PFC plasticity in the P65-85 age group. (c) Summary of the results shown in a and b. Each dot represents the mean LFP response obtained within the 30-40 min post-HFS (area marked in gray; **p<0.005, ***p<0.0005 vs. P25-40; +++p<0.0005 vs. picrotoxin, LSD post-hoc test). (d) Example traces of ventral hippocampal-evoked LFP taken from 5 min pre-HFS (-5) and 35 min post-HFS (+35) illustrating the age-dependent effects of picrotoxin on PFC plasticity (calibration bars: 3 mV, 50 ms). (e) Examples of coronal sections stained with cresyl violet showing the anatomical location of the recording (mPFC: medial PFC) and stimulating (vHC: ventral hippocampus) electrodes from a picrotoxin-infused P65-85 rat. Arrows indicate the track of the electrode placement.
In addition to the ventral hippocampus, the medial PFC also receives major glutamatergic inputs from the basolateral amygdala, a pathway that may undergo periadolescent regulation. To this end, the same HFS protocol used to elicit ventral hippocampal-dependent plasticity was delivered into the basolateral amygdala (Fig 3a), and changes in prefrontal LFP responses to single amygdala stimulation were determined. Conversely to the effects of hippocampal stimulation, HFS of the basolateral amygdala resulted in LTP of the prefrontal LFP response in both P30-40 (aCSF, n=5; p<0.005, baseline vs. 35-40 min post-HFS period, paired t-test; Fig 3b) and P65-85 (n=7; p<0.005, baseline vs. 35-40 min post-HFS period, paired t-test; Fig 3c) age groups. Relative to adults, the P30-40 PFC showed a small but significantly less potentiated LFP response within the 30-40 min post-HFS period (main age effect: F(1,20)=6.3, p=0.02, two-way ANOVA; Fig 3d). Interestingly, local PFC microinfusion of picrotoxin (P30-40, n=5; P65-85, n=7) did not alter the pattern or magnitude of LTP in both age groups (Fig 3), indicating that prefrontal GABAergic transmission is not recruited to sustain basolateral amygdala-dependent plasticity.
Figure 3.
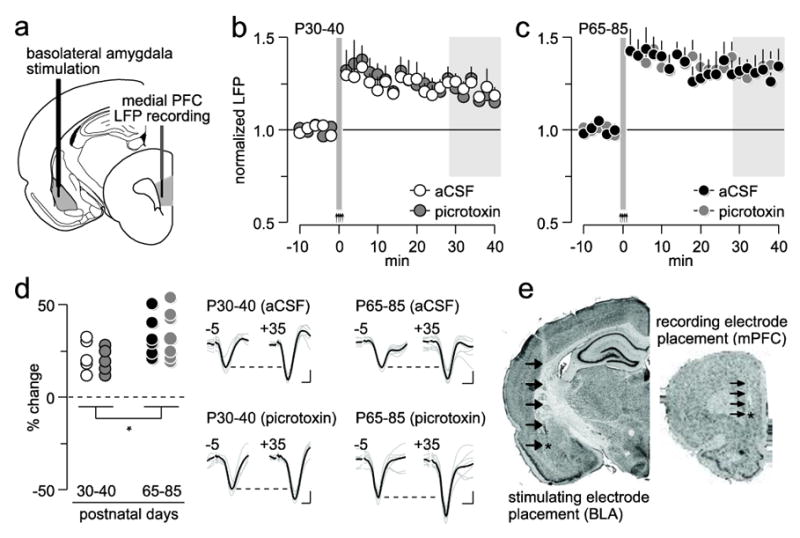
Role of local prefrontal GABAergic function in the regulation of basolateral amygdala-induced plasticity in the medial PFC. (a) Diagram depicting the recording arrangement used to study the effects of basolateral amygdala HFS on medial PFC LFP. (b) Effects of local PFC microinjection of aCSF (n=5) or picrotoxin (n=5) on basolateral amygdala HFS-induced prefrontal LTP in P30-40 rats. (c) Effects of local PFC microinjection of aCSF (n=7) or picrotoxin (n=7) on basolateral amygdala HFS-induced prefrontal LTP in P65-85 rats. (d) Summary of the mean LFP response obtained from the 30-40 min post-basolateral amygdala HFS period (area marked in gray shown in b and c; *p=0.02, main age effect). Insets traces are examples of basolateral amygdala-evoked LFP taken from 5 min pre-HFS (-5) and 35 min post-HFS (+35) illustrating the impact of picrotoxin on PFC plasticity (calibration bars: 3 mV, 50 ms). (e) Examples of cresyl violet-stained coronal sections obtained from a picrotoxin-infused P65-85 rat showing the location of the recording (mPFC: medial PFC) and stimulating (BLA: basolateral amygdala) electrodes. Arrows indicate the track of the electrode placement.
Discussion
In the present study, in vivo electrophysiological recordings and LFP measures of medial PFC responses revealed that plasticity originating from the ventral hippocampus undergoes distinct functional remodeling compared to that from the basolateral amygdala during adolescence. By adulthood, ventral hippocampal HFS typically results in prefrontal LTD whereas a robust LTP response emerges in the PFC following HFS of the basolateral amygdala. Recruitment of local prefrontal GABAergic transmission is required for the expression of ventral hippocampal-induced LTD, a form of PFC response that reaches maturity after P55. In contrast, prefrontal LTP elicited from the basolateral amygdala is already enabled by P30, which remains steady through adolescence and adulthood, and does not involve activation of PFC GABA-A receptors. Together, these results indicate that prefrontal GABAergic control of input-specific plasticity emerges late in adolescent, a mechanism that could contribute to the maturation of PFC processing of context-dependent inputs such as those from the ventral hippocampus.
The late adolescent expression of hippocampal-induced prefrontal LTD could result from the protracted maturation of PFC interneurons. Based on the expression of calcium-binding proteins, we have recently found that prefrontal GABAergic interneurons undergo differential regulation during adolescence (Caballero et al. 2013). Of particular interest are the parvalbumin-positive cells, which are the most abundant class of GABAergic interneurons in the rat PFC (Gabbott et al. 1997), and are functionally positioned to exert fast feed-forward control of cortical output responses due to their unique non-adapting and fast-spiking firing pattern (Bartos and Elgueta 2012). In addition, parvalbumin-positive/fast-spiking cells are of the only interneuronal subtype in the PFC that becomes functionally upregulated after P45 (Caballero et al. 2013). In this regard, the enhanced glutamatergic drive onto fast-spiking interneurons observed in the adolescent and adult PFC (Caballero et al. 2013) could increase the responsiveness of local GABAergic circuits and contribute to sustaining prefrontal LTD following ventral hippocampal HFS. Future studies are needed to determine the precise cellular mechanisms underlying the prefrontal GABAergic modulation of hippocampal-dependent plasticity.
While local GABAergic transmission is critical for sustaining prefrontal LTD, recordings conducted from the picrotoxin-treated PFC revealed a form of hippocampal-dependent LTP that also arises late in adolescence. These results indicate that ventral hippocampal HFS can elicit a long-lasting facilitation of prefrontal glutamatergic drive in tandem with the augmented PFC GABAergic inhibition. Accordingly, hippocampal stimulation has been known to result in a mix of inhibitory and excitatory responses in the PFC (Ishikawa and Nakamura 2003). Furthermore, the net LTD response observed in the PFC when local GABAergic transmission is intact suggests that the functional impact of ventral hippocampal inputs onto prefrontal interneurons is significantly greater than that onto pyramidal cells. Although the mechanisms underlying such differential modulation are unclear, one would have predicted the opposite based on the cellular distribution of glutamatergic inputs originating from the ventral hippocampus. In fact, the majority of hippocampal afferents to the PFC synapse onto pyramidal neurons (Carr and Sesack 1996; Gabbott et al. 2002) whereas only a minority of these inputs synapses onto local GABAergic interneurons (Gabbott et al. 2002). Interestingly, afferents originated from the amygdala follow a similar pattern of preferential innervations onto pyramidal cells (Bacon et al. 1996), and yet the prefrontal LTP elicited from the basolateral amygdala remains intact despite disruption of PFC GABAergic function. Nonetheless, the functionality of an amygdala-PFC interneurons connection has been recently validated (Dilgen et al. 2013). Results from this study show that amygdala stimulation with single pulses can drive a feedforward mechanism of inhibition in the PFC that involves activation of local interneurons (Dilgen et al. 2013). Thus, it is reasonable to conclude that the ventral hippocampus exerts a much more powerful control of prefrontal GABAergic plasticity than the basolateral amygdala, and that this function begins to emerge late in adolescence through a mechanism that has yet to be determined.
The pattern of prefrontal LTP elicited from the basolateral amygdala resembles that recorded in the picrotoxin-treated PFC following ventral hippocampal HFS, suggesting that both forms of plasticity may share common mechanisms of expression. However, the relative early onset of basolateral amygdala-dependent LTP (i.e., P30) compared to that induced from the ventral hippocampus (>P40) indicates that the underlying mechanism of prefrontal LTP is input-specific. In this regard, a number of molecular and functional changes known to enhance the response of PFC output neurons during adolescence could contribute to the onset of hippocampal-dependent LTP after P40. These include the periadolescent facilitation of NMDA response by D1 receptors (Tseng and O'Donnell 2005), the increased L-type Ca2+ function and PKA signaling after P40 (Heng et al. 2011b) concurrent with the functional expression of NR2B-containing NMDA transmission (Flores-Barrera et al. 2013). In addition to the acquisition of these postsynaptic excitatory functions, the periadolescent downregulation of presynaptic CB1 receptor inhibition of glutamatergic synaptic transmission onto prefrontal pyramidal neurons (Heng et al. 2011a) could also contribute to LTP in the PFC. Together, the late adolescent onset of prefrontal LTP could be attributable to an input-specific remodeling of pre and postsynaptic mechanisms of transmission that ultimately enhance prefrontal output to ventral hippocampal drive.
In conclusion, our results clearly demonstrate that not all mechanisms of synaptic plasticity in cortical circuits are acquired within the first 3-4 weeks of postnatal development in rodents as traditionally accepted (Dan and Poo 2006). Although the role of LTD and LTP at later stages of brain maturation remains elusive, the PFC and its known functions in working memory and decision making provide a good site for studying the role of synaptic plasticity in cognitive processing. Both the ventral hippocampus and the basolateral amygdala are critically involved in the development of these PFC actions, yet only plasticity originating from the former becomes enabled in late adolescence through a GABA-dependent mechanism. We speculate that the lack of normative acquisition of input-specific plasticity onto prefrontal GABAergic circuits could contribute to the adolescent onset of cognitive deficits as seen in schizophrenia and related psychiatric disorders.
Acknowledgments
This research was supported by Rosalind Franklin University and the National Institutes of Health Grant R01-MH086507 to KYT. All experiments reported here comply with the current laws of the United States of America.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- Abela AR, Dougherty SD, Fagen ED, Hill CJ, Chudasama Y. Inhibitory Control Deficits in Rats with Ventral Hippocampal Lesions. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs121. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–91. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Bacon SJ, Headlam AJ, Gabbott PL, Smith AD. Amygdala input to medial prefrontal cortex (mPFC) in the rat: a light and electron microscope study. Brain Res. 1996;720:211–9. doi: 10.1016/0006-8993(96)00155-2. [DOI] [PubMed] [Google Scholar]
- Bartos M, Elgueta C. Functional characteristics of parvalbumin- and cholecystokinin-expressing basket cells. J Physiol. 2012;590:669–81. doi: 10.1113/jphysiol.2011.226175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM. Myelination of cortical-hippocampal relays during late adolescence. Schizophr Bull. 1989;15:585–93. doi: 10.1093/schbul/15.4.585. [DOI] [PubMed] [Google Scholar]
- Best JR, Miller PH. A developmental perspective on executive function. Child Dev. 2010;81:1641–60. doi: 10.1111/j.1467-8624.2010.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A, Flores-Barrera E, Cass DK, Tseng KY. Differential regulation of parvalbumin and calretinin interneurons in the prefrontal cortex during adolescence. Brain Struct Funct. 2013 Feb 12; doi: 10.1007/s00429-013-0508-8. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Hippocampal afferents to the rat prefrontal cortex: synaptic targets and relation to dopamine terminals. J Comp Neurol. 1996;369:1–15. doi: 10.1002/(SICI)1096-9861(19960520)369:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54:241–57. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Cass DK, Thomases DR, Caballero A, Tseng KY. Developmental Disruption of Gamma-Aminobutyric Acid Function in the Medial Prefrontal Cortex by Noncontingent Cocaine Exposure During Early Adolescence. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol Psychiatry. 2001;50:71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Doobay VM, Liu Y. Hippocampal-prefrontal cortical circuit mediates inhibitory response control in the rat. J Neurosci. 2012;32:10915–24. doi: 10.1523/JNEUROSCI.1463-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressman VL, Balaban J, Steinfeld S, Shemyakin A, Graham P, Parisot N, Moore H. Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. J Comp Neurol. 2010;518:2693–709. doi: 10.1002/cne.22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453:116–30. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity: from synapse to perception. Physiol Rev. 2006;86:1033–48. doi: 10.1152/physrev.00030.2005. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Dilgen J, Tejeda HA, O'Donnell P. Amygdala inputs drive feedforward inhibition in the medial prefrontal cortex. J Neurophysiol. 2013 doi: 10.1152/jn.00531.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Barrera E, Thomases DR, Heng LJ, Cass DK, Caballero A, Tseng KY. Late adolescent expression of NR2B transmission in the prefrontal cortex is input-specific and requires postsynaptic PKA and D1 dopamine receptor signaling. J Neurosci. 2013 doi: 10.1016/j.biopsych.2013.07.033. (in revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–90. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott P, Headlam A, Busby S. Morphological evidence that CA1 hippocampal afferents monosynaptically innervate PV-containing neurons and NADPH-diaphorase reactive cells in the medial prefrontal cortex (Areas 25/32) of the rat. Brain Res. 2002;946:314–22. doi: 10.1016/s0006-8993(02)02487-3. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Dickie BG, Vaid RR, Headlam AJ, Bacon SJ. Local-circuit neurones in the medial prefrontal cortex (areas 25, 32 and 24b) in the rat: morphology and quantitative distribution. J Comp Neurol. 1997;377:465–99. doi: 10.1002/(sici)1096-9861(19970127)377:4<465::aid-cne1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Garcia R, Vouimba RM, Baudry M, Thompson RF. The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature. 1999;402:294–6. doi: 10.1038/46286. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, Helmstetter FJ. Trace and contextual fear conditioning require neural activity and NMDA receptor-dependent transmission in the medial prefrontal cortex. Learn Mem. 2010;17:289–96. doi: 10.1101/lm.1597410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng L, Beverley JA, Steiner H, Tseng KY. Differential developmental trajectories for CB1 cannabinoid receptor expression in limbic/associative and sensorimotor cortical areas. Synapse. 2011a;65:278–86. doi: 10.1002/syn.20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng LJ, Markham JA, Hu XT, Tseng KY. Concurrent upregulation of postsynaptic L-type Ca(2+) channel function and protein kinase A signaling is required for the periadolescent facilitation of Ca(2+) plateau potentials and dopamine D1 receptor modulation in the prefrontal cortex. Neuropharmacology. 2011b;60:953–62. doi: 10.1016/j.neuropharm.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–79. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Nakamura S. Convergence and interaction of hippocampal and amygdalar projections within the prefrontal cortex in the rat. J Neurosci. 2003;23:9987–95. doi: 10.1523/JNEUROSCI.23-31-09987.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroche S, Davis S, Jay TM. Plasticity at hippocampal to prefrontal cortex synapses: dual roles in working memory and consolidation. Hippocampus. 2000;10:438–46. doi: 10.1002/1098-1063(2000)10:4<438::AID-HIPO10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Maroun M, Richter-Levin G. Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. J Neurosci. 2003;23:4406–9. doi: 10.1523/JNEUROSCI.23-11-04406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–51. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109:681–8. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- O'Donnell P. Adolescent onset of cortical disinhibition in schizophrenia: insights from animal models. Schizophr Bull. 2011;37:484–92. doi: 10.1093/schbul/sbr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–57. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Bauman MD, Amaral DG. Abnormal structure or function of the amygdala is a common component of neurodevelopmental disorders. Neuropsychologia. 2011;49:745–59. doi: 10.1016/j.neuropsychologia.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. Functional differences between the prelimbic and anterior cingulate regions of the rat prefrontal cortex. Behav Neurosci. 1995;109:1063–73. doi: 10.1037//0735-7044.109.6.1063. [DOI] [PubMed] [Google Scholar]
- Spear LP. Heightened stress responsivity and emotional reactivity during pubertal maturation: Implications for psychopathology. Dev Psychopathol. 2009;21:87–97. doi: 10.1017/S0954579409000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomases DR, Cass DK, Tseng KY. Periadolescent exposure to the NMDA receptor antagonist MK-801 impairs the functional maturation of local GABAergic circuits in the adult prefrontal cortex. J Neurosci. 2013;33:26–34. doi: 10.1523/JNEUROSCI.4147-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res. 2009;204:295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. Post-pubertal emergence of prefrontal cortical up states induced by D1-NMDA co-activation. Cereb Cortex. 2005;15:49–57. doi: 10.1093/cercor/bhh107. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. The development of neural synchrony and large-scale cortical networks during adolescence: relevance for the pathophysiology of schizophrenia and neurodevelopmental hypothesis. Schizophr Bull. 2011;37:514–23. doi: 10.1093/schbul/sbr034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GW, Cai JX. Disconnection of the hippocampal-prefrontal cortical circuits impairs spatial working memory performance in rats. Behav Brain Res. 2006;175:329–36. doi: 10.1016/j.bbr.2006.09.002. [DOI] [PubMed] [Google Scholar]


