Abstract
Fgf8 exerts a strong effect on the mesenchymal cells of neural crest (NC) origin that are fated to form the facial skeleton. Surgical extirpation of facial skeletogenic NC domain (including mid-diencephalon down through rhombomere 2), which does not express Hox genes, results in the failure of facial skeleton development and inhibition of the closure of the forebrain neural tube, while Fgf8 expression in the telencephalon and in the branchial arch (BA) ectoderm is abolished. We demonstrate here that (i) exogenous FGF8 is able to rescue facial skeleton development by promoting the proliferation of NC cells from a single rhombomere, r3, which in normal development contributes only marginally to mesenchyme of BA1, and (ii) expression of Fgf8 in forebrain and in BA ectoderm is subjected to signal(s) arising from NC cells, thus showing that the development of cephalic NC-derived structures depends on FGF8 signaling, which is itself triggered by the NC cells.
Keywords: cephalic neural crest, facial skeleton, forebrain, regeneration, quail-chick chimeras
In vertebrates, the skeleton and connective components of the face are derived from the cephalic neural crest (NC), which can be divided into two domains. The first, a rostral domain, in which no Hox genes are expressed, extends from the presumptive level of the epiphysis down through the second rhombomere (r2); it yields the cartilages and membrane bones of the face (Fig. 1 A). It is referred to here as the facial skeletogenic NC (FSNC). The second, posterior domain (from r4 through r8) generates part of the hyoid cartilages and does not form any membrane bone. In this posterior domain of the NC, Hox genes of the four first paralogous groups are expressed both in neural tube and NC (1–3). A few NC cells (NCC) from r3 contribute to both domains. However, this contribution to branchial arches (BAs) is very small because most r3-derived NCC are undergoing apoptosis (4, 5).
Fig. 1.
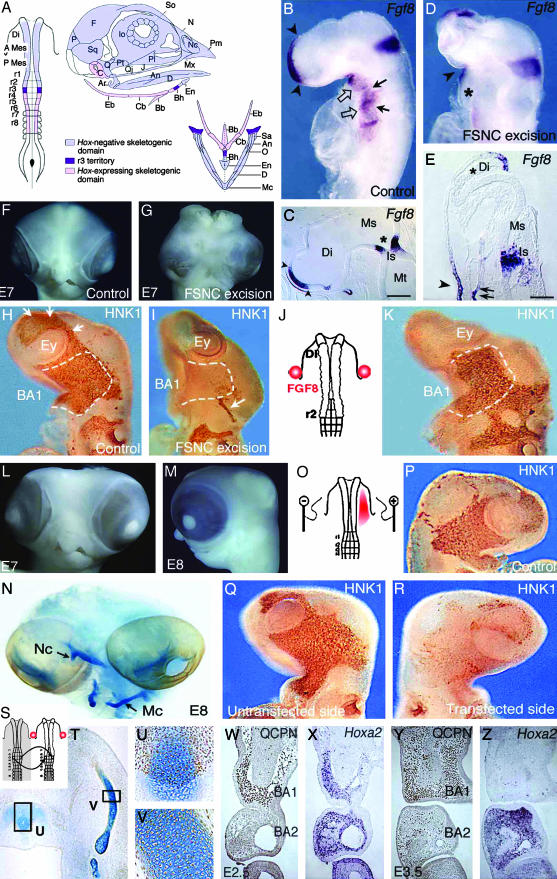
(On opposite page.) Influence of FSNC excision on Fgf8 expression in the embryonic head (A–E). (A) Fate map of the 5ss chicken embryo cephalic NF (2, 19). The anterior domain, extending from mid-diencephalon down through r2, yields Hox-negative NCC (in light blue); these NCC, which form the facial skeleton, are designated as FSNC. The posterior domain, extending from r4 through r8, generates Hox-positive NCC (in pink) and yields most of the hyoid cartilage. At the edge of both Hox-positive and Hox-negative domains, r3 NF is represented in violet. r3 NCC provide a minor contribution to both BA1 and BA2. Respective contributions of these domains to the craniofacial and hypobranchial skeleton are color-coded as described previously. (B) In situ hybridization with an Fgf8 riboprobe carried out on normal 24ss chick embryos in toto and (C) in sagittal section (C). The facial processes (nasal bud and BA1) are colonized by the FSNC. Fgf8 is expressed in the prosencephalon neuroepithelium and in the adjacent superficial ectoderm (arrowheads; B and C). A second site of Fgf8 expression is in the ectoderm of BA1, -2, and -3 through -4 (open arrows). First detected in the ventral ectoderm corresponding to the level of the presumptive BA1 from 13ss, Fgf8 expression becomes widely distributed at 24ss on the whole BA ectoderm with more intense foci of transcript accumulation facing the pharyngeal pouches (arrows). The third site of Fgf8 expression is in the neuroepithelium of the isthmus (B and C; *). In embryos in which resection of the FSNC is carried out bilaterally at 5–6ss from the mid-diencephalic level down to r3 (r3 excluded; D), striking changes are seen in Fgf8 expression during the 24 h after the resection. At 24ss, the prosencephalon is reduced in size, and the anterior Fgf8 expression site is less extended and displaced dorsally (D). On the sagittal section (E), FSNC extirpation affects the pattern of Fgf8 in the superficial ectoderm and precludes its expression in the prosencephalic neuroepithelium where transcript accumulation is completely abolished (*; E). The BAs are not properly developed, and expression of Fgf8 gene is very reduced (*) when compared to stage-matched controls (B and D). At the level of the isthmus, Fgf8 expression is less intense, more widespread, and essentially localized in the ventricular zone of the neural epithelium. Fgf8 expression is maintained in the stomodeal ectoderm (arrowhead on D and E) and in the endoderm of the foregut (double arrows on E). FSNC ablation completely abolishes the development of the face and brain [see E7 control (F) and operated (G) embryos]. Restoration of facial morphogenesis by exogenous FGF8 (H–N). Comparison of NC migration in control (H), FSNC-excised (I), and FSNC-excised subjected to exogenous FGF8 through beads placed at the presumptive level of BA1 ectoderm (J and K; HNK1 mAb staining at E3). In E3 control embryos, cephalic NCC, which appear as brown in toto, have spread laterally and massively populate the forming BA1 (dotted line). They encompass the optic vesicles and are in the process of migrating rostrally to colonize the nasofrontal bud (arrows). After FSNC excision, only rare HNK1-labeled cells are present in BA1. Note the labeling of the trigeminal ganglion (arrow) that attests to the contribution of midrhombencephalic NCC to the cranial peripheral nervous system. Treatment of FSNC-excised embryos with FGF8 rescues the colonization of BA1 by r3-derived cells. At E7 and E8, these embryos display a partial restoration of their facial morphology with the development of maxillary and mandibular components of the upper and lower beaks, respectively (L and M). (N) Alcian blue staining of the skeletal pieces reveals a reduced nasal capsule (Nc) in the upper face and the entire set of lower jaw cartilages among which Meckel's (Mc) is recognizable. Inhibition of RNA processing by RNAi using Fgf8-dsRNA (O–R). In normal chick embryos, Fgf8-dsRNA has been unilaterally electroporated in the BA1 presumptive ectoderm (red area; O). At E3 on both control (P) and experimental (Q, untransfected side) embryos, NCC fully populate the maxillo-mandibular processes. In contrast, on the transfected side (R), no (or very little) colonization of NCC occurs, suggesting a role of the FGF8 protein as an attractant for NCC migration. Maxillo-mandibular regeneration originates from r3-derived NCC (S–Z). After the bilateral excision of the FSNC, the r3 NF of the host embryo was bilaterally replaced by its quail counterpart and FGF8 beads were supplied as before (S). Quail cell detection using QCPN mAb combined with the Alcian blue counterstaining to evidence cartilaginous structures shows that r3-derived crest cells are the exclusive source of the chondroblasts that generate the anterior part of basihyal (T and U) and Meckel's cartilage (T and V) in the lower jaw. Note that the posterior part of basihyal is normally of r4 origin (2, 19). In this context, at E2.5, the r3-derived NCC that are in the process of populating the BA1 (W) express Hoxa2 (X). At E3.5, they turn to an Hox-negative status (Y and Z), although those that are in BA2 exhibit accumulation of the Hoxa2 transcript. An, angular; Ar, articular; A Mes, anterior mesencephalon; BA, branchial arch; Bb, basibranchial; Bh, basihyal; C, columella; Cb, ceratobranchial; D, dentary; Di, diencephalon; Eb, epibranchial; En, entoglossum; Ey, eye; F, frontal; Io, interorbital septum; Is, isthmus; J, jugal; Ms, mesencephalon; Mt, metencephalon; Mx, maxillary; N, nasal; Nc, nasal capsule; O, opercular; P, parietal; Pl, palate; Pt, pterygoid; P mes, posterior mesencephalon; Q, quadrate; Qj, quadratojugal; r, rhombomere; Sa, supraangular; So, sclerotic ossicles; Sq, squamosal. [Scale bars: 350 μm(C) and 100 μm(E).]
When the entire Hox-negative domain of the NC is removed, no facial structures develop, meaning that Hox-expressing NCC do not substitute for the Hox-negative ones (6, 7). In contrast, any fragment of the Hox-negative crest, grafted in the anterior cephalic region following the ablation of the FSNC, can regenerate a normal face (6). Thus, the Hox-negative crest behaves as an equivalence group showing that, at the early stages, the crest cells themselves do not possess the information to construct the specific bones and cartilages that constitute the facial skeleton. The ventrolateral endoderm of the foregut is able to provide the NCC with the information necessary for patterning the facial skeleton and also the hyoid cartilage (6, 8). Later in development of the facial structures, the ectoderm of the facial process also participates in the final patterning of the beak (9, 10).
The investigations reported here were prompted by the observation that removal of the FSNC resulted in a dramatic decrease of Fgf8 expression in the forebrain anlage, as well as in the BA ectoderm (Fig. 1 B–E). This was followed by the total (11) or partial (6, 7) failure of forebrain development, depending on the stage at which the excision was performed (Fig. 1 D, E, and G). Moreover, the role of Fgf8 in facial morphogenesis was previously stressed (12–18). This led us to explore whether beads soaked in FGF8 would be able to compensate for the loss of the endogenous factor and to promote the regeneration of NCC after excision of the FSNC. In our previous experiments, we observed that after excision of the FSNC domain, the r3-derived NCC were not able to generate skeletal tissues, although, in normal development, they provide BA1 with a small contingent of Hox-negative NCC (2, 19).
We could see that FGF8 exerts a trophic activity on NCC and that this exogenous source of recombinant protein is able to restore to a certain extent the development of the brain and of the facial skeleton. Another conclusion from these experiments is that in turn, the presence of NCC stimulates Fgf8 gene expression by the forebrain neuroepithelium and overlaying ectoderm, as well as by the superficial ectoderm of the BAs. These data account for an interdependent relationship between NCC and FGF8 production that is critical for head development.
Methods
Microsurgery. Quail and chick embryos were operated on at the 5- to 6-somite stage (5–6ss, corresponding to 24–29 h of incubation at 38°C) according to techniques previously described (20). The fate map of the cephalic neural fold (NF) (2, 3, 21–23) has served as reference for the operations throughout this study (Fig. 1 A). Bilateral excision of the FSNC was carried out from mid-diencephalon down through r2. Heparin acrylic beads about 120 μm in diameter (Sigma) were bilaterally implanted in contact with the presumptive ectoderm of BA1 (24) in embryos that had been subjected to the bilateral excision of the FSNC (Fig. 1J). Before implantation, beads were rinsed in PBS and soaked in a solution of 0.1 μg/μl FGF8 mouse recombinant protein (R&D Systems) overnight at 4°C.
Fgf8-dsRNA (Double-Stranded RNA) Synthesis and Electroporation. dsRNA (25) were synthesized from cDNA encoding Fgf8 (26); single strands of either sense or antisense Fgf8 RNA were used for control series. Delivery of RNA interference (RNAi) molecules was achieved by in ovo electroporation into the 5–6ss chicken embryo. Briefly, exogenous nucleic sequences mixed in a solution of Fast Green 0.01% (Sigma) were unilaterally blown under the ectoderm of the presumptive BA1 (Fig. 1O), leaving the controlateral side uninjected as an internal control. Platinum electrodes (Nepagene, Tokyo, Japan) were placed on the vitelline membrane flanking the head with a gap of 4 mm, the cathod facing the targeted ectoderm, to which dsRNA were headed by a series of 5 × 25 V pulses (T830 BTX, Genetronics, San Diego, CA). Embryos were collected 24 h after transfection and subjected to whole-mount immunocytochemistry with HNK1 mAb to evidence NCC migration (3).
Embryo Processing. Whole-mount and in situ hybridizations on sections with Fgf8 and Hoxa2 probes (26, 27) were performed as described (28, 29). Whole-mount preparations and paraffin sections were treated for immunocytochemistry with either QCPN or HNK1 mAb as described (7). When needed, cartilaginous structures subjected to immunochemistry with QCPN mAb on sections were counterstained with Alcian blue. Whole-mount skeleton was visualized according to standard staining protocol by using Alcian blue for cartilage and Alizarin red for bone.
Results
We have investigated the effect of exogenous FGF8 on the r3-derived NCC in chick embryos in which FSNC had been removed. NCC migration was traced either with the HNK1 mAb, which labels chick and quail NCC, or with QCPN mAb, which is quail-specific and was used in embryos for which quail r3 had been substituted for its chick counterpart (Figs. 1 H–K and P–R, and 2 B, D, I, and J). FGF8-soaked beads were disposed on the presumptive level of BA1 ectoderm on each side of the mesencephalon (24) (Fig. 1 J–N). Migration of NCC observed 24 h after the operation (at 20–25ss) showed that the invading capacities of r3 NCC were dramatically enhanced by FGF8 beads (Fig. 1 I and K). Crest cells invaded the BA1 territory and, when these embryos reached embryonic day 7 (E7) and E8, they exhibited facial structures, including nasal, maxillary, and mandibular processes (Fig. 1 L and M). The nasal as well as Meckel's cartilages were present although slightly reduced in length (Fig. 1N). In the five cases that survived up to E6, in which r3 was from quail, and that were subjected to FGF8 beads (Fig. 1S), articular, quadrate, and Meckel's cartilages were entirely formed by quail cells emanating from the implanted r3 (Fig. 1 T–V). Quail cells also gave rise to all of the other crest derivatives in BA1.
Fig. 2.
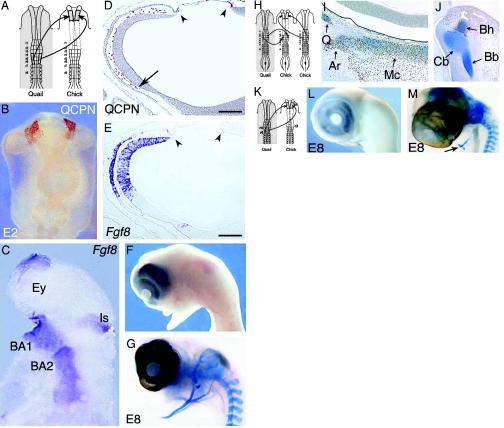
Hox-positive NC implanted at the diencephalic level in FSNC-deprived embryos restores Fgf8 expression in the brain and improves facial skeleto-genesis (A–G). (A) Implantation of r4–r6 NF in FSNC-excised embryos at the diencephalic level. (B) Twenty-four hours after grafting, whole-mount QCPN mAb immunodetection (in brown) reveals the presence of graft-derived cells at the diencephalic level. Note the absence of quail cells colonizing the presumptive BA1 territory. (C) The presence of r4–r6 NCC engrafted at the diencephalic level enables the restoration of Fgf8 expression in the forehead and BA territories. (D) On sagittal sections at E2.5, quail cell detection shows what remains of the transplant (arrowheads) from which the quail NCC have migrated in the nasofrontal bud (arrow) between the prosencephalic neuroepithelium and the overlaying ectoderm. (E) In situ hybridization for Fgf8 on an adjacent section reveals that the presence of NCC restores Fgf8 expression in the forehead superficial ectoderm and neuroepithelium. At E8, these embryos fail to develop an upper beak but show a fully developed lower jaw (F) in which the cartilaginous components of the mandible are evidenced (G). (H–M) Origin of the cells forming the mandibular skeleton. Labeling of the r3 NCC by quail grafting. r4–r6 NF from 5ss chicken embryo was placed at diencephalic level in a FSNC-extirpated stage-matched chick host, and its endogenous r3 NF were replaced by their quail counterparts (H). At E6, the quail r3 NF provide mesenchymal cells to the perioptic region and the BA1 where quail cells yield the quadrate (Q), articular (Ar), and Meckel's cartilages (Mc) (I). In the presumptive tongue (J), r3-derived cells contribute to the anterior part of the basihyal (Bh).(K) Removal of r3. Same experimental design as that in Fig. 2 A, but the excision of the cephalic NF includes r3. At E8, the nasofrontal and the lower beak are absent (L). Alcian blue staining shows the posterior part of hyoid cartilage (arrow) is the only crest-derived skeleton that develops in these embryos (M). Bb, basibranchial; Cb, ceratobranchial; Ey, eye; Is, isthmus. (Scale bars: 120 μmin D and E.)
FGF8 rescued to a large extent the development of not only facial structures but also the brain. In all of the embryos subjected to the ablation of the FSNC, the forebrain was abnormal and exhibited exencephaly (n = 18). In contrast, the encephalic vesicles were properly developed in FGF8-treated samples (n = 23; compare Fig. 1 G to L and M). We can conclude that the failure of facial development, resulting from the excision of the NC domain that normally forms the facial skeleton, can be overcome by administration of FGF8. In this situation, cells able to regenerate the jaws are provided by r3.
To see whether the strong proliferative effect of FGF8 on r3-derived NCC could also operate on r4-derived NCC, we performed an experiment in which the excision of the FSNC included r3. FGF8-soaked beads were similarly placed at the presumptive level of BA1 ectoderm. In this case, some r4-derived cells migrated to BA1, but no regeneration of the face occurred, showing that the NCC exiting from r4 do not have the ability, even in presence of FGF8, to respond to the developmental cues provided by the BA1 environment and cannot regenerate the face (data not shown).
We know that Hox-expressing NCC are unable to respond to the signals generating the facial skeleton (6, 7). It was thus relevant to explore the Hoxa2 expression pattern of r3-derived NCC in various situations: first, r3-derived NCC express Hoxa2 in NF before starting to migrate. However, in unoperated embryos, r3-derived NCC that colonized BA1 were Hoxa2-negative at E3, whereas those migrating to BA2 remained Hoxa2-positive (data not shown). In chick embryos subjected to FSNC excision followed by the in situ grafting of quail r3, Hoxa2 expression remained the same in r3-derived cells as that in the normal situation: they were Hoxa2-positive before and at the onset of their migration to BA1 and became rapidly Hoxa2-negative in the BA1 environment (Fig. 1 W–Z). NCC from r4 do not have such a plasticity, which may account for their inability to participate in the differentiation of the lower jaw skeleton.
We have next used a gene silencing strategy based on RNAi (25) directed to Fgf8 expression in the BA1 presumptive ectoderm (Fig. 1O). Migration of NCC toward the BA1 territory was clearly hampered in embryos in which the production of the endogenous FGF8 protein was reduced (Fig. 1 P–R).
Another striking result of these experiments was that removal of FSNC reduced dramatically the expression of Fgf8 by adjacent epithelial cells both in the prosencephalon (by the superficial ectoderm and the neural ectoderm; Fig. 1 B–E) and in the BAs (see BA1–BA2 in Fig. 1D). Therefore, it seems that the NCC exert a positive effect on the activity of Fgf8 gene in the adjacent ectodermal layer. To determine whether this action was restricted to the Hox-negative FSNC, quail Hox-positive NF from r4 through r6 were ectopically grafted at the level of the posterior diencephalon in embryos previously subjected to FSNC ablation (Fig. 2 A and H). Migration of quail NCC from the grafted r4–r6 NF was followed by means of their labeling with the QCPN mAb (Fig. 2 B and D), whereas NCC emigrating from the chick r3 were detected by means of the HNK1 mAb. The grafted r4–r6 NCC migrated around the anterior aspects of the eyes and into the nasofrontal bud, thus surrounding the prosencephalic vesicle. The crest cells from the remaining rhombencephalon covered the posterior aspect of the eyes and reached BA1, which they colonized abundantly. In situ grafting of quail r3 showed that this postero-anterior NCC migration stream is issued from r3 (Fig. 2 H–J).
Thus, the graft of the Hox-positive NC in the anterior domain of the FSNC mimicked to a certain extent the administration of FGF8 beads. In these embryos, Fgf8 gene expression was maintained at a normal level in the forebrain (Fig. 2 C and E). We thus consider that FGF8 produced in the nasofrontal bud is responsible for the stimulation of rhombencephalic NCC proliferation and migration to BA1. Notable is the fact that neither the explanted r4–r6 NF nor the NCC they gave rise to expressed Fgf8 themselves. Their presence, however, exerted a positive effect on the expression of this gene by both the neuroepithelium and the superficial ectoderm. Therefore, we show the inductive effect of NCC on expression of Fgf8 by ectodermal cells (both superficial ectoderm and neural ectoderm of the forebrain).
At E6 (n = 3), the embryos lacked a nasal bud but exhibited a rudimentary BA1 and a normally developed BA2. At E8 (n = 5), no nasal septum was visible, but the mandibular cartilages were present (Fig. 2 F and G). In one sample that reached E11, unpatterned cartilaginous nodules were present and covered the brain. In contrast, the lower jaw was normally developed and included, besides the quadrate, articular, and Meckel's cartilages, the entoglossum and the rest of the hyoid cartilage. In addition, the membrane bones of the mandibular and maxillary processes were present.
The proof that regeneration of the mandible arose from the NCC of r3 origin was further provided by an experiment in which excision of the FSNC included r3 (Fig. 2K). In this case, no facial skeleton developed (Fig. 2 L and M). The hyoid cartilage was truncated at the midbasihyal level as expected (see Fig. 1 A).
Discussion
A number of previous investigations have pointed out the role of fibroblast growth factors and particularly of FGF8 in the development of the facial structures of vertebrates (12–18). The results presented here bring about new facts on the expression of the Fgf8 gene in ectodermal structures and its effect on NC-derived cells. The first important notion revealed by these experiments is that Fgf8 expression in the prosencephalon and in the BA ectoderm depends on an interaction with NCC. The latter exert an induction on the expression of Fgf8 by the superficial ectoderm and the neuroepithelium of the forebrain and in the BA ectoderm. Although the construction of the facial skeleton can be achieved exclusively by the Hox-negative rostral cephalic NCC, Hox-positive NCC can fulfill this inductive function as well as Hox-negative ones. The signal involved in this induction is not known at the moment. Our experiments involving excision of the FSNC and addition of exogenous FGF8 show that there is a strong reciprocal effect between the FGF8 produced by epithelial structures and the NCC. FGF8 strongly promotes NCC proliferation. FGF8-soaked beads placed at the presumptive level of BA1 ectoderm are able to stimulate r3-derived NCC to a point that they construct a fully developed lower jaw, whereas in normal development r3-derived NCC contribute only little to BA1. It has been shown that odd-numbered rhombomeres (r3 and r5) yield only a small amount of NCC because of an inhibition exerted by their neighboring even-numbered counterpart (4, 5, 30). In the experiment performed in this study, the mesencephalic and r1–r2 NF were removed, thus alienating r3 NCC from this inhibition. Moreover, we have seen that, depending on their position in the FSNC-deprived embryos (data not shown), FGF8-soaked beads influence the directionality of the NCC migration stream from r3. This points to an attractive effect of FGF8 on NCC migration that further substantiated by RNAi experiments. By electroporating dsRNA of Fgf8 within the presumptive BA1 ectoderm, the NCC fail to colonize the mandibular and maxillary processes on the transfected side without disturbing the migration of crest cells on the opposite intact side.
In conclusion, these experiments show the considerable plasticity of the Hox-negative anterior cephalic NCC (to which the r3-derived NCC belong) and their strong dependency on FGF8 for survival, proliferation, and likely also the directionality of their migration to the facial processes. FGF8 is produced in multiple epithelial sites in the developing head. Activation of Fgf8 gene in the ectodermal epithelial sites located in the prosencephalon and BAs requires, at least for its maintenance, a signal arising from the NCC themselves.
Acknowledgments
We thank Sophie Gournet for illustrations, and Francis Beaujean and Michel Fromaget for photographs. S.C. received fellowships from the Fondation pour la Recherche Médicale (FRM) and the Fondation Lefoulon-Delalande. This work was supported by the Centre National de la Recherche Scientifique (CNRS) and the Association pour la Recherche contre le Cancer (ARC).
Abbreviations: BA, branchial arch; En, embryonic day n; FGF, fibroblast growth factor; FSNC, facial skeletogenic neural crest; NC, neural crest; NCC, NC cells; NF, neural fold; r, rhombomere; RNAi, RNA interference; nss, n-somite stage.
References
- 1.Couly, G. F., Coltey, P. & Le Douarin, N. M. (1993) Development (Cambridge, U.K.) 117, 409–429. [DOI] [PubMed] [Google Scholar]
- 2.Couly, G., Grapin-Botton, A., Coltey, P. & Le Douarin, N. M. (1996) Development (Cambridge, U.K.) 122, 3393–3407. [DOI] [PubMed] [Google Scholar]
- 3.Le Douarin, N. M. & Kalcheim, C. (1999) The Neural Crest (Cambridge Univ. Press, New York).
- 4.Graham, A., Heyman, I. & Lumsden, A. (1993) Development (Cambridge, U.K.) 119, 233–245. [DOI] [PubMed] [Google Scholar]
- 5.Graham, A., Francis-West, P., Brickell, P. & Lumsden, A. (1994) Nature 372, 684–686. [DOI] [PubMed] [Google Scholar]
- 6.Couly, G., Creuzet, S., Bennaceur, S., Vincent, C. & Le Douarin, N. M. (2002) Development (Cambridge, U.K.) 129, 1061–1073. [DOI] [PubMed] [Google Scholar]
- 7.Creuzet, S., Couly, G., Vincent, C. & Le Douarin, N. M. (2002) Development (Cambridge, U.K.) 129, 4301–4313. [DOI] [PubMed] [Google Scholar]
- 8.Ruhin, B., Creuzet, S., Vincent, C., Benouaïche, L., Le Douarin, N. M. & Couly, G. (2003) Dev. Dyn. 228, 239–246. [DOI] [PubMed] [Google Scholar]
- 9.Schneider, R. A. & Helms, J. A. (2003) Science 299, 565–568. [DOI] [PubMed] [Google Scholar]
- 10.Hu, D., Marcucio, R. S. & Helms, J. A. (2003) Development (Cambridge, U.K.) 130, 1749–1758. [DOI] [PubMed] [Google Scholar]
- 11.Etchevers, H. C., Couly, G., Vincent, C. & Le Douarin, N. M. (1999) Development (Cambridge, U.K.) 126, 3533–3543. [DOI] [PubMed] [Google Scholar]
- 12.Trumpp, A., Depew, M. J., Rubenstein, J. L., Bishop, J. M. & Martin, G. R. (1999) Genes Dev. 13, 3136–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trainor, P. A., Ariza-McNaughton, L. & Krumlauf, R. (2002) Science 295, 1288–1291. [DOI] [PubMed] [Google Scholar]
- 14.Shigetani, Y., Nobusada, Y. & Kuratani, S. (2000) Dev. Biol. 228, 73–85. [DOI] [PubMed] [Google Scholar]
- 15.Roehl, H. & Nusslein-Volhard, C. (2001) Curr. Biol. 11, 503–507. [DOI] [PubMed] [Google Scholar]
- 16.Schneider, R. A., Hu, D., Rubenstein, J. L., Maden, M. & Helms, J. A. (2001) Development (Cambridge, U.K.) 128, 2755–2767. [DOI] [PubMed] [Google Scholar]
- 17.Abu-Issa, R., Smyth, G., Smoak, I., Yamamura, K.-I. & Meyers, E. N. (2002) Development (Cambridge, U.K.) 129, 4613–4625. [DOI] [PubMed] [Google Scholar]
- 18.Firnberg, N. & Neubüser, A. (2002) Dev. Biol. 247, 237–250. [DOI] [PubMed] [Google Scholar]
- 19.Köntges, G. & Lumsden, A. (1996) Development (Cambridge, U.K.) 122, 3229–3242. [DOI] [PubMed] [Google Scholar]
- 20.Teillet, M.-A., Ziller, C. & Le Douarin, N. M. (1999) Molecular Embryology: Methods and Protocols (Humana Press, Totowa, NJ), pp. 305–318.
- 21.Couly, G. F. & Le Douarin, N. M. (1985) Dev. Biol. 110, 423–439. [DOI] [PubMed] [Google Scholar]
- 22.Couly, G. F. & Le Douarin, N. M. (1987) Dev. Biol. 120, 198–214. [DOI] [PubMed] [Google Scholar]
- 23.Grapin-Botton, A., Bonnin, M.-A., McNaughton, L. A., Krumlauf, R. & Le Douarin, N. M. (1995) Development (Cambridge, U.K.) 121, 2707–2721. [DOI] [PubMed] [Google Scholar]
- 24.Couly, G. F. & Le Douarin, N. M. (1990) Development (Cambridge, U.K.) 103 (Suppl.), 101–113. [DOI] [PubMed] [Google Scholar]
- 25.Pekarik, V., Bourikas, D., Miglino, N., Joset, P. & Stoekli, E. (2003) Nature Biotech. 21, 93–96. [DOI] [PubMed] [Google Scholar]
- 26.Crossley, P. H., Minowada, G., McArthur, C. A. & Martin, G. R. (1996) Cell 84, 127–136. [DOI] [PubMed] [Google Scholar]
- 27.Prince, V. & Lumsden, A. (1994) Development (Cambridge, U.K.) 120, 911–923. [DOI] [PubMed] [Google Scholar]
- 28.Henrique, D., Adam, J., Myat, A., Chitnis, A., Lewis, J. & Ish-Horowicz, D. (1995) Nature 375, 787–790. [DOI] [PubMed] [Google Scholar]
- 29.Etchevers, H. C., Vincent, C., Le Douarin, N. M. & Couly, G. F. (2001) Development (Cambridge, U.K.) 128, 1059–1168. [DOI] [PubMed] [Google Scholar]
- 30.Ellies, D. L., Church, V., Francis-West, P. & Lumsden, A. (2000) Development (Cambridge, U.K.) 127, 5285–5295. [DOI] [PubMed] [Google Scholar]