Abstract
The ability of the mammalian brain to modify existing memories through reconsolidation may be crucial for skill acquisition. The neural mechanisms of memory modification have been commonly studied at the cellular level. Yet surprisingly, the human brain systems-level mechanisms involved in day-to-day modification of existing procedural memories remain largely unknown. Here, we studied differences in fMRI regional signal activity and inter-regional functional connectivity in subjects in whom motor memory modification was interfered with by repetitive transcranial magnetic stimulation (rTMS), relative to subjects with intact memory modification. As a consequence, subjects with impaired memory modification had lower activity in the supplementary motor area (SMA) and weaker functional connectivity between M1, SMA, anterior cerebellum consistently engaged in early learning, and sensorimotor striatum active in later learning stages. These findings, identifying a link between engagement of this network and successful memory modification, suggest that memory reconsolidation may represent a transitional bridge between early and late procedural learning, underlying efficient skill acquisition.
Introduction
Following initial stabilization through consolidation (Glickman, 1961; Kandel, 2001) memories can be substantially modified (Lee 2008, 2009), possibly through reconsolidation mechanisms (Alberini, 2011; Nader and Hardt, 2009). Thus, memory modification enables additional learning with repetitive training sessions leading to strengthening or updating of memories, crucial for skill acquisition that impacts daily life activities (Walker et al., 2003; Censor et al., 2010, 2012). The neural mechanisms of memory modification have been studied at the cellular level (Lee, 2008; Dudai, 2012) and behavioral evidence has been accumulating for reconsolidation mechanisms in humans (Walker et al., 2003; Censor et al., 2010, Forcato et al., 2007; Hupbach et al., 2007; Schiller et al., 2010). Yet surprisingly, and in contrast to studies of initial consolidation (Karni et al., 1995; Doyon et al., 2002; Ungerleider et al., 2002; Lehéricy et al., 2005; Albouy et al., 2008; Debas et al., 2010), the human regional and network level neural mechanisms associated with modification of memories are poorly understood. Here, using a novel combination of noninvasive techniques we identify systems-level mechanisms associated with modification of procedural memories.
Modification of consolidated memories has been demonstrated across different animal models and memory types and predominantly studied by invasive administration of protein synthesis blockers during memory reactivation (Nader et al., 2000; Monfils et al., 2009). Since the use of such invasive approaches in humans is not feasible, we used a combination of noninvasive techniques: we studied changes in functional magnetic resonance imaging (fMRI) BOLD signal regional activity and inter-regional functional connectivity in subjects in whom a virtual lesion of the primary motor cortex (M1, a key region in acquisition of motor memories, Karni et al., 1995; Censor et al., 2010; Muellbacher et al., 2002) with transcranial magnetic stimulation (rTMS; Censor and Cohen, 2011) interfered with memory modification relative to subjects in whom memory modification was intact.
The behavioral paradigm that we used characterizes modification of previously consolidated motor memories in humans following their reactivation (Walker et al., 2003; Censor et al., 2010), a phenomenon that may reflect reconsolidation (Walker et al., 2003; Dudai and Eisenberg, 2004; Forcato et al., 2007; Hupbach et al., 2007; Lee, 2008; Monfils et al., 2009; Schiller et al., 2010). We reasoned that intact memory modification would involve large-scale neuronal networks and relate to differences in fMRI signal activity and functional connectivity relative to impaired memory modification. Thus, we expected that by impairing memory modification, alterations in fMRI signal activity and functional connectivity compared to when memory modification is not impaired, would enable us to identify the neuronal circuitry involved. Alternatively, it would have been possible that the impairment of memory modification expressed behaviorally would not result in alterations in the related circuitry.
Materials and methods
Subjects
26 naïve right-handed healthy subjects (11 men, 15 women; mean age 26.8±5.0 standard deviation) participated in the study. All subjects gave their written informed consent to participate in the project approved by the National Institute of Neurological Disorders and Stroke (NINDS) Institutional Review Board. Participation required a normal neurological examination, reporting at least 6 hours of sleep the night before each experimental session, not being an active musician, and ability to perform and learn the motor task (Karni et al., 1995; Korman et al., 2007; Censor et al., 2010).
Task and experimental design
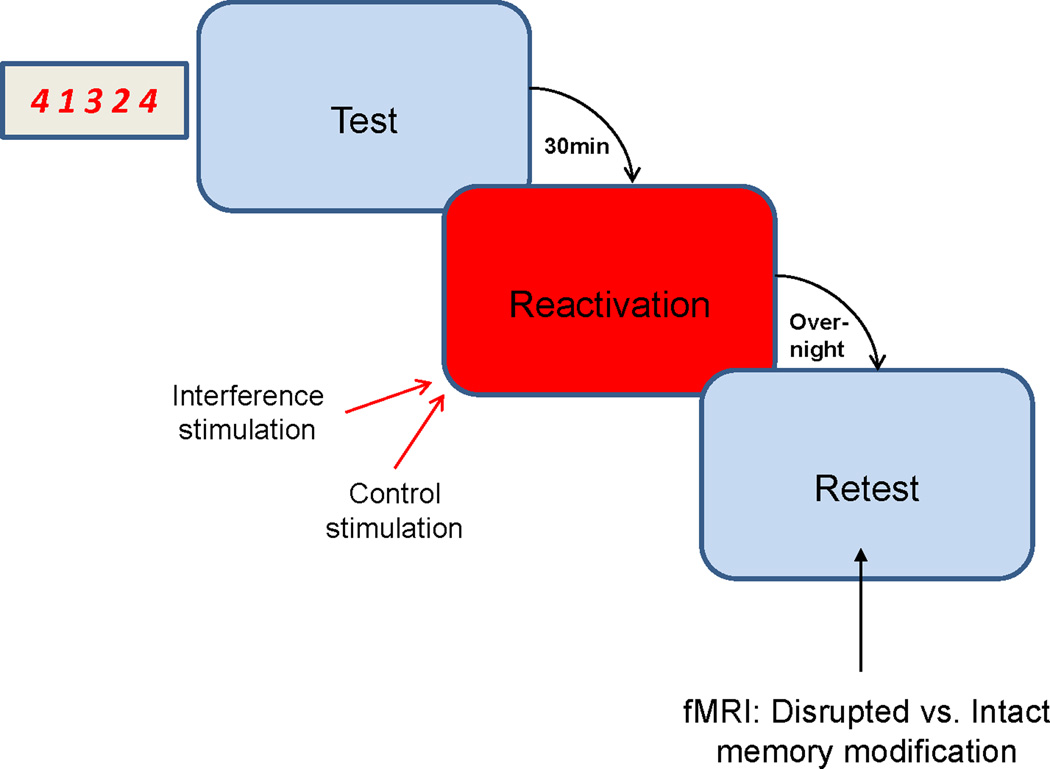
To measure memory modification, subjects performed a sequential finger-tapping task (Karni et al., 1995; Korman et al., 2007, Walker et al., 2003; Censor et al., 2010). All sessions were performed before 3 p.m., with subjects instructed to continue their usual daily routine after participation in the experiment. Each trial lasted for 10 seconds, in which participants had to repeatedly tap with their left hand as quickly and accurately as possible a sequence of 5 finger movements (4-1-3-2-4) using a 4-key response pad, with performance measured as the number of correct sequences during each trial (Karni et al., 1995; Korman et al., 2007; Censor et al., 2010) (Fig. 1). During the trial, the required sequence of finger taps was displayed on a monitor in front of the subject. Feedback was provided, with each key press producing a dot on the screen and with dots forming a row from left to right as the trial progressed. Intertrial interval was 10 seconds.
Figure 1.
Experimental design. All participants performed a sequential finger-tapping task, in which they were trained to repeatedly perform with their nondominant left hand a constant sequence of five finger movements, with performance measured as the number of correct sequences during each fixed 10 s trial (Karni et al., 1995; Korman et al., 2007; Censor et al., 2010). Following initial memory formation and test subjects were divided into 2 groups. To interfere with memory modification, one group was stimulated with 1-Hz rTMS applied to the primary motor cortex (M1) during memory reactivation trials. The second group received control rTMS to a vertex position applied simultaneously with peripheral nerve stimulation (ulnar nerve at the wrist) to mimic disruption of manual performance present when stimulating M1 without reducing memory modification (see Fig. 2). Memory modification and fMRI measurements were assessed on a retest the following day.
Subjects performed nine test trials of the consolidated motor skill (prior to the experiment, subjects consolidated the memory after training with 36 trials). After 30 minutes, participants were divided into two different groups which performed nine memory reactivation trials identical to the test trials, only now with rTMS applied over (a) M1 to interfere with memory modification (n=10) or (b) the vertex along with peripheral nerve stimulation (PNS) to the ulnar nerve at the wrist to mimic disruption of manual performance present when stimulating M1 (n=10). On the following day, all participants performed nine retest trials to measure memory modification (offline gains in the median number of correct sequences per trial between test and retest), and a repeated measures analysis of variance (ANOVA) was used with group (Interference, Control) as the between-subject variable and time (Test, Retest) as the within subject variable (Walker et al., 2003). Analysis was similar to our previous study (Censor et al., 2010). Bonferroni corrected post-hoc t-tests were applied for further analysis.
The experiment was designed to evaluate the functional consequences of blocking memory modification, relative to a control group in which memory modification was intact. Thus, to address our experimental question, we compared fMRI BOLD signal activity and functional connectivity during retest in subjects in whom memory modification was interfered with relative to subjects in whom memory modification was intact (Fig. 1). To evaluate if rTMS application during reactivation trials was critical to interfere with memory modification, we tested an additional group in whom rTMS over M1 was applied immediately after instead of simultaneously with reactivation trials. Subjects (n=6) performed the same task but received 15 minutes of 1 Hz rTMS after (instead of during) reactivation trials.
Stimulation and Neuronavigation
1 Hz rTMS was applied to M1 or vertex for 15 minutes while subjects performed the nine memory reactivation trials. Stimulus intensity was adjusted for each individual participant in order to elicit 5 out of 10 motor evoked potentials (MEPs) greater than 1 mV in the left first dorsal interosseous (FDI) muscle (Censor et al., 2010). Surface electromyogram (EMG) was recorded from surface electrodes positioned on the skin overlying the FDI muscle (bandpassed 25 Hz to 1 kHZ, sampled at 2 Hz). A Magstim (Magstim Company, Whitland, UK) standard double coil with loop diameter of 70 mm was connected to a rapid rate magnetic Magstim stimulator. The stimulating coil was positioned on the scalp over right M1 or the vertex. The coil was kept in position using a frameless stereotactic brain navigation system (Brainsight, Rogue Research, Montreal, Canada) and each subject’s MRI. The subjects receiving vertex stimulation also received 1 Hz ulnar nerve stimulation at the left wrist during the nine memory reactivation trials at an intensity required to elicit equivalent disruption of manual performance during reactivation trials as that seen in the M1-stimulated group (no significant difference in performance during reactivation trials between groups, unpaired t test, P=0.56; M1 group 4.1±1.1 correct sequences, control group 5.0±0.9).
Imaging data acquisition
fMRI was collected during retest (the day following rTMS stimulation) to assess BOLD signal activity in the impaired and intact memory modification groups. Scanning was performed on a 3T MRI scanner (GE Excite HDxt) with a standard 8-channel head coil. T1-weighted high-resolution (1 × 1 × 1 mm, MPRAGE sequence) anatomical images were acquired for each subject to allow volume-based statistical analysis. BOLD contrast was obtained with a gradient-echo echo-planar imaging sequence (EPI, repetition time = 2,000 ms; echo time = 35 ms; flip angle = 90°; matrix size 64 × 64; field of view 240 × 240; 3 × 3 × 3.75 mm resolution). The scanned volume included 34 axial slices of 4mm thickness.
Data analysis
Imaging data was analyzed using BrainVoyager QX (Brain Innovation). Preprocessing included correction of movement artifacts, high-pass filtering to remove low-frequency drifts over time (up to five cycles per experiment) and spatial smoothing using a Gaussian kernel of 6 mm full-width at half maximum (FWHM). The functional images were then superimposed on 2D anatomical images and incorporated into the 3D data sets through trilinear interpolation. The complete data set was transformed into Talairach space. Statistical analysis was based on the general linear model (GLM), with a regressor for the tapping condition modeled as a box-car function, convolved with a canonical hemodynamic response function. Multi-subject analysis was based on a random-effect GLM. To control for unspecific effects, a baseline measurement of tapping vs. rest BOLD contrast was performed in the same set of subjects. A cluster in the right IPL (X=57, Y=−42, Z=22) showed reduced baseline activity (measured at a different day, before any interference was applied) and was therefore not considered for further analysis. A cluster threshold adjustment method was used to control for family-wise type I error, and was based on Monte Carlo simulations performed with the BrainVoyager QX software. The simulations were based on a whole-brain mask and a connectivity radius of 4.0 mm. Therefore a minimum cluster size of 162 voxels was used to correct the statistical maps for multiple comparisons at P<0.05.
For the functional connectivity analysis, in each of the two groups, reference time courses were extracted from right M1 on the final retest (in ROIs, regions of interest, which were localized using a baseline measurement of tapping vs. rest BOLD contrast before the experiment as mentioned above). Correlations were then calculated between these reference time courses and the whole-brain BOLD activation obtained for each of the groups during retest. In order not to rely on a correlation value of a single voxel, correlations for each region were based on a cluster average of the 500 most correlated voxels.
Results
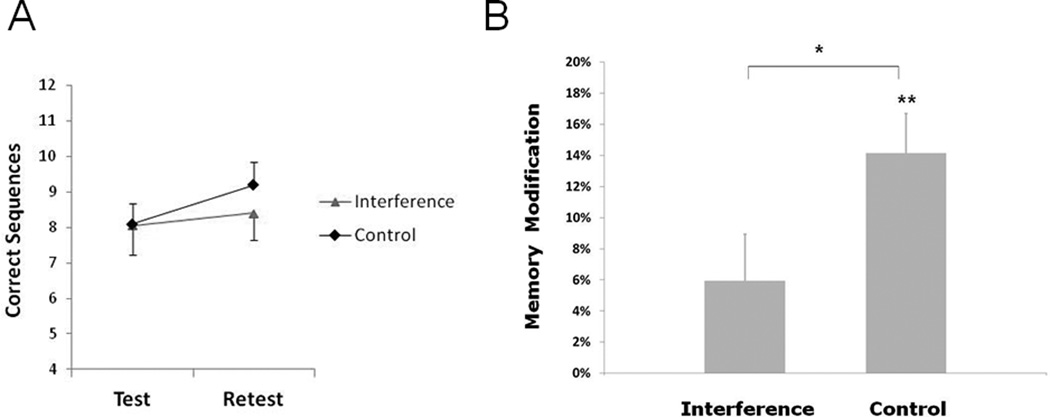
Repeated measures analysis of variance (ANOVA) showed a significant group (Interference, Control) by time (Test, Retest) interaction (F1,18=5.67, P<0.05), indicating that the external intervention applied here differentially affected memory modification. Memory modification in the group that received rTMS interference was disrupted relative to the group that received control stimulation (between group difference P<0.05, Fig. 2), in the absence of test baseline differences between the groups (Control 8.1±0.8 correct sequences, Interference 8.1±0.6, P=0.96, see also Censor et al., 2010). The control stimulation group showed intact memory modification (average performance improvement between test and retest of 14.2%±2.8%, P<0.002; retest 9.2±0.6 correct sequences) consistent with previous findings (Walker et al., 2003; Censor et al., 2010, 14.2%±2.5% with no stimulation), while rTMS interference impaired memory modification (5.9%±3.0%, P=0.20; retest 8.4±0.8 correct sequences). An additional control group that received rTMS interference immediately after (rather than during) the reactivation trials showed intact memory modification (average performance improvement between test and retest of 13.6%±4.1%, P<0.01; comparable to the control stimulation group, P=0.46, unpaired t test), suggesting that interference should be synchronous with reactivation in order to affect memory modification.
Figure 2.
Reducing memory modification. A, Test and Retest scores for the rTMS interference and control stimulation groups. B, Applying rTMS interference during reactivation of the existing memory reduced memory modification relative to the control stimulation group which exhibited intact, standard memory modification (Walker et al., 2003; Censor et al., 2010). Error bars depict standard errors of the mean.
BOLD signal activity following reduced and intact memory modification
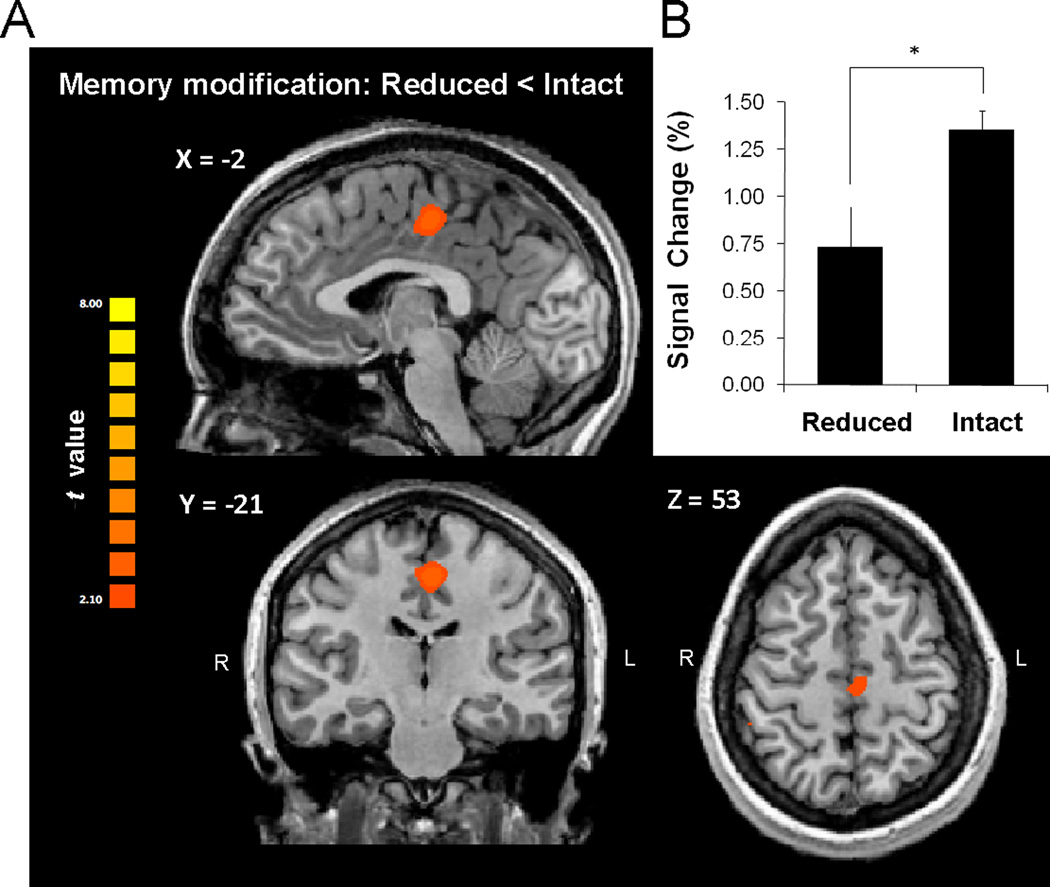
We contrasted the blood oxygenated level dependent (BOLD) signal change (sequence tapping vs. rest) of the group in which memory modification was reduced with the group in which it was intact. Whole brain analysis (corrected for multiple comparisons, see methods) showed that reduced memory modification was associated with reduced activity in the supplementary motor area (SMA proper, extending to the caudal cingulate zone) relative to controls (peak Talairach coordinates X=−2, Y=−21, Z=53, Brodmann area 6, Fig. 3A). BOLD signal change in the SMA cluster which was significantly lower in the group with reduced memory modification compared to the control group (Fig. 3B), did not correlate with performance at retest (r=0.15, P=0.53, across all subjects). Additionally, to control for performance confounds, a median split of all subjects’ retest performance (both groups pooled) showed in the whole brain analysis stronger activation in the higher performance subjects in a single cluster in the transverse temporal gyrus (X=59, Y=−11, Z=8, Brodmann area 42, t=3.36, P<0.004) but not in the SMA or any other region.
Figure 3.
Difference in BOLD signal activity following reduced and intact memory modification. A, Whole brain analysis identified reduced BOLD contrast of tapping vs. rest at retest specifically in the SMA proper extending to the caudal cingulate in the reduced memory modification relative to the intact modification group. Results are corrected for multiple comparisons at the cluster level (P<0.05). B, BOLD contrast activation in the SMA cluster when memory modification was reduced compared to the intact memory modification group. Error bars depict standard errors of the mean.
Functional connectivity following reduced and intact memory modification
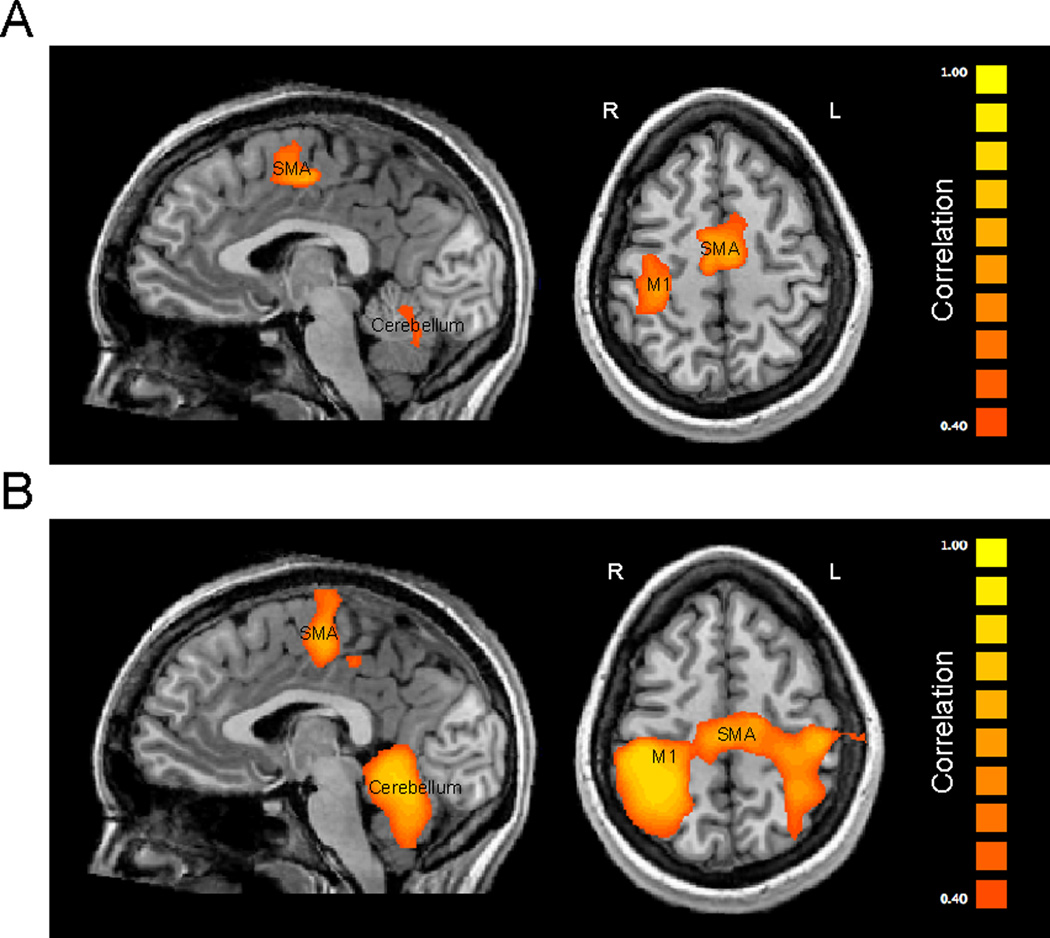
We then evaluated whole brain functional connectivity with M1 in both groups. Disrupted memory modification resulted in weaker correlation of BOLD signal change between the right M1 and the supplementary motor area (SMA proper, correlation of r=0.69 in the reduced memory modification group compared to r=0.77 in the intact memory modification group), the right anterior cerebellum (r=0.64 compared to r=0.79), the left anterior cerebellum (r=0.52 compared to r=0.84), the right dorsal striatum (r=0.44 compared to r=0.52) and the left M1 (r=0.51 compared to r=0.72) relative to the intact memory modification group (all at P<0.01, Fig. 4. and Table 1).
Figure 4.
Functional connectivity with M1 following reduced and intact memory modification. Brain areas showing weaker connectivity with the primary cortical region (M1) at retest after reduced (A) and intact (B) memory modification.
Table 1.
Summary of functional connectivity with right M1 following reduced and intact memory modification. For each cluster, Talairach coordinates at the center of gravity are specified along with the corresponding correlation coefficient for each of the groups. Correlations for each region were based on a cluster of the 500 most correlated voxels. For all regions, correlation coefficients r were different between the groups at a significant level of P<0.01, except for the left dorsal striatum (P=0.48). Results are corrected for multiple comparisons at the cluster level (P<0.05).
| Region | X | Y | Z | r (Reduced) | r (Intact) | P-Value |
|---|---|---|---|---|---|---|
| L M1 | −37 | −20 | 51 | 0.51 | 0.72 | P<0.01 |
| R-L SMA | −2 | −11 | 54 | 0.69 | 0.77 | P<0.01 |
| R dorsal Striatum | 22 | −2 | −1 | 0.44 | 0.52 | P<0.01 |
| L dorsal Striatum | −24 | −2 | 1 | 0.39 | 0.39 | P=0.48 |
| R anterior Cerebellum | 19 | −54 | −29 | 0.64 | 0.79 | P<0.01 |
| L anterior Cerebellum | −16 | −50 | −26 | 0.52 | 0.84 | P<0.01 |
Thus, these results identified a link between successful memory modification and efficient functional connectivity linking the SMA, the dorsal striatum, the anterior cerebellum and M1.
Discussion
Efficient memory modification is necessary to acquire skills that could be retained over time (Walker et al., 2003; Forcato et al., 2007; Hupbach et al., 2007; Lee, 2008; Censor et al., 2010; Karpicke and Roediger, 2008), yet the neural bases of successful memory modification in humans are largely unknown. The purpose of this investigation was to gain insight into the network mechanisms involved in modification of sequential motor memories. We reasoned that interference with memory modification relates to differences in fMRI regional signal activity and functional connectivity measured the following day relative to control stimulation. To that effect, we evaluated the consequences of interfering with modification of a previously consolidated motor memory on functional whole brain, regional BOLD activity and inter-regional functional connectivity. We posed that brain activation differences when memory modification was reduced relative to when it was intact could identify the functional connections involved.
First, we successfully reduced memory modification using a previously demonstrated approach through application of simultaneous 1Hz TMS over the primary motor cortex, a region involved in motor learning (Karni et al., 1995; Muellbacher et al., 2002), during retrieval of the memory (Censor et al., 2010). Our finding that rTMS applied to M1 after reactivation trials (control experiment) did not reduce memory modification is consistent with the view that the interaction between M1 function and reactivation of the memory must be synchronous for successful memory modification to occur. In addition, it has been previously shown that application of rTMS over M1 immediately before but not after adaptation learning alters motor memory consolidation (Richardson et al., 2006; Baraduc et al., 2004).
We then evaluated the consequences of reducing memory modification on whole brain BOLD signal activity. A cluster including the SMA proper that extended to the anterior cingulate showed reduced activity when memory modification was interfered with compared to when it was intact, providing evidence for the involvement of this region in successful memory modification. The SMA proper involvement in memory modification is in line with its role in planning memorized movement sequences in human (Gerloff et al., 1997) and non-human (Tanji and Shima, 1994) primates, in intermanual transfer of procedural motor learning and in successful motor memory recall (Perez et al., 2007). SMA is actively engaged during motor learning by integrating thalamic inputs from the basal ganglia and cerebellum (Doyon et al., 2002; Hikosaka et al., 2002; Lehéricy et al., 2005) and is strongly interconnected with M1 (Hikosaka et al., 2002; Picard and Strick, 2001).
We found a link between reduction in memory modification and weaker functional connectivity between M1 and the SMA, dorsal striatum and anterior cerebellum compared to the intact memory modification group. Thus, successful memory modification was associated with stronger dorsal striatum-M1 and anterior cerebellum-M1 functional connectivity. These results are in line with previous findings that motor learning relies on protein synthesis in the dorsal striatum in rodents (Wächter et al., 2010) and modulates population activity in striatal neurons in non-human primates (Jog et al., 1999). Importantly, reversible blockade of local neural activity in the dorsal striatum leads to disruption in the execution of previously learned sequences (Miyachi et al., 1997). In relation to the cerebellum, Purkinje cells that channel the output signals from the cerebellar cortex, have inhibitory connections with the dentate nucleus (DN), which in turn has a disynaptic excitatory connection through the ventral thalamus to M1 (Middleton and Strick, 2000; Kelly and Strick, 2003). Thus, Purkinje cells’ activity exerts an overall inhibitory tone over M1, also demonstrated in humans (Ugawa et al., 1991; Galea et al., 2009). Importantly, while the cerebellum is engaged in early stages of learning motor sequences as part of a cortico-cerebello-thalamo-cortical loop (Jenkins et al., 1994; Doyon et al., 1996; Shadmehr and Holcomb, 1997; Nezafat et al., 2001; Della-Maggiore and McIntosh, 2005), cortico-striato-thalamo-cortical loops have been reported active in later learning stages (Ungerleider et al., 2002; Costa et al., 2004; Lehéricy et al., 2005; Debas et al., 2010; Dayan and Cohen, 2011). It will be interesting in the future to evaluate memory modification over multiple reactivation sessions and in relation to other memory tasks, as well as its relation to retrieval (Walker et al., 2003; Dudai and Eisenberg, 2004; Lee 2008; Lasry et al., 2008). In addition, future studies based on the findings here may evaluate the use of motor cortex facilitatory non-invasive stimulation for improving motor learning in patients with neurological disorders such as stroke (Hummel et al., 2005; Hummel and Cohen, 2006). Thus, memory reactivation may be used as a valuable time window in order to subsequently reshape motor memory neural representations and consequently improve motor function.
Neural mechanisms underlying memory modification mediated by reconsolidation have been suggested at the cellular level (Lee, 2008; Dudai, 2012). The results here identify human regional and network level neural mechanisms associated with modification of procedural memories, possibly through reconsolidation processes. Our findings, identifying a network that includes regions involved in both early and late learning, suggest memory modification through reconsolidation as a transitional bridge between early and late procedural learning, reflecting the interaction between previously consolidated memories and additional environmental inputs present during memory reactivation in support of efficient skill acquisition.
Acknowledgements
Supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, National Institutes of Health. Nitzan Censor was supported by an NINDS Competitive Fellowship, the Ruth L. Kirschstein National Research Service Award (NRSA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
References
- Alberini CM. The role of reconsolidation and the dynamic process of long-term memory formation and storage. Frontiers in Behavioral Neuroscience. 2011;5:12. doi: 10.3389/fnbeh.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albouy G, Sterpenich V, Balteau E, Vandewalle G, Desseilles M, Dang-Vu T, Darsaud A, Ruby P, Luppi PH, Degueldre C, Peigneux P, Luxen A, Maquet P. Both the hippocampus and striatum are involved in consolidation of motor sequence memory. Neuron. 2008;58(2):261–272. doi: 10.1016/j.neuron.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Baraduc P, Lang N, Rothwell JC, Wolpert DM. Consolidation of dynamic motor learning is not disrupted by rTMS of primary motor cortex. Current Biology. 2004;14(3):252–256. doi: 10.1016/j.cub.2004.01.033. [DOI] [PubMed] [Google Scholar]
- Censor N, Cohen LG. Using repetitive transcranial magnetic stimulation to study the underlying neural mechanisms of human motor learning and memory. Journal of Physiology. 2011;589(Pt 1):21–28. doi: 10.1113/jphysiol.2010.198077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Censor N, Dimyan MA, Cohen LG. Modification of existing human motor memories is enabled by primary cortical processing during memory reactivation. Current Biology. 2010;20(17):1545–1549. doi: 10.1016/j.cub.2010.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Censor N, Sagi D, Cohen LG. Common mechanisms of human perceptual and motor learning. Nature Reviews Neuroscience. 2012;13(9):658–664. doi: 10.1038/nrn3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa RM, Cohen D, Nicolelis MA. Differential corticostriatal plasticity during fast and slow motor skill learning in mice. Current Biology. 2004;14(13):1124–1134. doi: 10.1016/j.cub.2004.06.053. [DOI] [PubMed] [Google Scholar]
- Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron. 2011;72(3):443–454. doi: 10.1016/j.neuron.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debas K, Carrier J, Orban P, Barakat M, Lungu O, Vandewalle G, Hadj Tahar A, Bellec P, Karni A, Ungerleider LG, Benali H, Doyon J. Brain plasticity related to the consolidation of motor sequence learning and motor adaptation. Proceedings of the National Academy of Science USA. 2010;107(41):17839–17844. doi: 10.1073/pnas.1013176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Maggiore V, McIntosh AR. Time course of changes in brain activity and functional connectivity associated with long-term adaptation to a rotational transformation. Journal of Neurophysiology. 2005;93(4):2254–2262. doi: 10.1152/jn.00984.2004. [DOI] [PubMed] [Google Scholar]
- Doyon J, Owen AM, Petrides M, Sziklas V, Evans AC. Functional anatomy of visuomotor skill learning in human subjects examined with positron emission tomography. European Journal of Neuroscience. 1996;8(4):637–648. doi: 10.1111/j.1460-9568.1996.tb01249.x. [DOI] [PubMed] [Google Scholar]
- Doyon J, Song AW, Karni A, Lalonde F, Adams MM, Ungerleider LG. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proceedings of the National Academy of Science USA. 2002;99(2):1017–1022. doi: 10.1073/pnas.022615199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y, Eisenberg M. Rites of passage of the engram: reconsolidation and the lingering consolidation hypothesis. Neuron. 2004;44(1):93–100. doi: 10.1016/j.neuron.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Dudai Y. The restless engram: consolidations never end. Annual Review of Neuroscience. 2012;35:227–247. doi: 10.1146/annurev-neuro-062111-150500. [DOI] [PubMed] [Google Scholar]
- Forcato C, Burgos VL, Argibay PF, Molina VA, Pedreira ME, Maldonado H. Reconsolidation of declarative memory in humans. Learning and Memory. 2007;14(4):295–303. doi: 10.1101/lm.486107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Jayaram G, Ajagbe L, Celnik P. Modulation of cerebellar excitability by polarityspecific noninvasive direct current stimulation. Journal of Neuroscience. 2009;29(28):9115–9122. doi: 10.1523/JNEUROSCI.2184-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff C, Corwell B, Chen R, Hallett M, Cohen LG. Stimulation over the human supplementary motor area interferes with the organization of future elements in complex motor sequences. Brain. 1997;120(Pt 9):1587–1602. doi: 10.1093/brain/120.9.1587. [DOI] [PubMed] [Google Scholar]
- Glickman S. Perseverative neural processes and consolidation of the memory trace. Psychological Bulletin. 1961;58:218–233. doi: 10.1037/h0044212. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Sakai K, Nakahara H. Central mechanisms of motor skill learning. Current Opinion in Neurobiology. 2002;12(2):217–222. doi: 10.1016/s0959-4388(02)00307-0. [DOI] [PubMed] [Google Scholar]
- Hupbach A, Gomez L, Hardt O, Nadel R. Reconsolidation of episodic memories: a subtle reminder triggers integration of new information. Learning and Memory. 2007;14(1-2):47–53. doi: 10.1101/lm.365707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, Cohen LG. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128(Pt 3):490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurology. 2006;5(8):708–712. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Brooks DJ, Nixon PD, Frackowiak RS, Passingham RE. Motor sequence learning: a study with positron emission tomography. Journal of Neuroscience. 1994;14(6):3775–3790. doi: 10.1523/JNEUROSCI.14-06-03775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building neural representations of habits. Science. 1999;286(5445):1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294(5544):1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377(6545):155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- Karpicke JD, Roediger HL., 3rd The critical importance of retrieval for learning. Science. 2008;319(5865):966–968. doi: 10.1126/science.1152408. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. Journal of Neuroscience. 2003;23(23):8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korman M, Doyon J, Doljansky J, Carrier J, Dagan Y, Karni A. Daytime sleep condenses the time course of motor memory consolidation. Nature Neuroscience. 2007;10(9):1206–1213. doi: 10.1038/nn1959. [DOI] [PubMed] [Google Scholar]
- Lasry N, Levy EM, Tremblay J. Making memories, again. Science. 2008;320(5884):1720. doi: 10.1126/science.320.5884.1720a. [DOI] [PubMed] [Google Scholar]
- Lee JL. Memory reconsolidation mediates the strengthening of memories by additional learning. Nature Neuroscience. 2008;11(11):1264–1266. doi: 10.1038/nn.2205. [DOI] [PubMed] [Google Scholar]
- Lee JL. Reconsolidation: maintaining memory relevance. Trends in Neurosciences. 2009;32(8):413–420. doi: 10.1016/j.tins.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehéricy S, Benali H, Van de Moortele PF, Pélégrini-Issac M, Waechter T, Ugurbil K, Doyon J. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proceedings of the National Academy of Science USA. 2005;102(35):12566–12571. doi: 10.1073/pnas.0502762102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Research Reviews. 2000;31(2-3):236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Miyachi S, Hikosaka O, Miyashita K, Kárádi Z, Rand MK. Differential roles of monkey striatum in learning of sequential hand movement. Experimental Brain Research. 1997;115(1):1–5. doi: 10.1007/pl00005669. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324(5929):951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallett M. Early consolidation in human primary motor cortex. Nature. 2002;415(6872):640–644. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406(6797):722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Nader K, Hardt OA. A single standard for memory: the case for reconsolidation. Nature Reviews Neuroscience. 2009;10(3):224–234. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- Nezafat R, Shadmehr R, Holcomb HH. Long-term adaptation to dynamics of reaching movements: a PET study. Experimental Brain Research. 2001;140(1):66–76. doi: 10.1007/s002210100787. [DOI] [PubMed] [Google Scholar]
- Perez MA, Tanaka S, Wise SP, Sadato N, Tanabe HC, Willingham DT, Cohen LG. Neural substrates of intermanual transfer of a newly acquired motor skill. Current Biology. 2007;17(21):1896–1902. doi: 10.1016/j.cub.2007.09.058. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Current Opinion in Neurobiology. 2001;11(6):663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Richardson AG, Overduin SA, Valero-Cabré A, Padoa-Schioppa C, Pascual-Leone A, Bizzi E, Press DZ. Disruption of primary motor cortex before learning impairs memory of movement dynamics. Journal of Neuroscience. 2006;26(48):12466–12470. doi: 10.1523/JNEUROSCI.1139-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463(7277):49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Holcomb HH. Neural correlates of motor memory consolidation. Science. 1997;277(5327):821–825. doi: 10.1126/science.277.5327.821. [DOI] [PubMed] [Google Scholar]
- Tanji J, Shima K. Role for supplementary motor area cells in planning several movements ahead. Nature. 1994;371(6496):413–416. doi: 10.1038/371413a0. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Day BL, Rothwell JC, Thompson PD, Merton PA, Marsden CD. Modulation of motor cortical excitability by electrical stimulation over the cerebellum in man. Journal of Physiology. 1991;441:57–72. doi: 10.1113/jphysiol.1991.sp018738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Doyon J, Karni A. Imaging brain plasticity during motor skill learning. Neurobiology of Learning and Memory. 2002;78(3):553–64. doi: 10.1006/nlme.2002.4091. [DOI] [PubMed] [Google Scholar]
- Wächter T, Röhrich S, Frank A, Molina-Luna K, Pekanovic A, Hertler B, Schubring-Giese M, Luft AR. Motor skill learning depends on protein synthesis in the dorsal striatum after training. Experimental Brain Research. 2010;200(3-4):319–323. doi: 10.1007/s00221-009-2027-7. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425(6958):616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]