Abstract
Objective
The effect of angiotensin converting enzyme (ACE) inhibitors on Alzheimer disease (AD) remains unclear, with conflicting results reported. We studied the interaction of the Apolipoprotein E (ApoE) genotype and ACE inhibitors on AD.
Methods
This was a cross-sectional study of homebound elderly with an AD diagnosis and documentation of medications taken. ApoE genotype was determined.
Results
A total of 355 subjects with status on ApoE alleles and cognitive diagnoses were studied. The average age (mean ± SD) of this population was 73.3 ± 8.3 years old, and 73% were female. Cross-sectionally, there was no difference in the number of AD cases between ApoE4 carriers and ApoE4 non-carriers or between ACE inhibitor users and non-users in the homebound elderly. ApoE4 carriers treated with ACE inhibitors, however, had more diagnoses of AD compared with those who did not have the treatment (28% versus 6%, p = 0.01) or ApoE4 non-carriers treated with an ACE inhibitor (28% versus 10%, p = 0.03). ACE inhibitor use was associated with AD diagnosis only in the presence of an E4 allele. Using multivariate logistic regression analysis, we found that in diagnosed AD cases there was a significant interaction between ApoE4 and ACE inhibitor use (odds ratio: 20.85; 95% confidence interval: 3.08–140.95; p = 0.002) after adjusting for age, sex, ethnicity, and education.
Conclusion
The effects of ACE inhibitors on AD may be different depending on ApoE genotype. A prospective study is needed to determine whether ACE inhibitor use accelerates or poorly delays AD development in ApoE4 carriers compared with ApoE4 non-carriers.
Keywords: Alzheimer disease, Apolipoprotein E4 allele (ApoE4), angiotensin converting enzyme (ACE), ACE inhibitor
The hallmark of Alzheimer disease (AD) is the presence of extracellular amyloid-β peptide (Abeta) in the form of brain amyloid plaques and angiopathy.1 The actual amount of neurotoxic Abeta in the brain is determined by Abeta production through amyloid precursor protein (APP) processing and Abeta degradation and clearance. Basic studies have demonstrated that multiple proteases including angiotensin converting enzyme (ACE) are involved in the catabolism of Abeta.2,3 ACE also converts Abeta42, the major component of amyloid plaques in the AD brain, to Abeta40, the major component of cerebral amyloid angiopathy,4 and reduces Abeta aggregation.2 On the other hand, ACE converts angiotensin I to angiotensin II, and high levels of angiotensin II cause cerebrovascular constriction and damage, which can increase the risk of AD.5,6 Because the ACE polymorphisms are associated with the AD risk,7 how ACE is involved in the AD pathogenesis is unclear. Some studies show that low blood ACE activity is associated with AD8 and the age at AD onset.9
ACE inhibitors are effective hypertension medications commonly used in the elderly.10,11 The relationship between ACE inhibitor use and the risk of AD is unclear, with conflicting results reported in the literature.12,13 One study found a positive association between peripheral ACE inhibitors and the risk of AD,14 whereas others showed that those ACE inhibitors are beneficial in reducing dementia risk.15,16 Using APP transgenic mice in an AD animal model, ACE inhibitors either had no effect or accelerated the development of AD pathology, depending on the duration of the drug use.17–19 It appears that longer-term treatment with ACE inhibitors (6 months versus 1 month) results in increased Abeta deposition in these mice.
Many clinical trial studies, especially in oncology, demonstrate the importance of personalized medicine by showing that different genetic profiles respond to certain chemotherapies differentially.20 We hypothesize that genetic risk factors of AD, such as Apolipoprotein E4 (ApoE4), which affects the Abeta clearance and deposition, may interact with ACE inhibitors to influence AD development. Using a homebound elderly population with ApoE genotyping and cross-sectional data on cognitive diagnoses and medications taken, we aimed to study whether an interaction between the ApoE4 allele and ACE inhibitor use is associated with AD. Using fluorogenic peptides, we also studied the relationship between ACE inhibitor use and ACE activities in these serum samples.
METHODS
Study Sample and Diagnoses
We studied a subgroup of 355 subjects who underwent clinical evaluation by physicians, including brain magnetic resonance imaging, from a population-based study, the Nutrition, Aging and Memory in the Elderly (NAME) study.21 The NAME study was based on the clients of four homecare agencies for the city of Boston. Anyone receiving homecare services is registered with one of these agencies if he/she lives in the city of Boston, has an annual income less than $18,890, and needs homecare service. All homebound elders aged 60 and older receiving services from the four agencies were invited to participate in the study. Of all eligible subjects, 66% enrolled in the study and gave informed consent approved by the institutional review board of Tufts University New England Medical Center.22 Those with Mini-Mental State Examination (MMSE) less than or equal to 10 or verbal IQ less than 75 were not eligible to continue in the study. A total of 1,262 subjects completed the neuropsychological evaluation during the home visits and were asked whether they would be willing to participate in the second phase of the study, which was to come to the hospital and undergo the examination by the physicians to receive a consensus diagnosis. Of this number, 355 agreed and came to the hospital by taxi. The data of subjects who came to the hospital were compatible with the whole study sample.21
Diagnosis of dementia
The diagnosis of dementia was based on the DSM-IV criteria. NINCDS-ADRDA guidelines23 were used to determine if criteria were met for a diagnosis of possible or probable AD as an outcome. Possible AD is marked by certain features related to clinical presentation and course, or magnetic resonance imaging changes, such that a more definitive diagnosis of AD (i.e., probable AD) cannot be made. The major feature leading to a diagnosis of possible AD in our study sample was the presence of cerebrovascular pathology.
Diagnosis of mild cognitive impairment
Diagnoses of mild cognitive impairment (MCI) were based on Petersen et al.24 guidelines, with some modifications to broaden the concept of MCI: 1) no dementia; 2) self-reported forgetfulness in daily activities or for recent events; 3) normal general cognitive functioning as assessed by the MMSE (i.e., a score less than 1 standard deviation [SD] below the mean of an age- and education-matched sample after exclusion of present dementia at entry); 4) objective memory impairment or impairment in other cognitive domains as assessed by performance on neuropsychological tests not more than 1.5 SD from the mean of an age- and education-matched sample; and 5) ability to independently perform basic activities of daily living. The neuropsychological battery included WMS-III Word List Learning, WMS-III Logical Memory, verbal fluency, WAIS-III Block Design, WAIS-III Digit Span, and Trails A and Trails B. Those with memory impairment only or cognitive impairment including memory and other domains were considered to have amnestic MCI; those without forgetfulness and with impairments in other cognitive domains, such as executive and visuospatial dysfunction, were considered to have non-amnestic MCI.
Characterization of normal cognition
Subjects were considered cognitively intact if they were not demented and scored no more than 1 SD below the mean of age-and education-defined strata on MMSE and no more than 1.5 SD below the mean of age-and education-defined strata on the neuropsychological tests.
ApoE Genotyping and the ACE Activity Assays
ApoE genotyping
A 244 bp fragment of the ApoE gene including the two polymorphic sites was amplified by PCR using a robotic Thermal Cycler (ABI 877, Perkin-Elmer/ Applied Biosystems), using oligonucleotide primers F4 (5′-ACAGAATTCGCCCCGGCCTGGTA-CAC-3’) and F6 (5′-TAAGCTTGGCACGGCTGTC-CAAGGA-3’). The PCR products were digested with five units of Hha I and the fragments separated by electrophoresis on 8% polyacrylamide non-denaturing gel. The specific allelic fragments were: E2; E3; and E4. ApoE epsilon 4 (ApoE4) was defined by E4/4, E3/4 or E2/4.25
ACE activity assays
To characterize ACE activity in human serum, we used 10 µM fluorogenic ACE specific substrates, Abz-SDK(Dnp)P-OH specific for ACE N-domain and Abz-LFK-(Dnp)-OH specific for ACE C-domain (Sigma), to incubate with 6 µL serum in the reaction buffer of 0.1 M Tris buffer, pH 7.0, at 37°C for 24 hours. Fluorometry with excitation at 320 nm and emission at 405 nm was used to measure fluorescent intensity. The reaction was triplicate for each sample. We used one sample as a positive control for every batch of measurement. To evaluate the ACE activity, we subtracted the background fluorescence (time 0) from the reaction generated fluorescence (time 24 hours) divided by the positive control fluorescence.
Medical Conditions and Medications
Subjects were classified as having cardiovascular disease (CVD) according to whether they had been previously informed by a doctor that they had congestive heart failure, coronary heart disease, angina pectoris, or a myocardial infarction. Stroke history per subject report was also recorded. Body mass index was measured and calculated as body mass (in kg) over the squared height (in m2). Diabetes was defined as the use of anti-diabetic medication or fasting glucose greater than 126 mg/dL.26 Current hypertension was defined by the average of systolic blood pressure greater than 140 mm Hg or diastolic blood pressure greater than 90 mm Hg at two determinations.
Subjects were asked to show all the medications they had been taking during a home visit, and research assistants documented the names of the medications according to the labels. All medications were coded. ACE inhibitors including captopril, fosinopril, lisinopril, perindopril, trandolapril, benazepril, enalapril, moexipril, quinapril, and ramipril were classified as one category.
Statistical Analysis
Statistical analysis was performed using SAS (version 9.1). The χ2 test was used to compare proportions for binary endpoints. Distributed variables were presented as mean ± SD and compared using t tests. Logistic regression was used to examine associations between AD and ApoE4 or ACE inhibitor use while adjusting for confounders of age, race, sex, school, and vascular diseases including CVD, diabetes, hypertension, and stroke. The interaction between ApoE4 and ACE inhibitor use applied to the logistic models. For all analyses, the two-sided significant level of 0.05 was used.
RESULTS
As previously noted, 355 subjects from the NAME study characterized for ApoE genotype and cognitive diagnoses were used in this analysis. The average age (mean ± SD) of this population was 73.3 ± 8.3 years old, and 73% were female. It was a mix of ethnicities with 62% white, 35% African American, and 3% other ethnicities. Seventy-five percent of subjects had at least high school level or above education. ApoE allele frequencies were ApoE2/2 or ApoE2/3 = 59/ 355 (17%); ApoE2/4 = 8/355 (2%); ApoE3/3 = 209/ 355 (59%); ApoE3/4 or ApoE4/4 = 79/355 (22%). Thus, there were 87 subjects (24%) carrying at least one ApoE4 allele. The majority of homebound elderly had hypertension (83%), and many of them had been using an ACE inhibitor (38%) to treat hypertension.
As shown in Table 1, other than ethnicity there were no differences in demographic variables between those with and without an ApoE4 allele. There was a higher proportion of African American subjects among ApoE4 allele carriers compared with ApoE4 non-carriers (χ2 test: 48% versus 30%, DF = 5, p = 0.03). The rates of CVD, diabetes, stroke and current hypertension were similar in those with and without ApoE4. At the cross-sectional level, the proportions of those with AD, including probable and possible AD cases, were similar in those with and without the ApoE4 allele. Compared with ApoE4 non-carriers, ApoE4 carriers tended to have a higher rate of ACE inhibitor use, but this did not reach statistical significance (χ2 test: 36% versus 44%, DF = 1, p = 0.19).
TABLE 1.
Demographic and Medical Status of non-ApoE4 and ApoE4 Carriers in the Homebound Elderly Population
| Demographic Data | ApoE4 − N = 268 | ApoE4 + N = 87 | DF | χ2 | p value |
|---|---|---|---|---|---|
| Age, years, mean ± SD | 73.6 ± 8.4 | 72.3 ± 8.2 | 1 | - | 0.22 |
| HS graduate and above, N/total (%) | 202/268 (75%) | 65/86 (75%) | 1 | 0.002 | 0.97 |
| BMI, kg/m2, mean ± SD | 30.7 ± 8.2 | 31.4 ± 7.0 | 1 | - | 0.23 |
| African American, N/total (%) | 80/264 (30%) | 42/87 (48%) | 5 | 12.4 | 0.03 |
| Female, N/total (%) | 195/268 (73%) | 61/86 (71%) | 1 | 0.11 | 0.74 |
| CAD, N/total (%) | 101/262 (39%) | 30/83 (36%) | 1 | 0.31 | 0.69 |
| Stroke, N/total (%) | 52/263 (20%) | 14/85 (16%) | 1 | 0.38 | 0.50 |
| Diabetes, N/total (%) | 79/262 (30%) | 31/84 (37%) | 1 | 1.39 | 0.24 |
| Hypertension, N/total (%) | 219/263 (83%) | 73/86 (85%) | 1 | 0.09 | 0.77 |
| Alzheimer disease, N/total (%) | 35/268 (13%) | 14/86 (16%) | 1 | 0.56 | 0.45 |
| MMSE Score, mean ± SD | 25.7 ± 3.2 | 25.1 ± 3.6 | 1 | - | 0.21 |
| ACE inhibitor use, N/total (%) | 97/268 (36%) | 38/87 (44%) | 1 | 1.76 | 0.19 |
Notes: Mean ± SD with t test or N/total (%) with χ2 test are presented. p values for statistical significance are shown. HS: high school; BMI: body mass index; CAD: cardiovascular disease; MMSE: Mini-Mental State Examination.
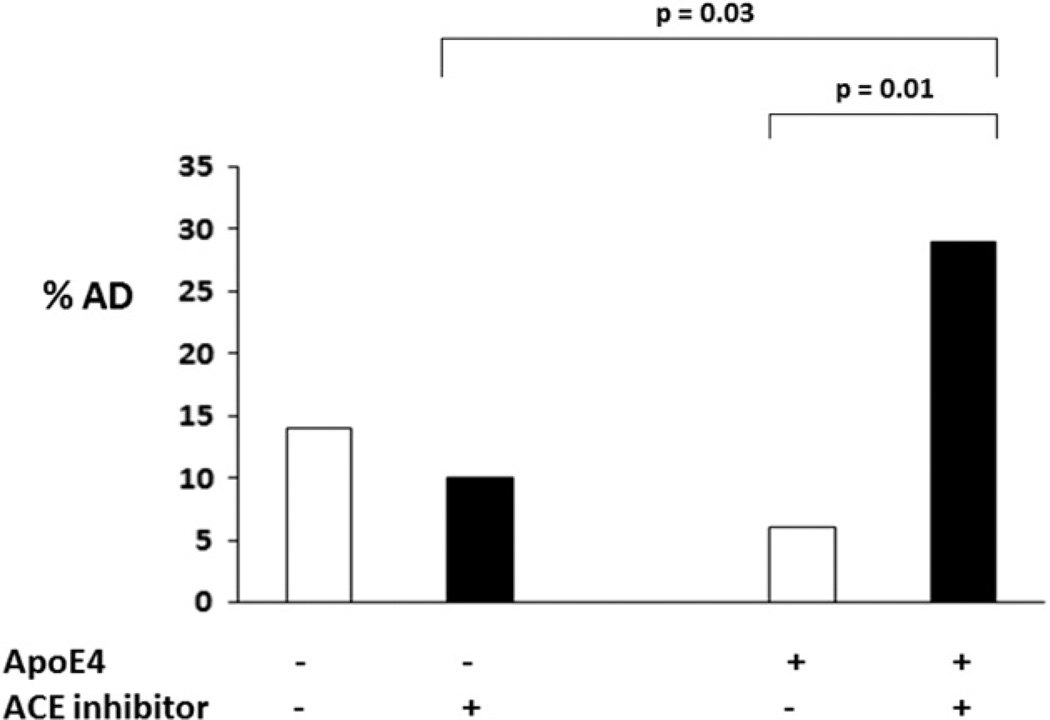
All subjects with ACE inhibitor use (100%) carried a diagnosis of hypertension and only 53% of them also had cardiovascular disease. Subjects were further divided into with (ACE inhibitor +) and without (ACE inhibitor −) ACE inhibitor use subgroups among both ApoE4 carriers and ApoE4 non-carriers. Figure 1 shows that the subgroup of ApoE4 carriers who were on an ACE inhibitor had a significantly higher number of AD cases than the group of those who were not on an ACE inhibitor (χ 2 test: 28% versus 6%, DF = 1, χ2 = 8.02, p = 0.01). In contrast, the ApoE4 non-carriers who were on an ACE inhibitor treatment had a similar or possibly lower rate of AD as those who were not on ACE inhibitor treatment (χ 2 test: 10% versus 14%, DF =1, χ2 = 1.23, p = 0.27). Consistently, among all the elderly who were on ACE inhibitor treatment, ApoE4 carriers had more AD cases than the ApoE4 non-carriers (χ2 test: 28% versus 10%, DF =1, χ2 = 6.21, p = 0.03). Using multivariate logistic analysis, Table 2 again shows that AD was not associated with ApoE4 or ACE independently although AD remained associated with the interaction of ApoE4 and the ACE inhibitor use after adjusting for age, ethnicity, and school (odds ratio: 20.85; 95% confidence interval: 3.08–140.95; DF = 1, Wald χ2 = 9.59, p = 0.002). Using the same model, this relationship was not found between vascular dementia and the interaction between ApoE4 and the ACE inhibitor use (data not shown).
FIGURE 1. Alzheimer disease among those with and without the ACE treatment in the absence and presence of ApoE4 allele.
The percentages of AD were compared between different subgroups: in the absence of ApoE4 (ApoE4 −) or presence of ApoE4 (ApoE4 +) and further divided into no ACE inhibitor use (ACE inhibitor −) and ACE inhibitor use (ACE inhibitor +). Chi square (χ2 test) was used to compare between any two subgroups or among the four subgroups. p values for the statistical significance between the two subgroups are shown. In the presence of ApoE4, those who were on an ACE inhibitor had a significantly higher number of AD cases than those who were not (χ2 test: 28% versus 6%, DF = 1, χ2 = 8.02, p = 0.01). Among all the elderly who were on ACE inhibitors, ApoE4 carriers had more AD cases than the ApoE4 non-carriers (χ2 test: 28% versus 10%, DF = 1, χ2= 6.21, p = 0.03)
TABLE 2.
Effects of ApoE4 Allele, ACE Inhibitor Use, and the Interaction Between ApoE4 Status and ACE Inhibitor Use on Alzheimer Disease
| Model I Alzheimer disease (N = 348) |
Model II Alzheimer disease (N = 308) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | DF | Wald χ2 | p value | Odds Ratio (95% CI) | DF | Wald χ2 | p value | |
| Age, years | 1.13 (1.08, 1.18) | 1 | 27.39 | <0.0001 | 1.14 (1.09,1.19) | 1 | 27.94 | <0.0001 |
| White | 0.81 (0.65, 1.02) | 1 | 2.57 | 0.07 | 0.79 (0.63, 1.00) | 1 | 2.94 | 0.05 |
| School, years | 0.89 (0.80, 1.00) | 1 | 3.99 | 0.05 | 0.88 (0.79, 0.99) | 1 | 4.36 | 0.04 |
| ApoE4 | 1.42 (0.66, 3.05) | 1 | 0.49 | 0.36 | 0.25 (0.05, 1.21) | 1 | 3.26 | 0.09 |
| ACE inhibitor use | 1.27 (0.64, 2.54) | 1 | 0.54 | 0.49 | 0.57 (0.24, 1.38) | 1 | 1.33 | 0.22 |
| ApoE4*ACE inhibitors | - | - | - | - | 20.85 (3.08, 140.95) | 1 | 9.59 | 0.002 |
Notes: Using multivariate logistic analysis, we first examined the associations between AD and ApoE4 + ACE inhibitor independently (model I), then the interaction of ApoE4 and the ACE inhibitor use (model II) after adjusting for age, ethnicity, and school. ApoE4*ACE inhibitor: interaction between ApoE4 and ACE inhibitor use. Odds ratios with 95% confidence interval (95% CI), DF, and Wald χ2 test were shown for each variable in the models. p values for statistical significance are shown.
The patterns of most demographic differences between the ACE inhibitor + and the ACE inhibitor -groups were similar across ApoE4 carriers and non-carriers (see Table 3). In terms of medical diseases, regardless of ApoE4 status, those with ACE inhibitor use had higher prevalence of stroke, hypertension, and diabetes than those without ACE inhibitor use. In the absence of the ApoE4 allele, however, ACE inhibitor + subjects had a significantly higher prevalence of cardiovascular disease than the ACE inhibitor - subjects (χ2 test: 57% versus 28%, DF = 1, p<0.0001). In contrast, in the presence of the ApoE4 allele, there was no statistical difference in the cardiovascular disease prevalence between the ACE inhibitor +/− subgroups (χ2 test: 40% versus 33%, DF = 1, p = 0.53). After adding each vascular diseases including diabetes, cardiovascular disease, hypertension, and stroke to a multivariate logistic regression model, the relationship between AD and the interaction of ApoE4 and the ACE inhibitor use remained (data not shown).
TABLE 3.
Demographic and Medical Status of Those Without and With ACE Inhibitor Use in the Content of ApoE4 Status
| Demographic Data | ApoE4 − |
ApoE4 + |
||||||
|---|---|---|---|---|---|---|---|---|
| ACEI − N = 170 | ACEI + N = 95 | DF | p values | ACEI − N = 49 | ACEI + N = 38 | DF | p values | |
| Age, years, mean ± SD | 73.7 ± 8.5 | 73.4 ± 8.2 | 1 | 0.79 | 72.4 ± 8.4 | 72.3 ± 8.0 | 1 | 0.90 |
| HS and above, N/total (%) | 133/170 (78%) | 64/95 (67%) | 1 | 0.01 | 38/48 (79%) | 26/37 (70%) | 1 | 0.25 |
| African American, N/total (%) | 48/169 (28%) | 32/95 (34%) | 5 | 0.02 | 21/49 (43%) | 21/38 (55%) | 5 | 0.23 |
| Female, N/total (%) | 131/170 (77%) | 63/95 (66%) | 1 | 0.06 | 35/49 (71%) | 26/38 (68%) | 1 | 0.76 |
| MMSE Score, mean ± SD | 25.9 ± 2.9 | 25.6 ± 3.6 | 1 | 0.41 | 25.5 ± 3.8 | 24.7 ± 3.8 | 1 | 0.13 |
| CAD, N/total (%) | 46/166 (28%) | 54/94 (57%) | 1 | <0.0001 | 16/48 (33%) | 14/35 (40%) | 1 | 0.53 |
| Stroke, N/total (%) | 27/166 (16%) | 25/95 (26%) | 1 | 0.05 | 3/48 (6%) | 11/37 (30%) | 1 | 0.003 |
| Hypertension, N/total (%) | 122/166 (73%) | 95/95 (100%) | 1 | <0.0001 | 35/48 (73%) | 38/38 (100%) | 1 | 0.0005 |
| Diabetes, N/total (%) | 33/165 (20%) | 45/95 (47%) | 1 | <0.0001 | 14/49 (29%) | 17/36 (47%) | 1 | 0.08 |
Notes: Mean ± SD with t test or N/total (%) with χ2 test are presented. DF and p values for statistical significance are shown. HS: high school; CAD: cardiovascular disease; MMSE: Mini-Mental State Examination.
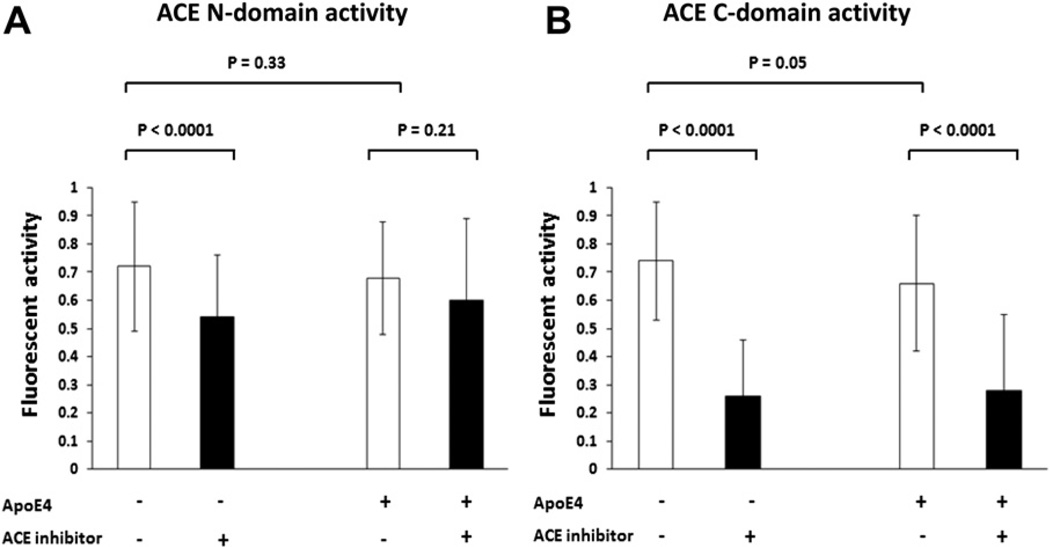
To examine ACE activity in serum, we used fluorogenic peptide substrates specific for ACE (Fig. 2) to examine the presence of ACE activity in human serum. Because ACE N- and C- domains have different functions in A β degradation,4 we thus used fluorogenic substrates specific for both ACE N -and C-domains to incubate with 6 µL of human serum at 37°C for 24 hours. The subjects who had prescribed ACE inhibitors had lower ACE activity than those who did not have this class of medication, suggesting that majority of them had been taking these medications (Fig. 2). Using t test analyses, we found that among the ApoE4 non-carriers, the ACE inhibitor + group had a lower activities of ACE N-domain (mean ± SD: 0.54 ± 0.22 versus 0.72 ± 0.23, DF = 1, p<0.0001) (Fig. 2A) and C-domain (mean ± SD: 0.26 ± 0.20 versus 0.74 ± 0.21, DF = 1, p<0.0001) (Fig. 2B) than ACE inhibitor − subgroup. In contrast, in the presence of ApoE4 allele, the activities of ACE N-domain did not show statistical difference between the ACE inhibitor + and − subgroups (Fig. 2A); but the activities of ACE C-domain showed a difference between the ACE inhibitor + and − subgroups (mean ± SD: 0.28 ± 0.27 versus 0.66 ± 0.24, DF = 1, p<0.0001) (Fig. 2B). In the absence of ACE inhibitor, the ApoE4 carriers had slightly lower average ACE C-domain activity than the ApoE4 non-carriers (mean ± SD: 0.66 ± 0.24 versus 0.74 ± 0.21, DF = 1, p = 0.05), and the difference of average ACE N-domain activities between the ApoE4 carriers and non-carriers did not reach statistical significance. Thus ApoE4 allele in itself had little or no impact on ACE activity when ACE inhibitors were not used. Differences in ACE activities were not found for the other commonly used antihypertensive medications including beta blockers or calcium-channel blockers in this population (data not shown).
FIGURE 2. Characterization of ACE activity in the serum samples.
6-µL human serum was incubated with fluorogenic substrates specific for either ACE N-domain (A) or C-domain (B) at 37°C for 24 hours and the generated fluorescence were measured. The ApoE4 status and ACE inhibitor usage for each subgroup are illustrated. Mean ± SD of fluorescence with t test used to compare the differences between any two subgroups, DF = 1 and p values for the statistical significance are shown.
DISCUSSION
Our study results suggest that the effect of ACE inhibitor use on AD in the elderly is linked with an individual’s ApoE genotype in the homebound elderly sample (Table 2 and Fig. 1). Sporadic late-onset AD may have multiple risk factors. Although the ApoE4 allele is the major genetic risk factor of late-onset AD27 and memory decline,28 not all of the ApoE4 carriers develop AD at very old age.29 Hypertension might be another risk factor for the development of AD, probably through an increase in cerebrovascular pathology,5,6 and ACE inhibitors are effective antihypertensive medications. We hypothesized that if an individual has the ApoE4 allele, ACE inhibitor use may accelerate or poorly delay AD development compared with ApoE2 or ApoE3; if an individual is an ApoE4 non-carrier, an ACE inhibitor may effectively delay AD development.
The ACE polymorphisms are associated with AD risk,7 and modulate renin-angiotensin system gene polymorphisms modify ACE inhibitors’ effect on cognitive function,30 suggesting ACE is probably involved in the AD pathogenesis.11 ACE activity was elevated in AD and correlated with Braak stage.31 The mechanism of possible interaction of ApoE4 and ACE inhibitor on AD is unclear, however. ApoE4 is shown to mediate increased Abeta42 in the AD brain through reduced clearance and accelerated aggregation of the peptide.32,33 Both ACE N- and C-domains are involved in the degradation of Abeta,2,34 and ACE N-domain also converts Abeta42 to Abeta40.4 One possibility is that both ApoE4 and ACE inhibitors are involved in inhibiting the Abeta clearance, 2,33 thus the presence of both factors may have a synergistically harmful effect on the AD risk. On the other hand, because ACE inhibitors reduce cerebrovascular damage caused by elevated angiotensin II, which could accelerate the AD pathogenesis, thus ACE inhibitor use may delay the development of AD, but less effectively when ApoE4 is present. Clinical trials using antihypertensives did not demonstrate their effectiveness in AD.15,16 Thus, hypertension in itself might not be crucial in development of AD. The actual reason that ACE inhibitors can play a role in modifying development or progression of AD may lie in their ability to alter or affect the clearance of Abeta in the brain.2
In this cross-sectional analysis, we did not find an independent relationship between ACE inhibitors and AD (Table 1 and Table 2). Because the effects of ACE inhibitor use had opposite effects between ApoE4 carriers and non-carriers (Fig. 1), however, only the interaction between ApoE4 and ACE inhibitor use was found to be associated with AD (Table 2). Prior reports on the relationship between ACE inhibitors and the risk of developing AD in the literature are not consistent and controversial,35 with reports showing no effect, a beneficial effect, and a harmful effect.14,15,36 ACE inhibitors pass through the blood-brain barrier (BBB) differently. For example, enalapril is a peripheral-acting ACE inhibitor and cannot pass through the BBB37; in contrast, trandolapril belongs to central-acting ACE inhibitors because it can completely pass through the BBB.38 One study showed that peripheral-acting ACE inhibitors increase dementia risk, but that central-acting ACE inhibitors reduce dementia risk.14 Using the APP transgenic mice, one study showed that long-term (6 months) use of the ACE inhibitor captopril increases Abeta deposition in the brain.17 The short-term use (1 month) of ACE inhibitors, however, does not change brain Abeta levels and plaques in these mice.18,19 Because ACE inhibitors are common hypertension medications, the use of ACE inhibitors is almost always long-term in humans.
This study demonstrated that ACE inhibitor users indeed had lower average ACE activities in their serum samples than those who did not use ACE inhibitors (Fig. 2). Our data showed that where ApoE4 had little or no impact on the ACE activity, ACE inhibitors were significantly associated with reduced ACE activities, especially the C-domain activity. Our previous study showed that lisinopril inhibited the degradation of substrate V, a substrate mimicking Abeta,39 by human serum ACE.40 A recent study demonstrated that sporadic AD patients had decreased clearance of Abeta in the central nervous system instead of increased Abeta production.41 Because Abeta can pass through the BBB into the blood,42 and plasma Abeta levels are associated with increased risk of AD,43,44 blood proteases mediating Abeta clearance may also be important for AD pathogenesis.
This study has limitations. Due to the cross-sectional study design, we were unable to determine and differentiate two possibilities in ApoE4 carriers: 1) an ApoE4–ACE inhibitor interaction accelerates the development of AD, or 2) ACE inhibitors delay the progression of AD in ApoE4 carriers, but less effectively than ApoE4 non-carriers, so that the elders could still live at home and were not placed in a nursing home. Likely due to the lack of adequate power and no longitudinal follow-up data, we did not find an independent relationship between ApoE4 and AD in the cross-sectional analysis. We did not have data on specific ACE inhibitors, peripheral- or central-acting ones, used by these elders, nor of the duration of drug use since some studies show that each ACE inhibitor drug has a unique effect on the brain depending on whether it can pass through the BBB.45 Nevertheless, this study showed the interaction between the ApoE genotype and ACE inhibitor use on AD, and suggested the importance of personalized medicine approaches to AD intervention and prevention, especially among hypertensive patients. It will also be interesting to study the influence of ACE inhibitors on the Abeta deposition in the brain by positron emission tomography scan46 based on ApoE4 genotype in the future.
Acknowledgments
We thank the NAME study staff and the Boston homecare agencies for their hard work and acquisition of subjects, and Xian Adiconis from Dr. Jose Ordovas’s laboratory for her characterization of ApoE alleles. This work was supported by grants from the National Institute on Aging (grants K23 AG022476 and RO1 AG031171 [WQQ]) and from the National Institute of Mental Health (grant K24 MH70027 [DCS]). Support was also provided through the General Clinical Research Center funded by the National Center for Research Resources of the National Institutes of Health under grant MO1-RR00054.
References
- 1.Selkoe DJ. Biochemistry and molecular biology of amyloid beta-protein and the mechanism of Alzheimer’s disease. Handb Clin Neurol. 2008;89:245–260. doi: 10.1016/S0072-9752(07)01223-7. [DOI] [PubMed] [Google Scholar]
- 2.Hu J, Igarashi A, Kamata M, et al. Angiotensin-converting enzyme degrades Alzheimer amyloid beta-peptide (A beta); retards A beta aggregation, deposition, fibril formation; and inhibits cytotoxicity. J Biol Chem. 2001;276:47863–47868. doi: 10.1074/jbc.M104068200. [DOI] [PubMed] [Google Scholar]
- 3.Qiu WQ, Folstein MF. Insulin, insulin-degrading enzyme and amyloid-beta peptide in Alzheimer’s disease: review and hypothesis. Neurobiol Aging. 2006;27:190–198. doi: 10.1016/j.neurobiolaging.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Zou K, Maeda T, Watanabe A, et al. Abeta42-to-Abeta40- and angiotensin-converting activities in different domains of angiotensin-converting enzyme. J Biol Chem. 2009;284:31914–31920. doi: 10.1074/jbc.M109.011437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leduc V, Jasmin-Belanger S, Poirier J. APOE and cholesterol homeostasis in Alzheimer’s disease. Trends Mol Med. 16:469–477. doi: 10.1016/j.molmed.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertram L. Alzheimer’s disease genetics current status and future perspectives. Int Rev Neurobiol. 2009;84:167–184. doi: 10.1016/S0074-7742(09)00409-7. [DOI] [PubMed] [Google Scholar]
- 8.Vardy ER, Rice PJ, Bowie PC, et al. Plasma angiotensin-converting enzyme in Alzheimer’s disease. J Alzheimers Dis. 2009;16:609–618. doi: 10.3233/JAD-2009-1002. [DOI] [PubMed] [Google Scholar]
- 9.Akatsu H, Ogawa N, Kanesaka T, et al. Higher activity of peripheral blood angiotensin-converting enzyme is associated with later-onset of Alzheimer’s disease. J Neurol Sci. 2011;300:67–73. doi: 10.1016/j.jns.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 10.Burgess E. Reviewing the benefits of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in diabetic nephropathy–are they drug specific or class specific? Can J Cardiol. 26(Suppl E):15E–19E. doi: 10.1016/S0828-282X(10)71169-7. [DOI] [PubMed] [Google Scholar]
- 11.Norris S, Weinstein J, Peterson K, et al. 2010 [PubMed] [Google Scholar]
- 12.Todd S, McGuinness B, Passmore AP. Designing appropriate clinical trials to assess ACEI use and cognitive decline in older adults with hypertension. Arch Intern Med. 2010;170:107. doi: 10.1001/archinternmed.2009.463. [DOI] [PubMed] [Google Scholar]
- 13.Anderson C, Teo K, Gao P, et al. Renin-angiotensin system blockade and cognitive function in patients at high risk of cardiovascular disease: analysis of data from the ONTARGET and TRANSCEND studies. Lancet Neurol. 2011;10:43–53. doi: 10.1016/S1474-4422(10)70250-7. [DOI] [PubMed] [Google Scholar]
- 14.Sink KM, Leng X, Williamson J, et al. Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the Cardiovascular Health Study. Arch Intern Med. 2009;169:1195–1202. doi: 10.1001/archinternmed.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah K, Qureshi SU, Johnson M, et al. Does use of antihypertensive drugs affect the incidence or progression of dementia? A systematic review. Am J Geriatr Pharmacother. 2009;7:250–261. doi: 10.1016/j.amjopharm.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Davies NM, Kehoe PG, Ben-Shlomo Y, et al. Associations of antihypertensive treatments with Alzheimer’s disease, vascular dementia, and other dementias. J Alzheimers Dis. 2011 doi: 10.3233/JAD-2011-110347. [DOI] [PubMed] [Google Scholar]
- 17.Zou K, Yamaguchi H, Akatsu H, et al. Angiotensin-converting enzyme converts amyloid beta-protein 1–42 (Abeta(1–42)) to Abeta(1–40), and its inhibition enhances brain Abeta deposition. J Neurosci. 2007;27:8628–8635. doi: 10.1523/JNEUROSCI.1549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckman EA, Adams SK, Troendle FJ, et al. Regulation of steady-state beta-amyloid levels in the brain by neprilysin and endothelin-converting enzyme but not angiotensin-converting enzyme. J Biol Chem. 2006;281:30471–30478. doi: 10.1074/jbc.M605827200. [DOI] [PubMed] [Google Scholar]
- 19.Hemming ML, Selkoe DJ, Farris W. Effects of prolonged angiotensin-converting enzyme inhibitor treatment on amyloid beta-protein metabolism in mouse models of Alzheimer disease. Neurobiol Dis. 2007;26:273–281. doi: 10.1016/j.nbd.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baehner FL, Lee M, Demeure MJ, et al. Genomic signatures of cancerbasis for individualized risk assessment, selective staging and therapy. J Surg Oncol. 103:563–573. doi: 10.1002/jso.21838. [DOI] [PubMed] [Google Scholar]
- 21.Scott TM, Peter I, Tucker KL, et al. The Nutrition, Aging, and Memory in Elders (NAME) study design and methods for a study: of micronutrients and cognitive function in a homebound elderly population. Int J Geriatr Psychiatry. 2006;21:519–528. doi: 10.1002/gps.1503. [DOI] [PubMed] [Google Scholar]
- 22.Qiu WQ, Sun X, Selkoe DJ, et al. Depression is associated with low plasma Abeta42 independently of cardiovascular disease in the homebound elderly. Int J Geriatr Psychiatry. 2007;22:536–542. doi: 10.1002/gps.1710. [DOI] [PubMed] [Google Scholar]
- 23.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 24.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 25.Lahoz C, Osgood D, Wilson PW, et al. Frequency of phenotypegenotype discrepancies at the apolipoprotein E locus in a large population study. Clin Chem. 1996;42:1817–1823. [PubMed] [Google Scholar]
- 26.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes APOE gene and the risk for dementia and related pathologiesthe Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 27.Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caselli RJ, Dueck AC, Locke DE, et al. Cerebrovascular risk factors and preclinical memory decline in healthy APOE epsilon4 homozygotes. Neurology. 2011;76:1078–1084. doi: 10.1212/WNL.0b013e318211c3ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanzi RE, Bertram L. New frontiers in Alzheimer’s disease genetics. Neuron. 2001;32:181–184. doi: 10.1016/s0896-6273(01)00476-7. [DOI] [PubMed] [Google Scholar]
- 30.Hajjar I, Kritchevsky S, Newman AB, et al. Renin angiotensin system gene polymorphisms modify angiotensin-converting enzyme inhibitors’ effect on cognitive function: the health, aging and body composition study. J Am Geriatr Soc. 2010;58:1035–1042. doi: 10.1111/j.1532-5415.2010.02860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miners S, Ashby E, Baig S, et al. Angiotensin-converting enzyme levels and activity in Alzheimer’s disease: differences in brain and CSF ACE and association with ACE1 genotypes. Am J Transl Res. 2009;1:163–177. [PMC free article] [PubMed] [Google Scholar]
- 32.Gearing M, Mori H, Mirra SS. Abeta-peptide length and apolipoprotein E genotype in Alzheimer’s disease. Ann Neurol. 1996;39:395–399. doi: 10.1002/ana.410390320. [DOI] [PubMed] [Google Scholar]
- 33.Holtzman DM. In vivo effects of ApoE and clusterin on amyloid-beta metabolism and neuropathology. J Mol Neurosci. 2004;23:247–254. doi: 10.1385/JMN:23:3:247. [DOI] [PubMed] [Google Scholar]
- 34.Hemming ML, Selkoe DJ. Amyloid beta-protein is degraded by cellular angiotensin-converting enzyme (ACE) and elevated by an ACE inhibitor. J Biol Chem. 2005;280:37644–37650. doi: 10.1074/jbc.M508460200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Todd S, McGuinness B, Passmore AP. Prevention of dementia by ACE inhibitors and angiotensin receptor blockers-potential but not proven. Int J Clin Pract. 2010;64:1595–1598. doi: 10.1111/j.1742-1241.2010.02490.x. [DOI] [PubMed] [Google Scholar]
- 36.Killiany RJ, Gomez-Isla T, Moss M, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer’s disease. Ann Neurol. 2000;47:430–439. [PubMed] [Google Scholar]
- 37.Jouquey S, Mathieu MN, Hamon G, et al. Effect of chronic treatment with trandolapril or enalapril on brain ACE activity in spontaneously hypertensive rats. Neuropharmacology. 1995;34:1689–1692. doi: 10.1016/0028-3908(95)00146-8. [DOI] [PubMed] [Google Scholar]
- 38.Tan J, Wang JM, Leenen FH. Inhibition of brain angiotensin-converting enzyme by peripheral administration of trandolapril versus lisinopril in Wistar rats. Am J Hypertens. 2005;18:158–164. doi: 10.1016/j.amjhyper.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Ralat LA, Ren M, Schilling AB, et al. Protective role of Cys-178 against the inactivation and oligomerization of human insulin-degrading enzyme by oxidation and nitrosylation. J Biol Chem. 2009;284:34005–34018. doi: 10.1074/jbc.M109.030627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Z, Zhu H, Fang GG, et al. Characterization of insulin degrading enzyme, and other amyloid-beta degrading proteases in human serum: a role in Alzheimer’s disease? J Alzheimers Dis. 2012;29:329–340. doi: 10.3233/JAD-2011-111472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mawuenyega KG, Sigurdson W, Ovod V, et al. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2011;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deane R, Wu Z, Sagare A, et al. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 43.Mayeux R, Tang MX, Jacobs DM, et al. Plasma amyloid beta-peptide 1–42 and incipient Alzheimer’s disease. Ann Neurol. 1999;46:412–416. doi: 10.1002/1531-8249(199909)46:3<412::aid-ana19>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 44.Graff-Radford NR, Crook JE, Lucas J, et al. Association of low plasma Abeta42/Abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol. 2007;64:354–362. doi: 10.1001/archneur.64.3.354. [DOI] [PubMed] [Google Scholar]
- 45.Yamada K, Uchida S, Takahashi S, et al. Effect of a centrally active angiotensin-converting enzyme inhibitor, perindopril, on cognitive performance in a mouse model of Alzheimer’s disease. Brain Res. 2010;1352:176–186. doi: 10.1016/j.brainres.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 46.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]